Abstract
Aims: Twist has been reported to play crucial roles for malignant aggressiveness; however, detailed pathological significance of Twist in renal cell carcinoma (RCC) is not fully understood. The present study was to clarify clinical significance and molecular functions of Twist in patients with RCC. Methods: Twist expression was examined by immunohistochemical techniques in 156 formalin-fixed specimens. Cell proliferation, angiogenesis, and apoptosis were measured as the percentage of Ki-67-positive cells (proliferation index, PI), CD31-stained vessels (microvessel density, MVD), and TUNEL-positive cells (apoptotic index, AI). In addition, semi-quantification of matrix metalloproteinase (MMP)-2 was performed. Macrophages were identified with anti-CD68 antibody, and the tumor associated macrophage (TAM) density was calculated as CD68-positive cells per high-power field. Results: Twist expression was positively associated with grade, pT stage, and metastasis (p<0.001). We also noticed that its expression was considerably higher in cancer cells of sarcomatoid RCC and in those at the edge of the tumors. Twist expression was positively correlated with PI, MVD, MMP2 expression, and TAM density (P<0.001), but not with AI, and MMP-2 expression and TAM density were independently correlate by multi-variate analyses. Kaplan-Meir survival curves showed high Twist expression was a worse predictor for cause-specific survival (P<0.001). Conclusions: Twist plays important roles in tumor growth, progression, and survival in patients with RCC patients. Such pathological mechanisms are significantly associated with increased cancer cell proliferation, angiogenesis, MMP2 expression, and macrophage recruitment. These findings are important information for discussion of treatment and observation strategies in these patients.
Keywords: Twist, cell proliferation, angiogenesis, matrix metalloproteinases, macrophage, renal cell carcinoma
Introduction
Radical nephrectomy is standard treatment in patients with renal cell carcinoma (RCC). However, about 30% of patients with RCC have distant metastases at diagnosis, and the presence of metastasis is a strong predictor of prognosis in these patients [1]. Therefore, the molecular mechanisms of cancer cell dissemination from the primary tumor are important in the considerations of treatment and observational strategies. Metastasis is a multi-step pathological process that is regulated by a wide variety of factors and molecules. For example, cancer cell proliferation and apoptosis influence cancer cell survival and tumor growth, and angiogenesis is closely associated with tumor growth and metastasis, acting as an in-flow channel for oxygen, and an out-flow pathway for cancer cells in almost all solid tumors. Furthermore, breakdown of the basement membrane barrier is essential for the movement and invasion of cancer cells, and such extra-cellular matrix (ECM) degradation is regulated by matrix metalloproteinases (MMPs). In addition, these pathological activities are also modulated by the presence of tumor-infiltrating macrophages [2]. These factors and molecules are reported to be associated with tumor malignancy and progression in patients with RCC [3-5]. On the other hand, epithelial-mesenchymal transition (EMT) has been reported to play a crucial role in promoting dissemination of “single cancer cells” from solid tumors [6]. However, the detailed regulatory mechanism of EMT in human RCC tissues are not fully understood.
Twist is a highly conserved basic helix-loop-helix transcription factor that has been reported as a master regulator of embryonic morphogenesis [7], and it governs cell migration and differentiation under various physiological conditions [8]. Moreover, Twist has been reported to significantly enhance EMT under various pathological conditions, including tumor malignancy [9,10]. Interestingly, Twist also plays an important role in tumor growth, cell invasion, and metastasis by regulating cancer-related functions such as angiogenesis, and the degradation of ECM in various malignancies [9,11-15]. In addition, apoptosis, angiogenesis, recruitment of macrophages, and MMP2 expression are known to be regulated by Twist under a variety of pathological conditions [14-17]. Therefore, Twist is speculated to be closely correlated with malignant potential, progression, and survival in patients with RCC. However, to our knowledge, there is a little information regarding the pathological roles and clinical significance of Twist expression in human RCC tissues.
The present study was designed to determine the relationships between Twist expression and malignant potential, clinicopathological features, and survival in patients with RCC. Furthermore, correlations between Twist expression and cell proliferation, apoptosis, angiogenesis, macrophage recruitment, and MMP2 expression in human RCC tissues were also investigated. Our results showed that Twist expression has a clinically significant pathological role in patients with RCC. This is the first report on the pathological role and molecular mechanism of Twist in metastasis in human RCC tissues.
Materials and methods
Patients and tissue samples
One hundred fifty-six consecutive patients who were diagnosed with RCC were reviewed retrospectively. As shown in Table 1, our study population included 108 men (69.2%) and 48 women (30.8%), and their median (interquartile range, IQR) age at the time of diagnosis was 62 (54-69) years. Patients who had received any pre-operative therapy were excluded from the study. All histological diagnoses were assessed using the 2002 TNM classification and Fuhrman grade. The mean (SD) follow-up period was 46.3 months (range, 3-168 months). The study protocol was approved by the Human Ethics Review Committee of Nagasaki University Hospital.
Table 1.
Relationship between Twist expression and clinicopathological features
| No. Pts. | Twist expression | P value | ||
|---|---|---|---|---|
|
|
||||
| Negative (n=96) | Positive (n=60) | |||
| Gender | 0.869 | |||
| Male | 108 | 66 (61.1) | 42 (38.9) | |
| Female | 48 | 30 (62.5) | 18 (37.5) | |
| Age at diagnosis | 0.510 | |||
| Median or less | 78 | 46 (59.0) | 32 (41.0) | |
| Over median | 78 | 50 (64.1) | 28 (35.9) | |
| Grade | <0.001 | |||
| 1 | 63 | 51 (81.0) | 12 (19.0) | |
| 2 | 68 | 41 (60.3) | 27 (39.7) | |
| 3+4 | 25 | 4 (16.0) | 21 (84.0) | |
| pT stage | <0.001 | |||
| pT1 | 98 | 79 (80.6) | 19 (31.7) | |
| pT2 | 22 | 12 (54.5) | 10 (45.5) | |
| pT3 | 31 | 4 (12.9) | 27 (87.1) | |
| pT4 | 5 | 1 (20.0) | 4( 80.0) | |
| Metastasis | <0.001 | |||
| Absence | 133 | 95 (71.4) | 1 (4.3) | |
| Presence | 23 | 38 (28.6) | 22 (95.7) | |
| Pathological type | 0.238 | |||
| Conventional | 135 | 84 (62.2) | 51 (37.8) | |
| Papillary | 12 | 5 (41.7) | 7 (58.3) | |
| Chromophobe | 10 | 7 (70.0) | 3 (30.0) | |
| Sarcomatous component | <0.001 | |||
| Absence | 141 | 94 (66.7) | 47 (33.3) | |
| Presence | 15 | 2 (13.3) | 13 (86.7) | |
Immunohistochemistry
The RCC tissues sections (5 µm thick) were deparaffinized and antigen retrieval was performed in 0.01 M sodium citrate buffer (pH 6.0), at 121°C for 15 min for the anti-Ki-67 antibody, and at 95°C for 40 min for all of the other antibodies. All tissue sections were immersed in 3% hydrogen peroxide for 30 min to block endogenous peroxidase activity. Primary antibodies were obtained from Abcam (Cambridge, UK; anti-Twist antibody), DakoCytomation (Glostrup, Denmark; anti-Ki-67 antibody), Daiichi Fine Chemical (Toyama, Japan; anti-MMP-2 antibody), and Novocastra Laboratories (Newcastle, UK; anti-CD31 and anti-CD68 antibodies). The sections were incubated with the primary antibodies at 4°C overnight. The sections were then washed extensively to remove all unbound primary antibodies prior to treatment with peroxidase using the labeled polymer method with DAKO EnVision+TM Peroxidase (Dako Corporation, Carpinteria, CA) for 60 min. The peroxidase reaction was visualized using the liquid DAB substrate kit (Zymed Laboratories Inc., San Francisco, CA). Sections were counterstained with hematoxylin. Two consecutive sections from each sample were processed without the primary antibody and used as a negative control. For the anti-Twist antibody, sarcoma tissues were used as the positive control in accordance with the data sheet from the manufacturer. For all other antibodies, immunohistochemical staining of the positive control was performed as described previously [3,4]. The method of in situ labeling for apoptosis was performed as described previously [18]. We used the ApopTag in situ apoptosis detection kit (Intergen, Purchase, NY), based on the terminal deoxynucleotidyl transferase-mediated nick and labeling (TUNEL).
Quantitative analysis and staining interpretation
All analyses of immunohistochemical staining were assessed by light microscopy within the tumor area. The staining intensity of Twist and MMP2 were graded semi-quantitatively as none, weak, moderate, and strong. In the present study, the expression was considered positive if staining intensity was moderate or strong. To determine the MVD and TAM density, the tumor sections were stained with anti-CD31 and anti-CD68 antibodies, respectively. For each tumor section, 3-5 fields with the greatest density of positively stained vessels (“hot spots”) were evaluated, irrespective of the tumor region. They were defined as the number of positively stained vessels and cells, per high-power field (HPF; magnification, ×200). The proliferation index (PI) and apoptotic index (AI) represented the percentage of Ki-67-positive and TUNEL-positive cells, respectively. For all variables, samples with staining greater than the median value were categorized into the higher group, and those with staining less than or equal to the median value were categorized into the lower group for statistical evaluation in at least 500 cancer cells. Slides were initially examined using an E-400 microscope (Nikon, Tokyo, Japan) producing digital images, and then examined using a computer-aided image analysis system (Win ROOF version 5.0; MITANI, Fukui, Japan). Slides were evaluated twice, at different times, by two different investigators (Y.M. and S.K.), who were blinded to the clinicopathological features and survival data.
Statistical analysis
Normality was evaluated by normal distribution and histograms for each variable, and the results were expressed as medians and IQR, unless specified otherwise. The Mann-Whitney U test was performed for continuous variables, and the chi-square test was used for the comparison of categorical data. The crude and adjusted effects were estimated by logistic regression analysis, and the values are reported as odds ratios (ORs) with 95% confidence intervals (95% CI) together with the P values. Variables that achieved statistical significance in a univariate analysis were subsequently entered into a multivariate analysis model. Cause-specific survival rates were compared with Kaplan-Meier analysis and the log rank test. Variables in these tests that achieved statistical significance in the univariate analysis were subsequently entered into a multivariate analysis using a COX proportional hazards analysis, and were described as hazard ratios (HRs) with 95% CI together with the p values. All statistical tests were two-sided, and significance was defined as P<0.050. All statistical analyses were performed using the statistical package StatView (version 5.0).
Results
Twist expression and its pathological significance
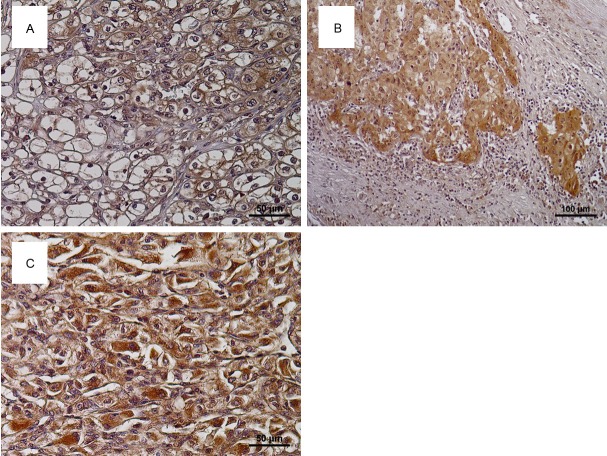
Representative examples of Twist expression in RCC tissues are shown in Figure 1. Twist expression was mainly detected in cytoplasm of cancer cells (Figure 1A), and 60 of 156 specimens (38.5%) were judged to have positive staining. With regard to the distribution of Twist-positive cancer cells, we noticed that intense staining of Twist was observed in cancer cells at edges of the tumor and at invasive fronts (Figure 1B). Table 1 shows the relationships between Twist expression and clinicopathological features. Twist expression was positively associated with grade, pT stage, and the presence of metastasis (P<0.001). Although Twist expression was not associated with pathological types of RCC, its expression in high grade cancer cells including SRCC cells showed strong expression for Fer (Figure 1C).
Figure 1.
Representative examples of Twist expression in human renal cell carcinoma (RCC) tissues. Twist was mainly detected in the cytoplasm of cancer cells (A). Cancer cells at the tumor edge (B) and high grade cancer cells including sarcomatoid RCC (C) often showed high expression of Twist.
Correlation with malignant aggressiveness and cancer-related factors
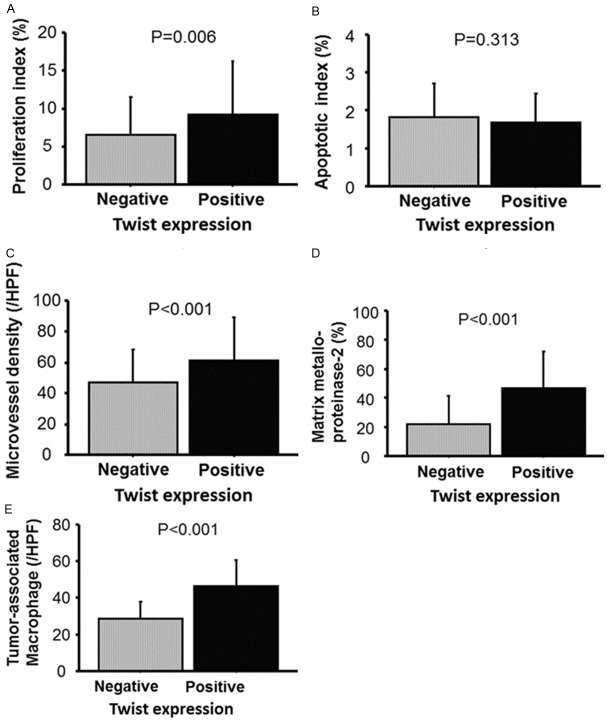
The mean and SD of PI, AI, MVD, TAM, and the positively stained ratio for MMP-2 according to Twist expression, are shown in Figure 2A-E. TAMs in Twist-positive specimens (46.4 and 13.8/HPF) were significantly higher (P<0.001) than those from Twist-negative ones (28.4 and 9.1/HPF). Similarly, Twist expression was positively correlated with MMP-2 (P<0.001), PI (P=0.006), and MVD (P<0.001). However, a significant correlation was not detected in the relationship between Twist expression and AI (P=0.313). When a multi-variate analysis model including pathological features was analyzed (Table 2), Twist expression was found to be independently associated with MMP-2 expression (OR=4.60, 95% CI=1.85-11.48, P<0.001) and TAM density (OR=5.83, 95% CI=2.31-14.74, P<0.001), but not with PI (OR=0.80, 95% CI=0.33-1.93, P=0.613) and MVD (OR=2.06, 95% CI=0.83-5.04, P=0.115).
Figure 2.
Correlation with proliferation index (PI), apoptotic index (AI), microvessel density, matrix metalloproteinase-2 expression, and tumor-associated macrophage density are shown in (A-E), respectively. Univariate analyses showing that PI, MVD, MMP-2 expression, and TAM density in Twist-positive tissues were significantly higher than in Twist-negative ones.
Table 2.
Multi-variate analyses for correlation with cancer-related factors
| Multi-variate analysis* | |||
|---|---|---|---|
|
|
|||
| Odds ratio | 95% confidential intervals | P value | |
| Proliferation index | 0.80 | 0.33-1.93 | 0.613 |
| Apoptotic index** | - | - | - |
| Microvessel density | 2.06 | 0.83-5.04 | 0.115 |
| Matrix metalloproteinase-2 expression | 4.60 | 1.85-11.48 | <0.001 |
| Tumor associated macrophage density | 5.83 | 2.31-14.74 | <0.001 |
Adjusted by pT stage, metastasis, grade, and presence of SRCC.
Apoptotic index was not significant predictor by univariate analysis.
Survival analyses
The Kaplan-Meir survival curve showed that patients with positive expression of Twist had a worse cause-specific survival (P<0.001), than those with negative Twist expression (Figure 3). On the other hand, multi-variate analysis demonstrated that high grade (HR=2.47, 95% CI=1.02-6.02, P=0.046), and presence of metastasis (HR=4.66, 95% CI=1.59-13.65, P=0.005), were independent predictors for cause specific survival, whereas Twist expression was not (HR=0.70, 95% CI=0.23-2.15, P=0.537).
Figure 3.
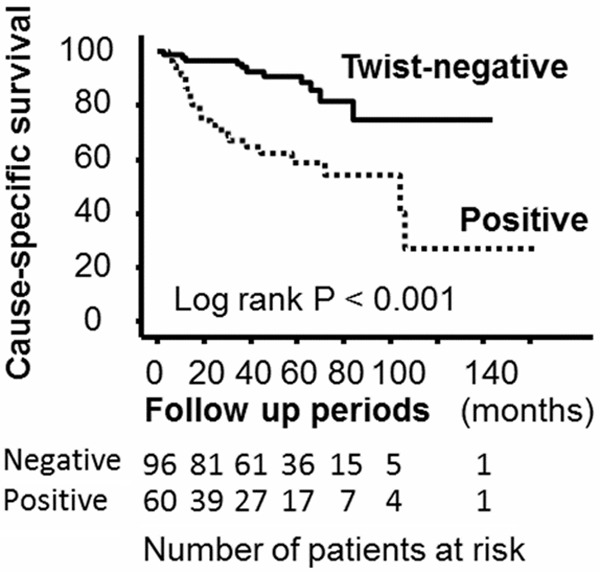
Kaplan-Meier survival curves showed that patients with Twist-positive have worse cause-specific survival compared to those with -negative (log rank p<0.001).
Discussion
Our study showed that Twist expression was positively associated with grade, pT stage, and metastasis in patients with RCC. With regard to Twist in RCC, previous reports demonstrated its role in pathological activities, such as the induction of EMT in RCC cells in vitro [19,20]. In addition, there is a report showing that Twist expression is significantly associated with prognosis in patients with RCC [21]. Our results are similar to these results and thus support these recent findings about Twist. However, this in vivo study investigated Twist expression only in patients with clinically localized RCC. So we do not have any information regarding the pathological significance of Twist in metastasis for RCC patients. Furthermore, the relationships between Twist expression and pathological types, and survival in patients with RCC are still unclear. In reports on other types of cancers, Twist expression has been shown to be significantly associated with metastasis and progression [9,12,13,15]. Thus, it was not surprising that we found Twist expression to be closely associated with metastasis and survival.
On the other hand, the strong expression of Twist in sarcomatoid RCC cells and cancer cells at edge of the tumors was a novel finding in our pathological examinations. We emphasize that these microscopic characteristics may reflect the biological activities and pathological roles of Twist in malignancies including RCC. With regard to Twist in sarcoma, several reports have shown that Twist expression is up-regulated and is significantly associated with malignancy [22,23]. We speculate that the up-regulated expression of Twist partly plays an important role in the extreme malignant aggressiveness of sarcomatoid RCC. However, our findings suggest that Twist expression at the edge of tumors is a strong indicator that Twist has a role in the invasiveness of RCC cells. Other investigators have reported higher expression of EMT including Twist in the invasive front in various cancers [24-26]. Our observations in RCC tissues support the idea that Twist is an important regulator of cancer cell invasion and sequential metastasis in malignancies. To examine this in greater detail, we performed further analysis of the more detailed potential pathological roles of the molecular changes of Twist in RCC tissues.
One of the more interesting results of this study was that Twist expression was positively associated with MMP2 expression and TAM density in a multivariate analysis model that included pathological features. As mentioned above, there have been several reports that Twist plays important roles in malignant aggressiveness and prognosis in RCC [19-21]. However, the detailed mechanism of such pathological activities is not yet fully understood. Although a previous in vitro study focused on the co-function of Twist and MMP2 in RCC cell lines [19], there is no information regarding correlation between Twist and TAM in RCC in vivo and in vitro. With regard to MMP2, other investigators have reported similar findings in breast cancer [14]. In addition, the up-regulation of MMP2 is important step in cancer cell invasion in human RCC tissues [3,27]. Based on these facts, we also suggest that MMP2 expression regulated by Twist affects cancer cell invasion and progression in patients with RCC. However, we did not find a similar staining pattern for MMP2 to that for Twist in our specimens. That is, although higher expression of Twist was detected in cancer cells at edge of tumors, such findings are not characteristics associated with MMP2 expression. Therefore, a further detailed investigation would be necessary to conclude that there is this correlation in RCC.
Our results also demonstrated that Twist expression was positively correlated with TAM density in human RCC tissues. Twist has been reported to recruit macrophages in cancers [19]. Macrophages in the tumor area can suppress the immune response by releasing of a variety of cytokines and cancer-related factors [28]. In addition to immune responses, recruited macrophages can also stimulate cell invasion and angiogenesis under pathological conditions, including cancers [4,29]. Among malignancies, the survival and malignant behavior of RCC cells are well known to be influenced by tumor immunity. In fact, TAM is associated with the pathological features and prognosis in patients with RCC [4]. Finally, we speculate that the release of various chemokines and cytokines from macrophages recruited by Twist, regulate the pathological circumstances in human RCC tissues.
In conclusion, high expression of Twist is significantly correlated with pathological features such as grade, pT stage, and the presence of metastasis in patients with RCC. In addition, its expression is also associated with cause- in specific survival these patients. In morphological analyses, Twist expression is speculated to play important roles in the gain of malignant potential and malignant aggressiveness, including cancer cell invasion. Multi-variate analyses showed that Twist expression was a significant stimulator for MMP2 expression, and the recruitment of macrophages. Finally, our results demonstrated that the up-regulation of Twist influences tumor growth, progression, and survival via the regulation of cell invasion, and immunity in patients with RCC. We suggest that Twist is useful predictor and potential therapeutic target for the treatment of RCC.
Acknowledgements
We are grateful to Mr. Takumi Shimogama for their outstanding support. This study was not supported financially by any private funding agency. However, this study was supported in part by a Grant-in-Aid from Japan Society for the Promotion of Science (to Y.M. and K.O.).
Disclosure of conflict of interest
None.
References
- 1.Rini BI, Rathmell WK, Godley P. Renal cell carcinoma. Curr Opin Oncol. 2008;20:300–306. doi: 10.1097/CCO.0b013e3282f9782b. [DOI] [PubMed] [Google Scholar]
- 2.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–6. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Miyata Y, Koga S, Kanda S, Nishikido M, Hayashi T, Kanetake H. Expression of cyclooxygenase-2 in renal cell carcinoma: correlation with tumor cell proliferation, apoptosis, angiogenesis, expression of matrix metalloproteinase-2, and survival. Clin Cancer Res. 2003;9:1741–9. [PubMed] [Google Scholar]
- 4.Ohba K, Miyata Y, Kanda S, Koga S, Hayashi T, Kanetake H. Expression of urokinase-type plasminogen activator, urokinase-type plasminogen activator receptor and plasminogen activator inhibitors in patients with renal cell carcinoma: correlation with tumor associated macrophage and prognosis. J Urol. 2005;174:461–465. doi: 10.1097/01.ju.0000165150.46006.92. [DOI] [PubMed] [Google Scholar]
- 5.Miyata Y, Iwata T, Maruta S, Kanda S, Nishikido M, Koga S, Kanetake H. Expression of matrix metalloproteinase-10 in renal cell carcinoma and its prognostic role. Eur Urol. 2007;52:791–7. doi: 10.1016/j.eururo.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 6.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 7.Thisse B, el Messal M, Perrin-Schmitt F. The twist gene: isolation of a Drosophila Zygotic gene necessary for the establishment of dorsoventral pattern. Nucleic Acids Res. 1987;15:3439–3453. doi: 10.1093/nar/15.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castanon I, Baylies MK. A Twist in fate: evolutionary comparison of Twist structure and function. Gene. 2002;287:11–22. doi: 10.1016/s0378-1119(01)00893-9. [DOI] [PubMed] [Google Scholar]
- 9.Lee TK, Poon RT, Yuen AP, Ling MT, Kwok WK, Wang XH, Wong YC, Guan XY, Man K, Chau KL, Fan ST. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res. 2006;12:5369–5376. doi: 10.1158/1078-0432.CCR-05-2722. [DOI] [PubMed] [Google Scholar]
- 10.Qin Q, Xu Y, He T, Qin C, Xu J. Normal and disease-related biological functions of Twist1 and underlying molecular mechanisms. Cell Res. 2012;22:90–106. doi: 10.1038/cr.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mironchik Y, Winnard PT Jr, Vesuna F, Kato Y, Wildes F, Pathak AP, Kominsky S, Artemov D, Bhujwalla Z, Van Diest P, Burger H, Glackin C, Raman V. Twist overexpression induces in vivo angiogenesis and correlates with chromosomal instability in breast cancer. Cancer Res. 2005;65:10801–10809. doi: 10.1158/0008-5472.CAN-05-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, play an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Gajula RP, Chettiar ST, Williams RD, Thiyagarajan S, Kato Y, Aziz K, Wang R, Gandhi N, Wild AT, Vesuna F, Ma J, Salih T, Cades J, Fertig E, Biswal S, Burns TF, Chung CH, Rudin CM, Herman JM, Hales RK, Raman V, An SS, Tran PT. The Twist Box Domain Is Required for Twist1-induced Prostate Cancer Metastasis. Mol Cancer Res. 2013;11:387–400. doi: 10.1158/1541-7786.MCR-13-0218-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao M, Hu HG, Huang J, Zou Q, Wang J, Liu MQ, Zhao Y, Li GZ, Xue S, Wu ZS. Expression and correlation of Twist and gelatinases in breast cancer. Exp Ther Med. 2013;6:97–100. doi: 10.3892/etm.2013.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang MH, Wu KJ. TWIST activation by hypoxia inducible factor-1 (HIF-1): implications in metastasis and development. Cell Cycle. 2008;7:2090–2096. doi: 10.4161/cc.7.14.6324. [DOI] [PubMed] [Google Scholar]
- 16.Sosic D, Richardson JA, Yu K, Ornitz DM, Olson EM. Twist regulates cytokine gene expression through a negative feedback loop that represses NF-κB activity. Cell. 2003;112:169–180. doi: 10.1016/s0092-8674(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 17.Low-Marchelli JM, Ardi VC, Vizcarra EA, van Rooijen N, Quigley JP, Yang J. Twist1 induces CCL2 and recruits macrophages to promote angiogenesis. Cancer Res. 2013;73:662–71. doi: 10.1158/0008-5472.CAN-12-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watanabe S, Miyata Y, Kanda S, Iwata T, Hayashi T, Kanetake H, Sakai H. Expression of X-linked inhibitor of apoptosis protein in human prostate cancer specimens with and without neo-adjuvant hormonal therapy. J Cancer Res Clin Oncol. 2010;136:787–93. doi: 10.1007/s00432-009-0718-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright TM, Brannon AR, Gordan JD, Mikels AJ, Mitchell C, Chen S, Espinosa I, van de Rijn M, Pruthi R, Wallen E, Edwards L, Nusse R, Rathmell WK. Ror2, a developmentally regulated kinase, promotes tumor growth potential in renal cell carcinoma. Oncogene. 2009;28:2513–23. doi: 10.1038/onc.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.An J, Liu H, Magyar CE, Guo Y, Veena MS, Srivatsan ES, Huang J, Rettig MB. Hyperactivated JNK is a therapeutic target in pVHL-deficient renal cell carcinoma. Cancer Res. 2013;73:1374–85. doi: 10.1158/0008-5472.CAN-12-2362. [DOI] [PubMed] [Google Scholar]
- 21.Harada K, Miyake H, Kusuda Y, Fujisawa M. Expression of epithelial-mesenchymal transition markers in renal cell carcinoma: impact on prognostic outcomes in patients undergoing radical nephrectomy. BJU Int. 2012;110:1131–1137. doi: 10.1111/j.1464-410X.2012.11297.x. [DOI] [PubMed] [Google Scholar]
- 22.D’Angelo E, Spagnoli LG, Prat J. Comparative clinicopathologic and immunohistochemical analysis of uterine sarcomas diagnosed using the World Health Organization classification system. Hum Pathol. 2009;40:1571–85. doi: 10.1016/j.humpath.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 23.Yin K, Liao Q, He H, Zhong D. Prognostic value of Twist and E-cadherin in patients with osteosarcoma. Med Oncol. 2012;29:3449–55. doi: 10.1007/s12032-012-0317-6. [DOI] [PubMed] [Google Scholar]
- 24.Fendrich V, Waldmann J, Feldmann G, Schlosser K, König A, Ramaswamy A, Bartsch DK, Karakas E. Unique expression pattern of the EMT markers Snail, Twist and E-cadherin in benign and malignant parathyroid neoplasia. Eur J Endocrinol. 2009;160:695–703. doi: 10.1530/EJE-08-0662. [DOI] [PubMed] [Google Scholar]
- 25.Malfettone A, Silvestris N, Paradiso A, Mattioli E, Simone G, Mangia A. Overexpression of nuclear NHERF1 in advanced colorectal cancer: association with hypoxic microenvironment and tumor invasive phenotype. Exp Mol Pathol. 2012;92:296–303. doi: 10.1016/j.yexmp.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Creighton CJ, Gibbons DL, Kurie JM. The role of epithelial-mesenchymal transition programming in invasion and metastasis: a clinical perspective. Cancer Manag Res. 2013;5:187–95. doi: 10.2147/CMAR.S35171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohba K, Miyata Y, Koga S, Kanda S, Kanetake H. Expression of nm23-H1 gene product in sarcomatous cancer cells of renal cell carcinoma: correlation with tumor stage and expression of matrix metalloproteinase-2, matrix metalloproteinase-9, sialyl Lewis X, and c-erbB-2. Urology. 2005;65:1029–34. doi: 10.1016/j.urology.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 28.Daurkin I, Eruslanov E, Stoffs T, Perrin GQ, Algood C, Gilbert SM, Rosser CJ, Su LM, Vieweg J, Kusmartsev S. Tumor-associated macrophages mediate immunosuppression in the renal cancer microenvironment by activating the 15-lipoxygenase-2 pathway. Cancer Res. 2011;71:6400–6409. doi: 10.1158/0008-5472.CAN-11-1261. [DOI] [PubMed] [Google Scholar]
- 29.Behnes CL, Bremmer F, Hemmerlein B, Strauss A, Ströbel P, Radzun HJ. Tumor-associated macrophages are involved in tumor progression in papillary renal cell carcinoma. Virchows Arch. 2013;464:191–196. doi: 10.1007/s00428-013-1523-0. [DOI] [PubMed] [Google Scholar]