Abstract
Renal solitary fibrous tumor (SFT) is a rare, and a large scale study on this topic is lacking to date. In this article, we summarize the previously reported cases. The symptoms and signs resemble those of renal cell carcinoma, including hematuria, flank/abdominal/lumbar pain and palpable abdominal mass. Grossly, the tumor demonstrates a well-circumscribed solid mass. Microscopically, the tumor consists of fusiform or ovoid spindle cells and a various amounts of collagen bundles with patternless, storiform, or fascicular arrangements with an occasional hemangiopericytomatous pattern. Immunohistochemically, CD34, CD99 and bcl-2 are often detected. Ultrastructurally, tumor cells contain irregular nuclei, prominent Golgi apparatus, branching rough endoplasmic reticulum, variable numbers of mitochondria. Surgical resection is considered to be the gold standard therapy. Most of renal SFT are benign, but cases of approximately 10 to 15% behave in an aggressive fashion. All patients need to be on long-term follow-up because clinical behavior is rather unpredictable. As the molecular genetic study of renal SFTs is lacking, a large scale study will be desirable in the future.
Keywords: Solitary fibrous tumor, kidney, CD34, STAT6
Introduction
Solitary fibrous tumor (SFT) is a rare spindle cell neoplasm generally arising from the pleura, but also occurs in various anatomic sites including orbital cavities, nasal cavities, paranasal sinuses, meninges, salivary glands, thyroid gland, liver, pancreas, adrenal gland, spermatid cord, kidney, urinary bladder, prostate, testis, uterine cervix, spinal cord, skin, periosteum, bone and soft tissue [1-3]. Approximately only 40 cases of renal SFTs have been reported in the English literature to date. In this article, we summarize the clinical and pathobiological characteristics of renal SFTs.
Epidemiology
The age of patients ranges from 28 to 83 years with a mean age of 52 years. Exceptionally, a case of 4-year-old boy with renal SFT has been described [4]. There is no sex predominance [3].
Clinical signs and symptoms
Patients with renal SFT present with hematuria, palpable mass, flank/abdominal/lumbar pain or abdominal discomfort [1,3,5-19]. These findings resemble those of renal cell carcinoma (RCC). The tumors are often incidentally detected [20,21]. Intermittent hypoglycemia due to the production of high molecular weight insulin-like growth factor may occur in some cases [22]. A case of renal SFT arising in chronic renal failure requiring dialysis has been reported [23]. A case of renal SFT associated with acute pyonephrosis has been also reported [3].
Imaging findings
Ultrasound sonography demonstrates a divergent pattern of echogenicity of hypo- or heterogeneous echogenic masses with a well-defined boundary [24]. The plain computed tomography (CT) scan reveals well-circumscribed lobulated soft-tissue attenuation and enhanced CT scan notes strong enhancement [3,14,24,25]. Magnetic resonance imaging shows predominant low or intermediate signal density on both T1- and T2-weighted-images. The low signal intensity on T2-weighted-images may be characteristics of SFT [24-26]. However, the high signal intensities on T2-weighted images correlate with myxoid or cystic degeneration as well as with hypercellularity and a small amount of collagenous matrix [24].
Pathological findings
Macroscopic findings
The tumor usually forms a well circumscribed mass and the cut surface of the tumor shows tan-gray, white-tan, brownish yellow, yellowish-white, gray-white, tan-pink or white in color with firm or fibrous consistency [3,5,6,8,9,12,13,20,23,27-31]. The capsule or lobulation can be observed [14,15,17,18]. Focal cystic change may be present [17,31]. Renal vein thrombus extending to inferior vena cava has been reported [17]. In malignant SFTs, necrosis or hemorrhage may be observed [15,31]. The size of renal SFTs ranges from 2 to 25 cm with a mean diameter of 8.75 cm [3]. Cases with two tumors in the same kidney or synchronous pleuro-renal SFTs have been described [22,32].
Microscopic findings
The tumor is composed of fusiform or ovoid spindle cells and a various amounts of thick, hyalinized or keloid-like collagen bands with patternless, storiform, or fascicular arrangements (Figure 1A) [2,9,10,27]. Hemangiopericytomatous pattern is frequently noted (Figure 1B) [2,7,9,10,22,27]. Neoplastic cells demonstrate vesicular chromatin, inconspicuous nucleoli and scant cytoplasm [6,9]. Focal myxoid change may be observed [5]. The stroma may contain a patchy lymphoplasmacytic or histiocytic infiltrate [5]. The tumor may infiltrate the wall of renal vein [7]. The diagnostic criteria for malignancy includes increased cellularity, pleomorphism, increased mitotic activity (more than 4 mitoses per 10 high power fields) [2,3,15,16,18,19,30]. The malignant variant can occur in de novo or through the transformation within a pre-existing benign SFT [15,23].
Figure 1.
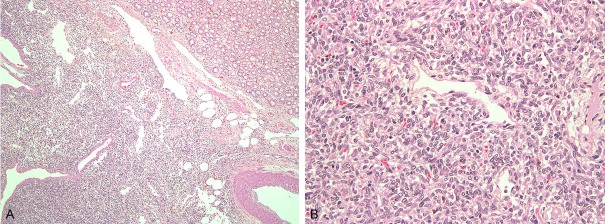
Histological findings. A: The tumor consists of spindle cell with patternless or fascicular arrangement. B: Hemangiopericytomatous growth pattern is seen.
Immunohistochemical findings
The tumor is usually positive for vimentin, CD34 (Figure 2A), CD99 and bcl-2 (Figure 2B), occasionally positive for epithelial membrane antigen (EMA), and may show a positive reaction for alpha smooth muscle actin, desmin, cytokeratin, or collagen IV in rare cases [1,3,6,7,9,11-13,15,17-19,21,23,30,31,33-35]. Rare reactivity to S-100 protein, Factor VIII or CD68 has been also reported [5,17,30,34]. Ki-67 and p53 labeling indices are generally low for benign SFTs [2,7,19,33], but is higher in malignant SFTs [15,30,34]. EBER-1 in situ hybridization is negative [5].
Figure 2.

Immunohistochemical findings. A: Spindle tumor cells are diffusely positive for CD34. B: Neoplastic cells stain diffusely for bcl-2.
Ultrastructural findings
Tumor cells contain irregular nuclei, prominent Golgi apparatus, branching rough-surfaced endoplasmic reticulum, variable numbers of mitochondria and scattered intermediate filaments [5,20,21]. Occasional intermediate filaments seem to be consistent with vimentin [21]. Rudimentary cells junctions may be observed [9]. These findings are consistent with characteristics of fibroblastic cells [5,9,20,21].
Flow cytometric findings
The DNA content of renal SFT without malignant histology shows the diploid histogram [6,7].
Presumed origin
The tumor is considered to originate from renal capsule, interstitial or peripelvic connective tissue [3,5,6,8,9,11,14,20,31,34,35].
Differential diagnosis
This tumor should be distinguished from fibroepithelial polyp of renal pelvis, renomedullary interstitial cell tumor (RMICT), schwannoma, perineurioma, leiomyoma, angiomyolipoma (AML), gastrointestinal stromal tumor (GIST), congenital mesoblastic nephroma, mixed epithelial and stromal tumor (MEST), inflammatory myofibroblastic tumor (IMT), fibrosarcoma, leiomyosarcoma, synovial sarcoma, or sarcomatoid RCC [5]. Fibroepithelial polyps of renal pelvis clinically form the small polypoid lesion in renal pelvis and usually occur in young adults [9,12]. The stromal cells are negative for CD34 [9]. RMICTs form the small lesion in the renal medulla and are usually found incidentally. In schwannoma, Antoni A and B patterns are generally observed with marked S-100 protein expression [5,12,33]. Perineurioma shows the divergent growth pattern such as storiform, lascicular, whorled and lamellar and exhibits the positivity for EMA [10]. Leiomyoma shows fascicular arrangement of spindle cells with eosinophilic cytoplasm and cigar-shaped nuclei, and is positive for alpha smooth muscle actin [12,23,33,37]. Renal AML contains adipose, smooth muscle components and thick walled vessels, and generally reacts for the HMB45 antigen, which detects the melanosome-associated antigen [5,12,33,38]. Renal SFT may contain the adipose tissue [36]. GISTs show the positivity for CD117 (c-kit), CD34 and DOG1 [12,33]. Congenital mesoblastic nephroma occurs within the first 3 months of life and bears the specific ETV6-NTRK3 gene fusion [23]. MEST generally occurs in middle-aged women and grossly show the multicystic appearance. The stroma has mostly Müllerian morphology and often contains smooth muscle cells [39]. In IMTs, chronic inflammatory cell infiltrate is focally prominent and most IMTs show the immunoreactivity to alpha smooth muscle actin, desmin or keratin [5,10]. Fibrosarcoma shows a herring-bone-like growth pattern, denser cellularity, greater nuclear pleomorphism, higher mitotic counts and lacks abundant collagen bands [9,10,23]. Leiomyosarcoma shows the fascicular arrangement of spindle cells with eosinophilic cytoplasm and significant cytologic atypia, and reacts to alpha smooth muscle actin [12,23,33]. Synovial sarcoma-mostly monophasic type can mimick SFT. Such tumor consists of primitive stromal cells show specific SYT-SSX gene fusion [9,10,12,23]. Finally, sarcomatoid RCC might show the area corresponding to the conventional RCC histology, and notes the positivity for Keratin, PAX2 and PAX8 [5,23,41,44]. Hemangiopericytoma is currently integrated into the concept of SFT.
Therapy
The complete surgical resection is the first choice of therapeutic modality if feasible. However, there is no evidence whether partial nephrectomy has the same outcome as radical nephrectomy or not [3]. Hypoglycemia can be resolved with the surgical resection [22]. The systemic chemotherapy is adopted in metastatic or non-resectable tumors, although there are no standard regimens. Radiation therapy may be applicable [22]. Immunotherapy such as interferon may be effective [35].
Prognosis
Although most cases of renal SFT pursue a benign clinical course [2,6,9,19,20,24,42], approximately 10 to 15% of renal SFT behave in an aggressive fashion [14,30]. Some tumors with atypical or malignant histologic features do not behave aggressively [2,34]. By contrast, renal SFT with benign histology may show distant metastasis [31]. Hence, SFT is regarded as an “intermediate malignant, rarely metastasizing neoplasm” [22]. Metastases may occur in the lung, liver and bone [30,31,35]. Retroperitoneal recurrence has been also reported [18]. All patients need to be on long-term follow-up because clinical behavior cannot be accurately predicted [2,15,18,29,34].
Future perspectives
Very recently, recurrent NAB2-STAT6 gene fusions have been identified in SFT and immunohistochemistry of STAT6 seems to be useful in the differential diagnosis of other soft tissue tumors [43-45]. Accordingly, a large scale study including molecular genetic examination will be required in order to elucidate the pathogenesis of renal SFTs.
Disclosure of conflict of interest
None.
References
- 1.Hasegawa T, Matsuno Y, Shimoda T, Hasegawa F, Sano T, Hirohashi S. Extrathoracic solitary fibrous tumors: their histological variability and potentially aggressive behavior. Hum Pathol. 1999;30:1464–1473. doi: 10.1016/s0046-8177(99)90169-7. [DOI] [PubMed] [Google Scholar]
- 2.Morimitsu Y, Nakajima M, Hisaoka M, Hashimoto H. Extrapleural solitary fibrous tumor: clinicopathologic study of 17 cases and molecular analysis of the p53 pathway. APMIS. 2000;108:617–625. doi: 10.1034/j.1600-0463.2000.d01-105.x. [DOI] [PubMed] [Google Scholar]
- 3.Khater N, Khauli R, Shahalt M, Degheili J, Khalifeh I, Aoun J. Solitary fibrous tumors of the kidneys: presentation, evaluation, and treatment. Urol Int. 2013;91:373–383. doi: 10.1159/000354394. [DOI] [PubMed] [Google Scholar]
- 4.Provance A, Ferrari ND III, Nield LS. Chronic cough. Clin Pediatr. 2006;45:871–873. [PubMed] [Google Scholar]
- 5.Gelb AB, Simmons ML, Weidner N. Solitary fibrous tumor involving the renal capsule. Am J Surg Pathol. 1996;20:1288–1295. doi: 10.1097/00000478-199610000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Fukunaga M, Nikaido T. Solitary fibrous tumour of the renal peripelvis. Histopathology. 1997;30:451–456. doi: 10.1046/j.1365-2559.1997.5570775.x. [DOI] [PubMed] [Google Scholar]
- 7.Leroy X, Copin MC, Coindre JM, Meria P, Wacrenier A, Gosset P. Solitary fibrous tumour of the kidney. Urol Int. 2000;65:49–51. doi: 10.1159/000064835. [DOI] [PubMed] [Google Scholar]
- 8.Cortes-Gutierrez E, Arista-Nasr J, Mondragon M, Mijangos-Parada M, Lerma-Mijangos H. Solitary fibrous tumor of the kidney. J Urol. 2001;166:602. [PubMed] [Google Scholar]
- 9.Wang J, Arber DA, Frankel K, Weiss LM. Large solitary fibrous tumor of the kidney: Report of two cases and review of the literature. Am J Surg Pathol. 2001;25:1194–1199. doi: 10.1097/00000478-200109000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Magro G, Cavallaro V, Torrisi A, Lopez M, Dell’Albani M, Lanzafame S. Intrarenal solitary fibrous tumor of the kidney. Report of a case with emphasis on the differential diagnosis in the wide spectrum of monomorphous spindle cell tumors of the kidney. Pathol Res Pract. 2000;198:37–43. doi: 10.1078/0344-0338-00182. [DOI] [PubMed] [Google Scholar]
- 11.Kunieda K, Tanaka Y, Nagao N, Yamaguchi K, Sano J, Osada S, Saji S, Shimokawa K. Large solitary fibrous tumor of the retroperitoneum: report of a case. Surg Today. 2004;34:90–93. doi: 10.1007/s00595-003-2632-1. [DOI] [PubMed] [Google Scholar]
- 12.Kohl S, Mathews K, Baker J. Renal hilar mass in an 85-year-old woman. Arch Pathol Lab Med. 2006;130:117–119. doi: 10.5858/2006-130-117-RHMIAY. [DOI] [PubMed] [Google Scholar]
- 13.Bozkurt SU, Ahiskali R, Kaya H, Demir A, Ilker Y. Solitary fibrous tumor of the kidney. APMIS. 2007;115:259–262. doi: 10.1111/j.1600-0463.2007.apm_500.x. [DOI] [PubMed] [Google Scholar]
- 14.Znati K, Chbani L, Fatemi HE, Harmouch T, Kamaoui I, Tazi F, Bennis S, Amarti A. Solitary fibrous tumor of the kidney: A case report and review of the literature. Rev Urol. 2007;9:36–40. [PMC free article] [PubMed] [Google Scholar]
- 15.Hsieh TY, ChangChien YC, Chen WH, Chen SC, Chang LC, Hwang CC, Chein HP, Chen JR. De novo malignant solitary fibrous tumor of the kidney. Digan Pathol. 2011;6:96. doi: 10.1186/1746-1596-6-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marzi M, Piras P, D’Alpaos M, Canessa S, Minervini MS, Di Zitti P. The solitary fibrous malignant tumour of the kidney: clinical and pathological considerations on a case revisiting the literature. Minerva Urol Nefrol. 2011;63:109–113. [PubMed] [Google Scholar]
- 17.Naveen HN, Nelivigi G, Venkatesh GK, Suriraju V. A case of solitary fibrous tumor of the kidney. Urol Ann. 2011;13:158–160. doi: 10.4103/0974-7796.84956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sfoungaristos S, Papatheodorou M, Kavouras A, Perimenis P. Solitary fibrous tumor of the kidney with massive retroperitoneal recurrence: A case presentation. Prague Med Rep. 2012;113:246–250. doi: 10.14712/23362936.2015.23. [DOI] [PubMed] [Google Scholar]
- 19.Demirtas A, Sabur V, Akgun H, Akinsal EC, Demirci D. Solitary fibrous tumor of the kidney: A case report. Case Rep Urol. 2013;2013:1–4. doi: 10.1155/2013/147496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yazaki T, Satoh S, Iizumi T, Umeda T, Yamaguchi Y. Solitary fibrous tumor of renal pelvis. Int J Urol. 2001;8:504–508. doi: 10.1046/j.1442-2042.2001.00360.x. [DOI] [PubMed] [Google Scholar]
- 21.Hirano D, Mashiko A, Murata Y, Satoh K, Ichinose T, Takahashi S, Jike T, Sugitani M. A case of solitary fibrous tumor of the kidney: an immunohistochemical and ultrastructural study with a review of the literature. Med Mol Morphol. 2009;42:239–244. doi: 10.1007/s00795-009-0456-9. [DOI] [PubMed] [Google Scholar]
- 22.Zhao G, Li G, Han R. Two malignant solitary fibrous tumors in one kidney: Case report and review of the literature. Oncol Lett. 2012;4:993–995. doi: 10.3892/ol.2012.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fine SW, McCathy DM, Chan TY, Epstein JI, Argani P. Malignant solitary fibrous tumor of the kidney. Report of a case and comprehensive review of the literature. Arch Pathol Lab Med. 2006;130:857–861. doi: 10.5858/2006-130-857-MSFTOT. [DOI] [PubMed] [Google Scholar]
- 24.Park SB, Park YS, Kim JK, Kim MH, Oh YT, Kim KA, Cho KS. Solitary fibrous tumor of the genitourinary tract. AJR. 2011;196:W132–W137. doi: 10.2214/AJR.09.3787. [DOI] [PubMed] [Google Scholar]
- 25.Katabathina VS, Vikram R, Nagar AM, Tamboli P, Menias CO, Prasad SR. Mesenchymal neoplasms of the kidney in adults: Imaging spectrum with radiologic-pathologic correlation. Radiographics. 2010;30:1525–1540. doi: 10.1148/rg.306105517. [DOI] [PubMed] [Google Scholar]
- 26.Johnson TR, Pedrosa I, Goldsmith J, Dewolf WC, Rofsky NM. Magnetic resonance imaging findings in solitary fibrous tumor of the kidney. J Comput Assist Tomogr. 2005;29:481–483. doi: 10.1097/01.rct.0000166637.24037.41. [DOI] [PubMed] [Google Scholar]
- 27.Constantinidis C, Koutalellis C, Liapis G, Stravodimos C, Alexandrou P, Adamakis I. A solitary fibrous tumor of the kidney in a 26-year-old man. Can J Urol. 2007;14:3583–3587. [PubMed] [Google Scholar]
- 28.MacLennan GT, Cheng L. Solitary fibrous tumor of the kidney. J Urol. 2009;181:2731–2731. doi: 10.1016/j.juro.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Makris A, Tabaza R, Brehmer B, Lindemann-Doctor K, Wildberger J, Jakse G. Solitary fibrous tumor of the kidney: A case report. Can J Urol. 2009;16:4854–4856. [PubMed] [Google Scholar]
- 30.Guo G, Zhang X, Zhou ZH. Clinical characteristics of malignant solitary fibrous tumors of the kidney with thoracic vertebral metastasis. Int J Urol. 2012;19:177–178. doi: 10.1111/j.1442-2042.2011.02921.x. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki H, Kurihara T, Katsuoka Y, Nakano T, Yoshioka M, Miyano S, Sato Y, Uejima I, Hoshikawa M, Takagi M, Chikaraishi T. Distant metastasis from benign solitary fibrous tumor of the kidney. Case Rep Nephrol Urol. 2013;3:1–8. doi: 10.1159/000346850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petrella F, Monfardini L, Musi G, Pelosi G, Veronesi G, Leo F, Solli P, Borri A, Galetta D, Gasparri R, Scanagatta P, Spaggiari L. Synchronous pleuro-renal solitary fibrous tumors: a new clinical-pathological findings. Minerva Chir. 2009;64:669–671. [PubMed] [Google Scholar]
- 33.Yamada H, Tsuzuki T, Yokoi T, Kobayashi H. Solitary fibrous tumor of the kidney originating from the renal capsule and fed by the renal capsular artery. Pathol Int. 2004;54:914–917. doi: 10.1111/j.1440-1827.2004.01772.x. [DOI] [PubMed] [Google Scholar]
- 34.Magro G, Emmanuele C, Lopez M, Vallone G, Greco P. Solitary fibrous tumor of the kidney with sarcomatous overgrowth. APMIS. 2008;115:1020–1025. doi: 10.1111/j.1600-0463.2008.01012.x. [DOI] [PubMed] [Google Scholar]
- 35.Cuello J, Bruges R. Malignant solitary fibrous tumor of the kidney: report of the first case managed with interferon. Case Rep Oncol Med. 2013;2013:1–6. doi: 10.1155/2013/564980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamaguchi T, Takimoto T, Yamashita T, Kitahara S, Omura M, Ueda Y. Fat-containing variant of solitary fibrous tumor (lipomatous hemangiopericytoma) arising on surface of kidney. Urology. 2005;65:175. doi: 10.1016/j.urology.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 37.Kuroda N, Inoue Y, Taguchi T, Tominaga A, Hes O, Michal M, Hayashi T, Hiroi M, Shuin T, Lee GH. Renal leiomyoma: An immunohistochemical, ultrastructural and comparative genomic hybridization study. Histol Histopathol. 2007;22:883–888. doi: 10.14670/HH-22.883. [DOI] [PubMed] [Google Scholar]
- 38.Kuroda N, Pan CC. Renal angiomyolipomas: clinical and histological spectrum. Urol Sci. 2011;22:40–42. [Google Scholar]
- 39.Michal M, Hes O, Bisceglia M, Simpson RW, Spagnolo DV, Parma A, Boudova L, Zachoval R, Suster S. Mixed epithelial and stromal tumors of the kidney: A report of 22 cases. Virchows Arch. 2004;445:359–367. doi: 10.1007/s00428-004-1060-y. [DOI] [PubMed] [Google Scholar]
- 40.Kuroda N, Toi M, Hiroi M, Enzan H. Review of sarcomatoid renal cell carcinoma with focus on clinical and pathobiological aspects. Histol Histopathol. 2003;18:551–555. doi: 10.14670/HH-18.551. [DOI] [PubMed] [Google Scholar]
- 41.Kuroda N, Tanaka A, Ohe C, Nagashima Y. Recent advances of immunohistochemistry for diagnosis of renal tumors. Pathol Int. 2013;63:381–390. doi: 10.1111/pin.12080. [DOI] [PubMed] [Google Scholar]
- 42.Fain JS, Eble JN, Nascimento AG, Farrow GM, Bostwick DG. Solitary fibrous tumor of the kidney: report of three cases. J Urol Pathol. 1996;4:227–238. [Google Scholar]
- 43.Robinson DR, Wu YM, Kalyana-Sundaram S, Cao X, Lonigro RJ, Sung YS, Chen CL, Zhang L, Wang R, Su F, Iyer MK, Roychowdhury S, Siddiqui J, Pienta KJ, Kinju LP, Talpaz M, Mosquera JM, Singer S, Schuetze SM, Antonescu CR, Chinnaiyan AM. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet. 2013;45:180–185. doi: 10.1038/ng.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schweizer L, Koelsche C, Sahm F, Piro RM, Capper D, Reuss DE, Pusch S, Habel A, Meyer J, Göck T, Jones DTW, Mawrin C, Schittenhelm J, Becker A, Heim S, Simon M, Herold-Mende C, Mechtersheimer G, Paulus W, König R, Wiestler OD, Pfister SM, von Deimling A. Meningeal hemangiopericytoma and solitary fibrous tumors carry the NAB2-STAT6 fusion and can be diagnosed by nuclear expression of STAT6 protein. Acta Neuropathol. 2013;125:651–658. doi: 10.1007/s00401-013-1117-6. [DOI] [PubMed] [Google Scholar]
- 45.Doyle LA, Vivero M, Fletcher CDM, Mertens F, Hornick JL. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol. 2014;27:390–395. doi: 10.1038/modpathol.2013.164. [DOI] [PubMed] [Google Scholar]


