Abstract
Besides STAT3 tyrosine 705 phosphorylation (pTyr705-STAT3), phosphorylation of STAT3 at serine 727 (pSer727-STAT3) is shown to contribute to tumorigenesis and be closely related with resistance to radiotherapy and chemotherapy in glioma, but there is currently no study regarding its relevance to prognosis in glioblastoma (GBM). Here, the expression of phosphorylated STAT3 was detected in tumor specimens from 88 patients with newly diagnosed GBM by immunohistochemistry, the Kaplan-Meier survival curve and COX proportional hazards regression model were applied to estimate its influences on progression-free survival (PFS) and overall survival (OS). Immunohistochemical assay showed elevated expression of pSer727-STAT3 in GBM compared with normal brain tissue. Univariate analysis indicated significant correlations of high percentage of pSer727-STAT3 positive tumor cells with shorter PFS (P = 0.006) and OS (P = 0.002). In multivariate analysis, high pSer727-STAT3 expression was demonstrated as an independent unfavorable prognostic indicator for PFS (HR 1.830, P = 0.022) and OS (HR 1.797, P = 0.040). And patients with high expression of both pTyr705-STAT3 and pSer727-STAT3 had a poorer prognosis compared with the remainder (P < 0.005). In conclusion, the high proportion of pSer727-STAT3 positive neoplastic cells in GBM is an independent unfavorable prognostic factor, and increased expression of both pTyr705-STAT3 and pSer727-STAT3 is predictive of poorer clinical outcome, thereby adding to the growing evidence that STAT3 inhibition may be a potential therapeutic strategy in glioblastoma.
Keywords: STAT3, serine, phosphorylation, prognosis, glioblastoma
Introduction
Glioblastoma (GBM) represents the most aggressive and frequent type of malignant glioma with a dismal clinical outcome. Given the molecular hub for multiple oncogenic signaling pathways-signal transducers and activators of transcription factor 3 (STAT3), it’s not surprising that a growing body of research has been centered on the oncogenic function of aberrant STAT3 activation in glioma in recent years.
It is well recognized that STAT3, acting as a cytoplasmic signaling molecule, is activated via phosphorylation at tyrosine 705 (pTyr705) in response to diverse stimuli. Then, after dimerization, pTyr705-STAT3 translocates to the cell nucleus and binds to specific DNA response elements, thereby activating the transcription of target genes. Under normal conditions, the activation of STAT3 is tightly controlled and usually last transitorily [1]. Compared with normal brain tissue, the expression of pTyr705-STAT3 is elevated and affects clinical outcome in patients with GBM [2].
In addition to tyrosine 705, STAT3 protein can also be phosphorylated at another site-serine 727 (pSer727). It has been reported that pSer727-STAT3 is required for the maximal transcriptional activity of STAT3 [3,4]. Moreover, phosphorylation of STAT3 at serine 727 may induce STAT3 activation independent of pTyr705, thereby driving tumorigenesis [5]. A growing body of evidence suggests that the constitutive phosphorylation of STAT3 at serine 727 is observed in various types of human cancer and is essential for tumor cell survival, growth and invasion (including glioma) [6-8], whereas blocking serine 727 phosphorylation impairs the tumorigenic capacity of STAT3 [5]. A recent result shows that pSer727-STAT3 mediates the effects of NOTCH signaling pathway which plays a key role in glioma carcinogenesis and development [9]. Furthermore, STAT3 is constitutively activated through phosphorylation preferentially at serine 727 in glioblastoma stem cells, and self-renewal of these cells depends upon the presence of STAT3 for their maintenance [10].
Nevertheless, some studies indicate that serine 727 phosphorylation negatively modulates the transcriptional activity of STAT3 through affecting its tyrosine phosphorylation and its binding to the promoter of target gene [11-13], thus suggesting that pSer727-STAT3 may harbor a tumor-suppressive function. One possible interpretation for these discrepancies could be the role of pSer727-STAT3 might depend on the cell context or the corresponding stimuli.
Given the controversial role of pSer727-STAT3, it would be interesting to elucidate its relationship with survival in patients with GBM, because such data could provide indirect evidence: whether or not to inhibit STAT3 would translate into clinical benefits to these patients. But so far there is no relevant study on that topic. The aim of this study was to detect the expression of phosphorylated STAT3 (especially serine 727 phosphorylation) in GBM tissues and further investigate its prognosis value.
Materials and methods
Patient description
This study was approved by the Ethics Committee of Fujian Medical University and conformed to the principles outlined in the Helsinki Declaration, each patient enrolled in the study had signed informed consent. In this retrospective study, tumor specimens were obtained from 88 patients with newly diagnosed supratentorial glioblastoma who underwent surgery in the First Affiliated Hospital of Fujian Medical University between May 2007 and August 2013. Fifty-three patients (60.2%) were male and thirty-five patients (39.8%) were female. The age at diagnosis ranged from 26 to 79 years (median age 56 years). Preoperative Karnofsky Performance Status (KPS) scores of patients ranged from 60 to 90 (median KPS 70). Postoperatively, radiotherapy of the contrast-enhanced lesion plus the area of peritumoral edema and a 2-cm-margin shown by magnetic resonance imaging was performed in 2-4 weeks after surgical resection. A total dose of 60 Gy was delivered in 30 fractions with concomitant chemotherapy (temozolomide, at a dose of 75 mg/M2/day). Temozolomide was applied in chemotherapy and was administered at a dose of 150-200 mg/M2/day for 4-6 cycles in the absence of irreversible blood toxicity, poor general conditions or death. Thirty-two patients treated with standard adjuvant chemoradiotherapy (radiotherapy plus chemotherapy), fifty-six received nonstandard chemoradiotherapy (including patients with radiotherapy or chemotherapy alone and patients without chemoradiotherapy). Tumor progression during the follow-up period was estimated according to the protocol of Wen and Macdonald [14]. All patients died from glioma-related causes.
Immunohistochemical assays
Immunostaining was performed on 4-μm-thick sections of formalin-fixed and paraffin-embedded tumor specimens by the 2-step peroxidase conjugated polymer detection system. Briefly, the sections were dewaxed by treatment in xylene and rehydrated by treatment in a graded ethanol series. Next, for the antigen retrieval of pSer727-STAT3, these sections were autoclaved in citrate buffer (pH 6.0) for 2.5 min; for pTyr705-STAT3, the antigen retrieval was done in ethylenediamine tetraacetic acid (EDTA, pH 9.0) for 20 min in a boiling water bath. The activity of endogenous peroxidase was quenched after incubation with methanol containing 3% hydrogen peroxide for 10 minutes. For immunohistochemical detection, according to the manufacturer’s instruction, the specimens were incubated at room temperature for 1 h with the following antibodies, respectively: phpspho(p)-STAT3 (serine 727, Santa Cruz, USA) at a dilution of 1:100; phpspho(p)-STAT3 (tyrosine 705, D3A7, Cell Signaling Technology, USA) at a dilution of 1:100. Then, the specimens were exposed to polyperoxidase-anti-rabbit/ mouse IgG (ZSGB-BIO, China) for 30 min, and diaminobenzidine detection kit (ZSGB-BIO, China) was used to develop peroxidase activity. Hematoxylin was used to counterstain the sections. Phosphate buffer solution (PBS, 0.01 M, pH 7.2) instead of the primary antibodies was applied to negative control slides. And according to the manufacturer’s instruction, positive control was performed using human breast carcinoma tissue and human lung carcinoma tissue for pSer727-STAT3 and pTyr705-STAT3, respectively.
Evaluation
Positive pSer727-STAT3 or pTyr705-STAT3 expression was considered when brown granules appeared in the nucleus of neoplastic cells. The high-power fields (×400) with the highest density of stained neoplastic cells was evaluated in order to determine the proportion of pSer727-STAT3 or pTyr705-STAT3 positive neoplastic cells in each sample, and the rate of positive expression in tumor cells was graded as previously described [2,15]: No positive expression and the positive expression rates which were ≤ 5% were determined as low expression, > 5% of stained neoplastic cells were considered as high expression. Immunohistochemical assessment was performed independently by two neuropathologists (Y.P.C., X.F.W.) without knowledge of patient clinical data.
Statistical methods
All the cases were followed up either by outpatient visit or telephone calling. Progression-free survival (PFS) was calculated according to the time (days) between surgery and first tumor progression (or death, or the latest follow-up). The period of time (days) between the date of surgical resection and death (or the latest follow-up) was determined as overall survival (OS). For statistical analyses, all calculations were performed using SPSS 19.0 statistical software. Correlation of pSer727-STAT3 with pTyr705-STAT3 expression and associations of pSer727-STAT3 expression with clinical variables (gender, age and KPS) were analyzed by non-parametric tests (Mann-Whitney U test and Spearman’s rank correlation, as appropriate). In univariate analysis, the associations of pSer727-STAT3 expression with PFS or OS were calculated using log-rank tests and displayed as Kaplan-Meier survival curves. COX proportional hazards model and stepwise regression analysis were applied to evaluate the influences of pSer727-STAT3 expression on PFS or OS in multivariate analysis, hazard ratios (HR) and the 95% confidence intervals (CI) were also computed. All results with a two-tailed P value less than 0.05 were considered as statistical significance.
Results
pSer727-STAT3 expression in glioblastoma tissues
Among 88 glioblastoma tissues, pSer727-STAT3 expression which mainly localized in the nucleus of neoplastic cells was detected in 62 (70.5%) cases; low expression of pSer727-STAT3 (Figure 1A) was 38 (43.2%) cases and high expression (Figure 1B) was 50 (56.8%) cases. Positive expression of pSer727-STAT3 was found in endothelial cells of some vessels (Figure 1C) as well, in addition to tumor cells. In some specimens, immunoreactivity was observed in the cytoplasm of tumor cells. No positive staining of normal brain tissue adjacent to tumor was observed.
Figure 1.
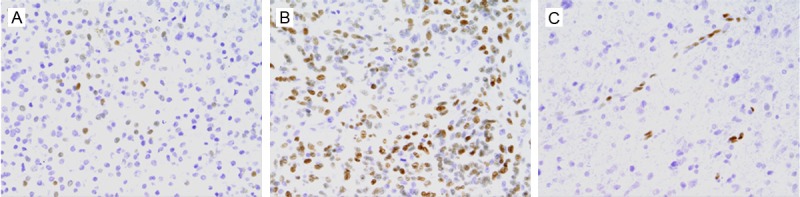
Immunohistochemical staining showing low (A) and high (B) expression of pSer727-STAT3 in glioblastoma tissues. Positive staining is also observed in endothelial cells of some vessels (C). Original magnification: ×400.
Correlations of pSer727-STAT3 expression with clinicopathological characteristics
To evaluate whether clinical characteristics would affect pSer727-STAT3 expression in glioblastoma, the relationships of pSer727-STAT3 expression with patient gender, age and preoperative KPS were analyzed. It was regretful that no statistically significant association was found between them in this study (Table 1).
Table 1.
Correlations of pSer727-STAT3 with clinical variables and pTyr705-STAT3 expression
| Variables | n | pSer727-STAT3 expression | ||
|---|---|---|---|---|
|
| ||||
| Median (%) | Rang (%) | P-value | ||
| Gender | ||||
| Male | 53 | 9 | 0-59 | 0.109a |
| Female | 35 | 5 | 0-42 | |
| KPS | ||||
| ≤ 70 | 46 | 8.5 | 0-59 | 0.251a |
| > 70 | 42 | 5 | 0-57 | |
| Age | 0.735b (r = 0.037) | |||
| pTyr705-STAT3 expression | 0.000b (r = 0.592) | |||
Results Mann-Whitney U test.
Results Spearman’s correlation coefficient.
Association between pSer727-STAT3 and pTyr705-STAT3 expression
The expression of pSer727-STAT3 and pTyr705-STAT3 in tumor cells exhibited considerable variability among GBM. In 88 cases, the percentage of pSer727-STAT3 positive neoplastic cells ranged from 0 to 59%, with a median of 8%, and the range of pTyr705-STAT3 expression was 0-78% (median 19%). A statistically significant positive correlation emerged among pSer727-STAT3 and pTyr705-STAT3 expression in glioblastoma (r = 0.592, P = 0.000) (Table 1).
pSer727-STAT3 expression and patient prognosis
During the follow-up period, 67 of the 88 patients with GBM (76.1%) showed tumor progression and 61 patients (69.3%) died. Follow-up showed that the median PFS time was 233 days (95% CI 183-283) and the median OS time was 334 days (95% CI 288-380) in the entire cohort. In univariate survival analysis (Tables 2 and 3), high percentage of pSer727-STAT3 positive neoplastic cells was shown to be significantly associated with a dismal PFS (P = 0.006) (Figure 2A) and OS (P = 0.002) (Figure 2B) of patients with GBM. The median PFS time for patients whose tumor exhibited high expression of pSer727-STAT3 was 186 days compared with 331 days for the remaining patients, and the median OS time was 201 days for patients highly expressing pSer727-STAT3 compared to 401 days for those displaying low expression. In addition, other parameters adversely influencing survival in the entire cohort included preoperative poor KPS and postoperative nonstandard chemoradiotherapy (P < 0.05), but patient gender and age were not related with their clinical prognosis (P > 0.05).
Table 2.
Univariate analysis and Multivariate analysis showing variables related to PFS in entire cohort
| Variables | n | Univariate analysis (PFS, days) | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Median | 95% CI | P-value | HR | 95% CI | P-value | ||
| Gender | |||||||
| Male | 53 | 216 | 170-262 | 0.580 | NS | ||
| Female | 35 | 246 | 185-307 | ||||
| Age | |||||||
| ≤ 55 years | 44 | 223 | 141-325 | 0.085 | NS | ||
| > 55 years | 44 | 225 | 176-274 | ||||
| KPS | |||||||
| ≤ 70 | 46 | 188 | 145-231 | 0.003 | 0.531 | 0.318-0.886 | 0.015 |
| > 70 | 42 | 275 | 199-351 | ||||
| Chemoradiotherapy | |||||||
| Nonstandard | 56 | 188 | 157-219 | 0.007 | 0.544 | 0.320-0.922 | 0.024 |
| Standard | 32 | 336 | 274-398 | ||||
| pSer727-STAT3 | |||||||
| Low expression | 38 | 331 | 246-416 | 0.006 | 1.830 | 1.090-3.074 | 0.022 |
| High expression | 50 | 186 | 149-224 | ||||
NS: no significance.
Table 3.
Univariate analysis and Multivariate analysis showing variables associated with OS in entire cohort
| Variables | n | Univariate analysis (OS, days) | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Median | 95% CI | P-value | HR | 95% CI | P-value | ||
| Gender | |||||||
| Male | 53 | 331 | 230-432 | 0.837 | NS | ||
| Female | 35 | 334 | 253-425 | ||||
| Age | |||||||
| ≤ 55 years | 44 | 382 | 178-584 | 0.067 | NS | ||
| > 55 years | 44 | 330 | 260-400 | ||||
| KPS | |||||||
| ≤ 70 | 46 | 269 | 198-338 | 0.001 | 0.447 | 0.225-0.783 | 0.005 |
| > 70 | 42 | 398 | 349-447 | ||||
| Chemoradiotherapy | |||||||
| Nonstandard | 56 | 218 | 156-280 | 0.000 | 0.336 | 0.187-0.606 | 0.000 |
| Standard | 32 | 549 | 370-728 | ||||
| pSer727-STAT3 | |||||||
| Low expression | 38 | 401 | 291-511 | 0.002 | 1.797 | 1.028-3.142 | 0.040 |
| High expression | 50 | 201 | 171-263 | ||||
NS: no significance.
Figure 2.
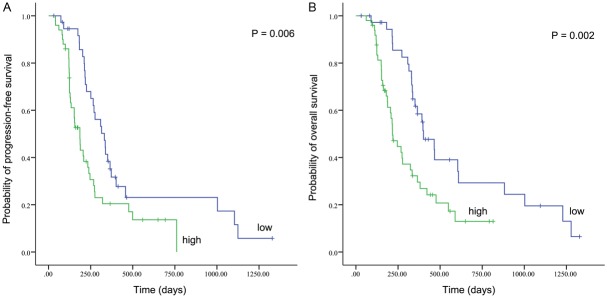
Kaplan-Meier survival curves showing a correlation of proportion of pSer727-STAT3 positive neoplastic cells with progression-free survival (A) and overall survival (B) in 88 patients with glioblastoma.
In multivariate survival analysis (Tables 2 and 3), COX proportional hazards regression model which was carried out on gender, age, KPS, chemoradiotherapy and the expression level of pSer727-STAT3 in GBM tissues showed that high percentage of pSer727-STAT3 positive neoplastic cells was a significant independent unfavorable prognostic indicator for PFS (HR 1.830, P = 0.022) and OS (HR 1.797, P = 0.040); similar results were obtained for poor KPS and nonstandard chemoradiotherapy (P < 0.05), but not for patient gender and age.
Our previous study have already confirmed pTyr705-STAT3 expression > 5% as an independent unfavorable prognostic factor [2]. Thus, we examined whether patients with two unfavorable factors (both high pTyr705-STAT3 expression and high pSer727-STAT3 expression) had a poorer prognosis compared with those with one unfavorable factor (highly expressed pTyr705-STAT3 or pSer727-STAT3). Out of 74 cases (excluding patients with lowly expressed both pTyr705-STAT3 and pSer727-STAT3), 47 patients displayed two unfavorable factors and 27 displayed only one unfavorable factor. The statistical results revealed that patients with two unfavorable factors exhibited a shorter PFS (P = 0.007) (Figure 3A) and OS (P = 0.005) (Figure 3B), compared to the remaining patients. When adjusted for gender, age, KPS and chemoradiotherapy and adjusted HR of patients with one unfavorable factor was 1.0 (as a reference), the adjusted HR of patients with two unfavorable factors was 2.405 (95% CI 1.319-4.384, P = 0.004) for PFS and 2.579 (95% CI 1.373-4.847, P = 0.003) for OS.
Figure 3.
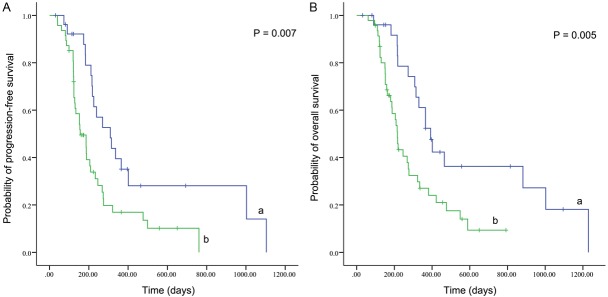
Kaplan-Meier survival curves of progression-free survival (A) and overall survival (B) in glioblastoma patientswith both high pTyr705-STAT3 expression and high pSer727-STAT3 expression (a) and patients with highly expressed pTyr705-STAT3 or pSer727-STAT3 (b).
Discussion
Even though enormous effort has been made to optimize the multimodality treatment in patient with glioblastoma, present therapeutic approaches provide little improvement in clinical outcome [16,17]. Hence, the knowledge of the signaling pathways that promote GBM tumorigenesis and progression is crucial. Recently, a considerable body of research has been focused on STAT3 molecule- a signaling node for carcinogenic pathways in glioma. Here we confirm for the first time that high percentage of pSer727-STAT3 positive neoplastic cells in GBM is an independent unfavorable predictor of survival, thereby implying an oncogenic capacity of STAT3 phosphorylation at serine 727 in glioblastoma.
Recent studies reveal that Ser727 phosphorylation of STAT3 play a significant role in tumorigenesis in various human cancers. Phosphorylation at serine 727 is sufficient to activate STAT3 independent of pTyr705, thus facilitating prostate carcinogenesis [5]; and pSer727-STAT3 confers full transcriptional activation of cyclin D1 and is required for breast cancer growth [6]. It has been reported that in glioma pSer727-STAT3 contributes to the constitutive activation of STAT3 and is essential for cell invasion [8]. Moreover, in glioblastoma stem cells STAT3 is shown to be constitutively phosphorylated at serine 727 preferentially, and the apoptotic effects of NOTCH signaling pathway blockade may be regulated through reducing the level of pSer727-STAT3 [9,10]. Recently a study shows that pSer727-STAT3 is closely associated with temozolomide-resistance in glioma cells [18]. Furthermore, the significant decrease of STAT3 phosphorylation at serine 727 by Stattic (a STAT3 inhibitor) could impair the proliferation of glioblastoma stem cells and sensitize them to the inhibitory effect of temozolomide with an obvious synergistic action [10], indicating that pSer727-STAT3 might be essential for the maintenance of self-renewal in glioblastoma stem cells which are highly resistant to temozolomide and ionizing radiations and could contribute to the cause of tumor recurrence [19-22]. Consequently, all of which have provided compelling evidence for the important role of pSer727-STAT3 in carcinogenesis, thus determining pSer727-STAT3 as an unfavorable prognostic factor of GBM.
Meanwhile, the statistical results show that patients with both high pTyr705-STAT3 expression and high pSer727-STAT3 expression exhibit a poorer clinical outcome compared with the remainder, suggesting that there might exist a synergistic effect between tyrosine and serine phosphorylation of STAT3. One possible explanation is that it might be associated with the regulation of STAT3 target genes involved in tumor progression. Previous studies indicate that pTyr705-STAT3 is required for the growth of glioblastoma cells [23,24], and serves as a pivotal driver of glioma angiogenesis, invasion and immunosuppression through regulating the expression of its corresponding target genes [25-27], thereby predicting a dismal prognosis [2]. Meanwhile, pSer727-STAT3 is necessary for the maximal transcriptional activity of STAT3 [3,4], and serine 727 phosphorylation is sufficient to activate STAT3 irrespective of tyrosine 705 phosphorylation [5]. Moreover, evidence shows that cooperation of phosphorylation at both serine and tyrosine residue contributes to full activation of STAT3 [4]. Taken together, these findings may explain that high expression of both pTyr705-STAT3 and pSer727-STAT3 is predictive of poorer prognosis in GBM patients.
More interestingly, recently pSer727-STAT3 has been reported to possess another function in addition to the role of modulating transcriptional activation. A pool of pSer727-STAT3 in mitochondria controls cellular respiration and supports altered oxidative phosphorylation activities characteristic of tumor cells, thereby regulating the metabolic shift important for tumor growth and sustaining Ras-dependent malignant transformation [28,29]. Furthermore, mitochondrial localized STAT3 contributes to tumorigenesis via phosphorylation of serine 727 [30]. In the present study, in some cases pSer727-STAT3 staining is also seen in the cytoplasm of tumor cells in accordance with a recent research [10], indicating that it is possible that in glioblastoma there is a similar mechanism for pSer727-STAT3-mediated tumorigenesis. Additionally, our results show that no significant associations are observed between the expression of pSer727-STAT3 and patient gender, age and preoperative performance status, suggesting that gender, age and KPS might not affect the expression of pSer727-STAT3 in glioblastoma.
To better understand the role of phosphorylation of STAT3 at serine 727 in glioblastoma pathogenesis, further studies focusing on investigating the nature of pSer727-STAT3 positive tumor cells are essential, especially the further insight into the regulatory mechanism of pSer727-STAT3 in glioblastoma stem cells.
In conclusion, this is the first study to demonstrate that the high percentage of pSer727-STAT3 positive neoplastic cells in GBM is an independent prognostic indicator for dismal clinical outcome, and increased expression of both pTyr705-STAT3 and pSer727-STAT3 is predictive of poorer prognosis. These data add to the growing evidence for the oncogenic role of STAT3 phosphorylation in GBM, implying that strategies centered upon the inhibition of STAT3 activation might be helpful for those patients.
Acknowledgements
This work was supported by key Clinical Special Discipline Construction Program of Fujian, China.
Disclosure of conflict of interest
None.
References
- 1.Swiatek-Machado K, Kaminska B. STAT signaling in glioma cells. Adv Exp Med Biol. 2013;986:189–208. doi: 10.1007/978-94-007-4719-7_10. [DOI] [PubMed] [Google Scholar]
- 2.Lin GS, Yang LJ, Wang XF, Chen YP, Tang WL, Chen L, Lin ZX. STAT3 Tyr705 phosphorylation affects clinical outcome in patients with newly diagnosed supratentorial glioblastoma. Med Oncol. 2014;31:924. doi: 10.1007/s12032-014-0924-5. [DOI] [PubMed] [Google Scholar]
- 3.Wen Z, Zhong Z, Darnell JE Jr. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Shaw PE. A STAT3 dimer formed by inter-chain disulphide bridging during oxidative stress. Biochem Biophys Res Commun. 2004;322:1005–1011. doi: 10.1016/j.bbrc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Qin HR, Kim HJ, Kim JY, Hurt EM, Klarmann GJ, Kawasaki BT, Duhagon Serrat MA, Farrar WL. Activation of signal transducer and activator of transcription 3 through a phosphomimetic serine 727 promotes prostate tumorigenesis independent of tyrosine 705 phosphorylation. Cancer Res. 2008;68:7736–7741. doi: 10.1158/0008-5472.CAN-08-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tkach M, Rosemblit C, Rivas MA, Proietti CJ, Diaz Flaque MC, Mercogliano MF, Beguelin W, Maronna E, Guzman P, Gercovich FG, Deza EG, Elizalde PV, Schillaci R. p42/p44 MAPK-mediated Stat3Ser727 phosphorylation is required for progestin-induced full activation of Stat3 and breast cancer growth. Endocr Relat Cancer. 2013;20:197–212. doi: 10.1530/ERC-12-0194. [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi M, Oka M, Iwasaki T, Fukami Y, Nishigori C. Role and regulation of STAT3 phosphorylation at Ser727 in melanocytes and melanoma cells. J Invest Dermatol. 2012;132:1877–1885. doi: 10.1038/jid.2012.45. [DOI] [PubMed] [Google Scholar]
- 8.Aziz MH, Hafeez BB, Sand JM, Pierce DB, Aziz SW, Dreckschmidt NE, Verma AK. Protein kinase Cvarepsilon mediates Stat3Ser727 phosphorylation, Stat3-regulated gene expression, and cell invasion in various human cancer cell lines through integration with MAPK cascade (RAF-1, MEK1/2, and ERK1/2) Oncogene. 2010;29:3100–3109. doi: 10.1038/onc.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan X, Khaki L, Zhu TS, Soules ME, Talsma CE, Gul N, Koh C, Zhang J, Li YM, Maciaczyk J, Nikkhah G, Dimeco F, Piccirillo S, Vescovi AL, Eberhart CG. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells. 2010;28:5–16. doi: 10.1002/stem.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villalva C, Martin-Lanneree S, Cortes U, Dkhissi F, Wager M, Le Corf A, Tourani JM, Dusanter-Fourt I, Turhan AG, Karayan-Tapon L. STAT3 is essential for the maintenance of neurosphere-initiating tumor cells in patients with glioblastomas: a potential for targeted therapy? Int J Cancer. 2011;128:826–838. doi: 10.1002/ijc.25416. [DOI] [PubMed] [Google Scholar]
- 11.Chung J, Uchida E, Grammer TC, Blenis J. STAT3 serine phosphorylation by ERK-dependent and -independent pathways negatively modulates its tyrosine phosphorylation. Mol Cell Biol. 1997;17:6508–6516. doi: 10.1128/mcb.17.11.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woetmann A, Nielsen M, Christensen ST, Brockdorff J, Kaltoft K, Engel AM, Skov S, Brender C, Geisler C, Svejgaard A, Rygaard J, Leick V, Odum N. Inhibition of protein phosphatase 2A induces serine/threonine phosphorylation, subcellular redistribution, and functional inhibition of STAT3. Proc Natl Acad Sci U S A. 1999;96:10620–10625. doi: 10.1073/pnas.96.19.10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain N, Zhang T, Fong SL, Lim CP, Cao X. Repression of Stat3 activity by activation of mitogen-activated protein kinase (MAPK) Oncogene. 1998;17:3157–3167. doi: 10.1038/sj.onc.1202238. [DOI] [PubMed] [Google Scholar]
- 14.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J. Clin. Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 15.Birner P, Toumangelova-Uzeir K, Natchev S, Guentchev M. STAT3 tyrosine phosphorylation influences survival in glioblastoma. J Neurooncol. 2010;100:339–343. doi: 10.1007/s11060-010-0195-8. [DOI] [PubMed] [Google Scholar]
- 16.Yang LJ, Zhou CF, Lin ZX. Temozolomide and radiotherapy for newly diagnosed glioblastoma multiforme: a systematic review. Cancer Invest. 2014;32:31–36. doi: 10.3109/07357907.2013.861474. [DOI] [PubMed] [Google Scholar]
- 17.Hadziahmetovic M, Shirai K, Chakravarti A. Recent advancements in multimodality treatment of gliomas. Future Oncol. 2011;7:1169–1183. doi: 10.2217/fon.11.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee ES, Ko KK, Joe YA, Kang SG, Hong YK. Inhibition of STAT3 reverses drug resistance acquired in temozolomide-resistant human glioma cells. Oncol Lett. 2011;2:115–121. doi: 10.3892/ol.2010.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, Parada LF. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Zhang W, Mao XG, Zhen HN, Cao WD, Hu SJ. Targeting role of glioma stem cells for glioblastoma multiforme. Curr Med Chem. 2013;20:1974–1984. doi: 10.2174/0929867311320150004. [DOI] [PubMed] [Google Scholar]
- 21.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 22.Kang MK, Kang SK. Tumorigenesis of chemotherapeutic drug-resistant cancer stem-like cells in brain glioma. Stem Cells Dev. 2007;16:837–847. doi: 10.1089/scd.2007.0006. [DOI] [PubMed] [Google Scholar]
- 23.Dasgupta A, Raychaudhuri B, Haqqi T, Prayson R, Van Meir EG, Vogelbaum M, Haque SJ. Stat3 activation is required for the growth of U87 cell-derived tumours in mice. Eur J Cancer. 2009;45:677–684. doi: 10.1016/j.ejca.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin L, Hutzen B, Li PK, Ball S, Zuo M, DeAngelis S, Foust E, Sobo M, Friedman L, Bhasin D, Cen L, Li C, Lin J. A novel small molecule, LLL12, inhibits STAT3 phosphorylation and activities and exhibits potent growth-suppressive activity in human cancer cells. Neoplasia. 2010;12:39–50. doi: 10.1593/neo.91196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Senft C, Priester M, Polacin M, Schroder K, Seifert V, Kogel D, Weissenberger J. Inhibition of the JAK-2/STAT3 signaling pathway impedes the migratory and invasive potential of human glioblastoma cells. J Neurooncol. 2011;101:393–403. doi: 10.1007/s11060-010-0273-y. [DOI] [PubMed] [Google Scholar]
- 26.Kang SH, Yu MO, Park KJ, Chi SG, Park DH, Chung YG. Activated STAT3 regulates hypoxia-induced angiogenesis and cell migration in human glioblastoma. Neurosurgery. 2010;67:1386–1395. doi: 10.1227/NEU.0b013e3181f1c0cd. discussion 1395. [DOI] [PubMed] [Google Scholar]
- 27.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 28.Gough DJ, Corlett A, Schlessinger K, Wegrzyn J, Larner AC, Levy DE. Mitochondrial STAT3 supports Ras-dependent oncogenic transformation. Science. 2009;324:1713–1716. doi: 10.1126/science.1171721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wegrzyn J, Potla R, Chwae YJ, Sepuri NB, Zhang Q, Koeck T, Derecka M, Szczepanek K, Szelag M, Gornicka A, Moh A, Moghaddas S, Chen Q, Bobbili S, Cichy J, Dulak J, Baker DP, Wolfman A, Stuehr D, Hassan MO, Fu XY, Avadhani N, Drake JI, Fawcett P, Lesnefsky EJ, Larner AC. Function of mitochondrial Stat3 in cellular respiration. Science. 2009;323:793–797. doi: 10.1126/science.1164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Q, Raje V, Yakovlev VA, Yacoub A, Szczepanek K, Meier J, Derecka M, Chen Q, Hu Y, Sisler J, Hamed H, Lesnefsky EJ, Valerie K, Dent P, Larner AC. Mitochondrial localized Stat3 promotes breast cancer growth via phosphorylation of serine 727. J Biol Chem. 2013;288:31280–31288. doi: 10.1074/jbc.M113.505057. [DOI] [PMC free article] [PubMed] [Google Scholar]


