Abstract
Cancer stem cells (CSCs), which have the abilities of tumor-initiating, self-renewal and differentiation, are thought to cause post-therapeutic recurrence and the progression of cancer. However, CSCs are commonly resistant to current cancer therapies including chemotherapy and radiotherapy. In this study, we isolated cancer stem celllike side population (SP) cells from human bladder cancer cell line SW780 by a flow cytometry-based SP technique. SP cells were only about 3.6% of SW780 cells and showed higher expression of ATP-binding cassette sub-family G member 2 (ABCG2) and CD133. In vitro assay of tumor sphere growth as well as in vivo assay of xenograft transplantation confirmed the higher tumorigenicity of isolated SP cells. These data indicated that SP cells were enriched with CSCs of bladder cancer. Furthermore, we determined the expression of melanoma antigen family A, 3 (MAGEA3), one of the most studied cancer testis (CT) antigens, in these SP and main population (MP) cells derived from SW780 cells. SW780 SP cells representing CSCs of bladder cancer showed an up-regulated expression of MAGE-A3 and a positive coexpression of MAGE-A3 and CD133, indicating that MAGE-A3 was a novel CT antigen preferentially expressed in the CSCs of bladder cancer. In summary, our findings confirmed the existence of cancer stem cell-like SP cells in bladder cancer cells, and further indicated that MAGE-A3 is a novel CSC antigen and therefore may serve as an immunotherapeutic target for CSCs of bladder cancer.
Keywords: Melanoma antigen family A, 3 (MAGE-A3), bladder cancer, cancer stem cells, side population, cancertestis, immunotherapy
Introduction
Bladder cancer is the fourth most common malignancy in the USA [1]. Although there are many treatment modalities, bladder cancer remains one of cancers with the highest recurrence rate, ranging from 60-90% [2]. Furthermore, bladder cancer is a very heterogeneous disease with a broad spectrum of clinical presentations.
The high frequency of recurrence of bladder cancer and its heterogeneous presentations have prompted us to suppose that cancer stem cells (CSCs) could be involved in the development of this kind of malignancy [3]. CSCs are defined as a small population of cancer cells that have high tumor-initiating, self-renewal and differentiation abilities [4]. With the detection of cell surface markers, CSCs have been isolated from leukemia and several solid tumors [5]. However, the stem cell markers of bladder cancer have not been clearly described yet [6]. Side population (SP) cells isolated from various cancer cells have emerged as an attractive alternative for CSCs. SP cells are a small subset of cancer cells detectable by dual-wavelength flow cytometry because they can efflux the DNA-binding dye Hoechst 33342 through ATP-binding cassette (ABC) transporters. Since SP cells are enriched in primitive and undifferentiated cells, they could be a useful tool for identifying putative CSCs, particularly those CSCs with uncertain surface markers [7,8].
Accumulating evidence has proposed that CSCs are responsible for the origin, progression and relapse of cancer. Based on the CSC hypothesis, cancer therapy should target CSCs rather than the cancer cells generated by them in an attempt to eradicate cancer cells. However, CSCs are commonly resistant to current cancer therapies, including chemotherapy and radiotherapy by various mechanisms [6]. More efficient CSC-targeting therapy thus is needed to cure cancer. Some recent studies have shown that immuno systems can efficiently recognize and kill CSCs [5,9-11]. Therefore, CSC-targeting immunotherapy might be a promising therapeutic approach for cancer, especially for bladder cancer which has well-documented responses to immunotherapy [12]. To this end, the tumor-associated antigens (TAAs) expressed in the CSCs of bladder cancer are needed to be identified.
Among TAAs identified to date, cancer-testis (CT) antigens have been recognized as a group of highly attractive targets for cancer immunotherapy because of their broad presentation in cancer tissues but restricted expression in normal tissues, namely only in the testis [13]. Of the CT antigens studied, melanoma antigen family A3 (MAGE-A3) is one of the most commonly expressed CT antigens in a variety of malignancies [14]. However, the expression and function of MAGE-A3 in CSCs, especially in the CSCs of bladder cancer, has not been documented.
In this study, we applied flow cytometry-based SP analysis to isolate cancer stem cell-like SP cells from human bladder cancer cell line SW780 and confirmed the enrichment of CSCs in these SP cells via in vivo and in vitro assays. Then, we investigated the expression of MAGE-A3 in the isolated SP cells representing CSCs of bladder cancer to test whether MAGE-A3 may serve as an immunotherapeutic target for CSCs of bladder cancer.
Materials and methods
Cell line and cell culture
The human bladder cancer cell line SW780 was purchased from the Type Culture Collection of Chinese Academy of Science (Shanghai, China). The cells were cultured in RPMI-1640 (Invitrogen, CA, USA) supplemented with 10% fetal bovine serum (FBS) at 37°C in incubator with humidified air and 5% carbon dioxide.
Flow cytometry-based SP analysis
SP cells were isolated as described previously with some modifications [8]. Briefly, cells were incubated with Hoechst 33342 (Molecular Probes, OR, USA) at a final concentration of 5 μg/ml for 45 min at 37°C in the absence or presence of 150 μM verapamil (Sigma-Aldrich, MO, USA) followed by staining with propidium iodine (PI) for the exclusion of dead cells. Fluorescence-activated cell sorting (FACS) was carried out with a FACSAria II cell sorter (Becton Dickinson, CA, USA). The fluorescence was measured using two filters, 450 nm (Hoechst blue) and 670 nm (Hoechst red). SP cells were then cultured in DMEM/F12 medium (Invitrogen) supplemented with 2% B27, 40 ng/ml basic fibroblast growth factor (bFGF; Sigma-Aldrich) and 20 ng/ml endothelial growth factor (EGF; Sigma-Aldrich).
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was isolated from homogenized SP and main population (MP) cells using RNeasy Mini Kit (Qiagen, TX, USA) following the manufacturer’s instructions with an additional step of treatment with DNase I (Invitrogen) to remove any genomic DNA contamination. cDNA was synthesized using iScript cDNA synthesis kit (BioRad, CA, USA). The housekeeping gene GAP DH was amplified using synthesized cDNA to confirm integrity. Primers for target genes were designed based on GenBank sequences. ATP-binding cassette sub-family G member 2 (ABCG2): F-5’-CCTGAGATCCTGAGCCTTTG-3’ and R-5’-AAGCCATTGGTGTTTCCTTG-3’, 124 bp; CD133: F-5’-ATGACAAGCCCATCACAACA-3’ and R-5’-AGCACTACCCAGAGACCAATG-3’, 197 bp; MAGE-A3: F-5’-ATGCTGACGCTCATTCAACCAT-3’ and R-5’-ATGCTGACGCTCATTCAACCAT-3’, 183 bp; and GAPDH: F-5’-GAGTCAACGGATTTGGTCGT-3’ and R-5’-GACAAGCTTCCCGTTCTCAG-3’, 185 bp. PCR was carried out under the following conditions: for ABCG2, denaturation for 30 s at 95°C followed by annealing for 30 s at 63°C, then elongation for 30 s at 70°C, 35 cycles total; CD133 was identical to ABCG2 except that 60°C was used as annealing temperature; MAGE-A3 and GAPDH were identical to CD133 except that only 30 cycles were used. Electrophoresis was done by loading 10 μL of each sample on a 2% agarose gel, and visualized by ethidium bromide staining using the Bio-Imaging System (Ultra-Violet Products, Cambridge, UK). Positive expression was defi-ned as those bands that electrophoresed with the expected size.
Western blot
Briefly, 25 μg protein was separated on a 10-20% SDS-PAGE and transferred onto PVDF membrane (Millipore, MA, USA). The membrane was incubated with primary antibody overnight at 4°C followed by incubation with horseradish peroxidase-conjugated secondary antibody, and developed with the Super Signal West Dura Extended Duration Substrate kit (Pierce, IL, USA). The primary antibody to MAGE-A3 (mouse monoclonal antibody; Santa Cruz Biotechnology, CA, USA) was incubated at 1:500 overnight then 1:2000 secondary antibody (horse anti-mouse antibody; Santa Cruz Biotechnology) was incubated for 2 h at room temperature. ß-actin was used as a loading control.
Immunofluorescence
Double immunofluorescence was applied to determine the coexpression of MAGE-A3 and CD133. Briefly, cells were fixed with 4% paraformaldehyde in PBS (Affymetrix, CA, USA) for 15 min at room temperature and blocked with 1% BSA (Thermo Fisher Scientific, MA, USA) in PBST (0.3% Triton X-100 in PBS) for 1 h. Slides were then incubated with the mixture of two primary antibodies including mouse anti-MAGE-A3 monoclonal antibody (Santa Cruz Biotechnology) and rat anti-CD133 monoclonal antibody (Novus, CO, USA) in 1% BSA in PBST overnight at 4°C. The mixture of two secondary antibodies including Alexa Fluor-555 labeled goat anti-mouse and Alexa Fluor-488 labeled goat anti-rat antibodies (Molecular Probes) in 1% BSA in PBST was applied and incubated for 2 h at room temperature in dark. The cells were then mounted with ProLong Gold antifade reagent (Invitrogen) with 4’, 6-diamidino-2-phenylindole (DAPI) detecting nuclei. The slides were then imaged using a confocal microscope (200 × magnification).
Xenograft transplantation
Female nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice at the age of 6 to 8 weeks were provided by the Model Animal Research Center of China Medical University, Shenyang, China. All mouse procedures were carried out in accordance with institutional protocol guidelines at Shengjing Hospital of China Medical University. Freshly sorted SP and MP cells were resuspended in 50 μl PBS in quantities ranging from 102 to 104 cells. Cells were then mixed with 50 μl Matrigel (BD Biosciences, CA, USA) and injected into the subcutaneous space of the back of NOD/SCID mice (5 mice per group). Tumor growth was quantified by caliper measurements and monitored weekly. Tumor volume was calculated by XY2/2 (X = long axis, Y = short axis). Survival curves were constructed according to the Kaplan-Meier method.
Statistical analysis
Data are presented as means ± SD. Statistical analysis was done with SPSS 16.0 (SPSS Inc., IL, USA). Student’s t test for two samples was performed for the differences between SP and MP groups. P < 0.05 was considered statistically significant.
Results
Identification of SP cells
SP cells were detectable in SW780 cells with a ratio of about 3.6% (Figure 1A) and these SP cells decreased dramatically in the presence of verapamil (Figure 1B). We then investigated the expression of ABCG2, which is one of the best characterized ABC transporters and is essential in the efflux of Hoechst 33342 from SP cells. As compared to MP cells, ABCG2 mRNA was found to be up-regulated in SP cells (Figure 2A). Furthermore, we evaluated the expression of CD133 which is an established CSC marker [3,9]. As shown in Figure 2B, SP cells showed a higher level of CD133 mRNA than did MP cells, suggesting these SP cells present stem cell-like phenotype.
Figure 1.
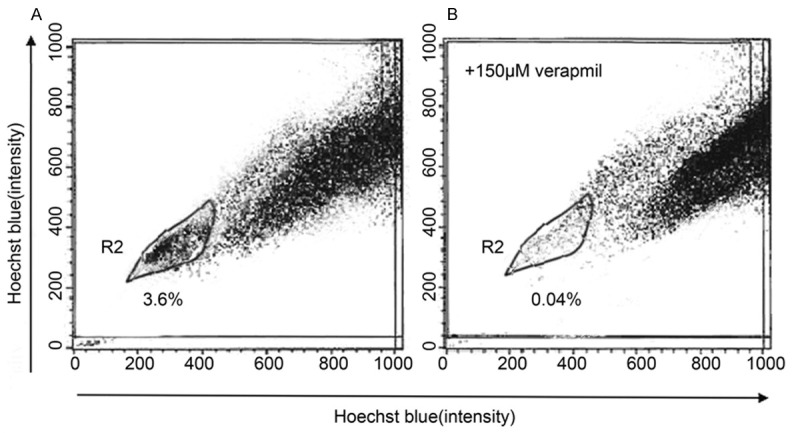
Isolation of SP cells from SW780 cell line by Hoechst 33342 SP analysis in the absence (A) or presence (B) of verapamil.
Figure 2.
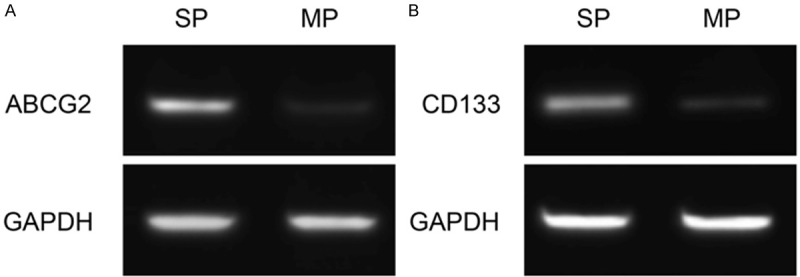
Expression of ABCG2 (A) and CD133 (B) detected by RT-PCR.
In vitro growth characteristics of SP cells
SP cells were cultured in serum-free medium for 2 weeks and then photographed while alive by a phase contrast microscope. As compared to MP cells, SP cells grew into sphere-like clusters/tumor spheres (i.e., they had round bodies and were barely attached to the dish), indicating the stem cell-like phenotype of these SP cells (Figure 3).
Figure 3.
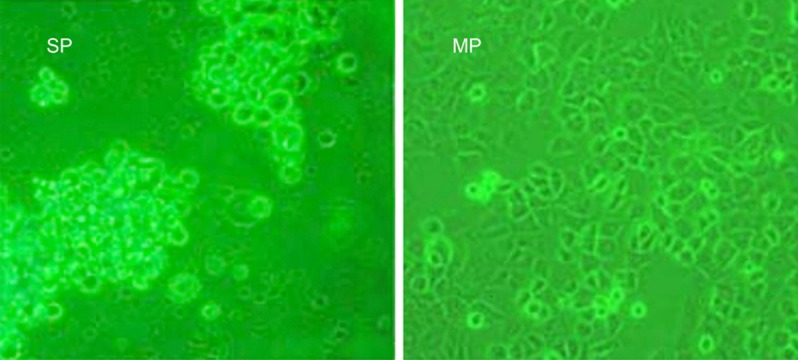
In vitro growth characteristics of SW780 SP cells. SP cells grew into sphere-like clusters/tumor spheres, as illustrated by phase contrast microscopy (20 ×).
Higher in vivo tumorigenicity of SP cells
The tumorigenicity of SP and MP cells was evaluated by inoculating serial dilutions of both subtypes of cells into NOD/SCID mice. As shown in Table 1 and Figure 4, SP cells initiated tumor formation with 103 (3 of 5 mice) and 104 (4 of 5 mice) cells, whereas MP cells needed 104 (1 of 5 mice) to initiate tumor formation. Therefore, SP cells have much more efficient tumorigenicity than MP cells. These data further validated the enrichment of CSCs in the isolated SP cells.
Table 1.
In vivo tumorigenicity of SP and MP cells derived from SW780 cells
| Cells | Cell number for inoculation | Tumor initiation | Tumor volume (mm3) |
|---|---|---|---|
| SP cells | 102 | 0/5a | |
| 103 | 3/5 | 96.8 ± 53.2 | |
| 104 | 4/5 | 186.8 ± 93.4 | |
| MP cells | 102 | 0/5 | |
| 103 | 0/5 | ||
| 104 | 1/5 | 16 |
The number of mice with a detected tumor; n = 5.
Figure 4.
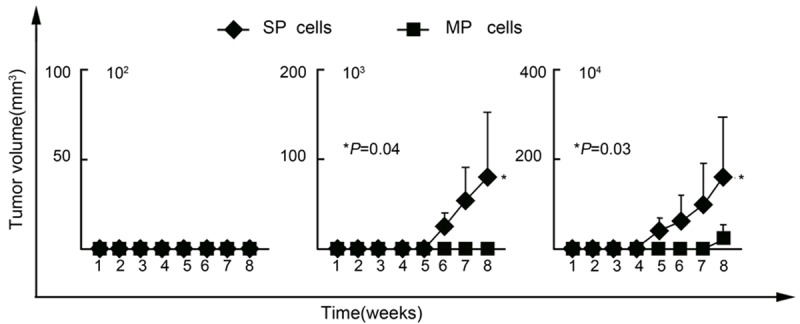
Higher in vivo tumorigenicity of SW780 SP cells. Data are means ± SD.
Expression of MAGE-A3 in cancer stem cell-like SP cells
After isolating cancer stem cell-like SP cells, we further examined the expression of MAGE-A3 in these SP cells representing CSCs of bladder cancer. As shown in Figure 5A, 5B, SP cells showed a higher expression of MAGE-A3 than did MP cells at both mRNA and protein levels. Furthermore, SP cells showed a positive coexpression of MAGE-A3 (red) and CD133 (green) (Figure 5C), suggesting the potential correlation of MAGE-A3 to the stem cell-like phenotype of these SP cells. These findings indicated that MAGE-A3 is predominantly expressed in cancer stem cell-like SP cells and therefore is a novel CT antigen preferentially expressed in the CSCs of bladder cancer.
Figure 5.
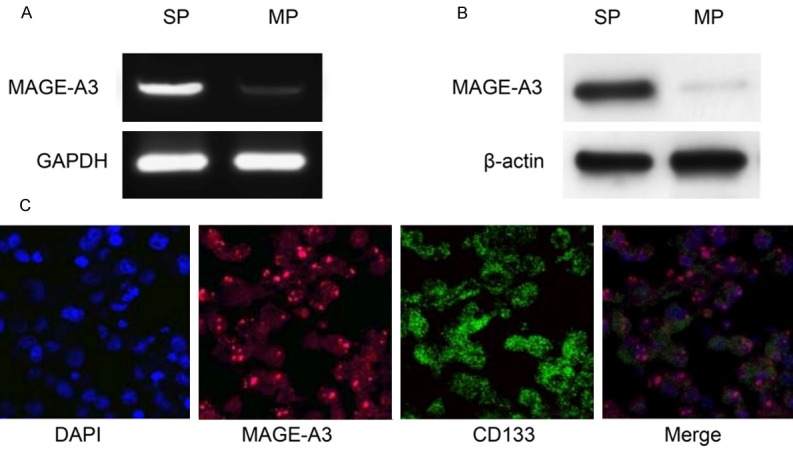
Expression of MAGE-A3 in cancer stem cell-like SP cells. Expression of MAGE-A3 was detected by RT-PCR (A) and Western blot (B); Double immunofluorescence analysis (C) of coexpression of MAGE-A3 (red) and CD133 (green) was performed in SW780 SP cells. DAPI was used to stain nuclei (200 × magnification).
Discussion
Although some cell surface markers have been reported useful for identification and characterization of the CSCs of bladder cancer, there is still no consensus on these markers [6]. SP cells isolated from cancer cells have proven to be an attractive alternative to study CSCs since these cells have been reported to be enriched with CSCs in several kinds of malignancies [8,11,15-18]. SP sorting thus is a good place to start the research for resident CSCs in a particular cancer, such as bladder cancer, especially when the phenotype of the cells in question is not known [8].
Higher tumorigenicity is the fundamental phenotype of CSCs and can be confirmed functionally by serial xenograft transplantation of a small number of putative CSCs in immunodeficient mice [11]. It can be complemented by in vitro assays of colony formation and tumor sphere growth [6]. Therefore, to further validate the enrichment of CSCs in the isolated SP cells, it is essential to confirm the higher tumorigenicity of SP cells via these in vivo and in vitro assays.
In the present study, we isolated CSCs of bladder cancer as SP cells from the bladder cancer cell line SW780. These SP cells decreased dramatically in the presence of verapamil and showed an up-regulation of ABCG2, indicating that this population was made up of bona fide SP cells. Furthermore, SP cells cultured in serum-free medium grew into sphere-like clusters/tumor spheres and showed higher tumorigenicity than that of MP cells by xenograft transplantation, suggesting that these SP cells possess CSC phenotype and therefore are suitable for analysis of CSCs of bladder cancer. Our study outlined a successful strategy for isolating cancer stem cell-like SP cells, which made it possible to further study the CSCs of bladder cancer.
Since CSCs are thought to cause post-therapeutic recurrence of cancer and make it untreatable, it is important to eliminate these CSCs to efficiently treat cancer. However, CSCs are commonly resistant to current cancer therapies including chemotherapy and radiotherapy by various mechanisms [19]. More efficient CSC-targeting therapy thus is needed to cure cancer.
With the identification of TAAs, cancer immunotherapy is becoming a reality [20]. However, can immunosystems recognize and kill therapy-resistant CSCs? Recently, some immunologic effector cells, including natural killer (NK) cells and γδT cells, have been reported to be able to efficiently recognize CSCs [5]. Furthermore, cytotoxic T lymphocytes (CTLs) have been shown to efficiently recognize and kill CSCs of human melanoma, colon cancer and renal cancer [9-11]. Therefore, immunotherapy targeting CSCs might be a reasonable candidate for CSC-targeting therapy, to possibly eliminate CSCs and cure cancer.
To apply efficient CSC-targeting immunotherapy, it is critical to identify the TAAs expressed in CSCs [11,21]. Among TAAs identified to date, CT antigens represent the most promising targets for cancer immunotherapy. In our previous study, we found that MAGE-A3 was one of the most commonly expressed CT antigens in bladder cancer [13]. However, the expression and function of MAGE-A3 in CSCs of bladder cancer has not been documented yet. To address this question, we then determined the expression of MAGE-A3 in those isolated SP cells representing CSCs of bladder cancer. We found that SP cells showed a higher expression of MAGE-A3 than MP cells at both mRNA and protein levels, indicating that MAGE-A3 was a novel CT antigen preferentially expressed in the CSCs of bladder cancer. Furthermore, a positive coexpression of MAGE-A3 and CD133, which is an established CSC marker, was detected in SP cells by double immunofluorescence, suggesting the potential correlation of MAGE-A3 with CSC phenotype.
Taken together, in this study, we isolated SP cells from human bladder cancer cell line SW780 and confirmed the higher tumorigenicity of these SP cells, indicating the enrichment of CSCs in the isolated SP cells. We further detected an up-regulation of MAGE-A3 in these SP cells representing CSCs of bladder cancer. Thus, we showed for the first time that MAGE-A3 is a novel CSC antigen of bladder cancer and therefore may serve as an immunotherapeutic target for CSCs of bladder cancer.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (No. 81372725).
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Irani J, Mottet N, Ribal Caparros MJ, Teillac P. New Trends in Bladder Cancer Management. Eur Urol Suppl. 2007;6:388–95. [Google Scholar]
- 3.Bentivegna A, Conconi D, Panzeri E, Sala E, Bovo G, Viganò P, Brunelli S, Bossi M, Tredici G, Strada G, Dalprà L. Biological heterogeneity of putative bladder cancer stem-like cell populations from human bladder transitional cell carcinoma samples. Cancer Sci. 2010;101:416–24. doi: 10.1111/j.1349-7006.2009.01414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 5.Hirohashi Y, Torigoe T, Inoda S, Takahashi A, Morita R, Nishizawa S, Tamura Y, Suzuki H, Toyota M, Sato N. Immune response against tumor antigens expressed on human cancer stem-like cells/tumor-initiating cells. Immunotherapy. 2010;2:201–11. doi: 10.2217/imt.10.10. [DOI] [PubMed] [Google Scholar]
- 6.van der Horst G, Bos L, van der Pluijm G. Epithelial plasticity, cancer stem cells, and the tumor-supportive stroma in bladder carcinoma. Mol Cancer Res. 2012;10:995–1009. doi: 10.1158/1541-7786.MCR-12-0274. [DOI] [PubMed] [Google Scholar]
- 7.She JJ, Zhang PG, Wang ZM, Gan WM, Che XM. Identification of side population cells from bladder cancer cells by DyeCycle Violet staining. Cancer Biol Ther. 2008;7:1663–8. doi: 10.4161/cbt.7.10.6637. [DOI] [PubMed] [Google Scholar]
- 8.Yin B, Yang Y, Zhao Z, Zeng Y, Mooney SM, Li M, Xu X, Song Y, Wu B, Yang Z. Arachidonate 12-lipoxygenase may serve as a potential marker and therapeutic target for prostate cancer stem cells. Int J Oncol. 2011;38:1041–6. doi: 10.3892/ijo.2011.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gedye C, Quirk J, Browning J, Svobodová S, John T, Sluka P, Dunbar PR, Corbeil D, Cebon J, Davis ID. Cancer/testis antigens can be immunological targets in clonogenic CD133+ melanoma cells. Cancer Immunol Immunother. 2009;58:1635–46. doi: 10.1007/s00262-009-0672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoda S, Hirohashi Y, Torigoe T, Morita R, Takahashi A, Asanuma H, Nakatsugawa M, Nishizawa S, Tamura Y, Tsuruma T, Terui T, Kondo T, Ishitani K, Hasegawa T, Hirata K, Sato N. Cytotoxic T lymphocytes efficiently recognize human colon cancer stem-like cells. Am J Pathol. 2011;178:1805–13. doi: 10.1016/j.ajpath.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishizawa S, Hirohashi Y, Torigoe T, Takahashi A, Tamura Y, Mori T, Kanaseki T, Kamiguchi K, Asanuma H, Morita R, Sokolovskaya A, Matsuzaki J, Yamada R, Fujii R, Kampinga HH, Kondo T, Hasegawa T, Hara I, Sato N. HSP DNAJB8 controls tumor-initiating ability in renal cancer stem-like cells. Cancer Res. 2012;72:2844–54. doi: 10.1158/0008-5472.CAN-11-3062. [DOI] [PubMed] [Google Scholar]
- 12.Miyazaki J, Nishiyama H, Yano I, Nakaya A, Kohama H, Kawai K, Joraku A, Nakamura T, Harashima H, Akaza H. The therapeutic effects of R8-liposome-BCG-CWS on BBN-induced rat urinary bladder carcinoma. Anticancer Res. 2011;31:2065–71. [PubMed] [Google Scholar]
- 13.Yin B, Liu G, Wang XS, Zhang H, Song YS, Wu B. Expression profile of cancer-testis genes in transitional cell carcinoma of the bladder. Urol Oncol. 2012;30:886–92. doi: 10.1016/j.urolonc.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Lonchay C, van der Bruggen P, Connerotte T, Hanagiri T, Coulie P, Colau D, Lucas S, Van Pel A, Thielemans K, van Baren N, Boon T. Correlation between tumor regression and T cell responses in melanoma patients vaccinated with a MAGE antigen. Proc Natl Acad Sci U S A. 2004;101(Suppl 2):14631–8. doi: 10.1073/pnas.0405743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kondo T, Setoguchi T, Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci U S A. 2004;101:781–6. doi: 10.1073/pnas.0307618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murase M, Kano M, Tsukahara T, Takahashi A, Torigoe T, Kawaguchi S, Kimura S, Wada T, Uchihashi Y, Kondo T, Yamashita T, Sato N. Side population cells have the characteristics of cancer stem-like cells/cancer-initiating cells in bone sarcomas. Br J Cancer. 2009;101:1425–32. doi: 10.1038/sj.bjc.6605330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakatsugawa M, Takahashi A, Hirohashi Y, Torigoe T, Inoda S, Murase M, Asanuma H, Tamura Y, Morita R, Michifuri Y, Kondo T, Hasegawa T, Takahashi H, Sato N. SOX2 is overexpressed in stem-like cells of human lung adenocarcinoma and augments the tumorigenicity. Lab Invest. 2011;91:1796–804. doi: 10.1038/labinvest.2011.140. [DOI] [PubMed] [Google Scholar]
- 18.Hadnagy A, Gaboury L, Beaulieu R, Balicki D. SP analysis may be used to identify cancer stem cell populations. Exp Cell Res. 2006;312:3701–10. doi: 10.1016/j.yexcr.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 19.Soltanian S, Matin MM. Cancer stem cells and cancer therapy. Tumour Biol. 2011;32:425–40. doi: 10.1007/s13277-011-0155-8. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg SA. A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity. 1999;10:281–7. doi: 10.1016/s1074-7613(00)80028-x. [DOI] [PubMed] [Google Scholar]
- 21.Mori T, Nishizawa S, Hirohashi Y, Torigoe T, Tamura Y, Takahashi A, Kochin V, Fujii R, Kondo T, Greene MI, Hara I, Sato N. Efficiency of G2/M-related tumor-associated antigen-targeting cancer immunotherapy depends on antigen expression in the cancer stem-like population. Exp Mol Pathol. 2012;92:27–32. doi: 10.1016/j.yexmp.2011.09.016. [DOI] [PubMed] [Google Scholar]


