Abstract
The Ca2+/calmodulin (CaM)-dependent protein kinase II (CaMKII) β has an essential function in dendritic spines via binding to and reorganization of the actin cytoskeleton during plasticity events not shared by CaMKIIα isoform. CaMKIIβ and CaMKIIα isoforms have remarkable structural differences within the variable region. Three exons (E1, E3, and E4) are present in CaMKIIβ but not in CaMKIIα gene. Four splice variants of CaMKIIβ isoforms (CaMKIIβ, β’, βe and β’e) were discovered in embryonic and adult brains. Exons E1 (lacked in βe and β’e) and E4 (lacked in β’ and β’e) are subject to differential alternative splicing. We hypothesized that the sequences encoded by exons E1, E3, and/or E4 are involved in CaMKIIβ-specific bundling to the F-actin cytoskeleton. We tested the colocalization and association of these CaMKIIβ variants within an F-actin-rich structure (microspike) in CaMKIIα free embryonic day 18 (E-18) rat cortical neurons. Our results showed that CaMKIIβ and CaMKIIβ’ containing exon E1 displayed an association with F-actin, while CaMKIIβe and CaMKIIβ’e lacking E1 did not. Moreover, CaMKIIβ’ lacking exon E4 but having E1 showed decreased actin bindingcapacity compared to WT CaMKIIβ. This suggested E1 is required for the association between CaMKIIβ and F-actin, while E4 assists CaMKIIβ to associate with F-actin better. Thus, alternative splicing of CaMKIIβ variants in developing neurons may serve as a developmental switch for actin cytoskeleton-associated isoforms and therefore correlated with dendritic arborization and synapse formation during LTP.
Keywords: CaMKIIβ, F-actin, developing neuron, FRAP
Introduction
The plasticity of neuronal synapses is essential for the formation and function of neural circuits, which is fundamental to learning and memory. Long-term potentiation (LTP) of synaptic function is crucial for the process of learning and memory by inducing the formation of new dendritic spines and increasing the volume of existing ones [1-4]. Calcium/calmodulin dependent protein kinase II (CaMKII) is a ubiquitously expressed serine/threonine protein kinase involved in a vast variety of cellular functions by phosphorylating a number of substrates including several cytoskeletal and signaling proteins [5-7]. Interestingly, CaMKII constitutes a substantial portion, 1-2%, of the total protein content of brain [8-11]. Especially in postsynaptic density (PSD), the percentage of CaMKII goes up to 10-30% [10]. It is much more abundant than any other signal transduction molecules and is comparable to the abundance of structural proteins found in the PSD, such as actin. Correspondingly, in addition to its signaling function, CaMKII also has a structural function to bundle actin filaments in coupling structural and functional plasticity of dendritic spines, which plays a critical role in the molecular mechanisms of LTP [12].
CaMKIIα and CaMKIIβ are the two major brain isoforms where they make up the vast majority of neuronal CaMKII [8]. CaMKIIβ was reported to have specific morphogenic functions in regulating dendritic arborization and synapse density which are not shared by CaMKIIα isoform [13]. This isoform specificity is very likely to be mediated by the specific binding of CaMKIIβ, but not CaMKIIα, to filamentous actin (F-actin) [13,14]. CaMKIIβ stabilizes the actin cytoskeleton in spines in a kinase activity independent manner and thereby maintains the stability of spine structure, while phosphorylation of CaMKIIβ reduces this bundling activity [15-17]. The most remarkable structural difference between CaMKIIβ and CaMKIIα isoforms is located within the variable region which connects the kinase domain and the association domain [9,18]. Three exons located in the variable region of CaMKIIβ, exon 1 (E1), exon 3 (E3), and exon 4 (E4), are not presented in the CaMKIIα gene (Figure 1), which leads to the speculation that sequences encoded by the exons E1, E3, and/or E4 are involved in CaMKIIβ-specific bundling to the F-actin cytoskeleton. In both embryonic and adult brains, four splice variants of CaMKIIβ isoforms are detected: wildtype (WT) CaMKIIβ, CaMKIIβ’, CaMKIIβe, and CaMKIIβ’e (a “e” refers to the embryonic form) [19]. Exons E1 (lacked in βe and β’e) and E4 (lacked in β’ and β’e) are subject to differential alternative splicing [18]. The regulation of alternative splicing may control the expression of actin-associated CaMKIIβ variants differently, thereby possibly affects the morphogenic functions of CaMKIIβ in dendritic arborization and/or synapse density.
Figure 1.
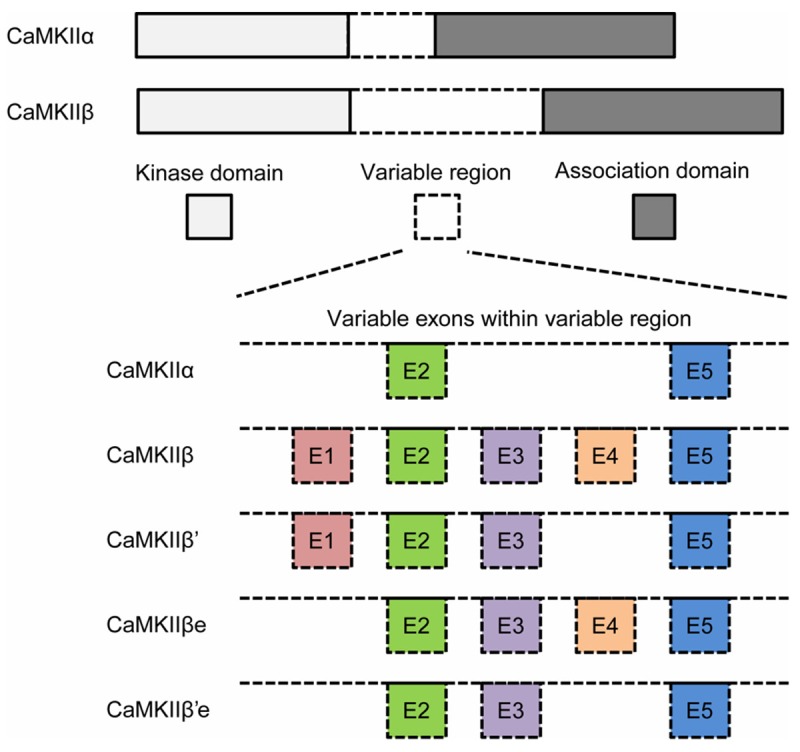
Schematic representation of individual isoforms of CaMKII variants. CaMKIIα and CaMKIIβ differ most in the variable region (They are products of different genes and have minor differences in the kinase and association domain which are not represented in this figure). Exon/intron structures of the CaMKIIα and β gene locus coding for the variable regions are shown in bottom. The variable region of CaMKIIα contains only exon 2 (E2) and exon 5 (E5). Both of these two exons are also presented in CaMKIIβ. Exon 3 (E3) is not identified in CaMKIIα but is contained in all CaMKIIβ variants. In CaMKIIβ variants, WT CaMKIIβ has all five axons. CaMKIIβ’ contains exon 1 (E1), E2, E3 and E5, but not exon E4. While CaMKIIβe lacks E1. E1 and E4 do not present in CaMKIIβ’e.
To better understand the underlying mechanism, we investigated how CaMKIIβ variants interact with F-actin in E-18 cortical neurons. In previous study, a type of F-actin-rich structure in cortical neurons from embryonic day 18 (E-18) rats was found and termed “Microspike” by our group [16,17]. Scanning electron microscopy results revealed that these microspikes were composed of bundled actin filaments, and confocal imaging showed that WT CaMKIIβ strongly colocalized to this F-actin-rich structure. CaMKIIβ was found to be the only CaMKII isoform enriched in microspikes in E-18 neocortex, where CaMKIIα was not detected until postnatal day 7 (P7). Meanwhile, cortical neuron cultures expressed a detectable level of CaMKIIβ at 4 days in vitro (DIV), while CaMKIIα was not detected until 12 DIV [16,17]. Therefore, this F-actin-rich structure, microspike, provides a powerful system in developing neurons to further characterize the association of CaMKIIβ and its variants with F-actin without the disturbance of CaMKIIα isoform.
Materials and methods
Cell culture
Embryonic day 18 cortical neurons from pregnant Long Evans Rat were cultured as described by Redmond et al. [20].
Dissection: Cortices of embryonic day 18 embryos were dissected from a sacrificed pregnant Long Evans rat (Charles River) and put in 1 × ice-cold Hanks Balanced Salt Solution (HBSS; Invitrogen) while waiting for further treatment. All cortices were incubated in enzyme solution containing 10 units/ml of papain (Worthington Biochemical Corp.) and 0.16 mg/ml of L-cysteine (Sigma-Aldrich) for 40 minutes, the enzymatic reaction was then stopped by trypsin inhibitor (Sigma).
Dissociation: Dissociation of cortical neurons was proceeded within BF media containing Basal Medium Eagle (Invitrogen), 1 mM L-glutamine (Invitrogen), 1 × Penicillin Streptomycin (Invitrogen), and 1% Fetal Bovine Serum (FBS; Invitrogen). Then dissociated cortical neurons were plated onto coverslips pre-coated by 20 μg/ml of poly-D-lysine (BD Biosciences) and 1 μg/ml of laminin (BD Biosciences). Cortical neurons were maintained in BNF media containing BF and N2 supplement (Invitrogen) or serum-free media (FSM) consisting of Neurobasal media (Invitrogen). After plated onto plates or dishes, the cortical neurons were cultured in a humidified incubator at 37°C in a 5% CO2 environment for 5 days in vitro (DIV). Cell densities were 0.25 × 106 cells per well in 24 well plates for immunostaining and 0.75 × 106 cells per 35-mm glass-bottom dish (Mat Tek) for live cell imaging.
DNA constructs
The green fluorescent protein (GFP)-tagged full-length CaMKIIα, CaMKIIβ constructs (β, β’, βe, β’e) and GFP-actin used in this study were described by Lin et al. [16,17].
Transfection
Cells were transfected with a modified calcium phosphate transfection procedure as described by Threadgill et al. [21]. Used culture medium was removed and saved 1 hr prior to transfection and replaced with Dulbecco’s Modified Eagle Medium (DMEM). The calcium phosphate/DNA precipitate was formed in HEPES buffered saline (pH 7.07) for 15-20 min when observed in light scattering. Then precipitate was added dropwise to neurons in DMEM. Following a 20-30 min transfection, during which a fine sandy precipitate covered the neurons, the cultures were washed 3-4 times until the precipitate disappeared in DMEM and then returned to the saved original culture media. Transfection efficiency via this method was typically between 1% and 5%, and there was no apparent toxicity to the cells. As early as 12 hr posttransfection the product of transfected gene could be detected.
Immunoblotting
Respectively transfected cortical cultures were harvested at 5 DIV with lysis buffer containing TBS, 1% Triton X-100, 10% glycerol, 1-mM PMSF, 1-μg/ml leupeptin, and 500-μM Na3VO4 on ice. Protein concentration was determined by BCA assay. Of the total protein, 10 μg from each was loaded onto SDS/PAGE and transferred to nitrocellulose. Blots were incubated in blocking buffer as follows: 5% BSA in TBST (0.1% Triton X-100) at 4°C overnight (CaMKIIβ, 1:1000). HRP-conjugated secondary antibodies were diluted in blocking buffer and visualized by chemiluminescence (Pierce).
Immunocytochemistry
Cultured cortical neurons were fixed with 4% paraformaldehyde (PFA; J.T. Baker) and 4% sucrose (Sigma-Aldrich) in 1 × phosphate buffer solution (PBS) for 15 min at room temperature at 5 DIV. Cultures were blocked with 3% bovine serum albumin (BSA; Fisher), 0.3% Triton X-100 (Sigma-Aldrich), 0.02% sodium azide (Sigma-Aldrich) in PBS for 2 hr at room temperature, then incubated with primary antibody GFP (1:1000; Molecular Probes), CaMKIIβ (1:250; Zymed) at 4°C overnight. F-actin was labeled with Alexa 488 or 568 conjugated phalloidin (1:25; Molecular Probes) to identify the microspike structures. Cell nuclei were stained with Hoechst. 25 × 75 mm glass micro slides (VWR) were used to mount coverslips with Aquamount (VWR) and sealed with fingernail polish.
Images used to quantify microspike/soma ratio were acquired from at least 3 independent experiments by using an Axiovert200 Zeiss fluorescent microscope with 40 × (for cell counts) or 63 × (for ratio measurement) objective. The mean intensities of GFP in three randomly chosen microspikes from one cell and the soma were measured and analyzed blind on GFP and phalloidin labeled cells with Improvision software Openlab. The ratio was calculated by (mean intensity in the microspike)/(mean intensity in the soma). The ratio values of three microspikes were averaged to generate one value per cell from 15-30 cells per construct.
Fluorescence recovery after photobleaching (FRAP)
E18 cortical neurons were cultured on 35-mm glass bottom dishes (MatTek) at 0.75 × 106 cells/dish and imaged at 5 DIV. Before imaging, media was replaced with Culture External Base which containing 2-mM MgCl2, 2-mM CaCl2, 150-mM NaCl, 2.5-mM KCl, 10-mM glucose, and 10-mM NaHEPES. Neurons were maintained in Culture External Base at room temperature during imaging for a maximum time of two hours. Images were taken with a LSM510Meta Zeiss confocal microscope by using 63 × objective at 16 × zoom. Images were captured every 1 sec for 200 sec without any treatment or 500 s when cells were incubated with 10-μg/ml Cytochalasin D for 30 min or 1-μM Jasplakinolide for 1 hr before imaging to stabilize actin filaments.
A circular region of interest (ROI) with radius of 0.43 μm was chosen for photobleaching. Neurons were photobleached after 10 frames of imaging with 10-12 iterations (20 iterations for GFP alone) of maximal excitation power. Background fluorescence, Fbkgd was determined in an unbleached area with similar initial fluorescence as the bleached ROI. F(t)ROI was normalized by background fluorescence with an equation of F(t)norm = (F(t)ROI/Fbkgd. The first time-point after the bleach was set to t = 0. The normalized fluorescence of the frame immediately before photobleaching, F(-1)norm, was set as 1. The fluorescence at other time points were normalized by F(-1)norm to generate the final fluorescence value, F(t)final = F(t)norm/F(-1)norm. Final fluorescence was plotted over time to generate the fluorescence recovery curve. When F(t)final reached 1, it was considered to have complete recovery. The 50% recovery time was then determined by the time required to reach 50% of final recovered fluorescence, F1/2 = (F(∞)-F (0))/2.
Statistical analysis
Data on all parameters were expressed as group mean ± SD. Differences between the experimental groups were analyzed by using the Student’s t-test. In all of the analyses, results were considered statistically significant when P < 0.05.
Results
WT CaMKIIβ colocalized with the actin cytoskeleton stronger than the other variants
The mGFP fusion proteins of WT CaMKIIβ and its splice variants CaMKIIβ’, CaMKIIβe and CaMKIIβ’e were expressed in cultured E-18 cortical neurons. Confocal images were taken at 4 DIV to observe mGFP fusion proteins (green) and fluorescent conjugated phalloidin (red) labeled F-actin. Examples of transfected and immunostained cortical neurons were shown in Figure 2A. Colocalization can be seen as yellow in the merged images.
Figure 2.
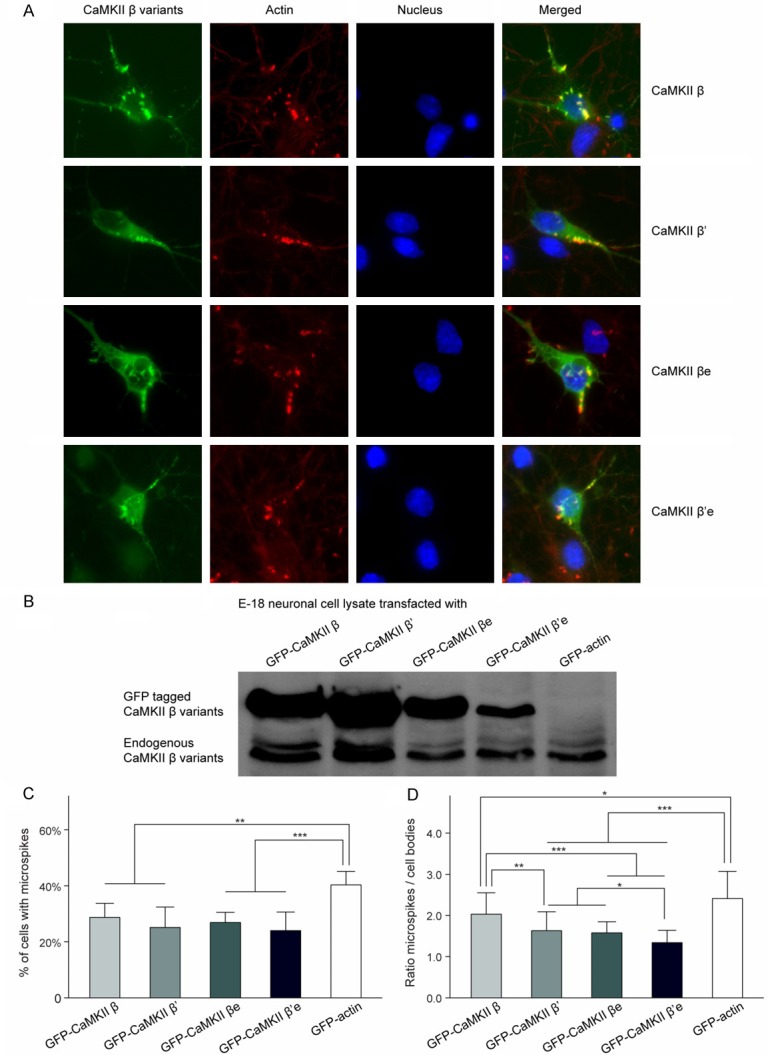
Colocalization of CaMKIIβ variants with F-actin in microspikes. A. CaMKIIβ variants preferentially localized to F-actin-rich structures: Confocal images of E-18 cortical neurons at 4 DIV with mGFP tagged CaMKIIβ variants (green) and F-actin labeled with fluorescent conjugated phalloidin (red). Colocalization can be seen as yellow in the merged images. B. Enrichment of GFP-tagged proteins as indicated in microspikes (ratio of microspike/soma mean intensity) *P < 0.05; ***P < 0.001; compared with GFP-actin. ##P < 0.01; ###P < 0.001; compared with CaMKIIβ. @P < 0.05; compared with CaMKIIβ’. %P < 0.05; compared with CaMKIIβe. C. Microspikes-containing cells percentage of respectively transfected E-18 cortical neurons. **P < 0.01; ***P < 0.001; compared with GFP-actin. D. Western blot analysis of E-18 cortical neuronal culture lysates 5 DIV after mGFP tagged CaMKIIβ variants transfection (upper panel). Endogenous CaMKIIβ were identified in cultured E-18 cortical neurons (lower panel).
No visible difference was observed between WT CaMKIIβ and the splice variants CaMKIIβ’, CaMKIIβe, and CaMKIIβ’e of their subcellular localization. All the three splice variants were observed mainly localized in the cytoplasm and processes, and displayed highly enriched colocalization with microspikes like WT CaMKIIβ as previously reported [16,17]. No significant differences among their colocalization patterns with microspikes in the E-18 cortical neurons could be determined with naked eyes through the microscope (Figure 2A).
To further quantitatively measure the F-actin colocalization of CaMKIIβ variants, we examined their subcellular localization by determining the enrichment (ratio of microspike/soma mean intensity) of these GFP-tagged variants in microspikes (Figure 2B). The enrichment of WT CaMKIIβ (2.03 ± 0.52) in microspikes was significantly higher than all the three variants, β’ (1.63 ± 0.46), βe (1.58 ± 0.27) and β’e (1.34 ± 0.30). The enrichment of WT CaMKIIβ in microspikes happened where F-actin was enriched, indicated strong colocalization of WT CaMKIIβ with F-actin and suggested a specific interaction between them. Although CaMKIIβ’, CaMKIIβe and CaMKIIβ’e were also localized to microspikes, they were significantly less enriched in the microspikes than WT CaMKIIβ. Among all the three variants, CaMKIIβ’e showed the lowest colocalization ratio with F-actin-based-structure microspikes which close to the non-binding GFP control (data not shown), indicating that CaMKIIβ’e had a very limited binding capacity with F-actin. Together, these data indicated that WT CaMKIIβ had the strongest association with actin, whereas CaMKIIβ’e had the weakest.
Our previous results showed that the binding ability of CaMKIIβ to F-actin was important for the formation and maintenance of microspikes [16]. To establish whether alternative splicing of CaMKIIβ affects microspikes formation or stability, we investigated the prevalence of the microspikes in CaMKIIβ variants transfected E-18 cortical neurons by calculating the percentage of microspike-containing cells. Our results showed that 28.7 ± 5.0% WT CaMKIIβ transfected neurons contained microspikes, which was significantly less compared to the 40.3 ± 4.8% of GFP-actin control transfected neurons (P < 0.05). However, no statistical significance was observed among the percentages of cells contained microspikes of WT CaMKIIβ and the other splice variants, β’ (25.1 ± 7.3%), βe (26.9 ± 3.6%) and β’e (24.0 ± 6.6%), although β had the highest percentage of microspike-containing cells than all the three variants (Figure 2C). Noticeablely, endogenous CaMKIIβ was co-expressed with the transfected GFP tagged CaMKIIβ variants in E-18 cortical neurons (Figure 2D), thus the ability of endogenous CaMKIIβ binding to F-actin might affect the number of microspikes-containing cells and resulted in no significant difference between all CaMKIIβ variants.
CaMKIIβ variants were less mobile within E-18 cortical neurons than CaMKIIα
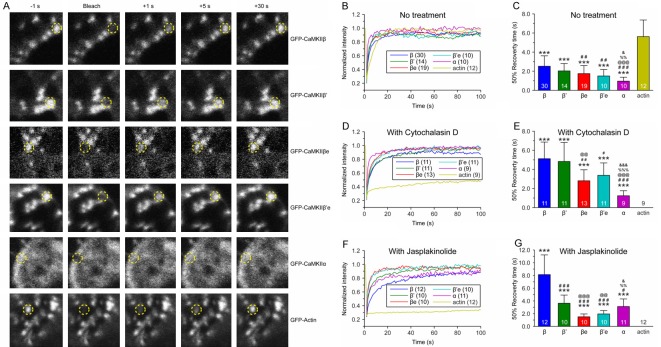
Fluorescence recovery after photobleaching (FRAP) analysis technology was employed to further investigate the strength and dynamics of CaMKIIβ splice variants and actin interaction specifically in the microspikes of live E-18 cortical neurons. A randomly selected single microspike from each E-18 cortical neuron transfected with mGFP fusion CaMKIIβ variants was photobleached at 5 DIV. The microspikes in neurons transfected with CaMKIIα and GFP-actin served as controls. Live cell images at 1 sec before bleach (-1 sec), bleach (0 sec), and 1, 5 and 30 sec after bleach (+ 1 sec, + 5 sec and + 30 sec, respectively) were chosen to represent the FRAP process (Figure 3A). Quantitative estimates of the association and dissociation rates of binding can be obtained from the FRAP [22-25].
Figure 3.
FRAP results Recovery patterns of GFP-CaMKIIβ variants, GFP-CaMKIIα and GFP-actin from photobleaching. A. Live confocal images of GFP-CaMKIIβ variants transfected neuronsneuron before bleaching (-1 sec), at bleach time 0 sec, and + 1 sec, + 5 sec and + 30 sec after bleaching were shown as examples, as well as control group GFP-CaMKIIα and GFP-actin. GFP tagged protein enriched areas can be seen as white pixels. Regions of interest (ROI) to be bleached were chosen from fluorescently intense areas (marked with yellow dashed circles). B. Fluorescence recovery curves of GFP-CaMKIIα, GFP-CaMKIIβ, β’, βe and β’e and GFP-actin without any treatment. C. Time required for the fluorescence of GFP-CaMKIIα, GFP-CaMKIIβ, β’, βe and β’e and GFP-actin to reache half-maximal recovery (50% recovery time). D. Fluorescence recovery patterns of GFP-CaMKIIα, GFP-CaMKIIβ, β’, βe and β’e and GFP-actin with Cytochalasin D treatment. E. 50% Recovery time of fluorescence of GFP-CaMKIIα, GFP-CaMKIIβ variants and GFP-actinafter Cytochalasin D treatment. F. Fluorescence recovery curves of GFP-CaMKIIα, GFPCaMKIIβ variants and GFP-actin with Jasplakinolide treatment. G. 50% Recovery time after Jasplakinolide treatment. ***p < 0.001; compared with GFP-actin. #P < 0.05; ##P < 0.01; ###P < 0.001; compared with CaMKIIβ. @@P < 0.01; @@@P < 0.001; compared with CaMKIIβ’. %%P < 0.01; %%%P < 0.001; compared with CaMKIIβe. &P < 0.05; &&&P < 0.001; compared with CaMKIIβ’e.
The recorded intensities at each time point were calculated as described in methods and pulled together to generate the recovery curve which reflected the molecular diffusion and binding dynamics. The recovery curve (Figure 3B) demonstrated that the recovery of CaMKIIβ’, CaMKIIβe and CaMKIIβ’e were barely distinguishable from each other but were noticeably faster than WT CaMKIIβ. It was also found that the GFP-actin control group regained full recovery much slower than all the CaMKIIβ variants, and the non-binding CaMKIIα control showed the fastest recovery than all the other groups.
Each group’s 50% recovery time was then calculated to further assess the recovery efficiency of each variants as well as controls (Figure 3C). Among all groups, GFP-actin control presented a standard level of how the actin fluorescence intensity recovered in this F-actin-rich structure. The 50% recovery time of CaMKIIα (5.62 ± 1.74 sec) was significant longer (P < 0.001) than all the CaMKIIβ variants and CaMKIIβ. CaMKIIα was the fastest to regain 50% recovery of its original fluorescence intensity at 0.96 ± 0.41 sec. This is consistent with previous reports that CaMKIIα did not directly bind to F-actin [15,16,26,27] and consequently more mobile than all CaMKIIβ variants within the E-18 cortical neurons. The 50% recovery time of WT CaMKIIβ (2.52 ± 1.09 sec) was almost two and half folds longer than non-binding CaMKIIα, indicating that the mobility of WT CaMKIIβ was restricted by binding to F-acin within the microspikes as reported [16,17]. The extended 50% recovery time in WT CaMKIIβ was revealed compared with variant CaMKIIβ’ (2.03 ± 0.77 sec), CaMKIIβe (1.75 ± 0.84 sec) and CaMKIIβ’e (1.50 ± 0.68 sec). Statistical significances were identified between CaMKIIβ and CaMKIIβe (P < 0.01), CaMKIIβ and CaMKIIβ’e (P < 0.01). CaMKIIβ’ did not present any significant difference in 50% recovery time compare to CaMKIIβe and CaMKIIβ’e, although its 50% recovery time was longer. The slower recovery after photobleaching suggested the presence of a binding partner restricted the mobility of CaMKIIβ variants or controls. These results suggested that all CaMKIIβ variants displayed a stronger binding ability with F-actin than CaMKIIα, although not as strong as that of WT CaMKIIβ.
CaMKIIβ presented stronger association with immobilized F-actin than all other variants
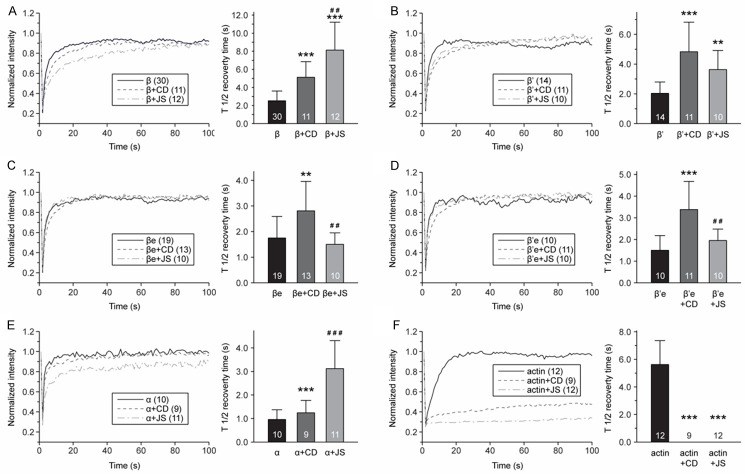
The continuous dynamic of both CaMKIIβ and actin existed in live E-18 cortical neurons makes it complicated to understand the interactions between them. Two drugs, Cytochalasin D and Jasplakinolide, were introduced to the system to inhibit actin movement. Cytochalasin D was reported to notably slow down but not completely prevent the recovery of actin, whereas Jasplakinolide completely immobilized actin, preventing any recovery of actin from photobleaching [16,28]. With the immobilized actin within live E-18 cortical neurons, it is easier to explore as well as interpret the FRAP results of the CaMKIIβ variants, which may have potential interactions with actin.
We sorted the Cytochalasin D or Jasplakinolide treated FRAP results of CaMKIIβ variants data together to build respective recovery curves (Figure 3D and 3F), and calculated their 50% recovery time (Figure 3E-G). GFP-actin group under respective drug treatments was set up as standard control for actin recovery rates. It presented significantly slower recovery compared to all CaMKIIβ variants groups with either Cytochalasin D or Jasplakinolide treatment, which strongly indicated that there was significant pool of CaMKIIβ variants which was not bound with the immobilized actin and remained mobile within the live cells. Because under either Cytochalasin D or Jasplakinolide, GFP-actins never regained their 50% recovery, no 50% recovery time result was shown for actin group in Figure 3E and 3G. CaMKIIβe and β’e recovery curves remained the same with either Cytochalisin D or Jasplakinolide treatment, and their 50% recovery time were significantly shorter compared to drug treated WT CaMKIIβ and CaMKIIβ’. These results indicated that the association of WT CaMKIIβ and CaMKIIβ’ with the F-actin were stronger than those of CaMKIIβe and CaMKIIβ’e. The FRAP recovery curve of WT CaMKIIβ and CaMKIIβ’ (Figure 3F) were separated with Jasplakionlide treatment but not with non-treatment nor with Cytochalisin D treatment (Figure 3B). Moreover, the 50% recovery time of WT CaMKIIβ (8.13 ± 3.09 sec) was almost one fold extended than CaMKIIβ’ (5.12 ± 1.74 sec) (Figure 3G), suggested that WT CaMKIIβ had stronger binding with F-actin than CaMKIIβ’.
WT CaMKIIβ displayed slower recovery with either Cytochalasin D or Jasplakinolide (Figure 4A), suggesting that it had strong binding with F-actin and hence took longer time for the bound ones to disassociate from the immobilized actin. CaMKIIβ’ also displayed similar results (Figure 4B), although not as significant as WT CaMKIIβ. CaMKIIβe and CaMKIIβ’e did not show a difference between Jasplakinolide treatment and non-treatment control (Figure 4C and 4D). Jasplakinolide was reported to completely immobilize the actin [28,29], and our GFP-actin with Jasplakinolide treatment also displayed the same result as the actin did not recover at all (Figure 4E). The completely immobilized F-acin did not affect the recovery of CaMKIIβe and CaMKIIβ’e, which indicated that these two variants did not bind to F-actin, otherwise delayed recovery curve and extended 50% recovery time would be noticed, as discovered in WT CaMKIIβ and CaMKIIβ’. GFP-actin control displayed different recovery patterns for actin under Cytochalasin D or Jasplakinolide treatment (Figure 4F) which was consistent with previous reports [16,28], and this might be responsible for the differences between Cytochalasin D and Jasplakinolide in most groups. Not surprisingly, non-binding control CaMKIIα also showed remarkable slow recovery curve when treated with Jasplakinolide (Figure 4E) as similar results were reported [16].
Figure 4.
Comparison of the effects with Cytochalasin D or Jasplakinolide treatment and without treatment in each GFP-CaMKIIβ variants groups, GFP-CaMKIIα and FPG-actin group. Fluorescence recovery patterns under non-treatment, with Cytochalasin D and Jasplakinolide treatment of WT CaMKIIβ (A), CaMKIIβ’ (B), CaMKIIβe (C) and CaMKIIβ’e (D), GFP-CaMKIIα (E) and GFP-actin (F). The recovery curves are shown on the left panels and the 50% recovery time plots are illustrated on the right. ***P < 0.001; compared with non-treatment. ##P < 0.01; ###P < 0.001; compared with under Cytochalasin D treatment.
Discussion
Some previous studies examined F-actin and CaMKIIβ interactions in nonneuronal cells or in dendrite spines where endogenous CaMKIIα and CaMKIIβ were both expressed [13-15,27]. F-actin was found to form thick bundled structures in vitro in the presence of the β-subunit of CaMKII but not in the presence of the α-subunit [15-17,27,30]. Protein structural studies indicate that CaMKII is an oligomer composed of 10-14 monomers arranged in rotational symmetry [31]. The CaMKIIα and β subunits coexist in neurons can associate and form hetero-oligomers, which may bind simultaneously to different actin filaments through multiple β-subunits and, thus may confer on CaMKII with the ability to bundle actin filaments together. In addition, CaMKIIα was addressed to have negative effect on CaMKIIβ and F-actin association [16], and therefore might affect the related binding outcomes. In this study, we performed in vivo analyses of dynamic molecular interactions in rat E-18 cortical neurons to explore how alternative splicing in the variable domain of CaMKIIβ affects the actin colocalization and association capability of CaMKIIβ variants in “microspikes”, a specific F-actin-rich structure previously identified and termed by our group [16,17]. CaMKIIα was not expressed at the age examined, while CaMKIIβ strongly colocalized with F-actin within microspikes in E-18 cortical neurons [16]. Therefore, potential interactions between endogenous CaMKIIα and CaMKIIβ variants were eliminated and we can further explore the possible structural, rather than enzymatic, role of CaMKIIβ variants and their interactions with actin in developing neurons without the disturbance of CaMKIIα.
WT CaMKIIβ contains the exons E1, E3 and E4 in its variable domain that CaMKIIα isoform doesn’t have. Correspondingly, WT CaMKIIβ but not CaMKIIα was proved to bind to F-actin [16]. Meanwhile, with the alternative splicing of exons E1 and E4 in the variable region, three CaMKIIβ variants, CaMKIIβ’, CaMKIIβe and CaMKIIβ’e, were produced in addition to WT CaMKIIβ [19]. The alternative splicing of CaMKIIβ mRNA is developmentally regulated and there appears to be a switch from the embryonic isoforms to the adult isoforms. In rat cortical neurons, CaMKIIβ and CaMKIIβ’ are the major expressed adult isoforms. CaMKIIβe and CaMKIIβ’e are embryonic isoforms expressed at high levels from birth until postnatal day 4 (P4) [19,32,33]. Notably, each of the new isoforms displayed identical protein kinase activity characteristic of WT CaMKIIβ (showed normal Ca2+/calmodulin-dependent phosphorylation of the specific CaMKII kinase) [19].However, their kinase independent actin binding ability [16] had not been explored in cortical neurons yet. We hypothesized that alternative splicing of exons E1, E3 and/or E4 may be necessary and serve as a developmental switch for the isoform-specific binding ability with F-actin, which plays an essential role in the process of neuronal maturation. Therefore, WT CaMKIIβ (contains E1, E3 and E4), CaMKIIβ’ (contains E1 and E3), CaMKIIβe (contains E3 and E4) as well as CaMKIIβ’e (contains E3 only) were investigated for their colocalization and association with microspikes in E-18 cortical neurons to determine the effect of alternative splicing in variable domain exons on their F-actin binding ability.
The measurement of CaMKIIβ varaints’ colocalization with actin allowed us to evaluate the respective binding ability in the fixed cells where both CaMKIIβ variants and actin were immobilized. In this condition, the intensity of colocalization should be consistent with the corresponding F-actin binding ability. The stronger colocalization represents the stronger binding with F-actin. Both variants WT CaMKIIβ and CaMKIIβ’ containing E1 presented higher colocalization ratios than the other two non-E1 variants CaMKIIβe and CaMKIIβ’e, indicating that E1 is essential for CaMKIIβ association with F-actin. The FRAP performed in live neurons allowed us to explore the interactions between CaMKIIβ variants and F-actin where both of them are dynamic. Furthermore, immobilization actin with drugs (Cytochalasin D or Jasplakinolide) helped us better understand the relative mobility and transferability of CaMKIIβ variants. If a molecule has a strong association with F-actin, it will take longer time for the bound ones to disassociate from the immobilized actin after the diffusion of limited free molecules. Consistent with the colocalization data, the significantly slowed recovery of WT CaMKIIβ and CaMKIIβ’ after the drug treatment indicates that exon E1 is responsible for the significant pool of remained persistently bound to the immobilized F-actin.
We noticed that all alternative splicing CaMKIIβ variants contain E3 yet they displayed significant enrichment differences within the microspikes. Besides, CaMKIIβ’e, which contains only E3, showed the lowest enrichment within F-actin rich microspikes. It suggested that possessing E3 alone was not enough for the association of CaMKIIβ variants with F-actin. The same conclusion can be drawn from the FRAP results of CaMKIIβ’e which showed a significantly faster recovery than CaMKIIβ’ (containing E1 and E3) under either Cytochalasin D or Jasplakinolide treatment.
CaMKIIβe, the dominant splice variant before birth [19], contains E4 in addition to E3. However, no significant difference of colocalization with microspikes was identified between CaMKIIβe and CaMKIIβ’e. FRAP recovery curves of CaMKIIβe and CaMKIIβ’e were almost identical to each other under all circumstances and no significant difference was observed for 50% recovery time between these two variants. These data indicated that E4 may not be sufficient enough to target CaMKIIβe to F-actin either. However, CaMKIIβ’, which only lacks E4 compare to WT CaMKIIβ, showed decreased colocalization within the microspikes, as well as faster FRAP recovery curve and significantly shorter 50% recovery time in most circumstances. These data referred that CaMKIIβ’ has decreased binding ability compared to WT CaMKIIβ, which suggested that the co-existence of E1 and E4 was needed for CaMKIIβ to better association with F-actin.
Interestingly, GFP-CaMKIIα also recovered more slowly from immobilized F-actin with drug treatments. Although we cannot rule out other mechanisms that may influence CaMKIIα mobility, the slowed recovery of GFP-CaMKIIα from immobilized F-actin is most likely due to CaMKIIα hetero-oligomerization via its association domain with endogenous CaMKIIβ [34], which was reported to co-assemble with non-actin binding isoforms and target such heteromeric holoenzymes to the actin cytoskeleton [26]. Oligomerization of CaMKIIβ, whether homo-oligomers or hetero-oligomers, is essential for actin binding, while monomeric CaMKIIβ can not bind to F-actin [35]. In CaMKIIα/β hetero-oligomers, the existence of CaMKIIα would decrease the ability of endogenous CaMKIIβ binding with F-actin [16], whereas homo-oligomers CaMKIIβe and CaMKIIβ’e did not binding to the F-actin, and did not affect the endogenous CaMKIIβ binding either. Therefore, CaMKIIβe and CaMKIIβ’e displayed slower recovery time than CaMKIIα.
Briefly, our results are consistent with a previous report performed on Cos 7 cells in vitro [27]. These results together clearly indicated that in the E-18 cortical neurons in the absence of CaMKIIα, the variable region exon E1, which is lacked in CaMKIIβe and CaMKIIβ’e, is necessary for localization of actin cytoskeleton within cells. CaMKIIβ’e containing only exon E3 in the variable region had no binding ability with F-actin. Meanwhile, since CaMKIIβe which contains exon E4 only was not able to bind to F-actin, while the variant CaMKIIβ’ containing E1 only showed a decreased actin binding ability compared to WT CaMKIIβ, suggesting that E4 may help E1 better associate with F-actin.
The actin cytoskeleton is remarkably dynamic and involved in many major cellular events such as LTP related synaptic plasticity [36], which may be differentially modulated via alternative splice of CaMKII isoforms with different actin association abilities [12,13,15-17,27]. Within living cells, there exsits a dynamic equilibrium between the monomeric form of globular-actin (G-actin) and the filamentous form of F-actin. It was reported that LTP could shift this F-actin/G-actin equilibrium toward F-actin, suggesting that modification of the F-actin/G-actin equilibrium was required to trigger the morphological reorganization during LTP [4]. The resemblance between the F-actin-rich microspikes we explored in embryonic neurons and the new F-actin complex formed at the dendritic spine during LTP hints that these F-actin-rich cellular structures also serve both as a scaffold for the mechanical stability of spine structure and as a major docking site for postsynaptic proteins that directly and indirectly bind to F-actin as reported [37,38]. This mechanism may be used to selectively captures the LTP-related proteins synthesized in the cell body and transported into dendrites [12]. In consideration that the binding ability of CaMKIIβ to F-actin was essential in mediating and regulating this F-actin-rich structure [16], The alternative splicing of CaMKIIβ variants in developing neurons which lead to differences in actin binding ability may serve as a developmental switch for actin cytoskeleton-associated isoforms and therefore correlates with dendritic arborization and synapse formation during LTP.
Acknowledgements
This work was supported by the Georgia Regants University (formerly Medical College of Georgia) and a National Institutes of Health grant to L.R.
References
- 1.Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- 2.Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- 3.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okamoto K, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat Neurosci. 2004;7:1104–1112. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- 5.Kelly PT. Calmodulin-dependent protein kinase II. Multifunctional roles in neuronal differentiation and synaptic plasticity. Mol Neurobiol. 1991;5:153–177. doi: 10.1007/BF02935544. [DOI] [PubMed] [Google Scholar]
- 6.Schulman H, Hanson PI. Multifunctional Ca2+/calmodulin-dependent protein kinase. Neurochem Res. 1993;18:65–77. doi: 10.1007/BF00966924. [DOI] [PubMed] [Google Scholar]
- 7.Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- 8.Erondu NE, Kennedy MB. Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain. J Neurosci. 1985;5:3270–3277. doi: 10.1523/JNEUROSCI.05-12-03270.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudmon A, Schulman H. Neuronal CA2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu Rev Biochem. 2002;71:473–510. doi: 10.1146/annurev.biochem.71.110601.135410. [DOI] [PubMed] [Google Scholar]
- 10.Peng J, Kim MJ, Cheng D, Duong DM, Gygi SP, Sheng M. Semiquantitative proteomic analysis of rat forebrain postsynaptic density fractions by mass spectrometry. J Biol Chem. 2004;279:21003–21011. doi: 10.1074/jbc.M400103200. [DOI] [PubMed] [Google Scholar]
- 11.Feng B, Raghavachari S, Lisman J. Quantitative estimates of the cytoplasmic, PSD, and NMDAR-bound pools of CaMKII in dendritic spines. Brain Res. 2011;1419:46–52. doi: 10.1016/j.brainres.2011.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okamoto K, Bosch M, Hayashi Y. The roles of CaMKII and F-actin in the structural plasticity of dendritic spines: a potential molecular identity of a synaptic tag? Physiology (Bethesda) 2009;24:357–366. doi: 10.1152/physiol.00029.2009. [DOI] [PubMed] [Google Scholar]
- 13.Fink CC, Bayer KU, Myers JW, Ferrell JE Jr, Schulman H, Meyer T. Selective regulation of neurite extension and synapse formation by the beta but not the alpha isoform of CaMKII. Neuron. 2003;39:283–297. doi: 10.1016/s0896-6273(03)00428-8. [DOI] [PubMed] [Google Scholar]
- 14.Shen K, Meyer T. Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science. 1999;284:162–166. doi: 10.1126/science.284.5411.162. [DOI] [PubMed] [Google Scholar]
- 15.Okamoto K, Narayanan R, Lee SH, Murata K, Hayashi Y. The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proc Natl Acad Sci U S A. 2007;104:6418–6423. doi: 10.1073/pnas.0701656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin YC, Redmond L. CaMKIIbeta binding to stable F-actin in vivo regulates F-actin filament stability. Proc Natl Acad Sci U S A. 2008;105:15791–15796. doi: 10.1073/pnas.0804399105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin YC, Redmond L. Neuronal CaMKII acts as a structural kinase. Commun Integr Biol. 2009;2:40–41. doi: 10.4161/cib.2.1.7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tombes RM, Faison MO, Turbeville JM. Organization and evolution of multifunctional Ca(2+)/CaM-dependent protein kinase genes. Gene. 2003;322:17–31. doi: 10.1016/j.gene.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Brocke L, Srinivasan M, Schulman H. Developmental and regional expression of multifunctional Ca2+/calmodulin-dependent protein kinase isoforms in rat brain. J Neurosci. 1995;15:6797–6808. doi: 10.1523/JNEUROSCI.15-10-06797.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redmond L, Kashani AH, Ghosh A. Calcium regulation of dendritic growth via CaM kinase IV and CREB-mediated transcription. Neuron. 2002;34:999–1010. doi: 10.1016/s0896-6273(02)00737-7. [DOI] [PubMed] [Google Scholar]
- 21.Threadgill R, Bobb K, Ghosh A. Regulation of dendritic growth and remodeling by Rho, Rac, and Cdc42. Neuron. 1997;19:625–634. doi: 10.1016/s0896-6273(00)80376-1. [DOI] [PubMed] [Google Scholar]
- 22.Sprague BL, McNally JG. FRAP analysis of binding: proper and fitting. Trends Cell Biol. 2005;15:84–91. doi: 10.1016/j.tcb.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 23.McNally JG. Quantitative FRAP in analysis of molecular binding dynamics in vivo. Methods Cell Biol. 2008;85:329–351. doi: 10.1016/S0091-679X(08)85014-5. [DOI] [PubMed] [Google Scholar]
- 24.Carrero G, McDonald D, Crawford E, de Vries G, Hendzel MJ. Using FRAP and mathematical modeling to determine the in vivo kinetics of nuclear proteins. Methods. 2003;29:14–28. doi: 10.1016/s1046-2023(02)00288-8. [DOI] [PubMed] [Google Scholar]
- 25.Pucadyil TJ, Chattopadhyay A. Confocal fluorescence recovery after photobleaching of green fluorescent protein in solution. J Fluoresc. 2006;16:87–94. doi: 10.1007/s10895-005-0019-y. [DOI] [PubMed] [Google Scholar]
- 26.Shen K, Teruel MN, Subramanian K, Meyer T. CaMKIIbeta functions as an F-actin targeting module that localizes CaMKIIalpha/beta heterooligomers to dendritic spines. Neuron. 1998;21:593–606. doi: 10.1016/s0896-6273(00)80569-3. [DOI] [PubMed] [Google Scholar]
- 27.O’Leary H, Lasda E, Bayer KU. CaMKIIbeta association with the actin cytoskeleton is regulated by alternative splicing. Mol Biol Cell. 2006;17:4656–4665. doi: 10.1091/mbc.E06-03-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Star EN, Kwiatkowski DJ, Murthy VN. Rapid turnover of actin in dendritic spines and its regulation by activity. Nat Neurosci. 2002;5:239–246. doi: 10.1038/nn811. [DOI] [PubMed] [Google Scholar]
- 29.Bubb MR, Spector I, Beyer BB, Fosen KM. Effects of jasplakinolide on the kinetics of actin polymerization. An explanation for certain in vivo observations. J Biol Chem. 2000;275:5163–5170. doi: 10.1074/jbc.275.7.5163. [DOI] [PubMed] [Google Scholar]
- 30.Sanabria H, Swulius MT, Kolodziej SJ, Liu J, Waxham MN. {beta}CaMKII regulates actin assembly and structure. J Biol Chem. 2009;284:9770–9780. doi: 10.1074/jbc.M809518200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoelz A, Nairn AC, Kuriyan J. Crystal structure of a tetradecameric assembly of the association domain of Ca2+/calmodulin-dependent kinase II. Mol Cell. 2003;11:1241–1251. doi: 10.1016/s1097-2765(03)00171-0. [DOI] [PubMed] [Google Scholar]
- 32.Burgin KE, Waxham MN, Rickling S, Westgate SA, Mobley WC, Kelly PT. In situ hybridization histochemistry of Ca2+/calmodulin-dependent protein kinase in developing rat brain. J Neurosci. 1990;10:1788–1798. doi: 10.1523/JNEUROSCI.10-06-01788.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bayer KU, Lohler J, Schulman H, Harbers K. Developmental expression of the CaM kinase II isoforms: ubiquitous gamma- and delta-CaM kinase II are the early isoforms and most abundant in the developing nervous system. Brain Res Mol Brain Res. 1999;70:147–154. doi: 10.1016/s0169-328x(99)00131-x. [DOI] [PubMed] [Google Scholar]
- 34.Colbran RJ. Targeting of calcium/calmodulin-dependent protein kinase II. Biochem J. 2004;378:1–16. doi: 10.1042/BJ20031547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brocke L, Chiang LW, Wagner PD, Schulman H. Functional implications of the subunit composition of neuronal CaM kinase II. J Biol Chem. 1999;274:22713–22722. doi: 10.1074/jbc.274.32.22713. [DOI] [PubMed] [Google Scholar]
- 36.Lamprecht R, LeDoux J. Structural plasticity and memory. Nat Rev Neurosci. 2004;5:45–54. doi: 10.1038/nrn1301. [DOI] [PubMed] [Google Scholar]
- 37.Dillon C, Goda Y. The actin cytoskeleton: integrating form and function at the synapse. Annu Rev Neurosci. 2005;28:25–55. doi: 10.1146/annurev.neuro.28.061604.135757. [DOI] [PubMed] [Google Scholar]
- 38.Cingolani LA, Goda Y. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci. 2008;9:344–356. doi: 10.1038/nrn2373. [DOI] [PubMed] [Google Scholar]