Abstract
The adapter protein growth factor receptor-bound 2 (GRB2) is essential for various basic cellular functions by mediating the regulation of receptor tyrosine kinase (RTK) signaling, however, little is known about GRB2 expression in esophageal squamous cell carcinoma (ESCC). We sought to characterize GRB2 expression and its relationship with clinicopathological parameters and prognostic significance in ESCC patients. Here, it was presented that GRB2 was overexpressed in cytoplasm in 58.1% (100/172) of ESCC cases by immunohistochemistry. Survival analysis demonstrated overexpression of GRB2 protein was significantly related to poor prognosis of ESCC patients (P = 0.021). Furthermore, overexpression of GRB2 was significantly associated with the lymph node metastases. In addition, subgroup analysis according to lymph node metastasis revealed a shorter disease-free survival (DFS) in the ESCC patients with GRB2 overexpression than the patients with GRB2 low-expression (Means for DFS months: 33.8 versus 52.1). Finally, the significant difference between overexpression of GRB2 and poor survival rates exhibited in univariate analysis (P = 0.022) and multivariate Cox analysis (close to significance, P = 0.065), demonstrated that GRB2 was an independent factor in prognosis of ESCC patients. In conclusion, GRB2 expression status could be as a positive biomarker of ESCC progression and lymph node metastasis.
Keywords: GRB2 expression, survival, lymph node metastasis, immunohistochemistry, esophageal squamous cell carcinoma
Introduction
Esophageal cancer is one of the most frequently occurring malignancies worldwide [1]. Among the common histological types of esophageal cancer, esophageal squamous cell carcinoma (ESCC) is the fourth most lethal malignancy in China due to the difficulty of early detection and metastatic recurrence in the advanced stage [2]. To properly address postoperative surveillance and treatment, it is necessary to develop prognostic markers to characterise the heterogeneity of ESCC. Some prognostic factors, such as lymph node status, have established a prognostic system [3]. However, the majority of ESCC patients with local lymph nodes metastasis or with cancers invading to the muscularis propria implicated poor prognosis [4,5]. Therefore, identification of useful biomarkers directed against metastasis of ESCC is needed.
Receptor protein tyrosine kinase (RTK) signaling pathways contributing to cell proliferation, angiogenesis, invasion, and metastasis, have been one of the most critical targets of intervention strategies [6]. Growth factor receptor-bound 2 (GRB2) as a ubiquitous adapter protein, provides a critical crosstalk between RTK signals and the intracellular signals [7,8]. The GRB2 adaptor protein contains one SH2 domain and two SH3 domains. The SH2 domain binds tyrosine phosphorylated sequences (pYXNX), and the two SH3 domains direct complex formation with proline-rich regions of other proteins such as SOS1 in close proximity to Ras at the plasma membrane [9-11]. Most importantly, it has been implicated that GRB2 overexpression can increase the activation of signaling pathways and malignant transformation, and correlates with poor prognosis in several human cancers, including of breast, bladder, gastric and colorectal cancers [12-14]. Previous investigation has reported that blocking GRB2 signaling inhibits cell motility in colorectal cancer [15]. However, little is known about the cellular distribution of GRB2 in human ESCC. Here, we explore the expression status of GRB2, and further evaluate its correlation with other clinicopathological features and disease-free survival in a large cohort of ESCC.
Materials and methods
Study patients
We obtained pathologically proven formalin fixed paraffin embedded (FFPE) specimens of 172 ESCC patients. All cases received curative surgery in Affiliated Shantou Hospital of Sun Yat-sen University (Shantou, China) between 2000 and 2006. Cases were selected for this study only if follow-up examinations and clinical data were available. The follow-up for patients after esophageal resection was continued until their deaths. Information on various clinicopathological characteristics including age, gender, stage of disease, and histopathologic factors summarized in Table 1, was obtained from the records of relevant Clinical Pathology Department. Meanwhile, the cases were classified according to the 7th edition of the tumor-node-metastasis (TNM) classification of the International Union against Cancer (UICC). The patients suffered from severpost-operative complications and died of other tumors or other causes were excluded. The study was approved by the ethical committee of the Central Hospital of Shantou City and the ethical committee of the Medical College of Shantou University, and a written informed consent was obtained from each patient to use resected samples for research.
Table 1.
Distribution of cases by clinical characteristics and survival information of the patients (Kaplan-Meier)
| Clinical parameter | Number | Means for DFS months (95% CI) | χ2 | * P value |
|---|---|---|---|---|
| Age (years) | ||||
| < 57.6 | 81 | 83.0 (68.2-97.7) | 1.297 | 0.255 |
| > 57.6 | 91 | 71.7 (58.3-85.1) | ||
| Gender | ||||
| female | 42 | 79.2 (59.8-98.7) | 1.121 | 0.728 |
| male | 130 | 75.4 (64.2-86.7) | ||
| Treatment | ||||
| Only surgery | 146 | 79.7 (68.9-90.6) | 1.134 | 0.287 |
| Surgery and adjuvant therapy | 26 | 59.7 (38.8-80.6) | ||
| Differentiation grade | ||||
| Well | 49 | 70.1 (54.3-85.9) | 0.535 | 0.765 |
| Moderately | 105 | 78.5 (65.5-91.4) | ||
| Poorly | 18 | 51.9 (33.3-70.5) | ||
| Invasive depth | ||||
| Tis, T1, T2 | 10 | 59.6 (39.3-79.9) | 0.517 | 0.472 |
| T3, T4 | 162 | 76.1 (65.8-86.3) | ||
| Lymph node metastasis | ||||
| N0 | 92 | 99.1 (86.0-112.1) | 26.103 | < 0.001 |
| N1, N2, N3 | 80 | 42.2 (33.3-51.4) | ||
| TNM classification | ||||
| I, IIa | 92 | 99.1 (86.0-112.1) | 26.103 | < 0.001 |
| IIb, III, IV | 80 | 42.2 (33.3-51.4) | ||
P value, significance level is 0.05;
CI, confidence interval.
Immunohistochemistry
All samples embedded in paraffin wax blocks underwent tissue microarray (TMA) construction and then were cut into 4 μm sections [16]. Immunohistochemical staining was performed using the SuperPicTure™ Polymer Detection Kit and Liquid DAB Substrate Kit (Zymed/Invitrogen, Carlsbad, CA, USA) as described [4,5,16]. Rabbit polyclonal antibodies to GRB2 (1:500, sc-255, Santa Cruz Biotechnology, Dallas, Texas, USA) were used for IHC according to protocols provided by the manufacturer. Scores were independently assessed by two researchers blinded to clinical data. Each separate tissue core was scored based on both intensity and percentage of positive cells [4,5,16]. Briefly, the intensity grade of staining was: 0, negative; 1, weak; 2, moderate; 3, strong. The percentage of positive cells was: 0, < 5%; 1, 5% to 25%; 2, 26% to 50%; 3, 51% to 75%; 4, > 75%. The final score was calculated by multiplying the intensity grade and the percentage of positive cells, producing a total range from 0 to 12.
Selection of cutpoint score and prognosis analysis
X-tile plots were applied to assess GRB2 expression and optimize the cutpoint score based on outcome [17]. The X-tile program divided the cohorts randomly into a matched training and validation set as a method for selecting optimal cutpoint score, respectively. Statistical significance was assessed by using the cutoff score derived from a training set to parse a separate validation set, using a standard log-rank method, with P values obtained from a lookup table. The X-tile plots allowed determination of an optimal cutoff value while correcting for the use of minimum P statistics by Miller-Siegmund P-value correction [18].
Statistical analysis
Data analysis was performed using SPSS 13.0 software (SPSS, Inc., Chicago, IL). Kaplan-Meier curves were constructed for disease-free survival (DFS) analysis using a log-rank test. DFS was defined as the period after curative treatment during which no recurrence was found. Associations of GRB2 protein level and other clinicopathological characteristics were assessed with the Kendall’s tau-b test. The univariate and multivariate Cox proportional-hazards regression models were developed to correlate the clinical characteristics, survival, and GRB2 protein level. Each P-value is two-tailed and significance level is 0.05.
Results
Clinicopathological characteristics of ESCC patients
Total of 172 ESCC specimens were examined by immunohistochemistry analysis in this study. As shown in Table 1, 75.6% were males and the mean age was 57.6 years in a range of 31 to 75 years with no significant gender difference. In addition, no survival advantage was found between the surgery combined with adjuvant therapy and the surgery-alone group. Hence, we analyzed the patients’ survival together. Kaplan-Meier survival analysis revealed that a poor DFS was significantly associated with lymph node metastasis and TNM stage (P < 0.001).
Expression patterns of GRB2 in ESCC specimens and X-tile analysis
Typical immunostainings are shown in Figure 1. GRB2 immunoreactivity was predominantly cytoplasmic in ESCC cells and almost all of the specimens were homogeneous staining. To assess statistical significance and avoid arbitrary cutoff point selection, the X-tile program was employed to determine cutoff scores for GRB2 expression. According to the X-tile plots, we divided the 172 cases into low and high populations were based on the cutoff point of over seven (Figure 2A and 2B). Therefore, scores of 0 to 7 were considered as low-expression, and scores of 8 to 12 were defined as overexpression. Among the 172 ESCC tissues, overexpression of GRB2 was observed in 58.1% (100/172), while another 72 cases presented relative weakly immunoreactivity. However, only 4 cases of 11 noncancerous-adjacent tissues (36.4%) exhibited GRB2 overexpression. Furthermore, there was significant difference in overexpression rate of GRB2 between ESCC tissues and noncancerous-adjacent tissues (P < 0.001).
Figure 1.
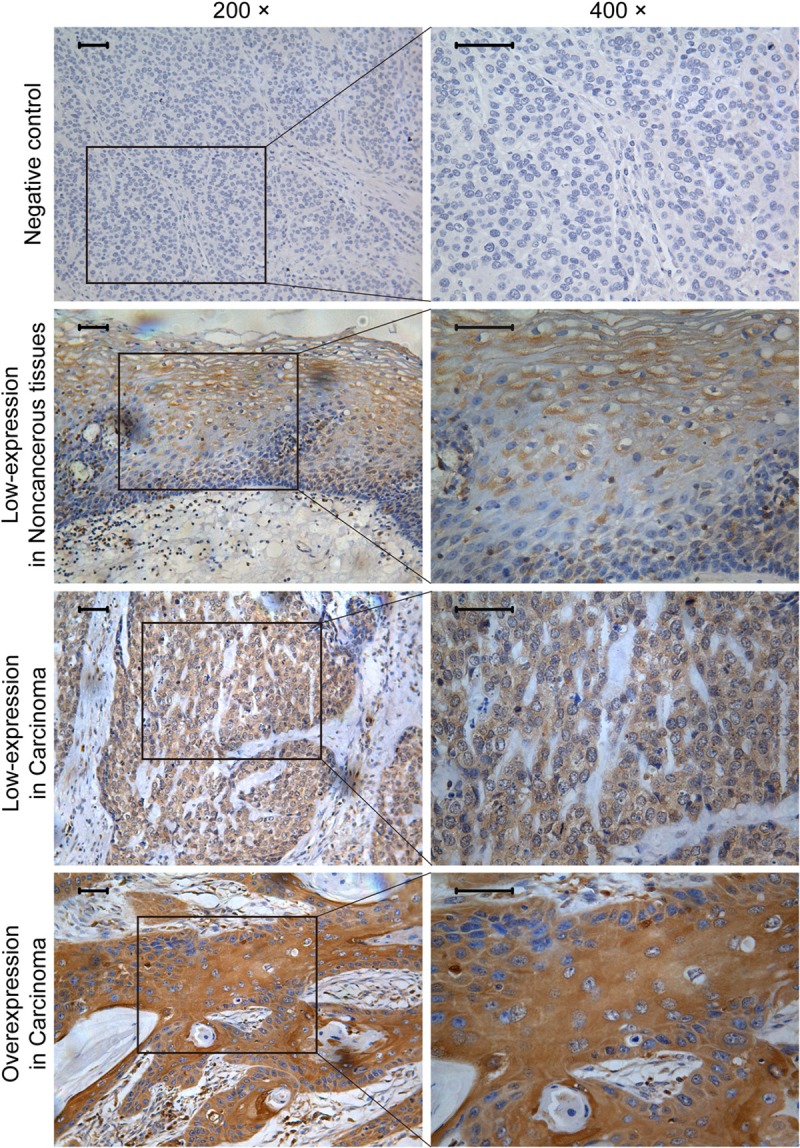
Immunohistochemistry expression pattern of GRB2 in ESCC. Negative immunoreactivity was observed when GRB2 antibody was replaced with PBS. The representative immunohistochemistry visual fields in this image were scored as follows: Immunoreactive scores for GRB2 was 4 in the cytoplasm of noncancerous tissues, 4 in cancer cytoplasm of low-expression group and 12 in cancer cytoplasm of overexpression group. The inserted bars are 50 μm.
Figure 2.
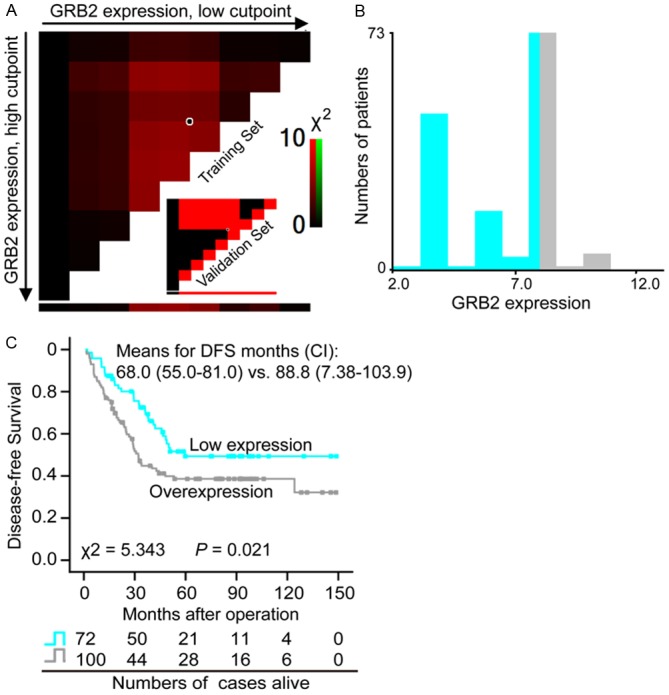
Disease-free survival (DFS) of patients with ESCC on the basis of GRB2 expression status. X-tile analysis was carried out on patient data, equally divided into training and validation subsets. A: The plot showed the chi-square log-rank values created when the cohort was divided into two populations, and the optimal cutpoint score highlighted by the black/white circle. B: The optimal cutpoint (0-7 vs. 8-12) was demonstrated on a histogram of the entire cohort. C: Kaplan-Meier survival analysis demonstrated that the DFS rate was significantly lower in the GRB2 overexpression group than that in the low expression group (P = 0.021). CI, 95% confidence interval; “vs.” means versus.
Correlations of GRB2 expression with various clinicopathological features in ESCC patients
To further investigate the clinical significance of GRB2 expression, the relationship between GRB2 expression status and various clinicopathological factors were shown in Table 2 by Kendall’s tau-b test. It was found that a positive correlation of GRB2 immunostaining with lymph node metastasis (P = 0.041). Overexpression of GRB2 was present in 53 of 80 cases (66.2%) with lymph node metastasis. However, there was no association between GRB2 expression and other clinicopathological variables, including age, gender, differentiation grade and invasive depth.
Table 2.
Correlation between GRB2 expression level and clinicopathologic features in ESCC patients (Kendall’s tau-b test)
| Clinical parameters | GRB2 | * P value | |
|---|---|---|---|
|
| |||
| Low-expression | Overexpression | ||
| Age (year) | |||
| < 57.6 | 33 (40.7%) | 48 (59.3%) | 0.779 |
| > 57.6 | 39 (42.9%) | 52 (57.1%) | |
| Gender | |||
| Female | 20 (47.6%) | 22 (52.4%) | 0.389 |
| Male | 52 (40.0%) | 78 (60.0%) | |
| Differentiation grade | |||
| Well | 22 (44.9%) | 27 (55.1%) | 0.931 |
| Moderately | 41 (39.0%) | 64 (61.0%) | |
| Poorly | 9 (50.0%) | 9 (50.0%) | |
| Invasive depth | |||
| Tis, T1, T2 | 3 (30.0%) | 7 (70.0%) | 0.415 |
| T3, T4 | 69 (42.6%) | 93 (57.4%) | |
| Lymph node metastasis | |||
| N0 | 45 (48.9%) | 47 (51.1%) | 0.041 |
| N1, N2, N3 | 27 (33.8%) | 53 (66.2%) | |
| TNM classification | |||
| I, IIa | 45 (48.9%) | 47 (51.1%) | 0.041 |
| IIb, III, IV | 27 (33.8%) | 53 (66.2%) | |
P value, significance level is 0.05.
Overexpression of GRB2 was associated with poor survival in ESCC patients
Giving the positive correlation of GRB2 overexpression with lymph node metastasis and the latter as the indicator of poor prognosis, we further analyzed whether GRB2 expression was a significant prognostic factor by Kaplan-Meier method. Indeed, as shown in Figure 2C, overexpression of GRB2 was significantly associated with poor survival (Means for survival time: 68.0 months versus 88.8 months; P = 0.021). Subsequently, subgroup analysis according lymph node metastasis was performed. As expected, patients with GRB2 overexpression predicted a shorter DFS than patients with GRB2 low-expression in the group with lymph node metastasis (Means for survival time: 33.8 months versus 52.1 months), however, this was not observed in those without lymph node metastasis (Means for survival time: 95.7 months versus 103.0 months) (Figure 3). Subsequently, we used univariate analysis and multivariate analysis to examine whether GRB2 is an independent high-risk predictor in ESCC (Table 3). In the univariate Cox regression analyses, GRB2 overexpression and lymph node metastasis was significantly associated with an unfavourable DFS (P = 0.022 and P < 0.001). However, in the multivariate Cox regression analysis, it was found that only lymph node metastasis was an independent predictor of overall survival (P < 0.001), the GRB2 protein level was close to significance (P = 0.065). The evidence provided clues that GRB2 was an independent predictor of DFS in ESCC patients.
Figure 3.
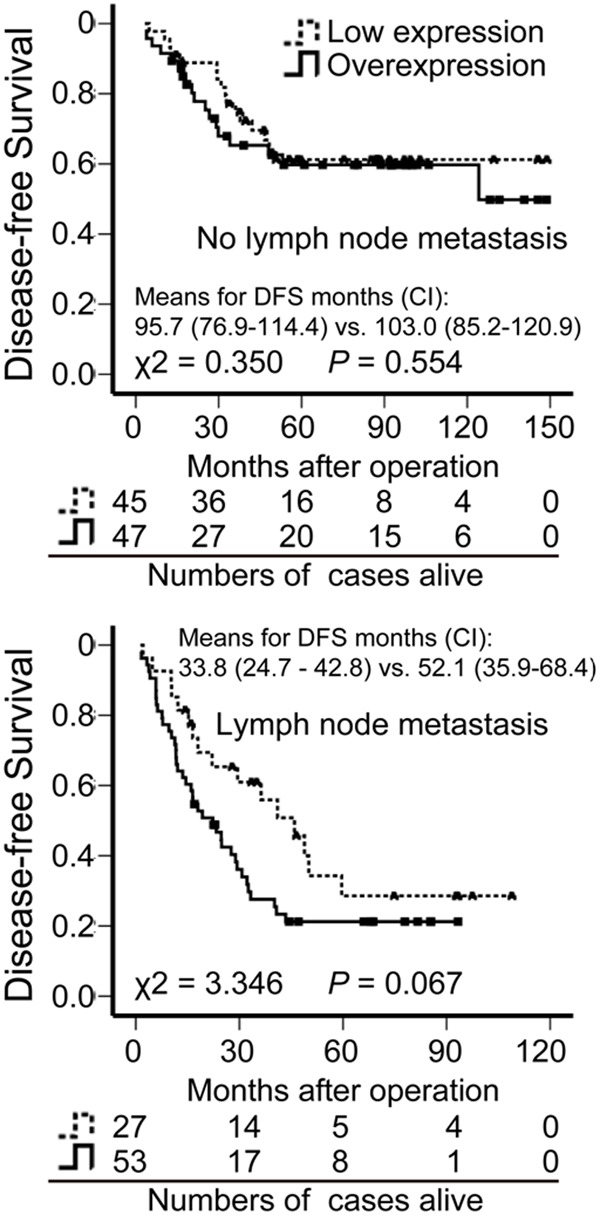
Disease-free survival (DFS) of patients with ESCC with or without lymph node metastasis, on the basis of GRB2 expression status. A shorter DFS only for the patients with lymph node metastasis was shown in the GRB2 overexpression group than that in the low expression group by Kaplan-Meier survival analysis (P = 0.067). CI, 95% confidence interval; “vs.” means versus.
Table 3.
Cox regression analysis to determine factors independently associated with unfavorable prognosis
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
|
|
||||
| Clinical parameter | * P value | Hazard ratio (95% CI) | * P value | Hazard ratio (95% CI) |
| Age (year) | 0.256 | 1.274 (0.839-1.937) | 0.666 | 1.098 (0.717-1.682) |
| Gender | 0.728 | 1.089 (0.673-1.762) | 0.809 | 1.063 (0.646-1.749) |
| Differentiation grade | 0.757 | 1.055 (0.750-1.485) | 0.619 | 0.911 (0.631-1.315) |
| Invasive depth | 0.475 | 1.442 (0.529-3.931) | 0.353 | 1.622 (0.584-4.502) |
| Lymph node metastasis | < 0.001 | 2.945 (1.908-4.545) | < 0.001 | 2.857 (1.824-4.476) |
| GRB2 expression | 0.022 | 1.657 (1.075-2.554) | 0.065 | 1.514 (0.974-2.355) |
P value, significance level is 0.05;
CI, confidence interval.
Discussion
The current study showed that GRB2 was widely expressed in the cytoplasm of ESCC cells and overexpression of GRB2 was a predictor of poor disease-free survival. A number of reports has been indicated that overexpression of GRB2 is related to poor survival in cancers including colorectal, gastric, and hepatocellular carcinoma [14,19,20]. These observations support the results of our study. In human cancers, GRB2 acts as a critical downstream intermediary in several oncogenic signaling pathways, such as RTK pathways [21,22]. Accumulating studies have demonstrated that GRB2 participates directly in the pathogenesis of several important human malignancies including leukemia and solid tumors [23,24]. Direct and indirect interactions between GRB2 and molecules involved in cytoskeleton remodeling, motility and other cellular processes, elucidated that GRB2 contribute to the multistep cascade of cancer cell proliferation and metastasis [8,25]. Moreover, inhibition of GRB2 expression could significantly inhibit proliferation and survival of cancer cells [15,26]. In view of the evidences, targeting disease-causing GRB2 protein to reduction may be an alternative therapeutic strategy for ESCC patients.
In our study, patients with GRB2 expression had a poor prognosis for disease-free survival. However, multivariate statistical analysis showed that GRB2 overexpression was not a prognostic factor by itself. Therefore, it can be assumed that this result is influenced by other clinicopathological factors, such as cell differentiation, tumour invasion, and lymph node metastasis. Indeed, our result demonstrated significant correlations between GRB2 overexpression and a traditional independent prognostic marker lymph node metastasis. Lymph node metastasis is one most important factor in determining prognosis of ESCC patients [3,27,28]. Thus, an effective means of treating lymph node metastasis is essential to avoid recurrence and would improve survival rates of most cancers [29,30]. As GRB2 overexpression was associated with lymph node metastasis, patients with GRB2 overexpression had a poor prognosis. Furthermore, subgroup analysis according to lymph node metastasis revealed a shorter DFS in the ESCC patients group with GRB2 overexpression. Therefore, these data hinted that GRB2 would play critical roles in human esophageal tumorigenesis and metastasis. In addition, further investigation is needed to confirm the roles of GRB2 in cell invasion and metastasis in ESCC cells.
In conclusion, overexpression of GRB2 is significantly related to lymph node metastasis in ESCC, which is consequently associated with a poor DFS, suggesting that GRB2 may serve as a prognostic factor in ESCC patients.
Acknowledgements
This work was supported by grants from the National 11th Five-Year Plan major scientific and technological issues of China (No.2009ZX9302-004), the National Basic Research Program (973 Program No. 2012CB526608), the National High Technology Research and Development Program of China (No. 2012AA02A503 and No. 2012AA02A209), and the Natural Science Foundation of China-GuangDong Joint Fund (No. U0932001 and No. U1301227).
Disclosure of conflict of interest
None.
References
- 1.Enzinger PC, Mayer RJ. Esophageal cancer. New Engl J Med. 2003;349:2241–52. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Ancona E, Rampado S, Cassaro M, Battaglia G, Ruol A, Castoro C, Portale G, Cavallin F, Rugge M. Prediction of lymph node status in superficial esophageal carcinoma. Ann Surg Oncol. 2008;15:3278–88. doi: 10.1245/s10434-008-0065-1. [DOI] [PubMed] [Google Scholar]
- 4.Li LY, Li EM, Wu ZY, Huang X, Shen JH, Xu XE, Wu JY, Huang Q, Xu LY. Connective tissue growth factor expression in precancerous lesions of human esophageal epithelium and prognostic significance in esophageal squamous cell carcinoma. Dis Esophagus. 2010 doi: 10.1111/j.1442-2050.2010.01147.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Zhao Q, Shen JH, Shen ZY, Wu ZY, Xu XE, Xie JJ, Wu JY, Huang Q, Lu XF, Li EM, Xu LY. Phosphorylation of fascin decreases the risk of poor survival in patients with esophageal squamous cell carcinoma. J Histochem Cytochem. 2010;58:979–88. doi: 10.1369/jhc.2010.955765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma WW, Adjei AA. Novel agents on the horizon for cancer therapy. CA Cancer J Clin. 2009;59:111–37. doi: 10.3322/caac.20003. [DOI] [PubMed] [Google Scholar]
- 7.Dharmawardana PG, Peruzzi B, Giubellino A, Burke TR Jr, Bottaro DP. Molecular targeting of growth factor receptor-bound 2 (Grb2) as an anti-cancer strategy. Anticancer Drugs. 2006;17:13–20. doi: 10.1097/01.cad.0000185180.72604.ac. [DOI] [PubMed] [Google Scholar]
- 8.Giubellino A, Burke TR Jr, Bottaro DP. Grb2 signaling in cell motility and cancer. Expert Opin Ther Targets. 2008;12:1021–33. doi: 10.1517/14728222.12.8.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chardin P, Cussac D, Maignan S, Ducruix A. The Grb2 adaptor. FEBS Lett. 1995;369:47–51. doi: 10.1016/0014-5793(95)00578-w. [DOI] [PubMed] [Google Scholar]
- 10.Lowenstein EJ, Daly RJ, Batzer AG, Li W, Margolis B, Lammers R, Ullrich A, Skolnik EY, Bar-Sagi D, Schlessinger J. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 1992;70:431–42. doi: 10.1016/0092-8674(92)90167-b. [DOI] [PubMed] [Google Scholar]
- 11.Hill RJ, Zozulya S, Lu YL, Ward K, Gishizky M, Jallal B. The lymphoid protein tyrosine phosphatase Lyp interacts with the adaptor molecule Grb2 and functions as a negative regulator of T-cell activation. Exp Hematol. 2002;30:237–44. doi: 10.1016/s0301-472x(01)00794-9. [DOI] [PubMed] [Google Scholar]
- 12.Verbeek BS, Adriaansen-Slot SS, Rijksen G, Vroom TM. Grb2 overexpression in nuclei and cytoplasm of human breast cells: a histochemical and biochemical study of normal and neoplastic mammary tissue specimens. J Pathol. 1997;183:195–203. doi: 10.1002/(SICI)1096-9896(199710)183:2<195::AID-PATH901>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe T, Shinohara N, Moriya K, Sazawa A, Kobayashi Y, Ogiso Y, Takiguchi M, Yasuda J, Koyanagi T, Kuzumaki N, Hashimoto A. Significance of the Grb2 and son of sevenless (Sos) proteins in human bladder cancer cell lines. IUBMB Life. 2000;49:317–20. doi: 10.1080/15216540050033195. [DOI] [PubMed] [Google Scholar]
- 14.Yu GZ, Chen Y, Wang JJ. Overexpression of Grb2/HER2 signaling in Chinese gastric cancer: their relationship with clinicopathological parameters and prognostic significance. J Cancer Res Clin Oncol. 2009;135:1331–9. doi: 10.1007/s00432-009-0574-8. [DOI] [PubMed] [Google Scholar]
- 15.Yu GZ, Chen Y, Long YQ, Dong D, Mu XL, Wang JJ. New insight into the key proteins and pathways involved in the metastasis of colorectal carcinoma. Oncol Rep. 2008;19:1191–204. [PubMed] [Google Scholar]
- 16.Zhang FR, Tao LH, Shen ZY, Lv Z, Xu LY, Li EM. Fascin expression in human embryonic, fetal, and normal adult tissue. J Histochem Cytochem. 2008;56:193–9. doi: 10.1369/jhc.7A7353.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–9. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 18.Raeside DE. Monte Carlo principles and applications. Phys Med Biol. 1976;21:181–97. doi: 10.1088/0031-9155/21/2/001. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Li Z, Yang M, Wang D, Yu L, Guo C, Guo X, Lin N. Identification of GRB2 and GAB1 Coexpression as an Unfavorable Prognostic Factor for Hepatocellular Carcinoma by a Combination of Expression Profile and Network Analysis. PLoS One. 2013;8:e85170. doi: 10.1371/journal.pone.0085170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang W, Gao X, Han Y, Du Y, Liu Q, Wang L, Tan X, Zhang Q, Liu Y, Zhu Y, Yu Y, Fan X, Zhang H, Zhou W, Wang J, Fu C, Cao G. Gene expression profiling-derived immunohistochemistry signature with high prognostic value in colorectal carcinoma. Gut. 2013 doi: 10.1136/gutjnl-2013-305475. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Margolis B, Skolnik EY. Activation of Ras by receptor tyrosine kinases. J Am Soc Nephrol. 1994;5:1288–99. doi: 10.1681/ASN.V561288. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed Z, George R, Lin CC, Suen KM, Levitt JA, Suhling K, Ladbury JE. Direct binding of Grb2 SH3 domain to FGFR2 regulates SHP2 function. Cell Signal. 2010;22:23–33. doi: 10.1016/j.cellsig.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Pendergast AM, Quilliam LA, Cripe LD, Bassing CH, Dai Z, Li N, Batzer A, Rabun KM, Der CJ, Schlessinger J, et al. BCR-ABL-induced oncogenesis is mediated by direct interaction with the SH2 domain of the GRB-2 adaptor protein. Cell. 1993;75:175–85. [PubMed] [Google Scholar]
- 24.Haines E, Saucier C, Claing A. The adaptor proteins p66Shc and Grb2 regulate the activation of the GTPases ARF1 and ARF6 in invasive breast cancer cells. J Biol Chem. 2014;289:5687–703. doi: 10.1074/jbc.M113.516047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giubellino A, Gao Y, Lee S, Lee MJ, Vasselli JR, Medepalli S, Trepel JB, Burke TR Jr, Bottaro DP. Inhibition of tumor metastasis by a growth factor receptor bound protein 2 Src homology 2 domain-binding antagonist. Cancer Res. 2007;67:6012–6. doi: 10.1158/0008-5472.CAN-07-0022. [DOI] [PubMed] [Google Scholar]
- 26.Modi H, Li L, Chu S, Rossi J, Yee JK, Bhatia R. Inhibition of Grb2 expression demonstrates an important role in BCR-ABL-mediated MAPK activation and transformation of primary human hematopoietic cells. Leukemia. 2011;25:305–12. doi: 10.1038/leu.2010.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuwano H, Sumiyoshi K, Sonoda K, Kitamura K, Tsutsui S, Toh Y, Kitamura M, Sugimachi K. Expression of p53 protein in glandular differentiation admixed with squamous cell carcinoma of the esophagus. Hepatogastroenterology. 1997;44:170–4. [PubMed] [Google Scholar]
- 28.Altorki N, Skinner D. Should en bloc esophagectomy be the standard of care for esophageal carcinoma? Ann Surg. 2001;234:581–7. doi: 10.1097/00000658-200111000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219–25. doi: 10.1007/pl00011720. [DOI] [PubMed] [Google Scholar]
- 30.Bird-Lieberman EL, Fitzgerald RC. Early diagnosis of oesophageal cancer. Br J Cancer. 2009;101:1–6. doi: 10.1038/sj.bjc.6605126. [DOI] [PMC free article] [PubMed] [Google Scholar]


