Abstract
The combined subchronic effects of exposure to lead acetate and cadmium chloride on apoptosis protein expression were detected in the liver and kidney of rats to investigate the hazards of environmentally relevant, low-dose exposure to these compounds. The TUNEL assay showed that there were increased numbers of apoptotic cells. Immunohistochemical tests showed increased numbers of positive cells under Bax and caspase-3 protein detection and decreased Bcl-2 protein. Furthermore, mitochondrial injury and increased numbers of apoptotic cells with condensed nuclei were observed by TEM. These results suggested that low-dose exposure to Pb and Cd can cause significant hepatic and renal apoptosis and finally impair their function. Hepatic and renal apoptosis induced by low-dose exposure is associated with mitochondrial injury and changes in levels of apoptogenic proteins, such as Bcl-2, Bax, and caspase-3.
Keywords: Pb and Cd, apoptosis, bax, Bcl-2, caspase-3, subchronic
Introduction
The Agency for Toxic Substances and Disease Registry classifies lead (Pb) and cadmium (Cd) as nonessential environmental pollutants and toxic compounds, which enter the environment because of natural and human-related activities [1,2]. The two elements are often released into the environment because of their wide industrial uses and untreated sewage from chemical plants. These elements then become concentrated in the environment. Thus, particular interest has been devoted to the safety of public food supplies for human health [2,3]. Given that the liver and kidney are the major targets of Pb and Cd, these elements are highly dangerous to hepatic and renal function. Furthermore, the synergism between the toxic effects of Pb and Cd has stimulated interest in the study of toxic substances in the biological system [4,5].
In the majority of previous animal studies, only one type of metal has been used in high concentration. However, in the environment, a population receives simultaneous multiple exposures [6], further experiments are needed on combinations of toxic metals. Historically, numerous models have been explored to predict the potential combined effects of chemicals for an individual species or population group. These models, especially concentration addition and independent action, have been applied to numerous mixtures [7,8]. However, limited data showed effects of Pb/Cd-induced on the apoptosis of the liver, kidney and testis in rats so far. Recently, we have that Pb combined with Cd induced oxidative damage in the liver and kidney of rats, and MT may be a biochemical environmental indicator [9].
In our previous research, Pb(NO3)2 and CdCl2∙2.5H2O were used in an equitoxic mixture ratio design. The fixed mixture ratio was based on the single toxicant LD50-value (median lethal dose value) for an oral acute study [5]. The result showed that Pb and Cd with the mixture toxicant LD50-value of 2696.54 mg/kg also have an additive effect for rat models. In this study, we chose low-dose exposure via the subchronic experiment because ingestion is the most important route of human and animal exposure that naturally occurs in the environment, and pollutants emanate mainly from industrial sources. The experimental model of rats treated with relatively low Pb and Cd levels were based on the mixture toxicant LD50-value of our oral acute study for a 90 d period as a model of Pb/Cd-induced subchronic toxicological evaluation.
The objective of this study was to examine the effects of simultaneous exposure to Pb and Cd on cellular apoptosis in liver and kidney of rats at exposure levels without anticipated overt clinical indicators of toxicity.
Materials and methods
Reagents
Lead acetate (Pb(NO3)2) and cadmium chloride (CdCl2∙2.5H2O), which were all analytical grade (AR > 99.0%), were obtained from Chengdu Kelong Chemical Co., Ltd (Chengdu, China).
Study design
This study was performed in accordance with the following references: (A) Food and Drug Administration Redbook [10]: Chapter IV.C.4.a Subchronic Toxicity Studies with Rodents; (B) Organization for Economic Cooperation and Development (OECD) Guidelines for the Testing of Chemicals, Section 4 Health Effects (Part 408) [11]: Repeated Dose 90 d Oral Toxicity Study in Rodents; and (C) Chinese Center for Disease Control and Prevention [12]: chemical-test method of repeated-dose oral toxicity study in rodents. The study was conducted in accordance with the OECD principles of Good Laboratory Practice.
Experimental animals
Four-week-old male and female specific pathogen-free Sprague-Dawley rats weighing 100 ± 5 g were purchased from the Chengdu Dossy Experimental Animals Co., Ltd. (License No. SCXK (Sichuan) 2013-24, China), and kept in the animal house at the Sichuan Agriculture University (Ya’an, China). Rats were housed separately in the laboratory animal house at 20-25°C, 50-60% humidity, and a 12 h light/dark cycle with the lights off at 7 PM. These conditions were based on the Guidelines of the International Committee on Laboratory Animals. Finally, rats were fed with a standard diet from Nuvital Nutrients (Colombo/PR, Brazil), allowed access to distilled water ad libitum, and had been acclimated to laboratory conditions for 7 d.
Treatments
Three groups of 20 rats, each containing 10 females and 10 males, consumed a daily dose of 29.96 (Group II, 29.25+0.71), 89.88 (Group III, 87.74+2.14), 269.65 (Group IV, 263.23+6.42) mg/kg b.w. with a mixture of Pb(NO3)2 and CdCl2∙2.5H2O for at least 90 consecutive days. The doses of our study aimed to chronically expose rats to relatively low and environmentally realistic concentrations of Pb/Cd [13-15]. In each case, the product volume administered by gavage was 10 mL/kg b.w. A vehicle-control group (Group I) formed by 20 rats consumed distilled water as drinking water during a 90-day period.
In each group, six rats (three males and three females) were anesthetized and perfused through the left ventricle with 300 mL of 0.9% saline preheated at 37°C, followed by 500 mL of ice-cold, freshly made 4% paraformaldehyde in 0.1 M PBS (pH 7.4) for terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL), immunohistochemistry (IHC) and Electron microscopic examination.
Analytical procedures
TUNEL assay
The DNA fragmentation indicative of apoptosis was examined using TUNEL. All liver and renal cortex were fixed by immersion for at least 1 d in 10% buffered formaldehyde phosphate, and subsequently dehydrated and embedded in paraffin wax. TUNEL assay was performed in the deparaffinized section (5 μm thick) with in situ cell apoptosis detection kit (MK1020, Boster, China) according to the manufacturer’s instructions, as described by Sun [16].
Bax, Bcl-2 and caspase-3 detected by IHC
The sections of liver and renal cortex were acquired according to the method mentioned above. Immunohistochemical staining against Bax, Bcl-2 and Caspase-3 was performed. For this purpose, briefly, a HRP/DAB detection IHC kit (ab80436 Abcam, China) was used according to the manufacturer’s protocol. The sections were then deparaffinized, and the formalin-fixed paraffin-embedded tissue sections were rehydrated. Hydrogen peroxide block was added to cover the sections, which were incubated for 10 min. After antigen retrieval (100 × Citrate Buffer, ab64236 Abcam, China) for 20 min in a domestic pressure cooker and blocking non-specific binding sites with protein block, the sections were immunoreacted with 10 μg/mL primary antibodies against Bax, Bcl-2 and Caspase-3 (AB20073b, AB20158b and AB20074b, Sangon Biotech, China) overnight at 4°C, respectively. For negative controls, the sections were immersed in PBS instead of the specific antibody. Then, mouse-specific HRP was applied to conjugate and incubate for 15 min at room temperature. DAB was applied to the tissue sections counterstained with hematoxylin.
For immunohistochemical quantification, two slices were selected from one integration receptor sample. Five 400 × microscopic views of cortex and medulla per slide were selected randomly and photographed using a Nikon ECLIPSE 80i microscope equipped with a Nikon DS-Ri1 camera (Nikon Corporation, Tokyo, Japan). The immunopositive reactions in the photographs were analyzed by Software Image Pro-Plus 6.0 (Media Cybernetics, Silver Spring, MD, USA), according to a previously introduced method [9,17]. All photographs were taken and measured in the same parameter setting to ensure that the data were comparable. The area of positive staining was measured in pixels using Image-Pro Software, which detected brown staining in the liver and renal cortex. The average optical density (AOD) was defined as the percentage of positive area in the total area of liver, renal cortex and testis sections.
Electron microscopic examination
For electron microscopy, small pieces of liver and renal cortex tissue (about 1 mm3) were rapidly fixed with 2.5 percent glutaraldehyde in 0.1 M sodium phosphate buffer (pH 7.2) for 3 h at 4°C, washed in the same buffer for 1 h at 4°C and postfixed with 1 percent osmium tetroxide in sodium phosphate buffer for 1 h at 4°C. The tissues were then dehydrated in graded series of ethanol, starting at 50 percent each step for 10 min, after two changes in propylene oxide. The tissue specimens were embedded in Araldite. Ultrathin sections were stained with Mg-uranyl acetate and lead citrate for transmission electron microscope (TEM) evaluation.
Statistical analysis
All data were expressed as the means ± standard error of the means, and analyzed by one-way ANOVA with Duncan’s multiple-range tests after using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). The significant values at either P < 0.05 (*) or P < 0.01 (**) were represented as asterisks.
Results
TUNEL assay
TUNEL staining is a method for detecting DNA fragmentation. The TUNEL-positive cells were mainly around hepatocyte in liver and around renal tubular epithelial cells in kidney (Figure 1). The results showed that the number of apoptotic hepatocyte and renal tubular epithelial cells were significantly higher in the group IV of Pb and Cd co-exposure administration than those in the control group.
Figure 1.
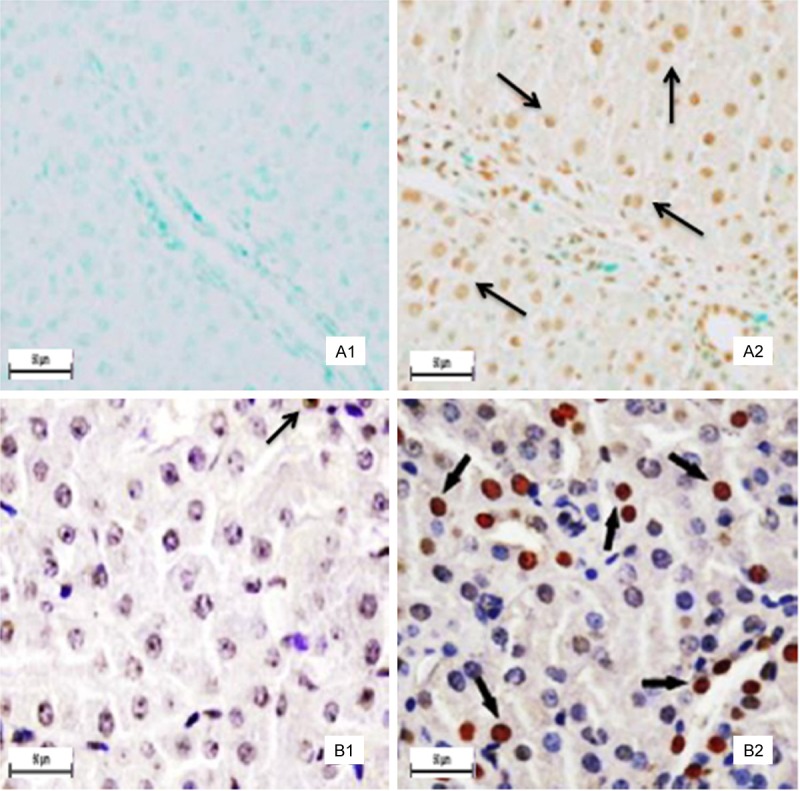
Positive cells stained by TUNEL. Panel A: Liver. No hepatocyte apoptosis was observed in the control group (A1). The positive cells brown stain) markedly increased in the group IV (A2). Panel B: Kidney. There are a few positive cells (brown stain) in the control group (B1). The positive cells markedly increased in the group IV (B2). The arrows demonstrate positive immunoreation. Final magnification × 400, bars = 50 μm.
Bax/Bcl-2 and caspase-3 protein detection
As shown in Figures 2 and 3 and Table 1, the positive cells containing Bcl-2 protein expression (brown stain) were decreased in Pb and Cd co-exposure administration (Groups III and IV) spread throughout the liver and renal cortex when compared with those of the control group. The intensity and amount of immunostaining of Group II showed a fewer decrease than those of the control group. Moreover, Bcl-2 immunostaining was primarily localized within hepatocytes and proximal convoluted tubules, and was weak or absent from distal convoluted tubules. The AOD of the Bcl-2 was much lower in the exposed rats (Groups II to IV) than that in the control group. However, the positive cells containing Bax and caspase-3 protein expression (brown stain) were increased in the high-fluorine groups II and III (Figures 2 and 3). Moreover, the AOD of the Bax and caspase-3 was higher in the exposed rats (Groups II to IV) than that in the control group (Table 1).
Figure 2.
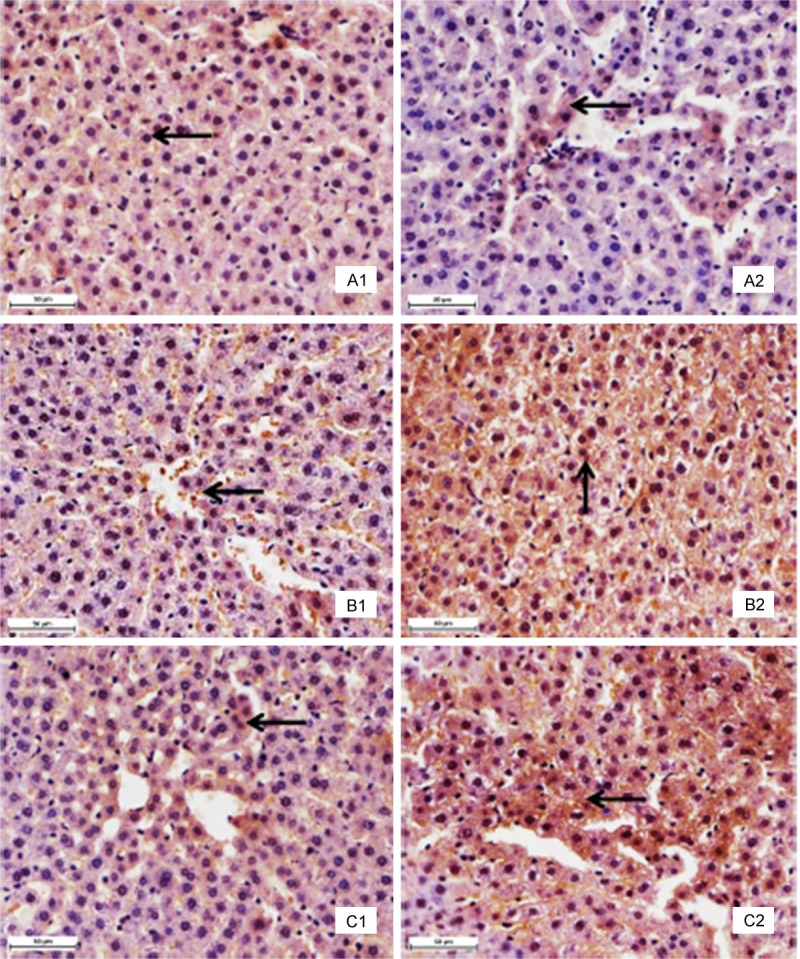
Immunohistochemistry with specific antibody against Bcl-2, Bax and caspase-3 in liver tissues. Panel A: Bcl-2 protein. There are many positive cells (brown stain) in the control group (A1). The positive cells markedly decreased in the group IV (A2). Panel B: Bax protein. There are several positive cells (brown stain) in the control group (B1). The positive cells markedly increased in the group IV (B2). Panel C: Caspase-3 protein. There are a few positive cells (brown stain) in the control group (C1). The positive cells markedly increased in the group IV (C2). The arrows demonstrate positive immunoreation. Immunohistochemical labeling of HRP-streptavidin detection and HE counterstain, final magnification × 400, bars = 50 μm.
Figure 3.
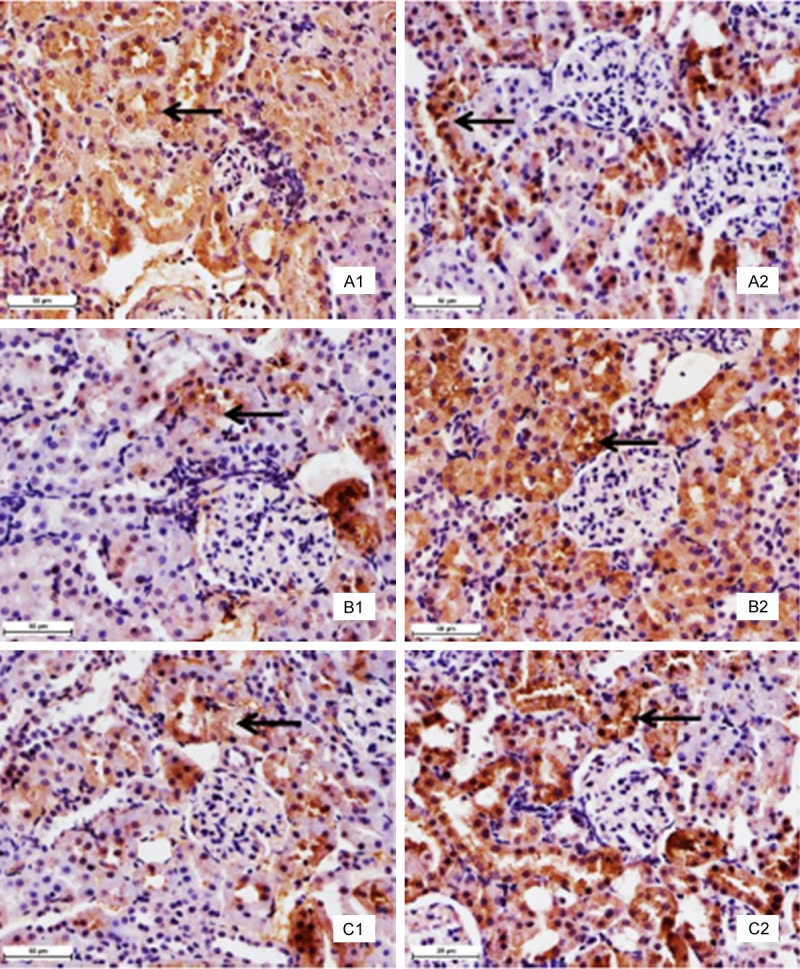
Immunohistochemistry with specific antibody against Bcl-2, Bax and caspase-3 in renal cortex tissues. Panel A: Bcl-2 protein. There are many positive cells (brown stain) in the control group (A1). The positive cells markedly decreased in the group IV (A2). Panel B: Bax protein. There are several positive cells (brown stain) in the control group (B1). The positive cells markedly increased in the group IV (B2). Panel C: Caspase-3 protein. There are a few positive cells (brown stain) in the control group (C1). The positive cells markedly increased in the group IV (C2). The arrows demonstrate positive immunoreation. Immunohistochemical labeling of HRP-streptavidin detection and HE counterstain, final magnification × 400, bars = 50 μm.
Table 1.
Average optical density (AOD) of Bax, Bcl-2, and caspase-3 positive cells in the liver and kidney
| organ | Parameter | mg/kg/day | |||
|---|---|---|---|---|---|
|
| |||||
| (G-I) | (G-II) | (G-III) | (G-IV) | ||
|
| |||||
| 0 | 29.96 | 89.88 | 269.65 | ||
| liver | Bcl-2 | 0.274 ± 0.010 | 0.266 ± 0.006* | 0.260 ± 0.005** | 0.244 ± 0.005** |
| Bax | 0.225 ± 0.007 | 0.229 ± 0.010* | 0.247 ± 0.021** | 0.272 ± 0.013** | |
| Caspase-3 | 0.253 ± 0.005 | 0.259 ± 0.008* | 0.271 ± 0.007** | 0.285 ± 0.006** | |
| kidney | Bcl-2 | 0.301 ± 0.010 | 0.286 ± 0.015* | 0.273 ± 0.011** | 0.268 ± 0.010** |
| Bax | 0.217 ± 0.011 | 0.233 ± 0.025* | 0.259 ± 0.010** | 0.266 ± 0.003** | |
| Caspase-3 | 0.263 ± 0.003 | 0.274 ± 0.010* | 0.296 ± 0.006** | 0.313 ± 0.026** | |
The values are presented as means ± standard deviation (n = 6). Compared with control group;
P < 0.05;
P < 0.01.
Ultrastructural observation
Electron microscope has revealed specific ultrastructural changes in hepatic cells and renal tubular epithelial cells under experimental conditions. Details of a normal hepatic cell and its organelles are illustrated in Figure 4A. After 90 days of introducing Pb and Cd co-exposure in rats, intracellular edema was manifested by distortion or fracture of the endoplasmic reticulum and swollen disorganized mitochondria (Figure 4B). The ribosomal fell off from the rough endoplasmic reticulum membrane and scattered into the cytoplasm. The mitochondria were found to be swollen and the cristae within the mitochondria were disorganized.
Figure 4.
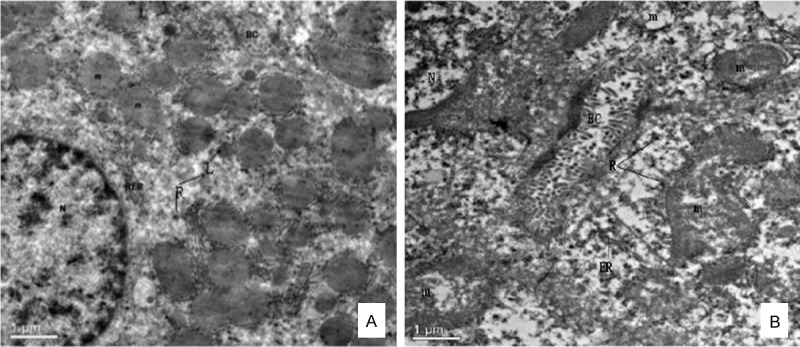
Effect of lead and cadmium on the TEM of liver of rats after administration for 90 days. Panel A: Normal liver, rat. Relationship of the hepatic cell to the sinusoids and characteristic organelles are seen. The bile canaliculus is located between adjoining cells, × 2550. Panel B: Detail of hepatic cell. Note ER fractured and membrane surface ribosome fallen off, marked intracellular edema and swollen disorganized mitochondria, × 2550. Nucleus (N), Mitochondria (m), Bile canaliculus (BC), Lysosomes (L), Ribosome (R), Rough endoplasmic reticulum (RER), Endoplasmic reticulum (ER).
The characterizations of intraluminal brush border of a normal proximal convoluted tubular cell are presented in Figure 5A. Pathological damage of the kidney produced by Pb/Cd were the changes of proximal convoluted tubules as well as the distal tubules. In some proximal convoluted tubule epithelial cells, the brush border was interrupted and escape from the cytoplasm to the lumen of the tubule (Figure 5B). The usually elongated mitochondria in the basilar portion of the distal convoluted tubule epithelial cells became more spherical, with marked disorganization and swelling of the cristae. However, the basement membrane of mitochondria was intact (Figure 5C). No significant alteration was observed in the glomeruli and the well preserved fine structure of these areas was illustrated in Figure 5D.
Figure 5.
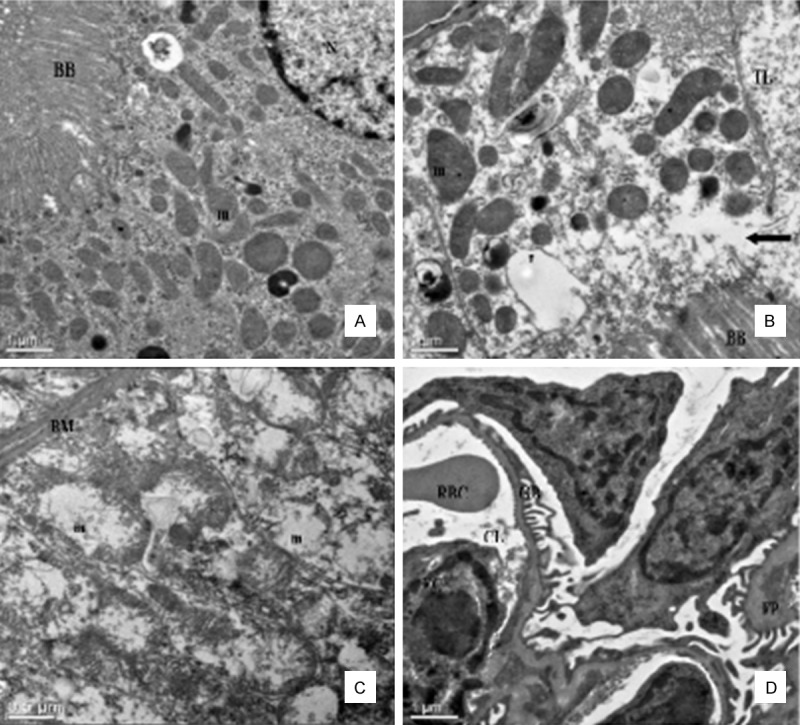
Effect of lead and cadmium on the TEM of kidney of rats after administration for 90 days. Panel A: Normal mice kidney. Detail of apical portion of proximal convoluted tubule cell, × 2,550. Panel B: Proximal tubular cell in group IV. Note swelling and disorganization of mitochondria, and discontinuity of brush border at arrow, with escape of cytoplasm into tubular lumen, × 2,550. Panel C: Distal tubule in group IV. Note swelling and disorganization of mitochondria (m), the fingerlike projections of the basement membrane (BM) of distal convoluted tubule epithelial cells appeared distorted by the mitochondrial swelling, marked disorganization of the cristae and stippling in the matrix, × 6,000. Panel D: Glomerulus in group IV. The fine structure appears well preserved. × 2,550. Brush border (BB), Mitochondria (m), Nucleus (N), Tubular lumen (TL), Glomerular Basement Membrane (GBM), Foot process (FP), Endothelial cells (EC), Capillary lumen (CL), Red blood cell (RBC).
Discussion
This study is the first attempt to report the equitoxic mixture ratio of Pb and Cd as the experimental animal model method for long-term toxicity experiments. After introducing Pb and Cd co-exposure in rats, no significant changes in body weight and intoxication were observed [5]. Thus, the general metabolic condition of the animals was within normal range. Moreover, this animal model may help in detecting the early events of chronic Pb/Cd intoxication with relatively low and environmentally realistic concentrations.
The main objective of our study was to establish whether Pb and Cd co-exposure in rats would increase apoptosis in hepatocyte and renal tubular epithelial cells. Based on the quantitative and qualitative evaluations of apoptosis in the liver and kidney, we found that the percentage of apoptotic cells in the liver and kidney showed no obvious changes in the control group, but were significantly increased in livers and kidneys that took in more than 29.96 mg/kg b.w. with a mixture of Pb(NO3)2 and CdCl2∙2.5H2O. TUNEL revealed that apoptotic cells with brown-stained nuclei were distributed within hepatocyte in liver and around renal tubular epithelial cells in kidney around the exposed rats (Groups II to IV) in comparison with that of the control group. Meanwhile, we also noted ultrastructural morphological characteristics, such as chromatin condensation and vacuolated mitochondria with degenerating cristae. Some reports [4] and our own data have supported that the Pb/Cd-induced apoptosis may be associated with mitochondrial injury. During the mitochondrial injury, mitochondrial apoptogenic proteins, such as those of Bcl-2 family, are released into the cytoplasm. Then, the apoptotic signal proceeds towards the final, execution phase of the apoptotic process [18].
It is well known that Bcl-2 protein is expressed in the inner mitochondrial membrane [18]. The members of the Bcl-2 protein family are known to regulate the release of apoptosis-activating factors that the ratio of Bcl-2 to Bax determines cell survival or cell death [19]. In the present study, Bax protein expression was found to be increased while Bcl-2 protein expression was depressed in the exposed rats (Groups II to IV). There is currently no report about the effects of Pb/Cd-induced on the expression of Bcl-2 family proteins. However, these findings in our study are similar to those of a study on vanadium-induced DNA damage and P53 expression [20]. At the same time, changes in the Bax and Bcl-2 expression also activate caspase-3 and induce apoptotic processes [21]. In the present study, the caspase-3 protein expression was found to be increased in the exposed rats (Groups II to IV). These changes resulted in an increased number of apoptotic hepatocyte in liver and around renal tubular epithelial cells in kidney of the exposed rats.
Mitochondrial injury and overexpression of proapoptotic proteins are associated with the production of free radicals. We have reported that deleterious effects of Pb and Cd co-exposed on anti-oxidative systems, which have been demonstrated in rat peripheral blood and in liver, kidney [9]. Lower levels of Pb/Cd-induced depressed the activities of antioxidant enzymes, and then free radicals accumulated in the body or organs and induced the lipid peroxidation of the membrane. It is reasonable to propose that oxidative damage could occur in mitochondria and cause the release of proapoptotic proteins into the cytosol, which resulted in cellular apoptosis [22].
In conclusion, it is confirmed that combined exposure to Pb and Cd disrupted apoptosis in the liver and kidney. Low-dose exposure to Pb and Cd can cause significant hepatic and renal apoptosis and finally impair their function. Hepatic and renal apoptosis induced by low-dose exposure is associated with mitochondrial injury and changes in levels of apoptogenic proteins, such as Bcl-2, Bax, and caspase-3.
Acknowledgements
This study was supported by the National Nature Science Foundation of China (NO. 31101860) and the Sichuan Youth Science and Technology Innovation Research Team for waterfowl disease prevention and control (NO. 2013TD0015).
Disclosure of conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Mol S. Levels of Heavy Metals in Canned Bonito, Sardines, and Mackerel Produced in Turkey. Biol Trace Elem Res. 2011;143:974–982. doi: 10.1007/s12011-010-8909-5. [DOI] [PubMed] [Google Scholar]
- 2.Bajpai R, Upreti D. Accumulation and toxic effect of arsenic and other heavy metals in a contaminated area of West Bengal, India, in the lichen Pyxine cocoes (Sw. ) Nyl. Ecotoxicol Environ Saf. 2012;83:63–70. doi: 10.1016/j.ecoenv.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Iwegbue CMA. Trace metal contents in some brands of canned beef in Nigeria. Toxicological & Environmental Chemistry. 2011;93:1368–1374. [Google Scholar]
- 4.Wang L, Li J, Li J, Liu Z. Effects of lead and/or cadmium on the oxidative damage of rat kidney cortex mitochondria. Biol Trace Elem Res. 2010;137:69–78. doi: 10.1007/s12011-009-8560-1. [DOI] [PubMed] [Google Scholar]
- 5.Yuan G, Dai S, Yin Z, Lu H, Jia R, Xu J, Song X, Li L, Shu Y, Zhao X. Toxicological assessment of combined lead and cadmium: Acute and sub-chronic toxicity study in rats. Food Chem Toxicol. 2014;65:260–268. doi: 10.1016/j.fct.2013.12.041. [DOI] [PubMed] [Google Scholar]
- 6.Teuschler LK, Hertzberg RC. Current and future risk assessment guidelines, policy, and methods development for chemical mixtures. Toxicology. 1995;105:137–144. doi: 10.1016/0300-483x(95)03207-v. [DOI] [PubMed] [Google Scholar]
- 7.Spurgeon DJ, Jones OA, Dorne JL, Svendsen C, Swain S, Sturzenbaum SR. Systems toxicology approaches for understanding the joint effects of environmental chemical mixtures. Sci Total Environ. 2010;408:3725–3734. doi: 10.1016/j.scitotenv.2010.02.038. [DOI] [PubMed] [Google Scholar]
- 8.Lu H, Yuan G, Yin Z, Dai S, Jia R, Xu J, Song X, Li L, Lv C. Effects of subchronic exposure to lead acetate and cadmium chloride on rat’s bone: Ca and Pi contents, bone density, and histopathological evaluation. Int J Clin Exp Pathol. 2014;7:640–647. [PMC free article] [PubMed] [Google Scholar]
- 9.Dai S, Yin Z, Yuan G, Lu H, Jia R, Xu J, Song X, Li L, Shu Y, Liang X, He C, Lv C, Zhang W. Quantification of metallothionein on the liver and kidney of rats by subchronic lead and cadmium in combination. Environ Toxicol Pharmacol. 2013;36:1207–1216. doi: 10.1016/j.etap.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 10.FDA U. Toxicological Principles for the Safety Assessment of Food Ingredients: Redbook 2000. 2000. Chapter IV. C. 4. a. Subchronic Toxicity Studies with Rodents. [Google Scholar]
- 11.OECD. Test No. 408: Repeated Dose 90-Day Oral Toxicity Study in Rodents. OECD Publishing; 1998. [Google Scholar]
- 12.CDC. Chemicals-Test method of repeated dose oral toxicity study in rodents. AQSIQ; 2008. [Google Scholar]
- 13.Antonio Garcia T, Corredor L. Biochemical changes in the kidneys after perinatal intoxication with lead and/or cadmium and their antagonistic effects when coadministered. Ecotoxicol Environ Saf. 2004;57:184–189. doi: 10.1016/S0147-6513(03)00063-0. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Wang G, Li X, Gan C, Zhu G, Jin T, Wang Z. Environmental level of cadmium exposure stimulates osteoclasts formation in male rats. Food Chem Toxicol. 2013;60:530–535. doi: 10.1016/j.fct.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 15.Thijssen S, Maringwa J, Faes C, Lambrichts I, Van Kerkhove E. Chronic exposure of mice to environmentally relevant, low doses of cadmium leads to early renal damage, not predicted by blood or urine cadmium levels. Toxicology. 2007;229:145–156. doi: 10.1016/j.tox.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Sun L, Lam W, Wong Y, Lam L, Tang H, Wai M, Mak Y, Pan F, Yew D. Permanent deficits in brain functions caused by long-term ketamine treatment in mice. Hum Exp Toxicol. 2011;30:1287–1296. doi: 10.1177/0960327110388958. [DOI] [PubMed] [Google Scholar]
- 17.JiangFeng F, Jiu YS, Wen ZZ, Ben L. The expression of Fas/FasL and apoptosis in yak placentomes. Anim Reprod Sci. 2011;128:107–116. doi: 10.1016/j.anireprosci.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Roset R, Ortet L, Gil-Gomez G. Role of Bcl-2 family members on apoptosis: what we have learned from knock-out mice. Front Biosci. 2007;12:4722–4730. doi: 10.2741/2421. [DOI] [PubMed] [Google Scholar]
- 19.Oltval ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 20.Xu J, Lian LJ, Wu C, Wang XF, Fu WY, Xu LH. Lead induces oxidative stress, DNA damage and alteration of p53, Bax and Bcl-2 expressions in mice. Food Chem Toxicol. 2008;46:1488–1494. doi: 10.1016/j.fct.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 21.Rana SVS. Metals and apoptosis: recent developments. J Trace Elem Med Bio. 2008;22:262–284. doi: 10.1016/j.jtemb.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Green DR. Apoptotic pathways: paper wraps stone blunts scissors. Cell. 2000;102:1–4. doi: 10.1016/s0092-8674(00)00003-9. [DOI] [PubMed] [Google Scholar]


