Abstract
Galectin-3 (Gal3) has been implicated in the development of different tumors because of its involvement in the Wnt signaling pathway by promoting beta-catenin translocation into the nucleus. The APC protein, a negative regulator of this pathway, has been strongly implicated in the development of colon cancer, but still has an undetermined role in the formation of oral cancer. Therefore, this study aimed to evaluate the relationship between Gal3, the Wnt signaling pathway, and APC expression in dysplasias and carcinomas developed experimentally in mice. Sixty galectin-3-deficient (Gal3-/-) and 60 wild-type (Gal3+/+) mice were early employed to be treated with the carcinogen 4NQO for 16 weeks and killed at either week 16 or week 32. Tongues were removed, processed and embedded in paraffin blocks. Sections 5 μm thick were made, and then stained by H&E to establish the diagnosis of dysplasia and carcinoma. Sections of 2 μm thickness were made to detect APC expression in these lesions by immunohistochemistry. Oral carcinogenesis occurred in both groups of mice, but no statistical difference was reached. APC expression was exclusively seen in the cytoplasm of all lesions studied. In the intragroup analysis, the majority of dysplasias and carcinomas exhibiting higher APC immunoreactivity was observed in Gal3-/- mice compared to Gal3+/+ mice, but no significant difference was found. However, a statistical difference was only observed between dysplastic lesions from two mice. Our results showed that neither the absence of Gal3 nor the APC protein appears to play a role in malignant transformation of the tongue.
Keywords: Oral carcinogenesis, immunohistochemistry, galectin-3, APC protein, tongue, mice
Introduction
Oral squamous cell carcinoma (OSCC) is a deadly malignancy, accounting for more than 90% of malignant tumors of the oral cavity worldwide [1]. Although there has been considerable progress in the understanding of genetic and molecular alterations related to its development, the exact molecular mechanism underlying its formation as well as progression has yet to be established in the literature [2]. For this purpose, a mouse model of oral carcinogenesis has been considered an important tool to underscore the events responsible for malignant transformation of keratinocytes from oral epithelium tissue [3,4]. Despite being reported to present some drawbacks, the mouse model allow to reproduce, at least in a reliable way, all sequential stages linked to fully malignant transformation, which range from hyperplasia to dysplasia and carcinoma [5-7]. Additionally, there are a few reports studying oral carcinogenesis in knockout mice for a targeted protein, which may be extremely valuable for determining the prime role of a specific protein inside the cell and its involvement in tumorigenesis through deregulating cellular homeostasis [8-12].
Galectin-3 (Gal3) is a carbohydrate-binding protein that has been implicated in tumor development [13]. The literature presents conflicting results regarding the importance of this lectin in tumor formation, suggesting that there is much more to be learned about its role in this context [14]. To shed light on this, in 2004 it was shown that Gal3 is an important key regulator of the Wnt signaling pathway, since then, several reports have spotted such regulation in different types of tumors, including OSCC [15-17]. For instance, an in vitro study recently reported that Gal3 may increase the potential of invasiveness of human tongue cancer cells through the Wnt/â-catenin signaling pathway and Akt phosphorylation [18]. In contrast, we previous showed in vivo that absence of Gal3 did not interfere in the incidence of tongue carcinomas in Gal3-/- mice challenged by the carcinogen 4-nitroquinoline-1-oxide (4NQO) [8]. Moreover, our group recently showed that beta-catenin expression during tongue malignant transformation did not differ between Gal3-/- and Gal3+/+ mice, indicating that the activated Wnt signaling pathway might be present in both groups of mice [19].
Due to the presence of conflicting results, it is necessary to continue studying the relationship between Gal3 and the Wnt signaling pathway and how this pathway may be affected by the absence of this lectin in the context of oral tumor development. In this way, the APC protein may be such a candidate especially because it acts as a negative regulator of this pathway and has been strongly associated with colon cancer [20,21]. In fact, it was from studies of this kind of tumor that a direct association was observed between mutated APC protein, an accumulation of beta-catenin inside the cell, and tumor formation [22,23]. However, its involvement in oral carcinogenesis is still poorly understood, therefore, determining its actual role in oral malignant transformation remains necessary.
As a result, in order to understand whether the absence of Gal3 may interfere in this process by modulating APC expression and the subsequent activity of the Wnt signaling pathway, this paper is aimed to describe, for the first time, APC expression in premalignant and malignant lesions experimentally developed in galectin-3 deficient (Gal3-/-) and wild-type (Gal3+/+) mice after challenge by the carcinogen 4NQO for 16 weeks.
Material and methods
Animals
Gal3-/- mice were generated through homologous recombination and backcrossed to C57BL/6 mice, as described by Hsu, et al. [24]. Thirty-six male Gal3-/- mice, weighing from 21 to 23 g, were employed in this study. Forty-one sex- and age-matched Gal3+/+ mice with the same genetic background were used as the control group. All mice were maintained under controlled conditions of temperature (22°C), light-dark periods of 12 h, and free access to commercial diet. The experiment was approved by the Committee on Animal Experimentation of the Universidade Federal de Uberlândia (protocol number 038/09).
Experimental protocol
To study APC expression in dysplasias and carcinomas, Gal3+/+ and Gal3-/- mice were submitted to a continuous ingestion of the carcinogen 4NQO (SIGMA CO, MO, USA). Then, all mice were randomly separated and killed at one of two time points (week 16 and week 32), which immediately followed 16 weeks of 4NQO ingestion. The protocol used was the same as that described by Tang, et al. [25]. Briefly, the carcinogen was diluted until a concentration of 100 μg/ml was achieved and then administered orally for 16 weeks. This solution was prepared weekly and the bottles were refilled once a week. At the end of the treatment, mice from each group scheduled to be killed at week 16 were immediately sacrificed, while the remaining group received only tap water until euthanasia, which occurred at week 32.
Histopathological analysis
All Gal3+/+ and Gal3-/- mice were killed by cervical dislocation under deep ether anesthesia. Tongues were removed and cut transversally into five fragments. When lesions were visible on the tongue surface, they were carefully cut to permit analysis using light microscopy. In this case, other parts of the same tongue were used to discard potential microscopic changes not detectable by the naked eye. All tissues were fixed in a 10% neutral-buffered formalin solution for 24 h, embedded in paraffin blocks, and stained with hematoxylin and eosin for histological examination. We used the criteria described by Lumerman, et al. [26] and Cardesa, et al. [27] to identify areas of dysplasia and carcinoma, respectively. All histological slides were blindly and independently examined by three well-trained pathologists (PRF, AML, and SVC). Subsequently, consensus scoring using a multihead microscope was used to solve any discrepancies. When two or more areas of epithelial alterations were present in the same histological slide, the highest grade epithelial lesion was taken as representative in order to determine which pathologic alteration was present in each mouse.
Immunohistochemistry for APC
All tongue sections from each group of mice were used to determine the pattern of APC protein expression. For this, an immunohistochemical assay using the avidin-biotin-peroxidase method was performed on 3 μm-thick sections mounted on 3-aminopropyltrietoxy-sylane-coated glass slides. After deparaffinization and hydration, sections were submitted to antigen retrieval with citrate buffer (10 mM, pH 6.0 using a microwave oven for three cycles of 5 min). Endogenous peroxidase activity was blocked with 3% H2O2 for 15 min, and then with a protein block solution (1% skim milk, 0.05% Triton X, and phosphate buffered saline [PBS]) for 20 min at room temperature to prevent nonspecific binding. Immunostaining was performed by incubating the primary monoclonal antibody against APC protein (Santa Cruz Biotechnology, CA, USA), diluted at 1:400, in a humid chamber at 4°C overnight. After amplification, the reaction was detected using the chromogen 3,3′-diamino-benzidinetetrahydrochloride (Sigma-Aldrich, USA). All sections were counterstained with Harris’ hematoxylin. As a negative control, the PBS solution was substituted for the primary antibody. Fragments of colon carcinoma tissue with known positivity for APC protein expression were used as a control.
Immunohistochemical evaluation
Expression was evaluated based on semiquantitative parameters. For this, two parameters were considered. First, the proportion of positive cells (PC) was assessed, which was graded in 0 (negative), 1 (1-25%), 2 (>25%-50%), 3 (>50%-75%), and 4 (>75%). Second, the intensity of stain (IS) was graded in 0 (negative), 1 (weak), 2 (intermediate), and 3 (strong). A final score was achieved by adding PC + IS. For statistical purpose, all lesions were categorized into two groups, as follows: 1, when PC + IS ≤2 (low expression), and 2, when PC + IS >2 (high expression).
Statistical analysis
The incidence of dysplasias and carcinomas between Gal3+/+ and Gal3-/- mice was determined by the Chi-square test. The relative risk (95% of confidence interval) was also calculated for the occurrence of dysplasia and carcinoma in Gal3-/- mice in comparison to Gal3+/+ animals. Fisher’s exact test was employed to evaluate APC protein expression between dysplasias and carcinomas from each group of mice. P-values less than 0.05 were considered significant to reject the null hypothesis. All analyses were performed using the software GraphPad Prism version 5.0 for Windows (GraphPad Software, San Diego, CA, USA).
Results
Incidence of papillomas, dysplasias and carcinomas
Unfortunately, some mice died during the experiment, which was interpreted as being not only a result of the carcinogen’s side-effects, but also due to starvation via tumor growth, especially in those mice scheduled to be killed at week 32, when the incidence of death was higher.
As depicted in Table 1, the chemical carcinogenesis procedure induced dysplasias and carcinomas and less frequently papillomas in Gal3+/+ and Gal3-/- mice. At week 16, 100% and 96.1% of Gal3+/+ and Gal3-/- mice developed dysplasia, respectively. In the same week, the incidence of carcinomas was 60% and 50% of the Gal3+/+ and Gal3-/- mice respectively. At week 32, on the other hand, the number of carcinomas increased in both groups of mice, but slightly more in Gal3+/+ (93.7%) than in Gal3-/- mice (90%). At this time, 100% of Gal3+/+ and Gal3-/- mice also presented dysplastic lesions. Statistically, neither dysplasia nor carcinoma incidence in the tongue showed a significant difference between Gal3+/+ and Gal3-/- mice. Moreover, the relative risk of the development of dysplasia and carcinoma in Gal3-/- mice was 0.97 (CI 95% 0.92-1.02) and 0.93 (CI 95% 0.60-1.15), respectively, which indicates that the absence of Gal3 is not pivotal for the occurrence of premalignant and malignant lesions when compared to mice that normally express this lectin. Likewise, the incidence of papillomas was somewhat similar in both groups of mice and increased from week 16 to week 32, but no significant difference was found.
Table 1.
Incidence of papillomas, dysplasias, and carcinomas diagnosed in Gal3+/+ and Gal3-/- mice at the end of the experiment
| Group of mice | Type of lesion | |||
|---|---|---|---|---|
|
| ||||
| Number of mice (%) | ||||
|
| ||||
| Papilloma | Dysplasia | Carcinoma | ||
| Week 16 | Gal3+/+ | 3/25 (12) | 25/25 (100)a | 15/25 (60)e |
| Gal3-/- | 0/26 (0) | 25/26 (96.1)b | 13/26 (50)f | |
| Week 32 | Gal3+/+ | 3/16 (18.7) | 16/16 (100)c | 15/16 (93.7)g |
| Gal3-/- | 2/10 (20) | 10/10 (100)d | 9/10 (90)h | |
Chi-square test: a + c vs. b + d: P = 0.46; e + g vs. f + h: P = 0.33; a + b: P > 0.05; c + d: P > 0.05; e + f: P = 0.57; g + h: P > 0.05.
APC protein expression
As the dimension of the lesions was very small, the number of lesions analyzed by immunohistochemistry was lower than those observed microscopically to establish the diagnosis of each lesion. In our study, APC protein expression was revealed to be exclusively cytoplasmic. In respect to intensity, it was variable, with a high incidence of lesions exhibiting a weak expression or even being negative. Dysplasias and carcinomas from Gal3-/- mice presented higher APC expression score than Gal3+/+ mice.
Figure 1A depicts the pattern of APC expression in both groups of mice and the number os lesions exhibiting low and high APC immunoreactivity. For the statistical purposes, we divided both dysplasia and carcinoma from each group of mice as presenting low and high expression according to the final score obtained for each lesion. When we compared APC expression in dysplasia between Gal3+/+ and Gal3-/- mice, a significant difference was found (P = 0.0004) (Figure 1B). However, no statistically significant difference was reached when carcinomas-expressing APC protein were compared between Gal3+/+ and Gal3-/- mice (Figure 1B), as seen in the intra-group analysis (Figure 2).
Figure 1.
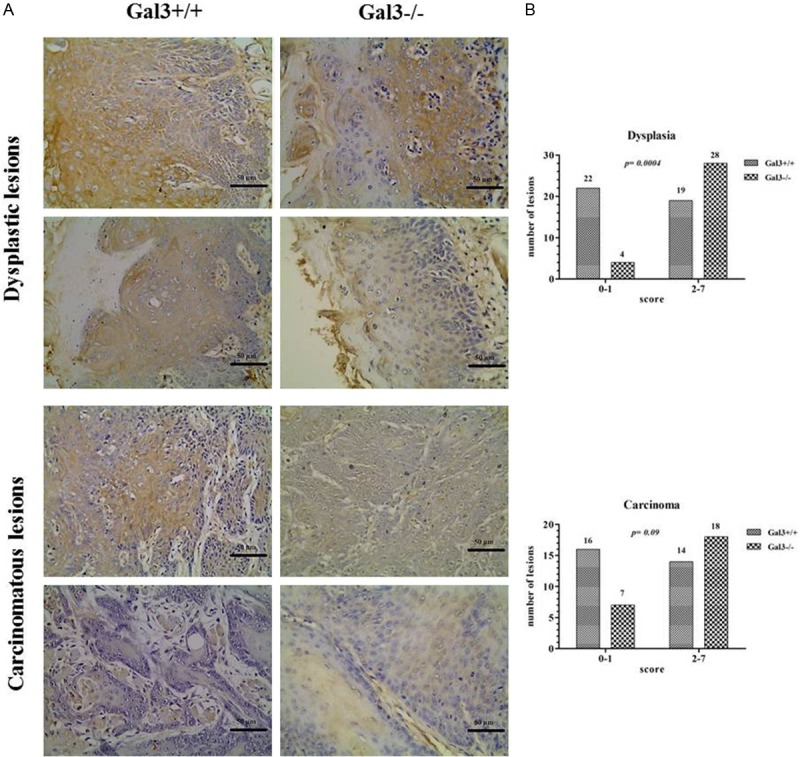
A. Immunohistochemical expression of the APC protein in dysplastic and carcinomatous lesions from Gal3+/+ and Gal3-/- mice exhibiting low (lower images of each lesion sample) and high (upper images of each lesion sample) immunoreactivity (dysplastic lesions: original magnification 100x; carcinomatous lesions: original magnification 400x). B. Comparative analysis in respect to the number of lesions expressing low (score: 0 to 1) and high (score: 2 to 7) APC protein in dysplasias and carcinomas from Gal3+/+ and Gal3-/- mice.
Figure 2.
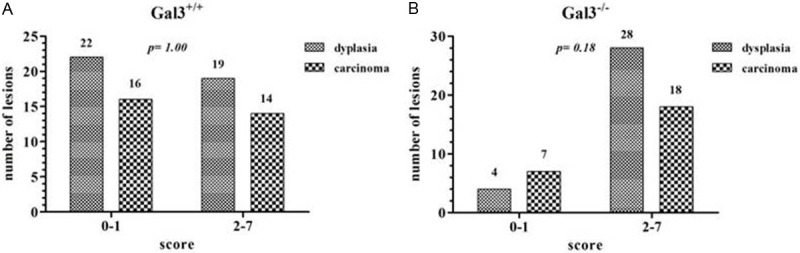
Intra-group analysis depicting the number of dysplastic and carcinomatous lesions expressing low (score: 0 to 1) and high (score: 2 to 7) APC protein in Gal3+/+ (A) and Gal3-/- (B) mice.
Discussion
This paper focused on finding a possible association between Gal3 and the development of tongue carcinoma, in particular to try to understand whether the absence of Gal3 could modulate expression of the APC protein. Confirming our previous study using the same mouse model of oral carcinogenesis, the present data showed that the APC protein, a key regulatory protein of the Wnt signaling pathway, was not involved in fully malignant transformation of tongue epithelium, supporting the hypothesis that during the tumor development in the mouse tongue, even in the absence of Gal3, this pathway seems not to be differently abrogated [8].
In respect to carcinogenesis, this was observed in both groups of mice. As expected, the number of Gal3-/- and Gal3+/+ mice that developed tongue carcinoma at week 32 was much higher than at week 16, whereas the incidence of dysplasia remained kept practically unchanged from week 16 to week 32, except in Gal3-/- mice, in which the level of dysplasia- affected mice reached 100% by week 32. Nevertheless, our findings did not show any statistical significance and the relative risk test indicated that the risk of the occurrence of both lesions in Gal3-/- mice is the same when compared to those normally expressing this lectin. These results are quite similar to those described by this group previously [8]. In that study, using the same mouse model, no difference was found. Therefore, these data reinforce our previous hypothesis that the absence of Gal3 does not have any influence on occurrence of tongue epithelium transformation in mice. A similar conclusion was reached by [28], who did not report any role for Gal3 in the development of breast and bowel tumors in mice.
It is well known that this lectin plays significant roles in several cellular processes including those with remarkable involvement in tumor formation, such as apoptosis, cell proliferation, angiogenesis, and adhesion [13,14,29]. Additionally, it was recently shown that Gal3 is a key member of the Wnt signaling pathway as it binds to and dislocates beta-catenin into the nucleus to cause the constitutive expression of Wnt target proteins, like cyclin D1 and c-myc [15,30]. Confirming this statement, it was reported that aberrant Wnt signaling activation was more prominent in lung tumors from Gal3+/+ when compared to Gal3-/- mice [16]. Although the results presented here are different from those demonstrated by Abdel-Aziz, et al. [16], constitutive activation of the Wnt pathway in tongue epithelium from Gal3+/+ and Gal3-/- might, at least in part, explain the figures obtained in the present study. In this regard, our group previously demonstrated that the expression of non-membranous (NM) beta-catenin was quite similar in dysplasia and carcinoma from each group of mice, indicating the aberrant activation of this pathway, which could be induce cell proliferation and angiogenesis, and thus tongue malignant transformation in Gal3+/+ and Gal3-/- mice [31]. In short, a persistent activation of the Wnt pathway in lesions from Gal3-/- mice might indicate that beta-catenin is not always shifted to the nucleus by binding to Gal3 and an independently mechanism of transportation without the involvement of this lectin should be investigated in oral tissues undergoing malignant transformation.
Among the downstream Wnt signaling components, the APC protein has a striking role. This protein acts as a tumor suppressor and may modulate a large number of functions like cell adhesion, signal transduction, and transcriptional activation of genes [32]. In the non-activated Wnt signaling pathway state or in the absence of a APC gene mutation, the APC protein together with Axin, Gsk3-beta and many other proteins form a multiprotein structure where beta-catenin is promptly phosphorylated and subsequently degraded through ubiquitin-proteosome complex [20,33]. In contrast, when the APC gene is mutated or the Wnt signaling pathway is dysregulated, the cytoplasmic complex is disassembled preventing beta-catenin phosphorylation and its subsequent destruction, and therefore elevating the free pool of this molecule in the cytoplasm [20]. As a result, beta-catenin, under Gal3 mediation, is transported to the nucleus where transcriptional activation of Wnt target genes, such as MYC and CCND1, is triggered, driving cell proliferation and inhibiting apoptosis [34,35].
The overall body of evidence collected so far has shown conflicting results relating the APC protein with OSCC development. In general, most studies have focused on genetic and epigenetic alterations of the APC gene in OSCC [36-43]. However, there have been few studies analyzing its expression by immunohistochemistry [44]. Tsuchiya, et al. [44] reported nuclear and cytoplasmic APC-expressing neoplastic cells in almost all human OSCC tissue samples evaluated, with the pattern of immunoreactivity being more remarkable in the nuclei of these cells than those in from normal and dysplastic epithelia. On the other hand, Uesugi, et al. [38] observed a significant reduction or loss of the APC protein in 15 (30%) out of 50 OSCC samples analyzed by immunohistochemistry, with the majority of them (70%) exhibiting high cytoplasmic expression. In contrast, nuclear APC expression was observed in 95% of squamous cell carcinomas of the skin studied, with no case presenting cytoplasmic immunoreactivity [45]. Here, APC expression was exclusively cytoplasmic in all dysplasias and carcinomas analyzed and there was a complete absence of nuclear immunoreactivity. In theory, it is plausible to suppose that these differences may be associated with the heterogeneity of each tumor, even with the same phenotype and location, and/or the presence of genetic changes in the APC gene in the samples studied in each work. Moreover, it has been reported that its localization seems to be related to cell proliferation, with quiescent cells presenting cytoplasmic and proliferative ones nuclear accumulation [44]. An interesting finding of our study, however, was in relation to the intensity of APC expression in dysplasias and carcinomas of both groups. In this regard, we observed a clear tendency of the number of both lesions from Gal3-/- mice showing a large increase in the intensity score when compared to Gal3+/+ ones, although no significance was found. Thus, these figures suggest that whether or not APC expression might be associated with cell proliferation, the cell proliferative rate between Gal3-/- and Gal3+/+ lesions might be somehow different. Therefore, further analysis should be performed to elucidate this matter.
We also aimed to comparatively evaluate APC expression in Gal3+/+ and Gal3-/- mice focusing on its involvement in the development of cancer in Gal3-deficient tongue epithelium. Here, a statistical difference was found in APC-expressing dysplastic lesions between two groups (P = 0.0004). On the other hand, there was no difference in APC-expressing carcinomas when comparing both groups, as well as when the intra-group analysis between dysplasias and carcinomas was carried out, indicating that tongue carcinoma development was not associated with altered expression of the APC protein. In the meantime, there is no explanation for why differential APC expression was only observed in dysplasia, whereas distinct misregulation of upstream Wnt components was seen during progression from normal to dysplastic epithelium in Gal3+/+ and Gal3-/- mice. In fact, there are studies showing that the altered expression of upstream canonical Wnt signaling components may affect the activity of this pathway independent of the presence of mutated and non-mutated downstream factors [46]. Additionally, mutation of the APC gene seems to occur during the first step of colon carcinogenesis [45]. In OSCC, on the other hand, the frequency of mutational changes in the APC gene has been reported to be approximately 12.5%, with some reports unable to find any mutations in the mutation cluster region of the gene, which, as the name suggest, is a region where mutational variation is frequently observed [44]. Although the APC gene status in the present study was not evaluated, both hypotheses could partially explain our findings in dysplastic lesions. Taking into consideration the aforementioned, it is worth evaluating not only the expression of the products of an activated Wnt signaling pathway, like beta-catenin, cyclin D1, and c-myc, but also the mechanisms governing its regulation at the upstream level in this mouse model of carcinogenesis.
In conclusion, our results describing APC expression in dysplasias and carcinomas from Gal3+/+ and Gal3-/- mice revealed that this protein seems not to be involved in tongue malignant transformation in both groups of mice, despite the fact that the number of APC overexpressing-carcinomas from Gal3-/- mice was greater than in Gal3+/+ mice. Other studies still need to be conducted to further elucidate the real relationship between this lectin, the Wnt signaling pathway, and its upstream and downstream components during tongue tumorigenesis in mice.
Acknowledgements
The authors would like to acknowledge the Research Supporting Foundation of Minas Gerais (FAPEMIG: CDS-APQ-00397-09) for the financial support.
Disclosure of conflict of interest
None.
References
- 1.Schwartz JL, Gu X, Kittles RA, Baptiste A, Shklar G. Experimental oral carcinoma of the tongue and buccal mucosa: possible biologic markers linked to cancers at two anatomic sites. Oral Oncol. 2000;36:225–235. doi: 10.1016/s1368-8375(99)00077-9. [DOI] [PubMed] [Google Scholar]
- 2.Molinolo AA, Amornphimoltham P, Squarize CH, Castilho RM, Patel V, Gutkind JS. Dysregulated molecular networks in head and neck carcinogenesis. Oral Oncol. 2009;45:324–334. doi: 10.1016/j.oraloncology.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanojia D, Vaidya MM. 4-nitroquinoline-1-oxide induced experimental oral carcinogenesis. Oral Oncol. 2006;42:655–667. doi: 10.1016/j.oraloncology.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Smith LP, Thomas GR. Animal models for the study of squamous cell carcinoma of the upper aerodigestive tract: a historical perspective with review of their utility and limitations. Part A. Chemically-induced de novo cancer, syngeneic animal models of HNSCC, animal models of transplanted xenogeneic human tumors. Int J Cancer. 2006;118:2111–2122. doi: 10.1002/ijc.21694. [DOI] [PubMed] [Google Scholar]
- 5.Vered M, Yarom N, Dayan D. 4NQO oral carcinogenesis: animal models, molecular markers and future expectations. Oral Oncol. 2005;41:337–339. doi: 10.1016/j.oraloncology.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Fracalossi AC, Silva Mde S, Oshima CT, Ribeiro DA. Wnt/beta-catenin signalling pathway following rat tongue carcinogenesis induced by 4-nitroquinoline 1-oxide. Exp Mol Pathol. 2010;88:176–183. doi: 10.1016/j.yexmp.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Herzig M, Christofori G. Recent advances in cancer research: mouse models of tumorigenesis. Biochim Biophys Acta. 2002;1602:97–113. doi: 10.1016/s0304-419x(02)00039-2. [DOI] [PubMed] [Google Scholar]
- 8.de Faria PR, Chammas R, de Melo TL, Hsu DK, Liu FT, Nonogaki S, Cardoso SV, Loyola AM. Absence of galectin-3 does not affect the development of experimental tongue carcinomas in mice. Exp Mol Pathol. 2011;90:189–193. doi: 10.1016/j.yexmp.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Ide F, Kitada M, Sakashita H, Kusama K, Tanaka K, Ishikawa T. p53 haploinsufficiency profoundly accelerates the onset of tongue tumors in mice lacking the xeroderma pigmentosum group A gene. Am J Pathol. 2003;163:1729–1733. doi: 10.1016/S0002-9440(10)63531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ide F, Oda H, Nakatsuru Y, Kusama K, Sakashita H, Tanaka K, Ishikawa T. Xeroderma pigmentosum group A gene action as a protection factor against 4-nitroquinoline 1-oxide-induced tongue carcinogenesis. Carcinogenesis. 2001;22:567–572. doi: 10.1093/carcin/22.4.567. [DOI] [PubMed] [Google Scholar]
- 11.Liu L, Tang XH, Scognamiglio T, Gudas LJ. Oral carcinogenesis induced by 4-nitroquinoline 1-oxide in lecithin:retinol acyltransferase gene knockout mice. J Nutr Biochem. 2010;21:975–982. doi: 10.1016/j.jnutbio.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fong LY, Jiang Y, Farber JL. Zinc deficiency potentiates induction and progression of lingual and esophageal tumors in p53-deficient mice. Carcinogenesis. 2006;27:1489–1496. doi: 10.1093/carcin/bgl012. [DOI] [PubMed] [Google Scholar]
- 13.Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 14.Dumic J, Dabelic S, Flogel M. Galectin-3: an open-ended story. Biochim Biophys Acta. 2006;1760:616–635. doi: 10.1016/j.bbagen.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 15.Shimura T, Takenaka Y, Tsutsumi S, Hogan V, Kikuchi A, Raz A. Galectin-3, a novel binding partner of beta-catenin. Cancer Res. 2004;64:6363–6367. doi: 10.1158/0008-5472.CAN-04-1816. [DOI] [PubMed] [Google Scholar]
- 16.Abdel-Aziz HO, Murai Y, Takasaki I, Tabuchi Y, Zheng HC, Nomoto K, Takahashi H, Tsuneyama K, Kato I, Hsu DK, Liu FT, Hiraga K, Takano Y. Targeted disruption of the galectin-3 gene results in decreased susceptibility to NNK-induced lung tumorigenesis: an oligonucleotide microarray study. J Cancer Res Clin Oncol. 2008;134:777–788. doi: 10.1007/s00432-007-0345-3. [DOI] [PubMed] [Google Scholar]
- 17.Song S, Mazurek N, Liu C, Sun Y, Ding QQ, Liu K, Hung MC, Bresalier RS. Galectin-3 mediates nuclear beta-catenin accumulation and Wnt signaling in human colon cancer cells by regulation of glycogen synthase kinase-3beta activity. Cancer Res. 2009;69:1343–1349. doi: 10.1158/0008-5472.CAN-08-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang D, Chen ZG, Liu SH, Dong ZQ, Dalin M, Bao SS, Hu YW, Wei FC. Galectin-3 gene silencing inhibits migration and invasion of human tongue cancer cells in vitro via downregulating beta-catenin. Acta Pharmacol Sin. 2013;34:176–184. doi: 10.1038/aps.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sant’ana JM, Chammas R, Liu FT, Nonogaki S, Cardoso SV, Loyola AM, de Faria PR. Activation of the Wnt/beta-catenin signaling pathway during oral carcinogenesis process is not influenced by the absence of galectin-3 in mice. Anticancer Res. 2011;31:2805–2811. [PubMed] [Google Scholar]
- 20.Aoki K, Taketo MM. Adenomatous polyposis coli (APC): a multi-functional tumor suppressor gene. J Cell Sci. 2007;120:3327–3335. doi: 10.1242/jcs.03485. [DOI] [PubMed] [Google Scholar]
- 21.Burgess AW, Faux MC, Layton MJ, Ramsay RG. Wnt signaling and colon tumorigenesis--a view from the periphery. Exp Cell Res. 2011;317:2748–2758. doi: 10.1016/j.yexcr.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Sayans M, Suarez-Penaranda JM, Herranz- Carnero M, Gayoso-Diz P, Barros-Angueira F, Gandara-Rey JM, Garcia-Garcia A. The role of the adenomatous polyposis coli (APC) in oral squamous cell carcinoma. Oral Oncol. 2012;48:56–60. doi: 10.1016/j.oraloncology.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Chaw SY, Majeed AA, Dalley AJ, Chan A, Stein S, Farah CS. Epithelial to mesenchymal transition (EMT) biomarkers--E-cadherin, beta-catenin, APC and Vimentin--in oral squamous cell carcinogenesis and transformation. Oral Oncol. 2012;48:997–1006. doi: 10.1016/j.oraloncology.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Hsu DK, Yang RY, Pan Z, Yu L, Salomon DR, Fung-Leung WP, Liu FT. Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. Am J Pathol. 2000;156:1073–1083. doi: 10.1016/S0002-9440(10)64975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang XH, Knudsen B, Bemis D, Tickoo S, Gudas LJ. Oral cavity and esophageal carcinogenesis modeled in carcinogen-treated mice. Clin Cancer Res. 2004;10:301–313. doi: 10.1158/1078-0432.ccr-0999-3. [DOI] [PubMed] [Google Scholar]
- 26.Lumerman H, Freedman P, Kerpel S. Oral epithelial dysplasia and the development of invasive squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:321–329. doi: 10.1016/s1079-2104(05)80226-4. [DOI] [PubMed] [Google Scholar]
- 27.Cardesa A, Gale N, Nadal A, Zidar N. Tumors of the Hypopharynx, Larynx and Trachea. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization Classification of Tumors. Pathology and Genetics of Head and Neck Tumors. Lyon: IARC; 2005. pp. 118–121. [Google Scholar]
- 28.Eude-Le Parco I, Gendronneau G, Dang T, Delacour D, Thijssen VL, Edelmann W, Peuchmaur M, Poirier F. Genetic assessment of the importance of galectin-3 in cancer initiation, progression, and dissemination in mice. Glycobiology. 2009;19:68–75. doi: 10.1093/glycob/cwn105. [DOI] [PubMed] [Google Scholar]
- 29.Smetana K Jr, Dvorankova B, Chovanec M, Boucek J, Klima J, Motlik J, Lensch M, Kaltner H, Andre S, Gabius HJ. Nuclear presence of adhesion-/growth-regulatory galectins in normal/malignant cells of squamous epithelial origin. Histochem Cell Biol. 2006;125:171–182. doi: 10.1007/s00418-005-0074-0. [DOI] [PubMed] [Google Scholar]
- 30.Shimura T, Takenaka Y, Fukumori T, Tsutsumi S, Okada K, Hogan V, Kikuchi A, Kuwano H, Raz A. Implication of galectin-3 in Wnt signaling. Cancer Res. 2005;65:3535–3537. doi: 10.1158/0008-5472.CAN-05-0104. [DOI] [PubMed] [Google Scholar]
- 31.Sant’ana JM, Chammas R, Liu F, Nonogaki S, Cardoso SV, Loyola AM, de Faria PR. Activation of the Wnt/beta-catenin signaling pathway during oral carcinogenesis process is not influenced by the absence of galectin-3 in mice. Anticancer Res. 2011;31:2805–2811. [PubMed] [Google Scholar]
- 32.Half E, Bercovich D, Rozen P. Familial adenomatous polyposis. Orphanet J Rare Dis. 2009;4:22. doi: 10.1186/1750-1172-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao S, Eiberg H, Krogdahl A, Liu CJ, Sorensen JA. Cytoplasmic expression of E-cadherin and beta-Catenin correlated with LOH and hypermethylation of the APC gene in oral squamous cell carcinomas. J Oral Pathol Med. 2005;34:116–119. doi: 10.1111/j.1600-0714.2004.00275.x. [DOI] [PubMed] [Google Scholar]
- 34.Lo Muzio L. A possible role for the WNT-1 pathway in oral carcinogenesis. Crit Rev Oral Biol Med. 2001;12:152–165. doi: 10.1177/10454411010120020501. [DOI] [PubMed] [Google Scholar]
- 35.Uraguchi M, Morikawa M, Shirakawa M, Sanada K, Imai K. Activation of WNT family expression and signaling in squamous cell carcinomas of the oral cavity. J Dent Res. 2004;83:327–332. doi: 10.1177/154405910408300411. [DOI] [PubMed] [Google Scholar]
- 36.Rigi-Ladiz MA, Kordi-Tamandani DM, Torkamanzehi A. Analysis of hypermethylation and expression profiles of APC and ATM genes in patients with oral squamous cell carcinoma. Clin Epigenetics. 2011;3:6. doi: 10.1186/1868-7083-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mao EJ, Schwartz SM, Daling JR, Beckmann AM. Loss of heterozygosity at 5q21-22 (adenomatous polyposis coli gene region) in oral squamous cell carcinoma is common and correlated with advanced disease. J Oral Pathol Med. 1998;27:297–302. doi: 10.1111/j.1600-0714.1998.tb01960.x. [DOI] [PubMed] [Google Scholar]
- 38.Uesugi H, Uzawa K, Kawasaki K, Shimada K, Moriya T, Tada A, Shiiba M, Tanzawa H. Status of reduced expression and hypermethylation of the APC tumor suppressor gene in human oral squamous cell carcinoma. Int J Mol Med. 2005;15:597–602. [PubMed] [Google Scholar]
- 39.Iwai S, Katagiri W, Kong C, Amekawa S, Nakazawa M, Yura Y. Mutations of the APC, beta-catenin, and axin 1 genes and cytoplasmic accumulation of beta-catenin in oral squamous cell carcinoma. J Cancer Res Clin Oncol. 2005;131:773–782. doi: 10.1007/s00432-005-0027-y. [DOI] [PubMed] [Google Scholar]
- 40.Sogabe Y, Suzuki H, Toyota M, Ogi K, Imai T, Nojima M, Sasaki Y, Hiratsuka H, Tokino T. Epigenetic inactivation of SFRP genes in oral squamous cell carcinoma. Int J Oncol. 2008;32:1253–1261. doi: 10.3892/ijo_32_6_1253. [DOI] [PubMed] [Google Scholar]
- 41.Uzawa K, Yoshida H, Suzuki H, Tanzawa H, Shimazaki J, Seino S, Sato K. Abnormalities of the adenomatous polyposis coli gene in human oral squamous-cell carcinoma. Int J Cancer. 1994;58:814–817. doi: 10.1002/ijc.2910580611. [DOI] [PubMed] [Google Scholar]
- 42.Largey JS, Meltzer SJ, Sauk JJ, Hebert CA, Archibald DW. Loss of heterozygosity involving the APC gene in oral squamous cell carcinomas. Oral Surg Oral Med Oral Pathol. 1994;77:260–263. doi: 10.1016/0030-4220(94)90295-x. [DOI] [PubMed] [Google Scholar]
- 43.Chang KW, Lin SC, Mangold KA, Jean MS, Yuan TC, Lin SN, Chang CS. Alterations of adenomatous polyposis Coli (APC) gene in oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2000;29:223–226. [PubMed] [Google Scholar]
- 44.Tsuchiya R, Yamamoto G, Nagoshi Y, Aida T, Irie T, Tachikawa T. Expression of adenomatous polyposis coli (APC) in tumorigenesis of human oral squamous cell carcinoma. Oral Oncol. 2004;40:932–940. doi: 10.1016/j.oraloncology.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Gray SE, Kay EW, Leader M, Mabruk M. Analysis of APC allelic imbalance/loss of heterozygosity and APC protein expression in cutaneous squamous cell carcinomas. Cancer Genomics Proteomics. 2011;8:149–155. [PubMed] [Google Scholar]
- 46.Shi Y, He B, You L, Jablons DM. Roles of secreted frizzled-related proteins in cancer. Acta Pharmacol Sin. 2007;28:1499–1504. doi: 10.1111/j.1745-7254.2007.00692.x. [DOI] [PubMed] [Google Scholar]


