Abstract
Introduction: Mesangial IgA deposition is the initiative factor in the pathogenesis of IgA nephropathy (IgAN). Glomerular IgA depositon leads to activation local complement system. C4d positivity shows that complement activation occurs via alternative pathway. C4d positivity at the time of renal biopsy can be associated with poor prognosis in IgA nephropathy. We aimed to evaluate C4d deposition and renal outcome in patients with IgA nephritis. Methods: Between January 2005 and December 2009, 40 patients with IgA nephritis were enrolled. Renal biopsy specimens of 33 patients have been evaluated. C4d immunohistochemical staining was performed 3-μm deparaffinized and rehydrated sections of formaldehyde-fixed renal tissues, using rabbit polyclonal anti-human C4d as the antibody. Baseline demographical, clinical and laboratory data were recorded retrospectively. Results: Mean age of the patients was 35.9 ± 12.9 years and female/male ratio was 19/21. Mean duration of follow-up was 32.8 (12-60) months. Baseline glomerular filtration ratio (GFR) and proteinuria were 55.8 ml/min and 2.44 gr/day respectively at the time of renal biopsy. Eleven patients were C4d positive. Presence of hypertension (p=0.133), proteinuria (p=0.007), serum creatinine levels (p=0.056) and glomerulosclerosis (p=0.004), mesengial hypersellularity (p=0.0001) and interstitial fibrosis (p=0.006) at the time of renal biopsy were higher in C4d positive group rather than negative group. Evolution to renal failure were 63.6% in C4d positive group and 13.6% in negative group (p=0.006). Renal survival at 3 years was 39% in C4d-positive patients versus 66.7% in the C4d-negative patients (log rank- p=0.0072).
Keywords: C4d, IgA nephropathy, renal prognosis
Introduction
IgA glomerulonephritis (IgAN) which is the most common primary glomerulonephritis in the world, is a proliferative glomerulonephritis characterised by aberrant IgA deposition in the mesangial areas [1]. The disease has a slow progression and is relatively a benign disorder, but 15-25% of the patients require renal replacement therapy at some point during the course of disease [2-4]. This variability in the clinical course justifies the efforts to determine clinical and histological features that predict poor prognosis in IgAN.
Several histological, laboratory and clinical parameters have been evaluated for the detection of high-risk patients at the time of diagnosis. Decreased renal function, the degree of proteinuria, the presence of hypertension at onset, familial history of the disease and age are the most important clinical predictors of poor outcome. Glomerular sclerosis and tubulointerstisial fibrosis are the strongest histological features that predict the development of renal failure in IgAN [5,6].
The initiating event in the pathogenesis of IgAN appears to be mesangial deposition of IgA and C3 complement. It is well known that complement activation commonly occurs via alternative pathway [6]. There is now increasing evidence that mannose-binding lectin (MBL) complement pathway also involves in the pathogenesis of IgAN [7].
Roos et al showed that activation of the lectin pathway of complement is associated with more severe renal damage at the time of renal biopsy in patients with IgAN [7]. Glomerular staining for C4d or C4d binding protein in the absence of C1q staining, indicates the activation of lectin complement pathway in IgAN. Therefore, we aimed to determine whether glomerular C4d immunofluorescence staining could be a useful prognostic marker for IgAN.
Materials and methods
This retrospective cross-sectional study included all patients with IgA nephropathy, who underwent renal biopsy at our center from January 2005 to December 2009. Forty patients included in the study, but 7 patients who had insufficient renal tissue (fewer than 6 glomeruli) remaining in the paraffin block were excluded. Therefore C4d staining was performed on the biopsy materials of 33 patients.
The diagnosis of IgAN was based on histological assesment of renal biopsy tissue with haematoxylin and eosine, Masson’s trichrome, periodic acid-Schiff, and methenamine silver for light microscopy and staining with IgG, IgA, IgM, C1q, C3, kappa and lambda for immunofluorescence microscopy. Mesangial hypercellularity, segmental glomerulosclerosis and adhesion, endocapillary proliferation, interstisiel fibrosis and tubular atrophy were evaluated according to “Oxford Classification” [8,9].
C4d immunohistochemical staining was performed on 4-µm deparaffinized and rehydrated sections of formaldehyde- fixed renal tissue, using rabbit polyclonal anti-human C4d (polyclonal antibody E17344, Spring Bioscience CA 94566 USA). C4d immunohistochemical staining was scored as negative (0) or positive (1). Patients were classified as ‘positive’ when >75% of the glomeruli were positive for C4d (Figure 1). This staining was classified as ‘global’ when >50% of mesangial area was affected and ‘segmental’ when <50% of the mesangial area was positive for C4d.
Figure 1.
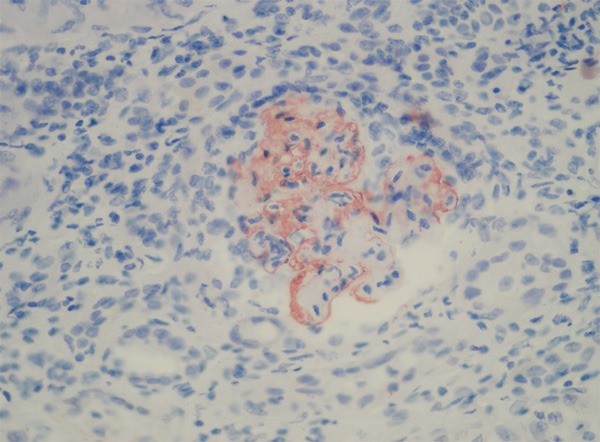
A glomerulus shows global staining C4d positivity in mesangial areas (Magnification, x 200).
33 patients with IgAN were evaluated for the study. The medical records were reviewed and the following information at the time of the renal biopsy was recorded: patient age, sex, presence or absence of hypertension (defined as blood pressure >140/90 mmHg or the use of antihypertensive agents), spot urine protein/ creatinine excretion and serum creatinine level. We calculated the estimated GFR (eGFR) using the abbreviated Modification of Diet in Renal Disease (MDRD) Study equation: eGFR (ml/min per 1.73 m2)=(186 x serum creatinine-1.154) x (age-0.203) x (0.742 if female) x (1.21 if black) [10].
Data of the patients were evaluated in April 2010, recording the last serum creatinine, eGFR and development of end-stage renal disease (ESRD) defined as chronic repetitive dialysis. The primary endpoint of this study was the onset of ESRD in the course of study.
Statistical analysis
Baseline demographical, clinical and laboratory data were recorded retrospectively. SPSS version 13.0 software (Chicago, IL, USA) was used for statistical analysis. Results were expressed as mean ± standard deviation. The Mann-Whitney U test was used to compare the continuous variables and the Chi-square test was used to compare categorical variables. Logistic regression analysis was used in multivariate analysis. P value of less than 0.05 was considered to be statistically significant. Kaplan-Meier analysis was also performed to evaluate the impact of C4d staining on the renal survival.
Results
C4d staining was performed on the renal biopsy specimens of 33 patients with IgAN. Their mean ± SD age was 35.9 ± 12.9 years and 52.5% were male. 50% of the patients had macroscopic haematuria, 45% were hypertensive, 65% had urine protein levels >1 g/day and 41% had eGFR <60 ml/min/1.73 m2 at the time of renal biopsy. Demographic and clinical features of the patients at the time of renal biopsy are presented in Table 1.
Table 1.
Clinical and pathological data of the patients at the time of renal biopsy
| Variable | Development of ESRD (N=13) | Non-development of ESRD (N=27) | P value |
|---|---|---|---|
| Age (years) | 40.54 ± 15.92 | 33.67 ± 10.92 | 0.224* |
| Gender (% female) | 53.8% | 44.4% | 0.413** |
| Hypertension (%) | 76.9% | 29.6% | 0.006** |
| Serum Creatinine (mg/dl) | 3.45 ± 2.30 | 1.68 ± 1.63 | 0.003* |
| Urinary protein excretion (g/day) | 4.28 ± 3.26 | 1.56 ± 1.36 | 0.009* |
| eGFR (ml/min/1.73 m2) | 29.46 ± 23.65 | 68.41 ± 32.69 | 0.0001* |
| Interstitial fibrosis/tubular atrophy moderate-severe (%) | 50% | 21.7% | 0.114** |
| Glomeruli showing sclerosis (%) | 60% | 52.2% | 0.488** |
| Mesangial hypercellularity moderate-severe (%) | 70% | 43.5% | 0.154** |
Mann-Whitney U;
Chi-square.
ESRD, end-stage renal disease; eGFR, estimated glomerular filtration rate. Values expressed as means ± SD.
Mean of 6.1 ± 1.06 glomeruli were identified in the renal biopsy specimens. Glomerular staining for Cd4 was segmental in 9 patients and global in 2 patients; both were accepted as C4d-positive. The rate of hypertension was greater in C4d-positive patients and they had a more impaired renal function at the time of renal biopsy compared to C4d-negative patients, but the difference was not significant (Table 2). Urinary protein extraction was more elevated in C4d-positive group at the time of renal biopsy (p=0.007). The renal biopsy showed that glomerulosclerosis and mesangial hypercellularity were present in most of the C4d-positive subjects and the difference was statistically significant (p=0.004 and p=0.0001). In addition, interstisiel fibrosis/tubular atrophy was more severe in C4d-positive patients compared to C4d-negative patients (p=0.006). The comparison of C4d-positive and C4d-negative patients are shown in Table 2.
Table 2.
Cmparison of C4d-positive and C4d-negative patients
| C4d+ (n=11) | C4d- (n=22) | P | |
|---|---|---|---|
| Age (years) | 32.18 ± 11.21 | 36.32 ± 14.03 | 0.541* |
| Gender (% female) | 45.5% | 50% | 0.549** |
| Presence of hypertension (%) | 63.6% | 36.4% | 0.013** |
| Proteinuria (gr/gün) | 3.95 ± 2.95 | 1.33 ± 1.44 | 0.007* |
| Serum creatinine (mg/dl) | 3.01 ± 2.60 | 1.75 ± 1.80 | 0.056* |
| eGFR (ml/min/1.73 m2) | 45.64 ± 32.95 | 68.68 ± 35.43 | 0.069* |
| Glomerulosclerosis (%) | 90.9% | 36.4% | 0.004** |
| Mesangial hypercellularity (%) | 100% | 27.3% | 0.0001** |
| Interstisiel fibrosis/ tubular atrophy (%) | 63.6% | 13.6% | 0.006** |
| Evolution to ESRD (%) | 63.6% | 13.6% | 0.006** |
Mann-whitney U;
Chi-square.
The patients were followed-up for 32.8 ± 20.7 months. 10 patients (30.3%) developed ESRD at the end of the follow up period. In the logistic regression model, serum creatinine (odds ratio [OR] 0.6), eGFR (OR 1.048), proteinuria (OR 0.571) at the time of renal biopsy and C4d+ glomerular staining (OR 11.083) were identified to be statistically significant contributors to the development of ESRD (Table 3). In multivariate analysis; only C4d-positivity (OR 15.022) and presence of hypertension (OR 33.082) were significantly associated with evolution to ESRD.
Table 3.
Univariate and multivariate regression analysis for evolution to ESRD
| Univariate | Multivariate | |||
|---|---|---|---|---|
|
|
||||
| R | P | R | p | |
| Age | 0.959 | 0.112 | - | - |
| Gender (female) | 1.458 | 0.578 | - | - |
| Serum creatinine | 0.625 | 0.025 | 2.033 | 0.142 |
| eGFR | 1.048 | 0.003 | 1.030 | 0.175 |
| Hypertension | 7.917 | 0.008 | 33.082 | 0.011 |
| Proteinuria | 0.571 | 0.008 | 1.382 | 0.420 |
| C4d+ glomerular staining | 11.083 | 0.006 | 15.022 | 0.027 |
| Glomerulosclerosis | 1.375 | 0.679 | - | - |
| Mesangial hypercellularity | 3.033 | 0.170 | - | - |
| Interstisiel fibrosis (%) | 3.600 | 0.114 | - | - |
Renal survival at 3 years was 39% in C4d-positive patients versus 66.7% in the C4d-negative patients (log rank- p=0.0072) (Figure 2).
Figure 2.
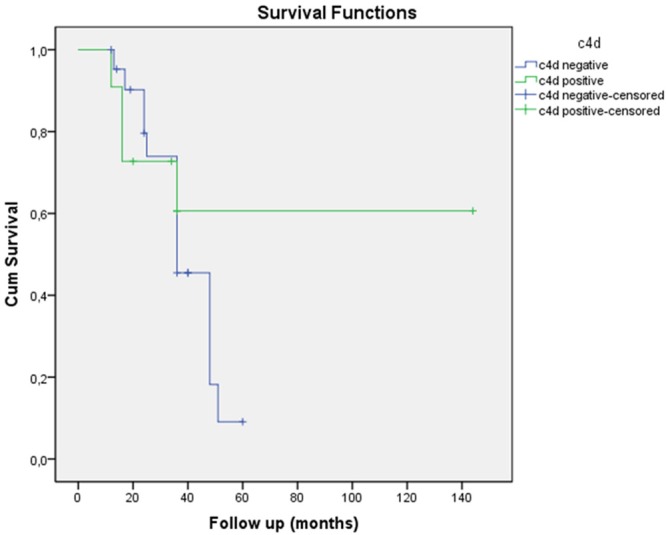
Kaplan-Meier renal survival according to C4d staining in patients with IgAN.
Discussion
Our study shows that glomerular C4d deposition may be used in the prognosis of disease activity in patients with IgA nephropathy. While renal survival at 3 years was 39% in C4d positive patients, this ratio was 66.7% in C4d negative patients.
Numerous attempts have been made to define clinical and laboratory findings that predict poor prognosis in patients with IgA nephropathy, including the presence of hypertension, the degree of proteinuria, the absence of macroscopic haematuria and decreased renal function [4,11]. Glomerular sclerosis and tubulointerstisial fibrosis are the strongest histological features that predict the development of renal failure in IgAN [12]. New histological parameters are needed to estimate the prognosis at the beginning of disease.
Roos et al have described that complement activation occurs by two ways in IgAN. The glomerular staining for mannose-binding lectin (MBL) or C4d in IgAN shows the activation of complement occurring via the lectin pathway and negative staining shows the activation of complement occurring via the alternative pathway [7]. They have also mentioned that glomerular activation by the lectin pathway of complement in IgAN is associated with a more severe renal disease at the time of renal biopsy.
Recently Espinosa et al reported that mesangial C4d deposition can be used as a prognostic tool in patients with IgAN [13]. Our study also showed that evolution to ESRD was significantly higher in the C4d-positive patients and C4d-positive patients had significantly more glomerulosclerosis, mesangial proliferation and more severe interstitial fibrosis. In the study of Espinosa et al. renal survival at 10 years was 43.9% in C4d-positive patients, in contrast to 90.9% in C4d-negative patients. In the present study, we found similar ratios for renal survival in C4d-positive and negative patients. They also showed that age, hypertension, serum creatinine levels, GFR, glomerular sclerosis, interstitial fibrosis and C4d-positive staining were all univariately associated with developing to ESRD. In the current study we found that hypertension, serum creatinine levels, GFR, the amount of proteinuria and C4d-positive staining were all univariately associated with evolving to ESRD. In multivariate analysis only C4d-positivity and presence of hypertension were significantly associated with evolution to ESRD. These findings suggest that C4d staining may be used to estimate the prognosis of IgAN.
There are several limitations for the present study. Firstly, this is an observational retrospective study. Other limitations are the small number of patients and the short period of follow-up. Besides these limitations, we found a strong relationship between C4d-positivity and poor prognosis of IgAN which supports the findings of Espinosa et al, even in small number of patients. Therefore this study highlights the role of glomerular C4d deposition as a prognostic factor in patients with IgAN.
In conclusion, we found a relationship between glomerular C4d staining in the renal tissues and prognosis of the patients with IgAN. Besides this, presence of hypertension, basal serum creatinine and proteinuria are the other clinical factors for poor prognosis in patients with IgAN.
References
- 1.Berger J, Hinglais N. Les depots intercapillaires d’IgA-IgG. J Urol Nephrol. 1968;74:694–5. [PubMed] [Google Scholar]
- 2.Bogenschütz O, Bohle A, Batz C, Wehrmann M, Pressler H, Kendziorra H, Gärtner HV. IgA nephritis: on the importance of morphological and clinical parameters in the long-term prognosis of 239 patients. Am J Nephrol. 1990;10:137–147. doi: 10.1159/000168068. [DOI] [PubMed] [Google Scholar]
- 3.Alamartine E, Sabatier JC, Guerin C, Berliet JM, Berthoux F. Prognostic factors in mesangial IgA glomerulonephritis: an extensive study with univariate and multivariate analyses. Am J Kidney Dis. 1991;18:12–19. doi: 10.1016/s0272-6386(12)80284-8. [DOI] [PubMed] [Google Scholar]
- 4.D’Amico G. Natural history of idiopathic IgA nephropathy: role of clinical and histological prognostic factors. Am J Kidney Dis. 2000;36:227–237. doi: 10.1053/ajkd.2000.8966. [DOI] [PubMed] [Google Scholar]
- 5.D’Amico G. Natural history of idiopathic IgA nephropathy and factors predictive of disease outcome. Semin Nephrol. 2004;24:179–196. doi: 10.1016/j.semnephrol.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Zwirner J, Burg M, Schulze M, Brunkhorst R, Gotze O, Koch KM, Floege J. Activated complement C3: A potentially novel predictor of progressive IgA nephropathy. Kidney Int. 1997;51:1257–1264. doi: 10.1038/ki.1997.171. [DOI] [PubMed] [Google Scholar]
- 7.Roos A, Rastaldi MP, Calvaresi N, Oortwijn BD, Schlagwein N, van Gijlswijk-Janssen DJ, Stahl GL, Matsushita M, Fujita T, van Kooten C, Daha MR. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol. 2006;17:1724–1734. doi: 10.1681/ASN.2005090923. [DOI] [PubMed] [Google Scholar]
- 8.Working Group of the International IgA Nephropathy Network and the Renal Pathology Society. Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, D’Agati V, D’Amico G, Emancipator S, Emma F, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Leung CB, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–545. doi: 10.1038/ki.2009.243. [DOI] [PubMed] [Google Scholar]
- 9.Working Group of the International IgA Nephropathy Network and the Renal Pathology Society. Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, Cattran DC, Coppo R, D’Agati V, D’Amico G, Emancipator S, Emma F, Feehally J, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76:546–556. doi: 10.1038/ki.2009.168. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 11.D’Amico G. Influence of clinical and histological features on actuarial renal survival in adult patients with idiopathic IgA nephropathy, membranous nephropathy, and membranoproliferative glomerulonephritis: survey of the recent literature. Am J Kidney Dis. 1992;20:315–323. doi: 10.1016/s0272-6386(12)70293-7. [DOI] [PubMed] [Google Scholar]
- 12.Daniel L, Saingra Y, Giorgi R, Bouvier C, Pellissier JF, Berland Y. Tubular lesions determine prognosis of IgA nephropathy. Am J Kidney Dis. 2000;35:13–20. doi: 10.1016/S0272-6386(00)70295-2. [DOI] [PubMed] [Google Scholar]
- 13.Espinosa M, Ortega R, Gómez-Carrasco JM, López-Rubio F, López-Andreu M, López-Oliva MO, Aljama P. Mesangial C4d deposition: a new prognostic factor in IgA nephropathy. Nephrol Dial Transplant. 2009;24:886–891. doi: 10.1093/ndt/gfn563. [DOI] [PubMed] [Google Scholar]


