Abstract
Hypertrophic scars are fibroproliferative disorders of excessive wound healing after skin injury. Vascular endothelial growth factor (VEGF)-induced angiogenesis plays a major role in fibrogenesis and hypertrophic scar formation. Over recent years, there has been a major interest in homeobox gene regulation of VEGF-VEGFR mediated angiogenesis in dermal tissue. In the current study, we investigated the role of homeobox genes in the epidermis, for their role in angiogenesis, with a focus on epidermal-mesenchymal interactions. As epidermal stem cells (ESCs) have a central role in epidermal homeostasis, we tested the hypothesis that these cells play a key role in the pathogenesis of hypertrophic scars through the HOXA9-VEGF/VEGFR signaling pathways. We found significant differences in the expression of homeobox A9 in hyperplastic scar tissue during different phases of development. These differences coincided with similar regulations in VEGF expression and with the distribution of ESCs. HOXA9 is expressed in cultured human ESCs in vitro. Antisense suppression of HOXA9 expression was found to suppress VEGF levels in ESCs. Together these findings indicate that homeobox A9 regulates the expression of VEGF in ESCs.
Keywords: Epidermal stem cells (ESCs), homeobox genes, vascular endothelial growth factor (VEGF), angiogenesis
Introduction
Hypertrophic scars, also named hyperplastic scars, are the result of pathological wound healing after skin injury. The detrimental effects of a hypertrophic scar are dysfunction and deformation. The exact pathogenesis of hypertrophic scar is still poorly understood and an effective therapy to prevent the formation of scars or treat existing scars is lacking.
Currently, the role of angiogenesis in the development of hyperplastic scar formation is gradually being clarified [1]. It has been shown that the VEGF-VEGFR2 axis plays a major role in scar formation [2,3]. There are many upstream factors that regulate VEGF-VEGFR2 transcription and expression, such as the hypoxia-inducible factor [4-7]. The effects of homeobox genes on angiogenesis by regulation of the VEGF-VEGFR2 pathway are also gradually becoming evident [8-11]. Homeobox genes are involved in physiological and pathological conditions in the skin [12], suggesting that these genes play a specific role in scar formation.
Wound healing and scar formation involve complex cascades of events involving epidermal, dermal and endothelial cells, as well as cells of the immune system. In various stages of tissue repair, these cells are involved in what is called the epidermal-mesenchymal interaction [13]. Although many studies have addressed this interaction, little is known about the epidermal biology of hypertrophic scars. Skin epithelium is comprised of epidermis and structures such as the hair follicles. Epidermal stem cells (ESCs) play a central role in homeostasis and repair of the epidermis [14]. We found that homeobox A9 protein was distributed in the basal layer of the epidermis and coincided with VEGF expression. ESCs are present in the epidermal basal layer and in the hair follicle outer root sheath [15].
We hypothesized that during the different wound healing phases, ESCs, the seed cells of the epidermis, regulate angiogenesis by means of homeobox A9 and VEGF-VEGFR pathways through epidermal-mesenchymal cellular interactions. Therefore, we investigated the mechanism by which homeobox A9 modulates the expression of VEGF in ESCs.
Material and methods
Ethics approval
The collection of tissue samples of foreskin, scar and skin tissues was approved by the local ethical review committee at Sun Yat-Sen University. All donors, or their parents, provided informed consent.
Immunohistochemistry and immunofluorescence
Hypertrophic scar samples and normal skin controls from the same individuals (n = 20, mean age 21.2 ± 6.1 years, female/male ratio 11/9) were collected from post-burn or post-traumatic (1 months to 23 years) patients. The tissue samples from patients undergoing scar revision operations were immediately fixed for 6-12 h with 4% paraformaldehyde, subsequently dehydrated, and embedded into paraffin. Four-μm serial sections were prepared for analysis.
In order to exclude the influence of endogenous pigment, sections were treated with 0.5% potassium permanganate solution and 2% oxalic acid after dewaxing and hydration. Immunostaining was performed according to the instructions of the EnVision™ Detection Kit (DAKO, Denmark). The primary antibodies used were anti–HOXA9 (Abcam, UK), anti–VEGF (Epitomics, USA), anti–integrin β1 (Bioworld, USA), and anti–cytokeratin 19 (Bioworld, USA).
Optical density measurements were performed on triplicate peroxidase-stained sections of hypertrophic scars and matched control skins (n = 20). The densitometry measurements were performed using Image-Pro Plus 6.0 (Media Cybernetics, USA) from images acquired by an Olympus microscope fitted with a CCD video camera module. A total of at least five high power fields from each section were analyzed.
Immunofluorescence analysis was performed according to the instructions of the DyLight488 kit (Jackson, USA). The primary antibodies used were anti–P63 (Abcam, UK), anti–cytokeratin 10 (Zymed, USA), anti–integrin β1 (Bioworld, USA), anti–cytokeratin 19 (Bioworld, USA), anti–HOXA9 (Abcam, UK), and anti–VEGF (Epitomics, USA).
Cell culture and siRNA transfection
The foreskin tissue samples from patients undergoing circumcision operations were used to isolate the ESCs. ESCs were isolated and cultured according to the procedure described by Rong-Hua Yang [16]. Scrambled siRNA (5’-AGCGUGUAGCUAGCAGAGG-3’) and siRNA (5’-UGCUGAGAAUGAGAGCGGC-3’) [17] corresponding to the human HoxA9 sequence were transfected in ESCs using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions.
RNA isolation, real time-PCR and ELISA
Total RNA was prepared using an RNeasy mini kit according to the manufacturer’s protocol (Qiagen Sciences, MD). Total RNA was prepared from cells transfected with HOXA9 or scrambled siRNA, and complementary DNA was obtained with SuperScript III (Invitrogen, CA). Two microliters of each complementary DNA reaction was used for real-time PCR using SYBR Green (Takara Bio, Japan).
The primers specific for HOXA9 (forward primer, 5’-CAGAACTGGTCGGTGATTTAGGTAG-3’ and reverse primer, 5’-CAACTGAAGTAATGAAGGGCAGTG-3’) and VEGF (forward primer, 5’-CGCAGCTACTGCCATCCAAT-3’ and reverse primer, 5’-GTGAGGTTTGATCCGCATAATCT-3’) included intronic sequences to avoid amplifying genomic DNA. PCR was performed using the Light Cycler system (Roche, CH) with a 95°C denaturation step for 10 seconds, followed by 40 cycles of denaturation at 95°C (10 seconds), annealing at 55°C (20 seconds), and extension at 72°C (15 seconds). HOXA9 and VEGF expression was normalized to that of actin. The PCR products were subjected to melting curve analysis, and the data were analyzed and quantified using Light Cycler software. Data are shown means ± standard deviation of two biological replicates (3–5 technical replicates each). The results are relative to the expression levels of intact controls.
Cultured 24 h, 48 h and 72 h, the medium fluid was aspirated, then stored under -20°C, ELISA method determined the concentration of VEGF (OD values were measured at an optimal wavelength of 450 nm).
Western blot
Cell lysates were run by SDS-PAGE (10% gels) and proteins present in the gels were transferred onto PVDF membranes. After blocking non-specific binding using 1% milk in TBS, Western-blots were probed with a 1:300 dilution of a monoclonal antibody against HOXA9 (Abcam, UK) or VEGF (Epitomics, USA), followed by a 1:1000 dilution of HRP conjugated goat anti-mouse secondary antibody (Zymed, USA). Excess antibody was removed by extensive washing, and blots were developed by ECL chemiluminescence (Thermo, USA).
Statistical analysis
The data were expressed as mean ± standard deviation. The student’s t-test was used for analyzing acquired data. Significant statistical difference was defined as P < 0.05.
Results
Modulation of homeobox A9 and VEGF in human scar and normal skin tissues
Histological analysis of hypertrophic scars by H&E confirmed the clinical diagnosis with the characteristic abnormal dermal collagen arrangement being found in all cases. Hypertrophic scar (HS) samples were divided into two groups: the proliferative HS group (N = 4) and the mature HS group (N = 16). The H&E staining showed an increased density of microvessels and compact collagen arrangement in the proliferative HS tissues, as compared to the normal skin tissues. Most of the microvessels were compressed and sometimes even occluded. In the mature HS group, the majority of microvessels were collapsed or occluded.
Homeobox A9 expression was mainly located in the basal layer of the epidermis in both hypertrophic scar tissues and in the normal skin tissues, while rarely expressed in the dermis. In proliferative HS (Figure 1B), the expression of HOXA9 was observed to be clearly stronger as compared with control skin (Figure 1A, P < 0.05, Table 1). In mature HS (Figure 1C), decreased HOXA9 expression was observed when compared with proliferative HS (P < 0.05, Table 1).
Figure 1.
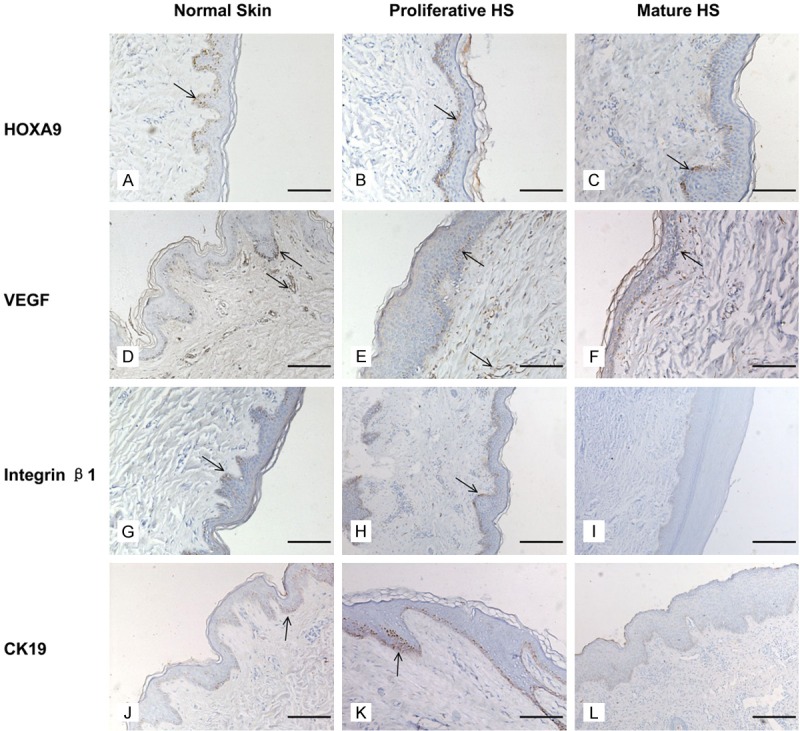
Immunohistochemical localization of HOXA9, VEGF, β1 integrin and CK19 expression in hypertrophic scars and control skin. Arrows indicate the specific staining. HOXA9 expression (A-C) shows a relative increase in the proliferative HS (B) compared with the mature HS (C) and the control skin (A). Counter staining was performed with hematoxylin. VEGF was expressed in both the epidermis and dermis. Differences in VEGF expression (D-F) mirrored the HOXA9 expression. Staining with an antibody against integrin β1 (G-I). CK19 was expressed in the epidermis (J-L). Scale bar = 250 μm.
Table 1.
Comparison of the optical density at different stages of scar formation
| Different optical density in each group | |||
|---|---|---|---|
|
|
|||
| Skin (n = 4) | Proliferative HS (n = 4) | Mature HS (n = 16) | |
| HOXA9 | 0.00179 ± 0.00305 | 0.01100 ± 0.00496* | 0.00433 ± 0.00526# |
| VEGF | 0.00724 ± 0.00904 | 0.02294 ± 0.00715* | 0.01239 ± 0.00857# |
| integrin β1 | 0.00834 ± 0.00947 | 0.02866 ± 0.01235* | 0.01220 ± 0.01403# |
| CK19 | 0.00173 ± 0.00167 | 0.00673 ± 0.00345* | 0.00298 ± 0.00304# |
P < 0.05 when compared with the skin group;
P < 0.05 when compared with the proliferative HS group.
Results are expressed as mean ± standard deviation.
VEGF was expressed in both the epidermis and dermis, mainly located in the basal layer of the epidermis and dermal blood vessels. The staining differences between the experimental groups were comparable to the homeobox A9 expression levels (Figure 1D-F, Table 1).
Distribution of ESCs in hypertrophic scar and normal skin tissue
Because specific markers for identification of ESCs are not available, there is the need to combine multiple markers with the biological characteristics. Several molecules such as β1 integrin, α6 integrin, P63, Cytokeratin-15 (CK15), Cytokeratin-19 (CK19), and β-catenin have been reported as putative ESC markers [18]. We used the markers β1 integrin and CK19 to locate the ESCs in the epidermis.
In normal skin tissue, integrin β1 mainly located in the epidermal ridges, this result is consistent with Michel M et al [19]. In proliferative HS (Figure 1H), there was stronger staining of integrin β1 in the basal layer of the epidermis when compared with control skin (Figure 1G, P < 0.05, Table 1). In contrast, a significant decrease in integrin β1 expression of the mature HS (Figure 1I, P < 0.05, Table 1) was observed when compared with hypertrophic HS.
CK19 was expressed in both epidermis and skin apendages [20]. The staining differences between the experimental groups were comparable to the integrin β1 expression levels (Figure 1J-L, Table 1).
ESC morphology and identification
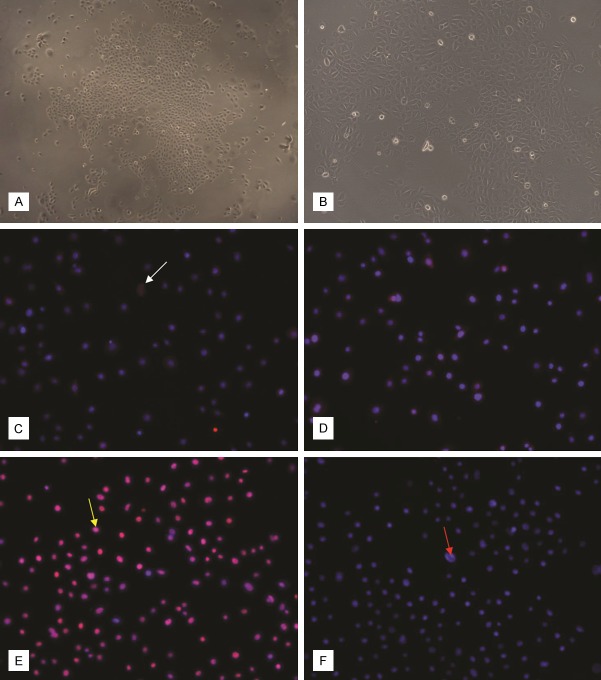
Isolated ESCs in culture show adherent and polygone cells with a pebble-like appearance (Figure 2A). The cells were observed to have large round nuclei and little cytoplasm (Figure 2B), meeting the general characteristics of stem cells. We used the second passage of the cells for our studies. Immunofluorescence of ESCs with antibodies against integrin β1 and CK19 revealed staining for both integrin β1 (Figure 2C) and CK19 (Figure 2D). The nuclei expressed P63 (Figure 2E), while CK10 was not observed in the cells (Figure 2F).
Figure 2.
Morphology of ESCs in culture. (A) ESC colonies in a primary 6-day culture (×40). (B) ESCs from second passage of culture. Small polygone shaped cells are observed to have a pebble-like appearance (×200). Cells positive for integrin β1, CK19 and P63 are considered ESCs. Integrin β1 (C), CK19 (D) were expressed in both the nucleus and cytoplasm. P63 (E) was mainly localized in the nucleus. CK10 (F), a marker for terminal differentiation of epidermal cells, was not observed to be expressed. Original magnification ×100 (C-F). The red arrow indicates nuclear staining, while the white arrow indicates the target protein staining. The yellow arrow indicates fusion staining.
Suppression of HOXA9 results in decreased levels of VEGF expression
We observed that HOXA9 protein was constitutively expressed in cultured human ESCs (Figure 3A). We addressed the role of HOXA9 in the induction of VEGF in ESCs by transfection of HOXA9-specific siRNA to ablate HOXA9 protein levels. Transfection of ESCs with HOXA9 siRNA led to an efficient partial suppression (60%) of HOXA9 mRNA expression compared to ESCs transfected with scrambled siRNA (Figure 4A). Transfection of ESCs with HOXA9 siRNA resulted in a significant reduction of VEGF mRNA (75%, Figure 4A). Western blot analysis showed a similar tendency at the protein level (Figure 4B). Consistent with the above data, we observed a significant downregulation of VEGF protein in supernatant when the ESCs were treated with HOXA9 siRNA compared with scrambled siRNA (Figure 4C).
Figure 3.
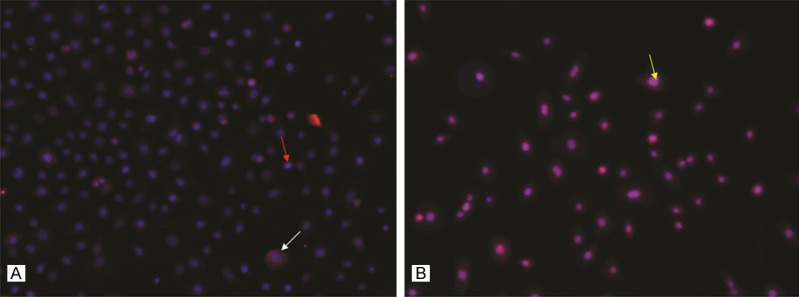
HOXA9 and VEGF protein are expressed in cultured human ESCs. HOXA9 (A) and VEGF (B) expression were observed in both the nucleus and cytoplasm. Original magnification ×100. The red arrow indicates nuclear staining, while the white arrow indicates the target protein staining. The yellow arrow indicates fusion staining.
Figure 4.
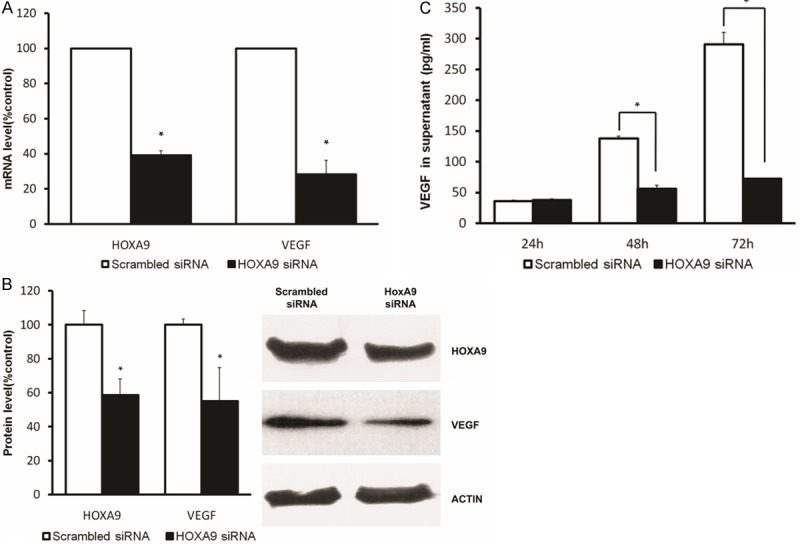
HOXA9 induces VEGF expression in human ESCs. Human ESCs were transfected with HOXA9 siRNA or scrambled siRNA. HOXA9 and VEGF mRNA (A), isolated from both the HOXA9 siRNA group and scrambled siRNA group, was measured by real-time PCR. The percent change was calculated relative to that of scrambled siRNA-transfected ESCs. Each mRNA expression level was normalized to that of actin mRNA expression. Data represent the mean ± SD for three replicate experiments. VEGF and HOXA9 protein levels (B) were detected in ESC whole cell lysates of both HOXA9 siRNA and scrambled siRNA groups by Western blot analysis. The blots were subsequently reprobed for actin as an internal loading control. The protein levels were quantified by densitometry. Secreted VEGF was measured by ELISA (C). The supernatant was taken 24, 48 and 72 hours after transfection. *P < 0.05 versus mock transfected cells.
The present results demonstrate that HOXA9 protein is constitutively expressed in human ESCs and that it is required for induction of VEGF gene expression.
Discussion
The etiology of hypertrophic scar formation is not fully known. Factors that are known to be involved are genetic factors (race, gender, etc.), cell proliferation and apoptosis in abnormal wound healing, abnormal secretion and action of cytokines and their receptors, metabolic disorders of the extracellular matrix, local biological stress, abnormal inflammation and other factors [21].
Recent studies present scar pathogenesis as a disorder of the dermis and do not consider a role for the epidermis [22]. However, reports on in-depth studies speculate on the fact that pathological scars result from abnormalities in the epidermal-mesenchymal interaction, rather than from isolated disorders in the dermis [23]. Epidermal-mesenchymal interactions occur at various stages of embryonic skin development and in response to injury during tissue repair [24]. Such cellular interactions are mediated by soluble growth factors and cytokines produced directly in an autocrine or a paracrine way [13].
In normal wound healing, the role of keratinocytes has been extensively studied. Activated keratinocytes are a feature of the early stages of wound healing, and produce growth factors that influence fibroblasts, endothelial cells, and cells of the immune system [23]. Thus, abnormal differentiation and proliferation of keratinocytes will cause a significant increase in epidermal thickness, and the formation of hypertrophic scar tissue [25]. In trauma or burn wounds, expression of the TGFβ-, FGF- and VEGF families of cytokines were described to be increased in keratinocytes which regulate re-epithelialization of epidermal cells and regeneration of dermal tissue by the autocrine or paracrine mechanism [26,27]. Recent evidence indicates that mesenchymal–epithetlial interactions play a critical role in the regulation of skin homeostasis and this cross-talk is mediated by soluble factors acting as autocrine/paracrine regulators of fibroblast and keratinocyte growth, function, and differentiation [28].
ESCs have a strong regenerative capacity and are the origin of the epidermal cell pool. ESCs exist in the epidermal basal layer and in the hair follicle outer root sheath [15]. ESCs do not only play a central role in homeostasis and wound repair, but also represent a major source of tumor initiation [14]. When the skin is subject to trauma, ESCs can migrate, proliferate and secrete different cytokines and growth factors. These factors are involved in epidermal-mesenchymal cellular interactions [29-31]. Zhao et al. found that ESCs and transit amplifying cells in mature scars were much less in number than in normal skin [32]. This report is consistent with our findings. The results indicated that the self-renewal ability of the scarred epidermis was disturbed and the differentiation process was in disorder. This may be a reason for the abnormality of structure and function of the epidermis in scar tissue [32]. We found that more β1 integrin- and CK19-positive cells are present in proliferative scar, as compared to normal skin and mature scar tissue. This observation is also consistent with the clinical phenomenon of proliferative scar hyperkeratosis.
In the skin and in scar tissue, VEGF is mainly secreted by macrophages, endothelial cells, keratinocytes, and fibroblasts [33]. The expression of VEGF is closely and positively related with vascular density and negatively with maturation of scar tissue [34]. We found that in the early post-injury scar, expression of VEGF in the basal layer of the epidermis is significantly increased, as compared to normal skin. With the maturation of hypertrophic scars, VEGF staining gradually decreased to levels below those seen the normal skin.
In recent years, there has been great interest in role of the homeobox gene family in the control of embryogenesis, adult tissue remodeling and the pathogenesis of the skin [35-37]. The genes encoding the Hox proteins, which contain a 180-bp homeodomain that facilitates DNA binding, are arranged in four major clusters (A through D), on four chromosomes, with each cluster containing between nine and thirteen genes [35,38]. Transcriptional targets of the Hox genes in both embryogenesis and in adult tissues include genes associated with the cell cycle and cell-ECM interactions [39-41]. Homeobox genes play an important role in the development of the cardiovascular system during embryogenesis as well as vessel remodeling in adults [8,9]. Sustained expression of HoxA5 in endothelial cells leads to downregulation of many pro-angiogenic genes including VEGFR2, ephrin A1, Hif1 and COX-2. In addition, HoxA5 also upregulates expression of anti-angiogenic genes, including thrombospondin-2 [42]. For HoxD10-overexpressing human endothelial cells, it was demonstrated that they failed to form new vessels when implanted into immunocompromised mice [43]. Also, several previous studies show that Homeobox genes can participate in the development, differentiation and tumorigenesis of the epithelium [12,37,44].
HoxA9 is expressed in multiple fetal and adult tissues [36]. It directly regulates a variety of key endothelial genes that are involved in the functional maturation and activity of endothelial cells [45]. For example, HOXA9 regulates endothelial cell proliferation, migration and angiogenesis via NK-κB [46], EphB4 [17] and other downstream genes. Although many studies investigated this angiogenesis link, the role that HOXA9 plays in the epidermis is much less addressed.
In summary, we have demonstrated that HOXA9 is involved in the transcriptional up-regulation of the VEGF gene in ESCs. The results of this study show that in addition to its multi-differentiation potential and highly proliferative properties, the molecule has an effect on ESCs resulting in regulation of dermal remodeling and homeostasis through the secretion of cytokines. These findings do not only contribute to a better understanding of scar formation, but also provide potential pathways for therapeutic intervention.
Disclosure of conflict of interest
None.
References
- 1.Senger DR. Molecular framework for angiogenesis: a complex web of interactions between extravasated plasma proteins and endothelial cell proteins induced by angiogenic cytokines. Am J Pathol. 1996;149:1–7. [PMC free article] [PubMed] [Google Scholar]
- 2.Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 3.Zhu KQ, Engrav LH, Armendariz R, Muangman P, Klein MB, Carrougher GJ, Deubner H, Gibran NS. Changes in VEGF and nitric oxide after deep dermal injury in the female, red Duroc pig-further similarities between female, Duroc scar and human hypertrophic scar. Burns. 2005;31:5–10. doi: 10.1016/j.burns.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Rosen LS. Clinical experience with angiogenesis signaling inhibitors: focus on vascular endothelial growth factor (VEGF) blockers. Cancer Control. 2002;9:36–44. doi: 10.1177/107327480200902S05. [DOI] [PubMed] [Google Scholar]
- 5.Cheng L, Zhang S, Sweeney CJ, Kao C, Gardner TA, Eble JN. Androgen withdrawal inhibits tumor growth and is associated with decrease in angiogenesis and VEGF expression in androgen-independent CWR22Rv1 human prostate cancer model. Anticancer Res. 2004;24:2135–2140. [PubMed] [Google Scholar]
- 6.Kobayashi T, Liu X, Wen F, Fang Q, Abe S, Wang XQ, Hashimoto M, Shen L, Kawasaki S, Kim HJ, Kohyama T, Rennard SI. Smad3 mediates TGF-β1 induction of VEGF production in lung fibroblasts. Biochem Biophys Res Commun. 2005;327:393–398. doi: 10.1016/j.bbrc.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 7.Stocks J, Bradbury D, Corbett L, Pang L, Knox AJ. Cytokines upregulate vascular endothelial growth factor secretion by human airway smooth muscle cells: Role of endogenous prostanoids. Febs Lett. 2005;579:2551–2556. doi: 10.1016/j.febslet.2005.02.083. [DOI] [PubMed] [Google Scholar]
- 8.Gorski DH, Walsh K. The role of homeobox genes in vascular remodeling and angiogenesis. Circ Res. 2000;87:865–872. doi: 10.1161/01.res.87.10.865. [DOI] [PubMed] [Google Scholar]
- 9.Cillo C, Faiella A, Cantile M, Boncinelli E. Homeobox genes and cancer. Exp Cell Res. 1999;248:1–9. doi: 10.1006/excr.1999.4451. [DOI] [PubMed] [Google Scholar]
- 10.Testori J, Schweighofer B, Helfrich I, Sturtzel C, Lipnik K, Gesierich S, Nasarre P, Hofer-Warbinek R, Bilban M, Augustin HG, Hofer E. The VEGF-regulated transcription factor HLX controls the expression of guidance cues and negatively regulates sprouting of endothelial cells. Blood. 2011;117:2735–2744. doi: 10.1182/blood-2010-07-293209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kodama A, Sakai H, Matsuura S, Murakami M, Murai A, Mori T, Maruo K, Kimura T, Masegi T, Yanai T. Establishment of canine hemangiosarcoma xenograft models expressing endothelial growth factors, their receptors, and angiogenesis-associated homeobox genes. BMC Cancer. 2009;9:363. doi: 10.1186/1471-2407-9-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcinkiewicz KM, Gudas LJ. Altered Histone Mark Deposition and DNA Methylation at Homeobox Genes in Human Oral Squamous Cell Carcinoma. J Cell Physiol. 2014;229:1405–16. doi: 10.1002/jcp.24577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan MB, Howard HG, Everett MA. Epithelial induction in dermatofibroma: a role for the epidermal growth factor (EGF) receptor. Am J Dermatopathol. 1997;19:35–40. doi: 10.1097/00000372-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Potten CS, Booth C. Keratinocyte stem cells: a commentary. J Invest Dermatol. 2002;119:888–899. doi: 10.1046/j.1523-1747.2002.00020.x. [DOI] [PubMed] [Google Scholar]
- 15.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang RH, Xie JL, Shu B, Liu XS, Chen XD, Ruan SB, Qi SH. An improved method for the isolation and culture of rat epidermal stem cells. Int J Clin Exp Pathol. 2013;6:2529–2534. [PMC free article] [PubMed] [Google Scholar]
- 17.Bruhl T, Urbich C, Aicher D, Acker-Palmer A, Zeiher AM, Dimmeler S. Homeobox A9 transcriptionally regulates the EphB4 receptor to modulate endothelial cell migration and tube formation. Circ Res. 2004;94:743–751. doi: 10.1161/01.RES.0000120861.27064.09. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Zhen G, Tsai SY, Jia X. Epidermal stem cells in orthopaedic regenerative medicine. Int J Mol Sci. 2013;14:11626–11642. doi: 10.3390/ijms140611626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu AJ, Haase I, Watt FM. Signaling via beta1 integrins and mitogen-activated protein kinase determines human epidermal stem cell fate in vitro. Proc Natl Acad Sci U S A. 1999;96:6728–6733. doi: 10.1073/pnas.96.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michel M, Torok N, Godbout MJ, Lussier M, Gaudreau P, Royal A, Germain L. Keratin 19 as a biochemical marker of skin stem cells in vivo and in vitro: keratin 19 expressing cells are differentially localized in function of anatomic sites, and their number varies with donor age and culture stage. J Cell Sci. 1996;109:1017–1028. doi: 10.1242/jcs.109.5.1017. [DOI] [PubMed] [Google Scholar]
- 21.Van den Kerckhove E, Stappaerts K, Boeckx W, Van den Hof B, Monstrey S, Van der Kelen A, De Cubber J. Silicones in the rehabilitation of burns: a review and overview. Burns. 2001;27:205–214. doi: 10.1016/s0305-4179(00)00102-9. [DOI] [PubMed] [Google Scholar]
- 22.O’Sullivan ST, O’Shaughnessy M, O’Connor TP. Aetiology and management of hypertrophic scars and keloids. Ann R Coll Surg Engl. 1996;78:168–175. [PMC free article] [PubMed] [Google Scholar]
- 23.Machesney M, Tidman N, Waseem A, Kirby L, Leigh I. Activated keratinocytes in the epidermis of hypertrophic scars. Am J Pathol. 1998;152:1133–1141. [PMC free article] [PubMed] [Google Scholar]
- 24.Ogawa R, Hsu CK. Mechanobiological dysregulation of the epidermis and dermis in skin disorders and in degeneration. J Cell Mol Med. 2013;17:817–822. doi: 10.1111/jcmm.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niessen FB, Schalkwijk J, Vos H, Timens W. Hypertrophic scar formation is associated with an increased number of epidermal Langerhans cells. J Pathol. 2004;202:121–129. doi: 10.1002/path.1502. [DOI] [PubMed] [Google Scholar]
- 26.Hakvoort T, Altun V, van Zuijlen PP, de Boer WI, van Schadewij WA, van der Kwast TH. Transforming growth factor-beta(1), -beta(2), -beta (3), basic fibroblast growth factor and vascular endothelial growth factor expression in keratinocytes of burn scars. Eur Cytokine Netw. 2000;11:233–239. [PubMed] [Google Scholar]
- 27.Wang X, Liu Y, Deng Z, Dong R, Liu Y, Hu S, Li Y, Jin Y. Inhibition of dermal fibrosis in self-assembled skin equivalents by undifferentiated keratinocytes. J Dermatol Sci. 2009;53:103–111. doi: 10.1016/j.jdermsci.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Ghahary A, Ghaffari A. Role of keratinocyte-fibroblast cross-talk in development of hypertrophic scar. Wound Repair Regen. 2007;15(Suppl 1):S46–S53. doi: 10.1111/j.1524-475X.2007.00225.x. [DOI] [PubMed] [Google Scholar]
- 29.Barrandon Y, Green H. Cell migration is essential for sustained growth of keratinocyte colonies: the roles of transforming growth factor-alpha and epidermal growth factor. Cell. 1987;50:1131–1137. doi: 10.1016/0092-8674(87)90179-6. [DOI] [PubMed] [Google Scholar]
- 30.Santoro MM, Gaudino G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp Cell Res. 2005;304:274–286. doi: 10.1016/j.yexcr.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 31.Borue X, Lee S, Grove J, Herzog EL, Harris R, Diflo T, Glusac E, Hyman K, Theise ND, Krause DS. Bone marrow-derived cells contribute to epithelial engraftment during wound healing. Am J Pathol. 2004;165:1767–1772. doi: 10.1016/S0002-9440(10)63431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao ZL, Fu XB, Sun TZ, Chen W, Sun XQ. [Study on the location and the expression characteristics of epidermal stem cells in normal adult skin and scar tissue] . Zhonghua Shao Shang Za Zhi. 2003;19:12–14. [PubMed] [Google Scholar]
- 33.Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol. 1998;152:1445–1452. [PMC free article] [PubMed] [Google Scholar]
- 34.He L, Marneros AG. Macrophages are essential for the early wound healing response and the formation of a fibrovascular scar. Am J Pathol. 2013;182:2407–2417. doi: 10.1016/j.ajpath.2013.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boncinelli E, Simeone A, Acampora D, Gulisano M. Homeobox genes in the developing central nervous system. Ann Genet. 1993;36:30–37. [PubMed] [Google Scholar]
- 36.Nunes FD, de Almeida FC, Tucci R, de Sousa SC. Homeobox genes: a molecular link between development and cancer. Pesqui Odontol Bras. 2003;17:94–98. doi: 10.1590/s1517-74912003000100018. [DOI] [PubMed] [Google Scholar]
- 37.Komuves LG, Ma XK, Stelnicki E, Rozenfeld S, Oda Y, Largman C. HOXB13 homeodomain protein is cytoplasmic throughout fetal skin development. Dev Dyn. 2003;227:192–202. doi: 10.1002/dvdy.10290. [DOI] [PubMed] [Google Scholar]
- 38.Botas J. Control of morphogenesis and differentiation by HOM/Hox genes. Curr Opin Cell Biol. 1993;5:1015–1022. doi: 10.1016/0955-0674(93)90086-6. [DOI] [PubMed] [Google Scholar]
- 39.Edelman GM, Jones FS. Outside and downstream of the homeobox. J Biol Chem. 1993;268:20683–20686. [PubMed] [Google Scholar]
- 40.Raman V, Martensen SA, Reisman D, Evron E, Odenwald WF, Jaffee E, Marks J, Sukumar S. Compromised HOXA5 function can limit p53 expression in human breast tumours. Nature. 2000;405:974–978. doi: 10.1038/35016125. [DOI] [PubMed] [Google Scholar]
- 41.Valerius MT, Patterson LT, Feng Y, Potter SS. Hoxa 11 is upstream of Integrin alpha8 expression in the developing kidney. Proc Natl Acad Sci U S A. 2002;99:8090–8095. doi: 10.1073/pnas.122229199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rhoads K, Arderiu G, Charboneau A, Hansen SL, Hoffman W, Boudreau N. A role for Hox A5 in regulating angiogenesis and vascular patterning. Lymphat Res Biol. 2005;3:240–252. doi: 10.1089/lrb.2005.3.240. [DOI] [PubMed] [Google Scholar]
- 43.Myers C, Charboneau A, Cheung I, Hanks D, Boudreau N. Sustained expression of homeobox D10 inhibits angiogenesis. Am J Pathol. 2002;161:2099–2109. doi: 10.1016/S0002-9440(10)64488-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Konradi S, Yasmin N, Haslwanter D, Weber M, Gesslbauer B, Sixt M, Strobl H. Langerhans cell maturation is accompanied by induction of N-cadherin and the transcriptional regulators of epithelial-mesenchymal transition ZEB1/2. Eur J Immunol. 2014;44:553–560. doi: 10.1002/eji.201343681. [DOI] [PubMed] [Google Scholar]
- 45.Kodama A, Sakai H, Murakami M, Murai A, Mori T, Maruo K, Yanai T, Masegi T. Immunohistochemical demonstration of angiogenesis-associated homeobox proteins in canine vascular tumours. J Comp Pathol. 2009;141:199–203. doi: 10.1016/j.jcpa.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Trivedi CM, Patel RC, Patel CV. Homeobox gene HOXA9 inhibits nuclear factor-kappa B dependent activation of endothelium. Atherosclerosis. 2007;195:e50–e60. doi: 10.1016/j.atherosclerosis.2007.04.055. [DOI] [PubMed] [Google Scholar]