Abstract
Carbonic anhydrase IX (CA IX), a hypoxia-inducible protein in tumors, has been shown to be valuable for the prognosis of nasopharyngeal carcinoma (NPC). However, the function and mechanism of CA IX has been not explored in NPC. Here, we found that CA IX was detected at higher levels in NPC cells and tissues than their corresponding partners. Furthermore, the cell growth, migration and invasion in vitro were altered with shRNA or overexpression of CA IX in NPC cells. More importantly, the metastatic ability of NPC cells stably expressing CA IX was significantly enhanced using the hepatic metastasis model of nude mice in vivo. Finally, the mTOR pathway was indicated to be involved in such effects of CA IX on NPC. This is the first evidence that CA IX may promote the NPC metastasis to potentially be a therapeutic target for NPC, and that the inhibitory molecules of CA IX and/or the mTOR pathway alone or combination with both may be worth to have a clinical trial for the patients with NPC.
Keywords: CA IX, NPC, growth, metastasis, mTOR
Introduction
Nasopharyngeal carcinoma (NPC), originating from the nasopharynx, is highly prevalent with an incidence rate of 15-50/100,000 in Southern China and Southeast Asia [1-3]. NPC, also often undifferentiated and highly sensitive to radiotherapy, has a high rate of distant metastasis, which makes it very different from other head and neck cancers [4,5]. But the molecular mechanisms of NPC is still poorly understood, although some progressions have been made recently [2,6-9]. So it is necessary to clarify the pathogenesis of NPC and to find new targets for the treatment of nasopharyngeal carcinoma.
Carbonic anhydrase IX (CA IX), a member of the carbonic anhydrase family, was first identified as an endogenous HeLa cells antigen [10], and it catalyzes the reversible conversion of carbon dioxide to bicarbonate and protons and is thus involved in ion transport and pH control [11,12]. It has been reported that CA IX were higher in tumor tissues, including clear cell renal cell carcinoma and breast cancer, than in normal tissues, and over-expression of CA IX predicts a poor clinical outcome in most cancer types including NPC [13-15]. But the function and mechanism of CA IX in NPC has not been explored, furthermore, whether CA IX can be used as a potential therapeutic target for NPC needs to be confirmed. The present study focuses on the expression and roles of CA IX in NPC. It has been found that CA IX is up-regulated in NPC cells. This study may provide new ideas and methods suitable for the treatment of nasopharyngeal carcinoma.
Materials and methods
Cell culture and sample collection
Six human NPC cell lines (Hone1, CNE1, CNE2, Sune1, 6-10B, 5-8F) were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen) supplemented with 10% fetal bovine serum (HyClone). The nasopharyngeal epithelial cell line (NP69) was grown in defined-KSFM medium supplemented with epidermal growth factor (EGF) (Invitrogen, Carlsbad, USA). All cell lines were incubated in a humidified chamber with 5% CO2 at 37°C. 10 fresh primary NPC tissues and 4 fresh NP tissues were obtained at the time of diagnosis before any therapy from Sun Yat-sen University cancer center (Guangzhou, China). The clinical processes were approved from the Ethics Committees of Sun Yat-sen University and the informed consent was collected from each patient.
Construction of stable lines overexpressing CA IX
Full-length human CA IX cDNA was cloned into pSin-puro vector, and CA IX was verified by DNA sequencing. The primers were as follows: 5’-GGAATTCCATATGACCATGGCTCCCCTGTGCCCCAG-3’ (forward), 5’-GGAATTCCATATGACCATGGCTCCCCTGTGCCCCAG-3’ (reverse). pSin-puro delivering CA IX or empty vector were co-transfected with pMD.2G and psPAX2 into HEK-293T cells for 48 hours. The recombinant virus were collected and added to 6-10B and Hone1 cells cultured with 8 μg/ml polybrene for 24 hours. The stable lines were selected with 1 μg/ml of puromycin for two weeks.
Construction of stable lines silencing CA IX
The vectors expressing either CA IX short hairpin RNAs (shRNAs) or a scrambled shRNA were generated using the Sigma shRNA system according to the manufacturer’s instructions. The targets of human CA IX shRNA#1, #2, #3 and #4 are 5’-TGCTGCCTCGCCTCTAGATAT-3’, 5’-CCTTAACTTCTGTGCCAACAA-3’, 5’-GCTGAACCATGCCTCCATCAT-3’, 5’-CATGCTGAAGAGAGGCATCTT-3’ and respectively. CA IX shRNAs or scrambled shRNA were co-transfected with pMD.2G and psPAX2 into HEK-293T cells for 48 hours. The recombinant virus were collected and added to 5-8F cells cultured with 8 μg/ml polybrene for 24 hours. The stable lines were selected with 1 μg/ml of puromycin for two weeks.
RNA extraction and quantitative real-time RT-PCR (qRT-PCR)
Quantitative real-time RT-PCR was performed as described previously [16,17]. Total RNA of tissue specimens was isolated using Trizol (Invitrogen) according to the manufacturer’s protocol. First-strand cDNA was synthesized using PrimeScript® RT reagent Kit with gDNA Eraser. And quantitative PCR was performed for detection of CA IX mRNA using SYBR® Premix Ex Taq™ II (Takara). The sequences of primers were as follows: for CA IX: 5’-GGATCTACCTACTGTTGAGGCT-3’ (forward), 5’-CATAGCGCCAATGACT CTGGT-3’ (reverse); for GAPDH: 5’-ACAGTCAGCCGCATCTTCTT-3’ (forward), 5’-GACAAGCTTCCCGTTCTCAG-3’ (reverse). The PCR condition was: 95°C for 10 minutes, followed by 40 cycles of 95°C for 20 s, 60°C for 20 and 70°C for 30 s.
CCK8 assay
The cell viability in vitro was assessed using CCK8 assay. Cells were seeded in 96-well plates at the density of 1,000 cells/well, the cells were incubated for 1, 2, 3, 4, or 5 days. Ten microliters of CCK8 (Cell Counting Kit-8, Beyotime, China) was added to each well and incubated for 1.5 hours. The absorbance value (OD) of each well was measured at 450 nm. For each experimental condition, 6 wells were used. Experiments were performed three times.
Colony formation assay
Cells were plated in the 6-well culture plates at 250 cells per well. Each group had 3 wells. After incubation for 15 days at 37°C, cells were washed twice with PBS and stained with Giemsa solution. The number of colonies containing ≥50 cells was counted under a microscope.
Wound-healing assay
Cell motility was assessed by measuring the movement of cells into a scraped, acellular area created by a 200 μl pipette tube, and the spread of wound closure was observed after 24 h and 48 h and photographed under a microscope.
Transwell assay
For the transwell migration assay, 3.5×104 cells in 200 μl of serum-free DMEM were added to the cell culture inserts with an 8-μm microporous filter without extracellular matrix coating (Becton Dickinson Labware, Bedford, MA). The DMEM medium containing 10% FBS was added to the bottom chamber. After 24 hours of incubation, the cells in the lower surface of the filter were fixed and stained followed by microscopic examination. The number of migrated cells in three random optical fields (×100 magnification) for each filter from triplicate filters was averaged. For the invasion assay, the inserts of the chambers to which the cells were seeded were coated with Matrigel (Becton Dickinson Labware, Bedford, MA). The number of invading cells in three random optical fields (×100 magnification) for each filter from triplicate inserts was averaged.
Western blotting
Western blotting was performed as described previously [16,17]. Briefly, cells were collected and lysed by RIPA buffer (150 mM NaCl, 0.5% EDTA, 50 mM Tris, 0.5% NP40) and centrifuged for 20 min at 12000 rpm at 4°C. Fifty micrograms of harvested total protein was loaded, separated in 8% sodium dodecyl sulfate-polyacrylamide gradient gels and transferred onto PVDF membranes followed by blocking with 5% non-fat milk for 2 hours at room temperature. Membranes were incubated with primary antibody and horseradish peroxidase-conjugated secondary antibody, and then detected using the ECL chemiluminescence system (Pierce, Rockford, USA). Antibodies against CA IX, mTOR, p-mTOR (Ser2448) were from Cell Signaling Technology. Antibody against Tubulin was from Bioworld Technology.
Animal experiments
All animal work was performed in accordance with protocols approved by Research Animal Resource Center of Sun Yat-sen University. Male athymic mice between 5 and 6 weeks of age were obtained from Shanghai Institutes for Biological Sciences (Shanghai, China). All the animal studies were conducted in accordance with the principles and procedures outlined in the guidelines of Institutional Animal Care and Use Committee at Sun Yat-sen University Cancer Center. The hepatic metastasis model of nude mice has been published previously [7]. Briefly, the total of 3×105 cells in 30 μl were injected into spleens of laparotomized mice using insulin syringes (Becton Dickinson). After tumor cell inoculation for 32 days, the experiment was terminated. The metastatic nodules in each liver were counted.
Results
CA IX is up-regulated in NPC cell lines and tissues
CA IX was reported to be expressed at higher levels in tumor tissues than in normal tissues and associated with prognosis in various cancers [13-15,18]. To evaluate the expression level in NPC, we detected the mRNA level of CA IX in NPC tissues and normal nasopharyngeal tissues using qRT-PCR. As shown in Figure 1A, the mRNA level of CA IX was significantly higher in 8 out of 10 tumor tissues than that in 4 normal nasopharyngeal tissues. Likewise, we found that the mRNA and protein levels of CA IX were higher in 4 out of 6 and all of 6 NPC cell lines tested, respectively, than in nasopharyngeal epithelial cell line (NP69) (Figure 1B, 1C). Notably, both the mRNA and protein levels of CA IX were much higher in 5-8F than in 6-10B, which is a pair of cell lines derived from the NPC Sune1 cells with high and low metastatic abilities, respectively. These results indicate that CA IX is up-regulated in NPC and may be related to the NPC progression and metastasis.
Figure 1.
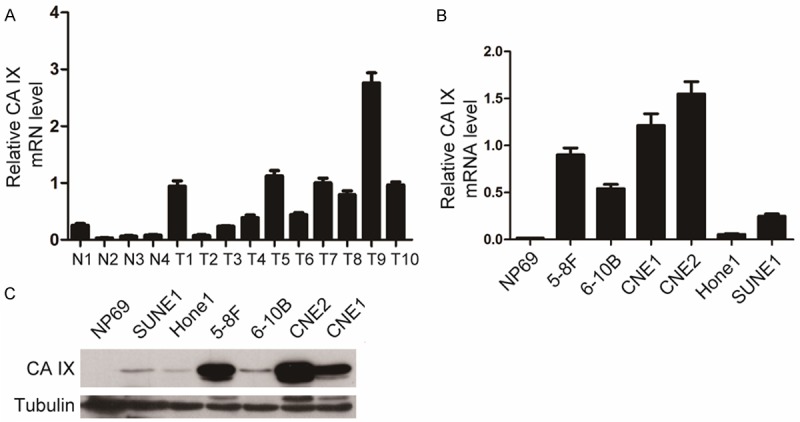
Up-regulation of CA IX was detected in NPC cell and tissues. A: The CA IX mRNA was detected in 10 human NPC primary tissues (T) and 4 normal nasopharyngeal tissues (N) by qRT-PCR. GAPDH was used as the internal control. Statistical analysis (Mann-Whitney test) was performed (*P<0.05). B: The mRNA levels of CA IX were determined in 6 NPC cell lines by qRT-PCR. GAPDH was used as the internal control. C: The protein levels of CA IX were analyzed in NPC cell lines. Tubulin was used as the internal control.
CA IX increases the cell growth and colony formation of NPC cells
In order to explore the function of CA IX in NPC, we generated the stable cell lines expressing ectopic CA IX in Hone1 and 6-10B cells (Figure 2A). On the other hand, four different shRNAs specifically targeting different CA IX coding regions were used in 5-8F cells, and sh#2 and sh#3 were more efficient to silence CA IX (Figure 2A). Then we evaluated the effect of CA IX on cell growth using CCK8. As shown in Figure 2B, overexpression of CA IX in 6-10B and Hone1 cells increased the cell growth compared with the control cells. On the country, knockdown of CA IX in 5-8F cells decreased the cell growth compared with the control cells (Figure 2C). In addition, overexpression of CA IX increased the ability of colony formation of 6-10B and Hone1 cells (Figure 2D), whereas knockdown of CA IX decreased the ability of colony formation of 5-8F cells (Figure 2E). Taken together, these results indicate that CA IX play an important role in regulation of the cell growth and colony formation of NPC cells.
Figure 2.
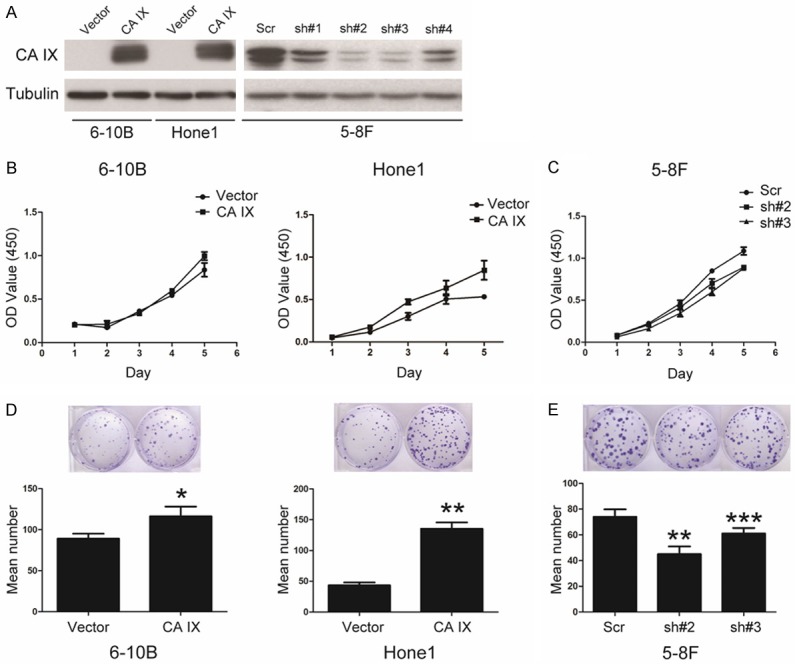
CA IX promotes cell growth and colony formation in NPC cells. A: The generation of stable cell lines in 6-10B, Hone1, and 5-8F cells overexpressing or knocked down of CA IX, as indicated, and the protein level was analyzed by western blotting. B, C: Cell growth of the indicated stable cell lines in vitro was measured in different time points as indicated by CCK8 assay. D, E: Colony formation of the indicated stable cell lines in vitro was measured for 14 days, as described in “Materials and methods”. Bars correspond to mean + standard error, with P value calculated using Student’s t-test. *P<0.05, **P<0.001, ***P<0.0001.
CA IX promotes the cell migration and invasion of NPC cells in vitro
Our results showed CA IX expression higher levels in NPC cells than normal nasopharyngeal cells. Furthermore, CA IX expression higher levels in high metastatic cells 5-8F than low metastatic cells 6-10B. So we evaluated the effect of CA IX on the abilities of migration and invasion in NPC cells using wound-healing and transwell assay. As shown in Figure 3A, 3B the cell ability of wound-healing was dramatically enhanced and suppressed by both overexpressing and knocking down CA IX, respectively. And the transwell assays showed the abilities of migration and invasion were dramatically enhanced and suppressed by both overexpressing and knocking down CA IX, respectively (Figure 3C, 3D). These results suggest that CA IX play a crucial role in regulation of the cell migration and invasion of NPC cells in vitro.
Figure 3.
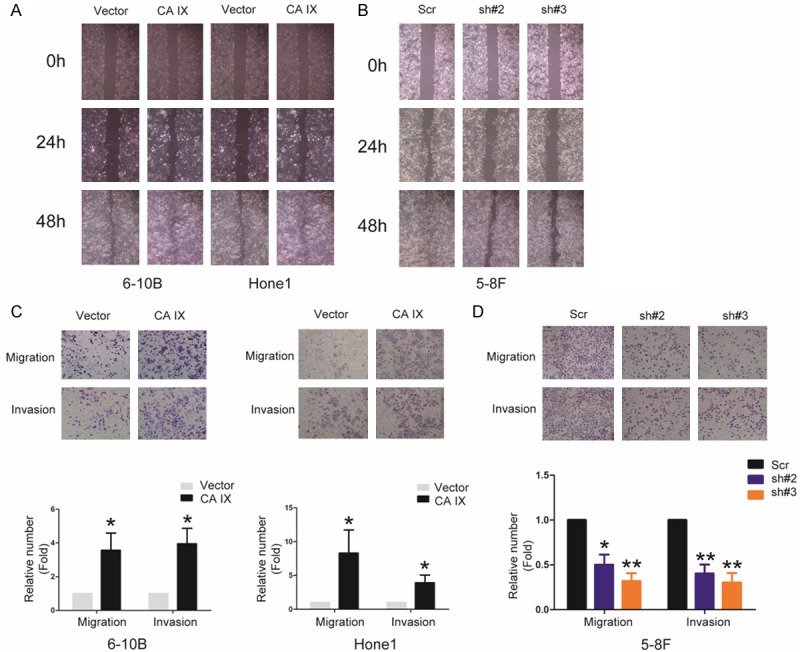
CA IX strongly promotes the cell migration, invasion of NPC in vitro. A, B: The cell ability of wound gap closure were dramatically enhanced and suppressed by both overexpressing and knocking down CA IX, respectively. C, D: Migratory and invasive abilities of the indicated stable cell lines in vitro were measured by Transwell assay as described in “Materials and methods”. Bars correspond to mean + standard error, with P value calculated using Student’s t-test. *P<0.05, **P<0.001.
CA IX promotes metastasis of NPC cells in vivo
Based on these findings that CA IX dramatically enhanced the abilities of migration and invasion of NPC cells in vitro, we detected the metastatic ability through the hepatic metastasis model of nude mice in vivo. Strikingly, Overexpression of CA IX markedly increases metastasis of NPC cells in vivo (Figure 4). These results indicate that CA IX strongly enhances metastasis of NPC cells in vivo, consistent with the previous reports showing that CA IX takes part in pH regulation facilitating acidification of the microenvironment, enhancing cell growth and migration in most cancer cells [19,20].
Figure 4.
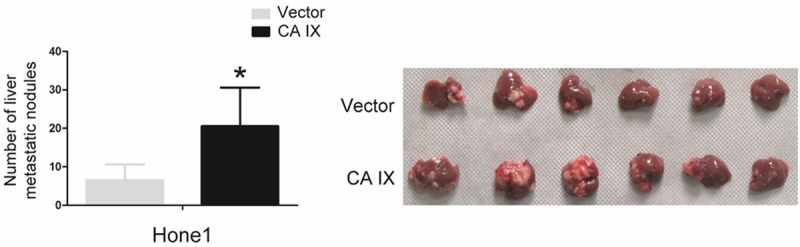
CA IX strongly promotes metastasis of NPC in vivo. The liver metastatic ability of Hone1 cells expressing CA IX or empty vector In vivo was evaluated in nude mice. Bars correspond to mean + standard error, with P value calculated using Student’s t-test. *P<0.05.
CA IX may activate the mTOR pathway in NPC cells
Given that mTOR is a key regulator for cancer progression and metastasis including NPC [21,22], As showed in Figure 5A, both the p-mTOR (Ser2448) level was increased, while the total mTOR protein level was constant, in both Hone1 and 6-10B cells stably expressing of ectopic CA IX. On the other hand, knockdown of CA IX decreased the p-mTOR (Ser2448) level in 5-8F cells (Figure 5B). These results suggest that CA IX may activate the mTOR pathway in NPC cells.
Figure 5.
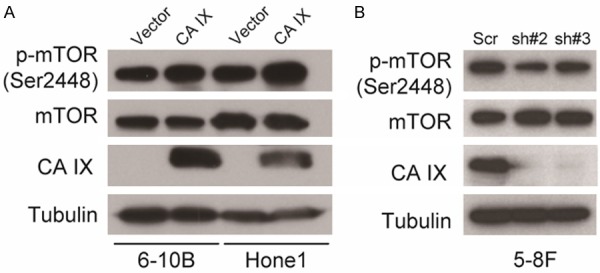
CA IX may activate the mTOR pathway in NPC cells. A, B: Immunoblots of whole-cell lysates from the indicated stable cell lines using the indicated antibodies, as described in “Materials and methods”.
Discussion
Although it has been reported that tumor hypoxia can induce CA IX expression and the high level of CA IX predicts a poor outcome in NPC [15,18], its function and mechanism in NPC has not been characterized. Furthermore, whether it can be used as a potential therapeutic target for NPC needs to be confirmed. In this study, we demonstrated that CA IX plays an oncogenic role in NPC probably through the mTOR pathway.
We demonstrated that CA IX is higher in NPC cell lines and tissues than in its counterpartner’s (Figure 1). This is constant with the literature showing that CA IX is expressed at higher levels in tumor tissues than in normal tissues in various cancer types [13-15,18]. Furthermore, we provided the first evidence that CA IX can promote NPC cells growth and colony formation but dramatically enhances NPC cells migration and invasion in vitro and metastasis in vivo (Figures 2, 3 and 4). These results indicate that CA IX may play oncogenic roles in NPC progression, especially in NPC metastasis, providing a reasonable explanation that the high level of CA IX predicts a poor outcome in NPC.
Notably, CA IX has been proposed to be an attractive therapeutic target [23], as some inhibitory molecules, such as BAY 79-4620, for CA IX have been developed for anticancer therapies [24]. Our results indicated that CA IX can activate the mTOR pathway in NPC (Figure 5), and the preclinical animal models have been showed that targeting the mTOR pathway is an efficient strategy for NPC [25-28]. Therefore, we speculate that the inhibitory molecules of CA IX and/or the mTOR pathway alone or combination with both may be worth to have a clinical trial for the patients with NPC.
Disclosure of conflict of interest
None.
References
- 1.Bei JX, Li Y, Jia WH, Feng BJ, Zhou G, Chen LZ, Feng QS, Low HQ, Zhang H, He F, Tai ES, Kang T, Liu ET, Liu J, Zeng YX. A genome-wide association study of nasopharyngeal carcinoma identifies three new susceptibility loci. Nat Genet. 2010;42:599–603. doi: 10.1038/ng.601. [DOI] [PubMed] [Google Scholar]
- 2.Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet. 2005;365:2041–2054. doi: 10.1016/S0140-6736(05)66698-6. [DOI] [PubMed] [Google Scholar]
- 3.Chen X, Li P, Yang Z, Mo WN. Expression of fragile histidine triad (FHIT) and WW-domain oxidoreductase gene (WWOX) in nasopharyngeal carcinoma. Asian Pac J Cancer Prev. 2013;14:165–171. doi: 10.7314/apjcp.2013.14.1.165. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Xia W, Lu X, Sun R, Wang L, Zheng L, Ye Y, Bao Y, Xiang Y, Guo X. A novel statistical prognostic score model that includes serum CXCL5 levels and clinical classification predicts risk of disease progression and survival of nasopharyngeal carcinoma patients. PLoS One. 2013;8:e57830. doi: 10.1371/journal.pone.0057830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang M, Liu WQ, Qin YT, Wei ZX, Wang RS. Long-term efficacy of microwave hyperthermia combined with chemoradiotherapy in treatment of nasopharyngeal carcinoma with cervical lymph node metastases. Asian Pac J Cancer Prev. 2013;14:7395–7400. doi: 10.7314/apjcp.2013.14.12.7395. [DOI] [PubMed] [Google Scholar]
- 6.Hwang CF, Shiu LY, Su LJ, Yu-Fang Y, Wang WS, Huang SC, Chiu TJ, Huang CC, Zhen YY, Tsai HT, Fang FM, Huang TL, Chen CH. Oncogenic fibulin-5 promotes nasopharyngeal carcinoma cell metastasis through the FLJ10540/AKT pathway and correlates with poor prognosis. PLoS One. 2013;8:e84218. doi: 10.1371/journal.pone.0084218. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Li XJ, Peng LX, Shao JY, Lu WH, Zhang JX, Chen S, Chen ZY, Xiang YQ, Bao YN, Zheng FJ, Zeng MS, Kang TB, Zeng YX, Teh BT, Qian CN. As an independent unfavorable prognostic factor, IL-8 promotes metastasis of nasopharyngeal carcinoma through induction of epithelial-mesenchymal transition and activation of AKT signaling. Carcinogenesis. 2012;33:1302–1309. doi: 10.1093/carcin/bgs181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo DH, Chen QY, Liu H, Xu LH, Zhang HZ, Zhang L, Tang LQ, Mo HY, Huang PY, Guo X, Mai HQ. The independent, unfavorable prognostic factors endothelin A receptor and chemokine receptor 4 have a close relationship in promoting the motility of nasopharyngeal carcinoma cells via the activation of AKT and MAPK pathways. J Transl Med. 2013;11:203. doi: 10.1186/1479-5876-11-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun XJ, Liu H, Zhang P, Zhang XD, Jiang ZW, Jiang CC. miR-10b promotes migration and invasion in nasopharyngeal carcinoma cells. Asian Pac J Cancer Prev. 2013;14:5533–5537. doi: 10.7314/apjcp.2013.14.9.5533. [DOI] [PubMed] [Google Scholar]
- 10.Pastorekova S, Zavadova Z, Kostal M, Babusikova O, Zavada J. A novel quasi-viral agent, MaTu, is a two-component system. Virology. 1992;187:620–626. doi: 10.1016/0042-6822(92)90464-z. [DOI] [PubMed] [Google Scholar]
- 11.Svastova E, Hulikova A, Rafajova M, Zat’ovicova M, Gibadulinova A, Casini A, Cecchi A, Scozzafava A, Supuran CT, Pastorek J, Pastorekova S. Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett. 2004;577:439–445. doi: 10.1016/j.febslet.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 12.Takacova M, Bartosova M, Skvarkova L, Zatovicova M, Vidlickova I, Csaderova L, Barathova M, Breza J Jr, Bujdak P, Pastorek J, Breza J Sr, Pastorekova S. Carbonic anhydrase IX is a clinically significant tissue and serum biomarker associated with renal cell carcinoma. Oncol Lett. 2013;5:191–197. doi: 10.3892/ol.2012.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chia SK, Wykoff CC, Watson PH, Han C, Leek RD, Pastorek J, Gatter KC, Ratcliffe P, Harris AL. Prognostic significance of a novel hypoxia-regulated marker, carbonic anhydrase IX, in invasive breast carcinoma. J. Clin. Oncol. 2001;19:3660–3668. doi: 10.1200/JCO.2001.19.16.3660. [DOI] [PubMed] [Google Scholar]
- 14.Giatromanolaki A, Koukourakis MI, Sivridis E, Pastorek J, Wykoff CC, Gatter KC, Harris AL. Expression of hypoxia-inducible carbonic anhydrase-9 relates to angiogenic pathways and independently to poor outcome in non-small cell lung cancer. Cancer Res. 2001;61:7992–7998. [PubMed] [Google Scholar]
- 15.Hui EP, Chan AT, Pezzella F, Turley H, To KF, Poon TC, Zee B, Mo F, Teo PM, Huang DP, Gatter KC, Johnson PJ, Harris AL. Coexpression of hypoxia-inducible factors 1alpha and 2alpha, carbonic anhydrase IX, and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clin Cancer Res. 2002;8:2595–2604. [PubMed] [Google Scholar]
- 16.Hong J, Hu K, Yuan Y, Sang Y, Bu Q, Chen G, Yang L, Li B, Huang P, Chen D, Liang Y, Zhang R, Pan J, Zeng YX, Kang T. CHK1 targets spleen tyrosine kinase (L) for proteolysis in hepatocellular carcinoma. J Clin Invest. 2012;122:2165–2175. doi: 10.1172/JCI61380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu S, Wu Y, Chen Q, Cao J, Hu K, Tang J, Sang Y, Lai F, Wang L, Zhang R, Li SP, Zeng YX, Yin Y, Kang T. hSSB1 regulates both the stability and the transcriptional activity of p53. Cell Res. 2013;23:423–435. doi: 10.1038/cr.2012.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sung FL, Hui EP, Tao Q, Li H, Tsui NB, Dennis Lo YM, Ma BB, To KF, Harris AL, Chan AT. Genome-wide expression analysis using microarray identified complex signaling pathways modulated by hypoxia in nasopharyngeal carcinoma. Cancer Lett. 2007;253:74–88. doi: 10.1016/j.canlet.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Chiche J, Ilc K, Brahimi-Horn MC, Pouyssegur J. Membrane-bound carbonic anhydrases are key pH regulators controlling tumor growth and cell migration. Adv Enzyme Regul. 2010;50:20–33. doi: 10.1016/j.advenzreg.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Gieling RG, Williams KJ. Carbonic anhydrase IX as a target for metastatic disease. Bioorg Med Chem. 2013;21:1470–1476. doi: 10.1016/j.bmc.2012.09.062. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Hu CF, Hou JH, Shao Q, Yan LX, Zhu XF, Zeng YX, Shao JY. Epstein-Barr virus encoded latent membrane protein 1 regulates mTOR signaling pathway genes which predict poor prognosis of nasopharyngeal carcinoma. J Transl Med. 2010;8:30. doi: 10.1186/1479-5876-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang XM, Dai CB, Mou ZL, Wang LJ, Wen WP, Lin SG, Xu G, Li HB. Overproduction of cyclin D1 is dependent on activated mTORC1 signal in nasopharyngeal carcinoma: implication for therapy. Cancer Lett. 2009;279:47–56. doi: 10.1016/j.canlet.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 23.McDonald PC, Winum JY, Supuran CT, Dedhar S. Recent developments in targeting carbonic anhydrase IX for cancer therapeutics. Oncotarget. 2012;3:84–97. doi: 10.18632/oncotarget.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrul HM, Schatz CA, Kopitz CC, Adnane L, McCabe TJ, Trail P, Ha S, Chang YS, Voznesensky A, Ranges G, Tamburini PP. Therapeutic mechanism and efficacy of the antibody-drug conjugate BAY 79-4620 targeting human carbonic anhydrase 9. Mol Cancer Ther. 2012;11:340–349. doi: 10.1158/1535-7163.MCT-11-0523. [DOI] [PubMed] [Google Scholar]
- 25.Cai Y, Xia Q, Su Q, Luo R, Sun Y, Shi Y, Jiang W. mTOR inhibitor RAD001 (everolimus) induces apoptotic, not autophagic cell death, in human nasopharyngeal carcinoma cells. Int J Mol Med. 2013;31:904–912. doi: 10.3892/ijmm.2013.1282. [DOI] [PubMed] [Google Scholar]
- 26.Ma BB, Lui VW, Hui CW, Lau CP, Wong CH, Hui EP, Ng MH, Cheng SH, Tsao SW, Tsang CM, Cheung CS, Ho K, Chan AT. Preclinical evaluation of the mTOR-PI3K inhibitor BEZ235 in nasopharyngeal cancer models. Cancer Lett. 2014;343:24–32. doi: 10.1016/j.canlet.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Wong CH, Loong HH, Hui CW, Lau CP, Hui EP, Ma BB, Chan AT. Preclinical evaluation of the PI3K-mTOR dual inhibitor PF-04691502 as a novel therapeutic drug in nasopharyngeal carcinoma. Invest New Drugs. 2013;31:1399–1408. doi: 10.1007/s10637-013-0007-z. [DOI] [PubMed] [Google Scholar]
- 28.Yang C, Peng J, Jiang W, Zhang Y, Chen X, Wu X, Zhu Y, Zhang H, Chen J, Wang J, Cho WC, Jin K. mTOR activation in immature cells of primary nasopharyngeal carcinoma and anti-tumor effect of rapamycin in vitro and in vivo. Cancer Lett. 2013;341:186–194. doi: 10.1016/j.canlet.2013.08.004. [DOI] [PubMed] [Google Scholar]


