Abstract
Although CD44 was overexpressed and considered as a useful prognostic marker in renal cell carcinoma, the prognostic role of CD44s in clear cell renal cell carcinoma (ccRCC) remains controversial. Moreover, the correlation and prognostic significance of CD44s and its downstream signaling target pSTAT3 are unclear in ccRCC. In this study, 75 pairs of carcinoma and paired adjacent non-tumor renal tissue samples were collected from patients with localized ccRCC who underwent a nephrectomy. The expression levels of CD44s and pSTAT3 were analyzed using immunohistochemistry. Correlations between CD44s/pSTAT3 expression and clinical and pathological characteristics were determined using x2 test, Kaplan-Meier analysis and Cox’s proportional hazards model. We found that CD44s is highly expressed in 46.67% of tumor tissues, and its high expression was significantly associated with high tumor grade (P < 0.001), large tumor size (P = 0.009) and advanced T stage (P = 0.004). A strong correlation exists between high expression of CD44s and pSTAT3 (r = 0.4013, P = 0.0004). The joint over expression of CD44s and pSTAT3 was present in 42.66% of tumor specimens and had an additive negative impact on overall survival. Patients with CD44shighpSTAT3high expression had significantly poor survival as compared to patients with CD44slowpSTAT3low tumor expression (P = 0.024), though the concurrent overexpression of CD44s and pSTAT3 was not an independent prognostic factor for overall survival. Our data indicate that expression of both CD44s and pSTAT3 in ccRCC is associated with advanced tumor stage and patient survival. The conclusions from this study may improve the prediction of ccRCC prognosis information when CD44s and pSTAT3 expression are evaluated together with classical clinicopathological parameters.
Keywords: CD44s, pSTAT3, ccRCC, prognosis, survival
Introduction
Renal cell carcinoma (RCC) is the most lethal urologic cancers, with more than 200,000 new cases diagnosed worldwide annually [1]. The most common form of kidney cancer is RCC of which 70-80% of cases are defined as clear cell renal cell carcinoma (ccRCC) [2]. Currently, the only potential curative treatment is surgery. However, 20-30% of patients with RCC experience local or distant recurrence within 5 years after an initial nephrectomy [3]. Therefore, the recurrence of RCC after surgery is a major factor limiting patient survival. Identification of new biomarkers to predict tumor relapse and patient survival remains a subject of fundamental importance.
To date, the traditional assessment of prognosis for RCC patients relies mainly on tumor stage, nuclear grade, and histological features [4]. However, these parameters are neither reliable nor accurate for predicting the therapeutic response [5,6], and studies to discover additional prognostic markers is greatly needed. More importantly, the ability to predict which patients will develop recurrence after surgery is valuable, allowing physicians to make more informed decisions on the follow-up schedule and additional treatment.
CD44, a ubiquitous multifunctional cell surface adhesion molecule, has been implicated in tumorigenesis, progression and tumor recurrence. Additionally, CD44 is recognized as a potential biomarker in a variety of human cancers [7]. Several isoforms of CD44 have been identified as the result of alternative post-transcriptional splicing. The standard form, CD44s, is the main receptor for hyaluronate acid (HA) and has been implicated in lymphocyte homing, tissue regeneration, and tumor cell dissemination [7]. Moreover, CD44s has been identified as a marker of tumor initiating cells (TICs) [7], and plays an essential role in cells undergoing epithelial-mesenchymal transition (EMT) [8]. We recently identified CD44+ subpopulation (CD133+/CD44+) as the putative pancreatic TICs; and found that CD44s plays an important role in TICs maintenance, radiation resistance and tumor recurrence [9,10]. Although CD44s acts as a potential prognostic factor, contradictory results have been reported concerning CD44s overexpression in relation to tumor development and progression in different tumors types. Patients with CD44s overexpression were reported to have better survival in bladder cancer, myxofibrosarcoma and gastrointestinal cancer [11,12], while CD44s overexpression was associated with poor survival in pharyngeal and laryngeal cancer patients [13]. In oral cancer, prostate cancer and ovarian carcinoma, no clear association was revealed between CD44s expression and prognosis [13-15]. In RCC, elevated CD44 expression was reported, but its prognostic role in RCC remains controversial; some studies support the role of CD44 as a prognostic marker [1,4,16] while other studies were unable to confirm the importance of CD44 including CD44s as a predictor of survival [17,18]. Thus, the role of CD44s as a biomarker for ccRCC warrants further investigation to confirm its utility in patients with ccRCC, which stimulated our current study.
CD44 can activate a number of signaling molecules, including signal transducer and activator of transcription 3 (STAT3). STAT3 is structurally and functionally coupled in the HA/CD44 signaling pathway and known as a proximal mediator of CD44-dependent chemo-resistance in stem cell like cells [19,20]. As a oncogenic transcription factor, STAT3 modulates the transcription of responsive genes involved in cell differentiation, proliferation, apoptosis, angiogenesis, metastasis, immune responses, orchestrating tumor-associated inflammation [21] and maintaining self-renewal of CD44+CD24- stem cell like cells [22]. Tumor cell lines bearing constitutively activated STAT3 require continued STAT3 activation, a phenotype that has been termed “oncogene addiction” [23]. The majority of human malignancies demonstrate elevated levels of constitutively activated STAT3 and the elevated levels of activated STAT3 have been associated with poor prognosis including in RCC patients [23,24]. We recently showed that pSTAT3 acts downstream of CD44s activation, and that CD44s and pSTAT3 were co-overexpressed in pancreatic cancer, suggesting their potential connection in tumor development and progression [10,25]. However, the correlation of CD44s co-overexpression with pSTAT3 and their clinical relevance in ccRCC is currently unknown.
In this paper, we examined CD44s and pSTAT3 co-overexpression in carcinoma and paired adjacent non-tumor renal tissue from 75 patients with ccRCC, and determined the correlation between their individual expression and co-expression with various clinicopathological features, and analyzed their prognostic value for survival of patients with ccRCC.
Materials and methods
Patient samples
Tissue samples were collected from 75 patients with ccRCC who underwent a radical or partial nephrectomy between 2006 and 2012. The clinical, pathological, and treatment information, together with follow-ups were obtained through medical record review. The following histopathological factors were evaluated: tumor histologic subtype, grade, tumor size, location, TNM stage, and pathological stage according to the 2009 American Joint Committee on Cancer (AJCC) TNM staging system (7th edition). Follow-up data was included: date of nephrectomy, survival status, date of death, and/or date of last follow-up. This study was reviewed and approved by the Institutional Review Board of the Fourth Military Medical University.
Tissue microarray
A tissue microarray (TMA) constructed from formalin-fixed, paraffin-embedded tissue blocks from 75 patients with ccRCC was purchased from the National Engineering Center for Biochip (Shanghai, China). First, representative tumor areas and paired adjacent non-neoplastic tissues were carefully selected by a trained pathologist from a hematoxylin and eosin-stained section of each donor block. Each tissue sample was represented by three cylindrical core tissue biopsies (diameter 0.6 mm) collected using a manual tissue arrayer (MTA1 Tissue Arrayer SY010103, Beecher instruments Inc, Sun Prairie, Wisconsin, USA). After tissue cores were extracted from tissue blocks, 4-μm thick sections were cut and transferred to an adhesive-coated slide. The presence of tumor tissue on the arrayed samples was verified on a hematoxylin-eosin-stained section.
Immunohistochemistry
TMA slides were dried at 63°C for one hour before staining. All procedures were performed at room temperature as previously described [26]. Briefly, sections were dewaxed in xylene and rehydrated in a graded alcohol series. Sections were then washed in water before antigen retrieval using a Leica ST5010 Autostainer (Leica Microsystems Inc., Buffalo Grove, IL, United States) with 10 mM sodium citrate buffer (pH 5.96) at 100°C in an autoclave for 5 minutes. The sections were then treated with 3% hydrogen peroxide for 15 min to block endogenous peroxidase. Primary antibody was prepared as: mouse CD44s monoclonal antibody (Abnova, Taipei City, Taiwan) diluted at 1:200, and rabbit pSTAT3 monoclonal antibody (Epitomics, Inc., California, USA) diluted at 1:50 with background-reducing diluents (Dako, Carpinteria, CA, USA). After 30 minutes of incubation with primary antibody in a humidity chamber, the slides were incubated for 30 minutes with an EnVision™/HRP anti-rabbit or anti-mouse solution (Dako, Glostrup, Denmark). Reaction products were visualized with diaminobenzidine plus substrate-chromogen solution applied for 5 minutes. The slides were counterstained with Meyer’s hematoxylin and mounted. Careful rinses with several changes of phosphate-buffered saline (PBS) were performed between each stage of the procedure. Negative controls were prepared by excluding the primary antibody.
TMA scoring
The immunohistochemical staining results were scored by two independent pathologists, who were unaware of clinicopathological details of the patients. Expression staining was scored based on the localization of CD44s on the surface of cells and nuclear pSTAT3. The percentage of positive stained cells was scored as: group 0 had no positive stained cells; group 1, 2, and 3 had 1-25%, 26-75% and > 75% positive stained cells. The intensity of positive stained cells was scored into four groups: group 0 displayed no visible difference as compared to the negative control sample; the positive stained cells of group 1, 2 and 3 were light brown, mid-brown and dark brown, with the same intensity covering more than 75% of the staining area. For tissue sample with surface bound CD44s and phospho-STAT3-positive nuclei: the samples with no staining were defined as negative expression; the samples with 1+ staining ≤ 50% of cells or 2+ staining ≤ 25% of cells were defined as weak expression; the samples with 1+ staining > 50% of cells or 2+ staining between 26-75% of cells or 3+ staining ≤ 25% of cells were defined as moderate expression; the samples with 2+ staining > 75% of cells or 3+ staining > 25% of cells were defined as strong expression [25].
Statistical analysis
Pearson’s x2 test was used to assess the correlation between CD44s/pSTAT3 expression and clinicopathological features as well as patient survival. The Kaplan-Meier method and the log-rank test were used to compare overall survival, defined as the time from surgery until death (living patients were censored at the time of their last follow-up). A Cox’s proportional hazards model was performed to analyze the correlation of CD44s/pSTAT3 expression on patient survival and determine the independent prognostic effects of these factors. A P-value of less than 0.05 was considered statistically significant. All statistical analyses were conducted using SPSS statistical software (vers. 16.0; SPSS, Inc., Chicago, IL, USA).
Results
Patient characteristics
The patient characteristics are summarized in Table 1. The patients ranged from 29 to 82 years of age (average 58.7 years), and 66.7% of the patients were male. Of the patients, 44, 32, 22.7 and 1.3% were presented with AJCC clinical stage 1-4 respectively; and 49.4, 33.3 and 17.3% had pT1-pT3 primary tumors respectively with average tumor size of 6.7 cm (range, 2.3-15 cm). Tumor grading demonstrated grade 1-4 lesions in 5.3, 60, 28 and 6.7% of patients, respectively. Over 6 years, 14 (32.6%) patients died of the ccRCC.
Table 1.
Patient’s characteristics
| Features | No. patients (%) |
|---|---|
| Age, year | |
| Mean, Median (range) | 58.7, 57 (29~82) |
| Gender | |
| Male | 50 (66.7%) |
| Female | 25 (33.3%) |
| Tumor size, cm | |
| Mean, Median (range) | 6.7, 6 (2.3~15) |
| Tumor grade | |
| I | 4 (5.3%) |
| II | 45 (60%) |
| III | 21 (28%) |
| IV | 5 (6.7%) |
| T stage | |
| T1 | 37 (49.3%) |
| T2 | 25 (33.3%) |
| T3 | 13 (17.3%) |
| N stage | |
| N0 | 69 (93.2%) |
| N1+N2 | 5 (6.8%) |
| AJCC clinical stage | |
| 1 | 33 (44%) |
| 2 | 24 (32%) |
| 3 | 17 (22.7%) |
| 4 | 1 (1.3%) |
| Overall Survivala, month | |
| Mean, Median (range) | 52.6, 63 (2~74) |
| live | 29 (67.4%) |
| dead | 14 (32.6%) |
Only 43 cases have available survival data.
CD44s expression and its correlation with clinicopathological features in ccRCC
In resected primary tumors from 75 patients with ccRCC, CD44s expression patterns were investigated by immunohistochemistry. As shown in Figure 1, CD44s was diversely stained in the membrane and cytoplasm of cells. In non-neoplastic tissues, CD44s has negative to weak expression; while in ccRCC its expression is greatly enhanced. The percentage of strong CD44s expression was significantly higher in cancer as compared to that of paired non-neoplastic tissue (26.67% vs. 1.33%, P < 0.001, Table 2). For further analysis, we divided patients into CD44s low expression group (negative to weak expression) and CD44s high expression group (moderate to strong expression), with the ratio of CD44s high expression in ccRCC significantly greater than that of in non-neoplastic tissue (46.67% vs. 9.33%, P < 0.001).
Figure 1.
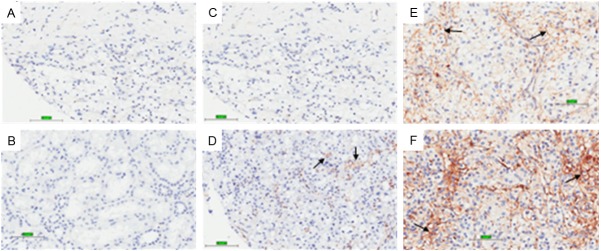
Representative images of CD44s immunohistochemistry staining in clear cell renal cell carcinoma tissues. (A) Negative control, (B) non-neoplastic tissue, (C) no staining, (D) weak staining intensity, (E) intermediate staining intensity and (F) strong staining intensity. Bar, 101.3 μm. Magnification: 400X.
Table 2.
CD44s expression in non-neoplastic tissue and ccRCC
| CD44s expression levels† | Non-neoplastic tissue (n = 75) | ccRCC (n = 75) |
|---|---|---|
| Negative | 68% (51/75) | 18.67% (14/75) |
| Weak | 22.67% (17/75) | 34.66% (26/75) |
| Moderate | 8% (6/75) | 20% (15/75) |
| Strong | 1.33% (1/75) | 26.67% (20/75)* |
Expression levels of CD44s are scored as: the samples with no staining were defined as negative expression (Negative); the samples with 1+ staining ≤ 50% of cells or 2+ staining ≤ 25% of cells were defined as weak expression (Weak); the samples with 1+ staining > 50% of cells or 2+ staining between 26-75% of cells or 3+ staining ≤ 25% of cells were defined as moderate expression (Moderate); the samples with 2+ staining > 75% of cells or 3+ staining > 25% of cells were defined as strong expression (Strong).
Statistically significant (P < 0.05); Estimated by χ2 test as compared to non-neoplastic tissue.
We next investigated the correlation between CD44s expression and clinicopathological parameters. First, we examined the association of CD44s expression with survival time in available 43 ccRCC patients using Kaplan-Meier survival analysis. As shown in Figure 2A, 75% of patients with high CD44s expression survived on average 32 months as compared to longer than 61 months for patients with low expression of CD44s (P = 0.086) [hazard ratio (HR) = 2.673, 95% confidence interval (CI) = 0.83 to 8.611, P = 0.099]. The 5-year survival rate for patients whose tumors expressed low and high levels of CD44s was of 85% and 60.9%, respectively. No significant correlation exists in age, gender, N stage, AJCC clinical stage and patient survival (Table 3). However, high expression of CD44s was significantly correlated with high tumor grade (P < 0.001), large tumor size (P = 0.009) and advanced T stage (P = 0.004).
Figure 2.
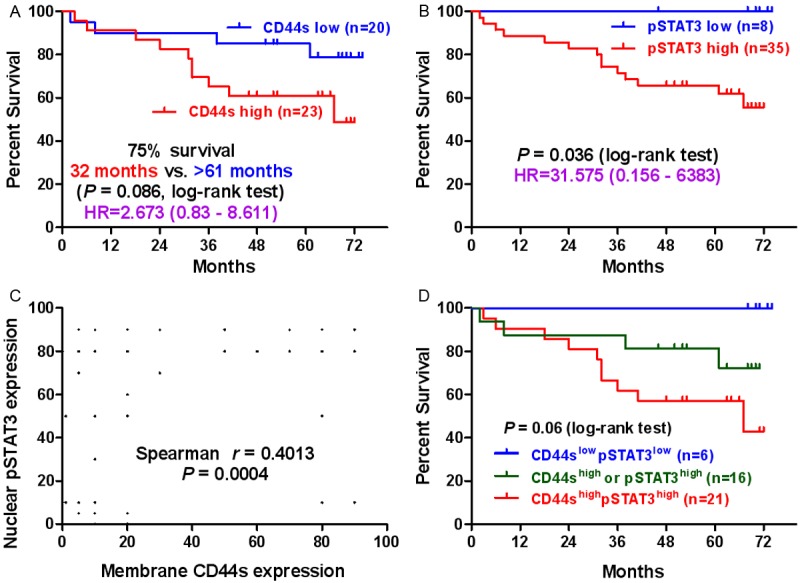
CD44s/pSTAT3 expression associated with patients’ overall survival. A: Kaplan-Meier analysis of overall survival in 43 patients comparing high and low CD44s expression groups. B: Kaplan-Meier analysis of overall survival in 43 patients comparing high and low pSTAT3 expression groups. C: Correlation analysis between CD44s and pSTAT3 expression in ccRCC patients. D: Kaplan-Meier analysis of overall survival for 43 patients based on CD44s and pSTAT3 expression. CD44shighpSTAT3high: patients with tumors overexpressing both proteins; CD44shigh or pSTAT3high: patients with tumors overexpressing only one of the two proteins; CD44slowpSTAT3low: patients with tumors low expressing both proteins.
Table 3.
Correlation between CD44s/pSTAT3 expression and clinicopathological features
| Characteristics | CD44s expression* | pSTAT3 expression* | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Low (n = 40) | High (n = 35) | P value † | Low (n = 17) | High (n = 58) | P value † | |
| Age | 0.716 | 0.975 | ||||
| ≤ 60 | 25 | 21 | 10 | 36 | ||
| > 60 | 14 | 14 | 6 | 22 | ||
| Gender | 0.870 | 0.435 | ||||
| Male | 27 | 23 | 10 | 40 | ||
| Female | 13 | 12 | 7 | 18 | ||
| Tumor size | 0.009 | 0.043 | ||||
| ≤ 7 (cm) | 30 | 16 | 14 | 32 | ||
| >7 (cm) | 10 | 19 | 3 | 26 | ||
| Tumor grade | < 0.001 | 0.001 | ||||
| I+II | 34 | 15 | 17 | 32 | ||
| III+IV | 6 | 20 | 0 | 26 | ||
| T stage | 0.004 | 0.002 | ||||
| T1 | 26 | 11 | 14 | 23 | ||
| T2+T3 | 14 | 24 | 3 | 35 | ||
| N stage | 1 | 0.475 | ||||
| N0 | 36 | 23 | 17 | 52 | ||
| N1+N2 | 3 | 2 | 0 | 5 | ||
| AJCC clinical stage | 0.386 | 0.021 | ||||
| 1+2 | 32 | 25 | 17 | 40 | ||
| 3+4 | 8 | 10 | 0 | 18 | ||
| pSTAT3 | 0.006 | - | ||||
| Low | 14 | 3 | - | - | ||
| High | 26 | 32 | - | - | ||
Low (negative to weak expression), High (moderate to strong expression).
Estimated by χ2 test.
Correlation between concurrent CD44s/pSTAT3 expression and clinicopathological features in ccRCC
We previously reported that CD44s promotes tumor-initiation and post-radiotherapy recurrence through pSTAT3 signaling [10]. However, their relationship in ccRCC was unclear. Thus, we first studied the nuclear pSTAT3 expression in the same 75 pairs of ccRCC tissue samples in order to understand better the correlation between CD44s and pSTAT3 expression on patient outcome. Differently from CD44s, both tumor tissues and adjacent non-neoplastic tissues have high level of pSTAT3 expression in ccRCC due to the role of pSTAT3 as an inflammatory factor. The percentage of tumor tissue samples with high nuclear expression of pSTAT3 was 76.33%, which was significantly greater than that of CD44s (46.67%, P < 0.001). Similarly, high expression of nuclear pSTAT3 was significantly associated with poor survival (P = 0.036), high tumor grade (P = 0.001), large tumor size (P = 0.043), advanced T stage (P = 0.002) and AJCC stage (P = 0.021) (Figure 2B, Table 3). However, univariate analyses showed that pSTAT3 was not a survival predictor for patients with localized ccRCC [hazard ratio (HR) = 31.575, 95% confidence interval (CI) = 0.156 to 6383, P = 0.202].
Remarkably, high expression of membrane bound CD44s was significantly associated with high expression of nuclear pSTAT3 (P = 0.006) with strong correlation between them (r = 0.4013, P = 0.0004, Figure 2C). In 46 of 75 (61.33%) tumour specimens, concurrent CD44s and pSTAT3 expression was found, which includes concurrent high expression in 42.66% and concurrent low expression in 18.67% (Table 4). Taken together, neither CD44s nor pSTAT3 alone significantly affect patient survival and prognosis in patients with localized ccRCC although they are significantly correlated with a variety of clinicopathological parameters.
Table 4.
Co-expression of CD44 and pSTAT3 in ccRCC
| Groups | Percentage |
|---|---|
| CD44lowpSTAT3low | 18.67% (14/75) |
| CD44highpSTAT3low | 4% (3/75) |
| CD44slowpSTAT3high | 34.67% (26/75) |
| CD44shighpSTAT3high | 42.66% (32/75) |
Pearson r = 0.4013 (95% confidence interval: 0.1917-0.5753), P = 0.0004.
As CD44s and pSTAT3 were highly correlated in ccRCC, we thus divided patients into three distinct subgroups, identified by the expression of CD44s and pSTAT3 antigens. As shown in Figure 2D, patients with tumors highly expressing both antigens experienced the poorest clinical outcome. In this population of patients (CD44shigh/pSTAT3high), the average survival time for 75% of patients was 32 months; in contrast to an intermediate prognosis in 75% of patients highly expressing either CD44s or pSTAT3 with an average survival of 61 months. Finally, patients with low expression of both antigens in their tumors carried the best prognosis. In our patient cohort, no death occurred in the 6 patients with low expression of CD44s/pSTAT3 after monitoring over 6 years post operation. Statistically, patients with high expression of both antigens had a poor overall survival as compared to patients with low expression of both antigens (P = 0.024, log-rank test).
In regards to the pathological features of the tumor samples, there were significant differences in tumor grade (P < 0.001), tumor size (P = 0.006) and T stage (P = 0.001) among patients with high expression of both antigens, high expression of either CD44s or pSTAT3 and low expression of both antigens (Table 5). Using the univariate analyses method, we discovered that age (P = 0.028), tumor grade (P = 0.024) and CD44s/pSTAT3 co-expression (P = 0.028) were significant risk factors affecting the overall survival of patients with ccRCC (Table 6). However, multivariate analysis using Cox’s proportional hazards model determined that only tumor grade proved to be an independent prognostic variable for overall survival, but not the co-expression of CD44s/pSTAT3 and age.
Table 5.
Correlation between CD44s/pSTAT3 co-expression and clinicopathological features
| Characteristics | CD44lowpSTAT3low * (n = 14) | CD44high or pSTAT3high * (n = 29) | CD44shighpSTAT3high * (n = 32) | P value † |
|---|---|---|---|---|
| Age | 0.884 | |||
| ≤ 60 (y) | 8 | 19 | 19 | |
| > 60 (y) | 5 | 10 | 13 | |
| Gender | 0.130 | |||
| Male | 7 | 23 | 20 | |
| Female | 7 | 6 | 12 | |
| Tumor size | 0.006 | |||
| ≤ 7 (cm) | 11 | 22 | 13 | |
| > 7 (cm) | 3 | 7 | 19 | |
| Tumor grade | <0.001 | |||
| I+II | 14 | 23 | 12 | |
| III+IV | 0 | 6 | 20 | |
| T stage | 0.001 | |||
| T1 | 11 | 18 | 8 | |
| T2+T3 | 3 | 11 | 24 | |
| N stage | 0.422 | |||
| N0 | 14 | 25 | 30 | |
| N1+N2 | 0 | 3 | 2 | |
| AJCC clinical stage | 0.062 | |||
| 1+2 | 14 | 21 | 22 | |
| 3+4 | 0 | 8 | 10 |
Low (negative to weak expression), High (moderate to strong expression);
Estimated by χ2 test.
Table 6.
Multivariate analysis of various prognostic markers including concurrent CD44s and pSTAT3 high expression
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 1.060 (1.007-1.115) | 0.028* | 1.050 (0.933-1.111) | 0.086 |
| Gender (female/male) | 0.944 (0.327-2.723) | 0.915 | - | - |
| Tumor size (> 7/≤ 7 cm) | 2.129 (0.713-6.357) | 0.176 | - | - |
| Tumor grade (III-IV/I-II) | 3.829 (1.195-12.267) | 0.024* | 3.637 (1.136-11.646) | 0.030* |
| CD44s expression (high/low) | 2.673 (0.830-8.611) | 0.099 | - | - |
| pSTAT3 expression (high/low)+ | 31.575 (0.156-6383) | 0.202 | - | - |
| CD44spSTAT3 co-expression (CD44shighpSTAT3high/CD44shigh or pSTAT3high/CD44slowpSTAT3low) | 3.113 (1.130-8.575) | 0.028* | 2.337 (0.849-6.434) | 0.100 |
HR hazard ratio, CI confidence interval.
Discussion
Renal cell cancer (RCC) constitutes a group of epithelial tumors that are highly heterogeneous with respect to morphology and clinical behavior. We focused on patients with localized clear cell RCC (ccRCC) who underwent surgery. One reason is that ccRCC is the most prevalent type of kidney cancer and its molecular pathogenesis is not completely understood [27]. Another reason is the relative homogeneous features within the population.
In this study, we show that the overexpression of CD44s was significantly correlated with important clinical variables such as tumor grade, tumor size and T stage, but not with patient survival time, suggesting a limited prognostic role of CD44s alone in patients with ccRCC. Additionally, our study revealed a significant correlation between the expression of CD44s and pSTAT3 in ccRCC. The co-expression of CD44shigh/pSTAT3high had an additive negative impact on patient survival, as compared to patients with CD44slow/pSTAT3low expression in tumors.
Although it’s limited role in prognosis, CD44s could be a promising therapeutic target. In our study, we found a significant correlation between high expression of CD44s and high tumor grade, reflecting a functional role for CD44s in maintaining a more embryonic and dedifferentiated state of neoplastic renal cells [18], possibly by maintaining TICs in ccRCC. Apart from its role as a TICs marker, CD44s functionally regulates TICs by activating stem cell genes (Nanog/Sox2/Rex1) and maintaining TICs features (metastasis and drug/radiation resistance) [8,10]. CD44s is also required for the formation of EMT-associated recurrent breast tumors [8], and regulates the mesenchymal phenotype in HCC cells [28,29]. Our results here reveal a potential role of CD44s in ccRCC; however the detailed function of CD44s in ccRCC initiation and progression needs to be studied further.
Our observation of a significant positive correlation between CD44s and pSTAT3 overexpression in ccRCC is consistent with previous literature. Several studies demonstrated evidence supporting the role of CD44/STAT3 signaling in cancer progression. Recently, studies reported that CD44+ TICs had preferential activation of STAT3 [22,30,31], implying a potential role of pSTAT3 in maintaining CD44s+ ccRCC TICs which needs to be studied further. STAT3 act as a downstream target of CD44(s) during cancer cell invasion [32], chemoresistance [19,20,31] and radio-resistance [10]. Additional studies have found that CD44 and STAT3 directly interact. For example, CD44 translocates into the nucleus and directly interacts with STAT3 in response to osteopontin [33], or CD44 interacts with STAT3 in the absence of exogenous ligands on the membrane, functioning as a scaffold protein for the CD44-STAT3-JAK2 complex [32]. In this paper, patients with concurrent CD44shigh/pSTAT3high tumor expression had a significant reduction in overall survival, although the prognostic value was not confirmed in the survival multivariate analysis.
Several studies using CD44(s) monoclonal antibodies have demonstrated the therapeutic potential of targeting CD44 by inhibiting TICs, such as monoclonal antibody H90 for eliminating leukaemia stem cells [34], and H4C4 for eliminating pancreatic cancer stem cells [10]. Our study here suggests that CD44s monoclonal antibodies like H4C4 and pSTAT3 inhibitors like FLLL32 [35] may be efficacious for the treatment of patients diagnosed with CD44shigh/pSTAT3high expressing ccRCC. The prognostic value of concurrent CD44s/pSTAT3 expression in ccRCC needs to be validated in a larger cohort of patients in the future.
In summary, our study revealed that membrane CD44s and nuclear pSTAT3 were both overexpressed and significantly correlated in ccRCC. Co-expression of CD44shigh/pSTAT3high was associated with advanced ccRCC, indicated by features such as, high tumor grade, large tumor size and advanced T stage. Taken together, our study indicates that the combination of CD44s and pSTAT3 high expression may significantly improve the prediction of ccRCC prognosis. Furthermore, the co-expression of CD44s/pSTAT3 could be regarded as novel targets for therapeutic interventions in ccRCC.
Acknowledgements
This work was partly supported by Young Talents Support Program from Fourth Military Medical University awarded to LL.
Disclosure of conflict of interest
None.
References
- 1.Jeong BJ, Liang ZL, Huang SM, Lim JS, Kim JM, Lee HJ. CD44 is associated with tumor recurrence and is an independent poor prognostic factor for patients with localized clear cell renal cell carcinoma after nephrectomy. Exp Ther Med. 2012;3:811–817. doi: 10.3892/etm.2012.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dutcher JP. Recent developments in the treatment of renal cell carcinoma. Ther Adv Urol. 2013;5:338–353. doi: 10.1177/1756287213505672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili R, Bjarnason GA, Garcia-del-Muro X, Sosman JA, Solska E, Wilding G, Thompson JA, Kim ST, Chen I, Huang X, Figlin RA. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 2009;27:3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim SD, Young AN, Paner GP, Amin MB. Prognostic role of CD44 cell adhesion molecule expression in primary and metastatic renal cell carcinoma: a clinicopathologic study of 125 cases. Virchows Arch. 2008;452:49–55. doi: 10.1007/s00428-007-0530-4. [DOI] [PubMed] [Google Scholar]
- 5.Saroufim A, Messai Y, Hasmim M, Rioux N, Iacovelli R, Verhoest G, Bensalah K, Patard JJ, Albiges L, Azzarone B, Escudier B, Chouaib S. Tumoral CD105 is a novel independent prognostic marker for prognosis in clear-cell renal cell carcinoma. Br J Cancer. 2014;110:1778–1784. doi: 10.1038/bjc.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bex A, Fournier L, Lassau N, Mulders P, Nathan P, Oyen WJ, Powles T. Assessing the Response to Targeted Therapies in Renal Cell Carcinoma: Technical Insights and Practical Considerations. Eur Urol. 2014;65:766–777. doi: 10.1016/j.eururo.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 7.Zoller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer. 2011;11:254–267. doi: 10.1038/nrc3023. [DOI] [PubMed] [Google Scholar]
- 8.Brown RL, Reinke LM, Damerow MS, Perez D, Chodosh LA, Yang J, Cheng C. CD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progression. J Clin Invest. 2011;121:1064–1074. doi: 10.1172/JCI44540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji Q, Hao X, Zhang M, Tang W, Yang M, Li L, Xiang D, Desano JT, Bommer GT, Fan D, Fearon ER, Lawrence TS, Xu L. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One. 2009;4:e6816. doi: 10.1371/journal.pone.0006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L, Hao X, Qin J, Tang W, He F, Smith A, Zhang M, Simeone DM, Qiao XT, Chen ZN, Lawrence TS, Xu L. Antibody Against CD44s Inhibits Pancreatic Tumor Initiation and Postradiation Recurrence in Mice. Gastroenterology. 2014;16:1108–1118. doi: 10.1053/j.gastro.2013.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matuschek C, Lehnhardt M, Gerber PA, Poremba C, Hamilton J, Lammering G, Orth K, Budach W, Bojar H, Bolke E, Peiper M. Increased CD44s and decreased CD44v6 RNA expression are associated with better survival in myxofibrosarcoma patients: a pilot study. Eur J Med Res. 2014;19:6. doi: 10.1186/2047-783X-19-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang YM, Li XH, Li WM, Lu YY. Prognostic significance of PTEN, Ki-67 and CD44s expression patterns in gastrointestinal stromal tumors. World J Gastroenterol. 2012;18:1664–1671. doi: 10.3748/wjg.v18.i14.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J, Zhou J, Lu J, Xiong H, Shi X, Gong L. Significance of CD44 expression in head and neck cancer: a systemic review and meta-analysis. BMC Cancer. 2014;14:15. doi: 10.1186/1471-2407-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tei H, Miyake H, Harada KI, Fujisawa M. Expression profile of CD44s, CD44v6, and CD44v10 in localized prostate cancer: Effect on prognostic outcomes following radical prostatectomy. Urol Oncol. 2014;32:694–700. doi: 10.1016/j.urolonc.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Chang B, Liu J. CD44 standard form expression is correlated with high-grade and advanced-stage ovarian carcinoma but not prognosis. Hum Pathol. 2013;44:1882–1889. doi: 10.1016/j.humpath.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kroeze SG, Bijenhof AM, Bosch JL, Jans JJ. Diagnostic and prognostic tissuemarkers in clear cell and papillary renal cell carcinoma. Cancer Biomark. 2010;7:261–268. doi: 10.3233/CBM-2010-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tawfik OW, Kramer B, Shideler B, Danley M, Kimler BF, Holzbeierlein J. Prognostic significance of CD44, platelet-derived growth factor receptor alpha, and cyclooxygenase 2 expression in renal cell carcinoma. Arch Pathol Lab Med. 2007;131:261–267. doi: 10.5858/2007-131-261-PSOCPG. [DOI] [PubMed] [Google Scholar]
- 18.Costa WH, Rocha RM, Cunha IW, Guimaraes GC, Zequi Sde C. Immunohistochemical expression of CD44s in renal cell carcinoma lacks independent prognostic significance. Int Braz J Urol. 2012;38:456–465. doi: 10.1590/s1677-55382012000400004. [DOI] [PubMed] [Google Scholar]
- 19.Bourguignon LY, Earle C, Wong G, Spevak CC, Krueger K. Stem cell marker (Nanog) and Stat-3 signaling promote MicroRNA-21 expression and chemoresistance in hyaluronan/CD44-activated head and neck squamous cell carcinoma cells. Oncogene. 2012;31:149–160. doi: 10.1038/onc.2011.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bourguignon LY, Peyrollier K, Xia W, Gilad E. Hyaluronan-CD44 interaction activates stem cell marker Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells. J Biol Chem. 2008;283:17635–17651. doi: 10.1074/jbc.M800109200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li N, Grivennikov SI, Karin M. The unholy trinity: inflammation, cytokines, and STAT3 shape the cancer microenvironment. Cancer cell. 2011;19:429–431. doi: 10.1016/j.ccr.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marotta LL, Almendro V, Marusyk A, Shipitsin M, Schemme J, Walker SR, Bloushtain-Qimron N, Kim JJ, Choudhury SA, Maruyama R, Wu Z, Gonen M, Mulvey LA, Bessarabova MO, Huh SJ, Silver SJ, Kim SY, Park SY, Lee HE, Anderson KS, Richardson AL, Nikolskaya T, Nikolsky Y, Liu XS, Root DE, Hahn WC, Frank DA, Polyak K. The JAK2/STAT3 signaling pathway is required for growth of CD44(+)CD24(-) stem cell-like breast cancer cells in human tumors. J Clin Invest. 2011;121:2723–2735. doi: 10.1172/JCI44745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnston PA, Grandis JR. STAT3 signaling: anticancer strategies and challenges. Mol Interv. 2011;11:18–26. doi: 10.1124/mi.11.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo C, Yang G, Khun K, Kong X, Levy D, Lee P, Melamed J. Activation of Stat3 in renal tumors. Am J Transl Res. 2009;1:283–290. [PMC free article] [PubMed] [Google Scholar]
- 25.Li L, Tang W, Wu X, Karnak D, Meng X, Thompson R, Hao X, Li Y, Qiao XT, Lin J, Fuchs J, Simeone DM, Chen ZN, Lawrence TS, Xu L. HAb18G/CD147 promotes pSTAT3-mediated pancreatic cancer development via CD44s. Clin Cancer Res. 2013;19:6703–6715. doi: 10.1158/1078-0432.CCR-13-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai Y, DeSano JT, Meng Y, Ji Q, Ljungman M, Lawrence TS, Xu L. Celastrol potentiates radiotherapy by impairment of DNA damage processing in human prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74:1217–1225. doi: 10.1016/j.ijrobp.2009.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato Y, Yoshizato T, Shiraishi Y, Maekawa S, Okuno Y, Kamura T, Shimamura T, Sato-Otsubo A, Nagae G, Suzuki H, Nagata Y, Yoshida K, Kon A, Suzuki Y, Chiba K, Tanaka H, Niida A, Fujimoto A, Tsunoda T, Morikawa T, Maeda D, Kume H, Sugano S, Fukayama M, Aburatani H, Sanada M, Miyano S, Homma Y, Ogawa S. Integrated molecular analysis of clear-cell renal cell carcinoma. Nat Genet. 2013;45:860–867. doi: 10.1038/ng.2699. [DOI] [PubMed] [Google Scholar]
- 28.Mima K, Okabe H, Ishimoto T, Hayashi H, Nakagawa S, Kuroki H, Watanabe M, Beppu T, Tamada M, Nagano O, Saya H, Baba H. CD44s Regulates the TGF-beta-Mediated Mesenchymal Phenotype and Is Associated with Poor Prognosis in Patients with Hepatocellular Carcinoma. Cancer Res. 2012;72:3414–3423. doi: 10.1158/0008-5472.CAN-12-0299. [DOI] [PubMed] [Google Scholar]
- 29.Okabe H, Ishimoto T, Mima K, Nakagawa S, Hayashi H, Kuroki H, Imai K, Nitta H, Saito S, Hashimoto D, Chikamoto A, Ishiko T, Watanabe M, Nagano O, Beppu T, Saya H, Baba H. CD44s signals the acquisition of the mesenchymal phenotype required for anchorage-independent cell survival in hepatocellular carcinoma. Br J Cancer. 2014;110:958–966. doi: 10.1038/bjc.2013.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin L, Hutzen B, Lee HF, Peng Z, Wang W, Zhao C, Lin HJ, Sun D, Li PK, Li C, Korkaya H, Wicha MS, Lin J. Evaluation of STAT3 Signaling in ALDH+ and ALDH+/CD44+/CD24- Subpopulations of Breast Cancer Cells. PLoS One. 2013;8:e82821. doi: 10.1371/journal.pone.0082821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Wang G, Zhao Y, Liu X, Ding Q, Shi J, Ding Y, Wang S. STAT3 mediates resistance of CD44(+)CD24(-/low) breast cancer stem cells to tamoxifen in vitro. J Biomed Res. 2012;26:325–335. doi: 10.7555/JBR.26.20110050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.So JY, Smolarek AK, Salerno DM, Maehr H, Uskokovic M, Liu F, Suh N. Targeting CD44-STAT3 signaling by Gemini vitamin D analog leads to inhibition of invasion in basal-like breast cancer. PLoS One. 2013;8:e54020. doi: 10.1371/journal.pone.0054020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee JL, Wang MJ, Chen JY. Acetylation and activation of STAT3 mediated by nuclear translocation of CD44. J Cell Biol. 2009;185:949–957. doi: 10.1083/jcb.200812060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charrad RS, Li Y, Delpech B, Balitrand N, Clay D, Jasmin C, Chomienne C, Smadja-Joffe F. Ligation of the CD44 adhesion molecule reverses blockage of differentiation in human acute myeloid leukemia. Nat Med. 1999;5:669–676. doi: 10.1038/9518. [DOI] [PubMed] [Google Scholar]
- 35.Lin L, Hutzen B, Zuo M, Ball S, Deangelis S, Foust E, Pandit B, Ihnat MA, Shenoy SS, Kulp S, Li PK, Li C, Fuchs J, Lin J. Novel STAT3 phosphorylation inhibitors exhibit potent growth suppressive activity in pancreatic and breast cancer cells. Cancer Res. 2010;70:2445–2454. doi: 10.1158/0008-5472.CAN-09-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]


