Abstract
Lipoblastoma is a rare benign mesenchymal tumor that composes of embryonal white fat tissue and typically occurs in infants or young children under 3 years of age. It usually affects the extremities, trunk, head, and neck. The perineum is a rare location with only 7 cases reported in the literature. We describe a case of 3-year-old girl with a lipoblastoma arising from perineum. An approximately 4.5 cm × 3.5 cm × 2.5 cm nodule was resected in left perineum with satisfied results. Pathological examination showed that it was composed of small lobules of mature and immature fat cells, separated by fibrous septa containing small dilated blood vessels. The left perineal lipoblastoma, although rare, should be differentiated from some other mesenchymal tumors with similar histologic and cytological features.
Keywords: Perineum, lipoblastoma, myxoid liposarcoma, childhood, pathology
Introduction
Lipoblastoma was first described by Jaffe in 1926 [1]. It was not identified as a distinctive tumor until Vellios et al. discovered that the adipose tissue of three-week embryos which disappeared later in foetal development that was the basis for the pathogenesis of lipoblastoma in 1958 [2]. The localization of lipoblastomas is mostly in the extremities but may be also found in other less common sites including the head and neck, trunk, mediastinal, kidney, mesenterial, retroperitoneum and perineum [3], while perineum is a rare location of lipoblastomas with only 7 cases reported in the literature. The correct preoperative diagnosis of perineal lipoblastoma is difficult because of atypical symptom except a rapidly enlarging mass. It mainly depends on postoperative pathological examination and cytogenetics and molecular biology studies. The best surgical treatment is a complete resection including the edge of the tumor in case of recurrences, and long-term follow-up is recommended. We herein report a case of perineal lipoblastoma and review the previous reported cases, and then discuss the possible differential diagnosis.
Case report
A 3-year-old girl was admitted to the Department of General Surgery with a soft tissue mass in the left perineal region for more than 6 months. The swelling, painless mass was first noticed at 2-year old, but the lesion had a rapidly progressive course with asymptomatic over the next 6 months. There was no history of trauma, pain or discharge related to the swelling. She denied any relevant previous or family history. On physical examination, there was a nontender, soft, rounded, perineal mass measuring approximately 3 cm × 2 cm × 2 cm. No overlying skin changes and vaginal discharge were noted. Abdominal and rectal examinations were unremarkable. Computerized tomography (CT) scan revealed a well-defined soft tissue mass and low density tissue (-35 HU~-90 HU), which suggested lipoma (Figure 1). With a presumed diagnosis of lipoblastoma the patient was taken.
Figure 1.
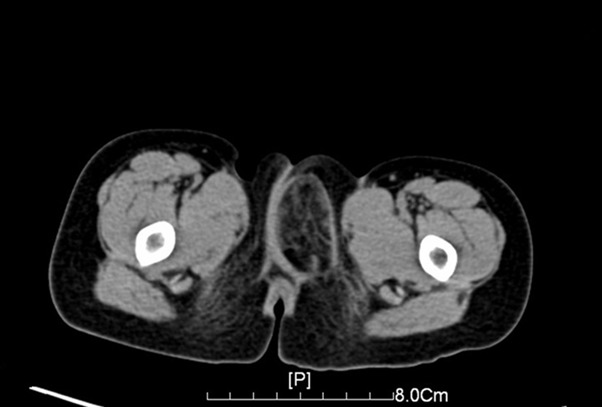
Computerized tomography (CT) revealed a well-defined soft tissue mass and low density tissue (-35 HU~-90 HU).
At laparotomy, a lobulated, yellow, solid fatty mass was identified and gross total resection was performed. The excised mass was approximately 4.5 cm × 3.5 cm × 2.5 cm (Figure 2). Pathological examination, the resected specimen was composed of small lobules of mature and immature fat cells, separated by fibrous septa containing small blood dilated vessels (Figure 3). Immunohistochemically, these small lobules of fat cells showed diffusely positive for S-100 (Figure 4), CK and CD34 were negative. Based on pathological examination and immunohistochemistry, the diagnosis of lipoblastoma was rendered. Postoperative course was uneventful; the patient had an uncomplicated recovery and was free of disease at 2 years postoperative follow-up.
Figure 2.
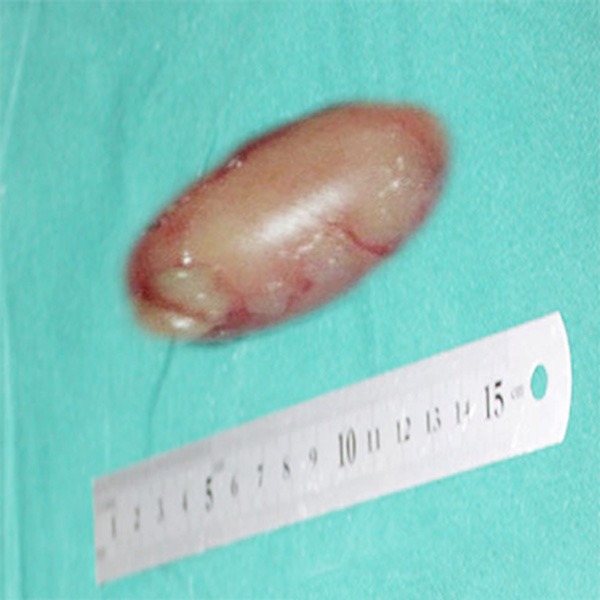
Gross appearance of the resected specimen, which measured 4.5 cm × 3.5 cm × 2.5 cm in left perineum region.
Figure 3.
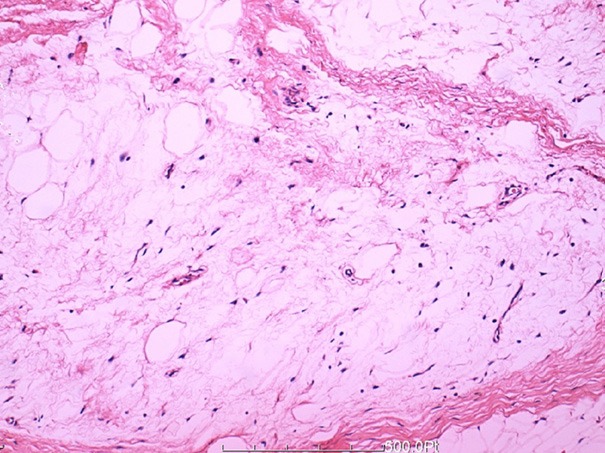
Histologic features of the lesion showed the tumor was composed of small lobules of mature and immature fat cells, separated by fibrous septa containing small blood dilated vessels (HE × 200).
Figure 4.
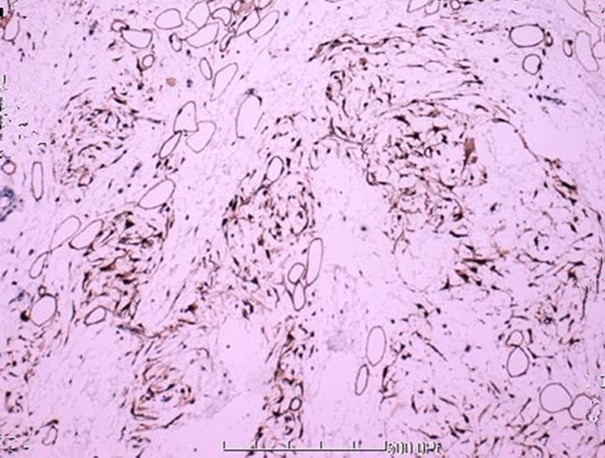
Tumor cells showing diffuse immunohistochemical positivity for S-100.
Discussion
Perineal lipoblastoma is an uncommon neoplasm of mesenchymal origin that primarily affects the extremities. It may occur elsewhere in the body including head and neck, trunk, mediastinum, kidney, mesenterial, retroperitoneum and perineum. Lipoblastoma was first described by Jaffe in 1926, since then, nearly 200 cases had been documented in the literature [4]. However, there are only 7 reported cases of lipoblastoma originating from the perineum (Table 1) [5-11].
Table 1.
Clinical date of perineal lipoblastomas in 7 patients previously reported in the literature
| No | Author | Year | Age/gender | Treatment | Size, cm | Recurrence |
|---|---|---|---|---|---|---|
| 1 | Tsai H | 1997 | 2 M/F | Excision | 4.0 × 2.0 × 1.5 | Not reported |
| 2 | Ito M | 2004 | 18 D/F | Excision | Not reported | No |
| 3 | Moreiro C | 2005 | 9 D/M | Excision | 3.0 | Not reported |
| 4 | Al-Momani | 2005 | 18 M/M | Excision | 4.0 × 0.12 | Yes |
| 5 | McVay MR | 2006 | 30 M/M | Excision | Not reported | Yes |
| 6 | Antoniou D | 2009 | 7 M/M | Excision | 3.5 × 3.0 | No |
| 7 | Ahn KH | 2010 | At birth/F | Excision | 3.0 × 2.0 × 2.1 | No |
M, months; D, days; F, female; M, male.
Lipoblastoma is a rare, benign mesenchymal tumor of fetal white fat tissue appearing most commonly in infancy and early childhood, and usually accounting for less than 1% of all childhood neoplasms [12]. It mostly occurs in infants or young children under 3 years of age. The morbidity of sex ratio is 3:2 male to female (4 cases were male in 7 patients). This type of neoplasm was presented in two different forms described by Chung and Enzinger in 1973. One is lipoblastoma which is a localized, capsular, and well-circumscribed, the other is lipoblastomatosis which is a multi-centric, noncapsulated, diffuse type with the tendency to infiltrate adjacent tissues [13]. Clinical presentation is asymptomatic except a slowly enlarging painless soft tissue mass. The patient suffered a rapidly progressive lesion with asymptomatic over the next 6 months, Computerized tomography (CT) image suggested lipoma, which led then to the misdiagnosis. We resected the mass, and diagnosed the tumor as the lipoblastoma by postoperative pathological examination.
Many iconography examinations are available, but none of them can give a definitive diagnosis, they only suggest likely diagnosis as in our case. Ultrasound with color Doppler usually demonstrates single or multiple echogenic mass appearing as a solid lesion with vascularisation and a well-defined margin [14]. Computerized tomography (CT) scan reveals a well-defined soft tissue mass and low density tissue (-60 HU~-120 HU), which suggests fat [15]. It is difficult to distinguish this type of lipomatous tumor by CT, just as this patient. MRI is also of little use in diagnosis of lipomatous tumors. Only increased cellularity of lipoblastoma makes lower density on T1-weighted MRI images and can be helpful in differentiating from lipoma [16]. In a word, ultrasonography, computed tomography and MRI all provide useful information regarding the position, depth and anatomical relationships of the lesion and complement each other, but seldom, can make a definitive lipoblastoma diagnosis.
Although imaging examination will certainly help for lipoblastoma, definitive diagnosis mostly depend on postoperative pathological histological features. Moreover, the cytogenetics and molecular biology methods can be used in unclear complicated cases. General histological observation shows that grossly soft and smooth mass, well circumscribed and lobulated in lipoblastoma, the lobules are separated by thin fibrous tissue septa containing gaping, thick-walled blood vessels [17]. Besides, there are characteristic cytogenetic changes on chromosome 8. Translocations with chromosome 8 usually involve chromosomes 3, 7 or 8.
Lipoblastoma should be differentiated from some other tumors with similar histologic and cytological features: myxoid or well-differentiated liposarcomas, lipoma, hibernomas and fibrous hamartoma of infancy. At first, high morbidity of lipoblastoma are younger than 3 years of age in children, but liposarcomas are frequently found in adults, especially in third decennium, and are very rarely diagnosed in adolescents [18]. Besides, lipoblastoma is characterized by small lobules of mature and immature fat cells separated by fibrous septa with or without a myxoid stroma. In lipoblastomatosis the lobular pattern is less pronounced [19]. However, liposarcoma is lack of nuclear atypia and infiltrates the surrounding structures and has solid or streaky components. What is more, the chromosomal studies find out the detection of a rearrangement of 8q11-q13 in lipoblastomas, while the myxoid or well-differentiated liposarcomas are characterized by the translocation t(12;16)(q13;p11) [20]. Hibernomas is another soft tissue tumor with similar histologic and cytological features that may be confused with lipoblastoma. We can see the granular brown fat cells which have more intracytoplasmic vacuoles compared with lipoblastoma and a characteristic gray-brown, lobulated structure [21]. Fibrous hamartoma of infancy must also be considered in the differential diagnosis. It is composed of well-defined bundles of fibrous tissue and primitive mesenchyme arranged in nests, concentric whorls or bands, mature adipose tissue intimately mixed with other components may also be noted [22].
There are only 7 reported cases of lipoblastoma originating from the perineal region, but it should be differentiated from some other tumours originating from this region. The differential diagnoses of perineal mass includes hernias, infectious or inflammatory processes, congenital abnormalities, perianal sepsis, skin tags, condylomas, hymenal and paraurethral cysts, and vulvar hydroceles [23]. They can be easily differentiated from perineal lipoblastoma by its clinical presentation. Lesion of anogenital mammary-like glands (AGMLG) is another likely to be confused with perineal lipoblastoma. AGMLG, can be found in the anogenital area of both sexes, a newly recognized variant of cutaneous adnexal glands with characteristics of modified eccrine and apocrine glands. Furthermore, it show a typically prominent homology with lesions in the breast. So it plays an important role in distinguishing lesions of AGMLG from perineal lipoblastoma by the appearance of glandular structures and the histological homology to its counterpart breast lesions [24]. Some stromal tumors, such as angiomyofibroblastoma, aggressive angiomyxoma, cellular angiofibroma, leiomyosarcoma, rhabdomyosarcoma and epithelioid sarcoma, can be ruled out by their histopathological and immunohistochemical findings [25].
The treatment of lipoblastoma is complete surgical excision. Lipoblastoma and lipoblastomatosis are generally benign, but local recurrence may occur, particularly with lipoblastomatosis, when the excision is incomplete. However, we do not recommend an aggressive surgical approach. The recurrence rate is between 0 and 25% and the average period to recurrence is 3 years [26]. So the follow-up as long as possible is necessary. The patient reported was free of disease at 2 years postoperative follow-up.
In conclusion, there is no clear diagnostic criteria for lipoblastoma, Its diagnosis should be made by their microscopical and histopathological features. Lipoblastoma should be considered in the differential diagnosis of lesions with myxoid or well-differentiated liposarcomas, lipoma, hibernomas, fibrous hamartoma of infancy and some other tumors originating from mesenchymal tissues.
Disclosure of conflict of interest
None.
References
- 1.Jaffe RH. Recurrent lipomatous tumors of the groin: liposarcoma and lipoma pseudomyxomatodes. Arch Pathol. 1926;1:381–387. [Google Scholar]
- 2.Vellios F, Baez J, Shumacker HB. Lipoblastomatosis: a tumor of fetal fat different from hibernoma. Report of a case with observations on the embryogenesis of human adipose tissue. Am J Pathol. 1958;134:1149–1459. [PMC free article] [PubMed] [Google Scholar]
- 3.Dilley AV, Patel DL, Hicks MJ, Brandt ML. Lipoblastoma: pathophysiology and surgical management. J Pediatr Surg. 2001;36:229–231. doi: 10.1053/jpsu.2001.20060. [DOI] [PubMed] [Google Scholar]
- 4.Kok KY, Telisinghe PU. Lipoblastoma: clinical features, treat-ment, and outcome. World J Surg. 2010;34:1517–1522. doi: 10.1007/s00268-010-0466-8. [DOI] [PubMed] [Google Scholar]
- 5.Tsai H, Whitney K, Kogan S. Perineal lipo-blastoma in a neonate. J Urol. 1997;158:2272–73. doi: 10.1016/s0022-5347(01)68234-x. [DOI] [PubMed] [Google Scholar]
- 6.McVay MR, Keller JE, Wagner CW, Jackson RJ, Smith SD. Surgical management of lipoblastoma. J Pediatr Surg. 2006;41:1067–1071. doi: 10.1016/j.jpedsurg.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 7.Ito M, Hashizume K, Kanamori Y. New phenotype of accessory scrotum with perineal lipoblastoma: coexistence of midperineal and lateral accessory scrotums. Int J Urol. 2004;11:125–127. doi: 10.1111/j.1442-2042.2004.00744.x. [DOI] [PubMed] [Google Scholar]
- 8.Moreiro C, Rapella A, Rosanda C, Tassano E. PLAG1-HAS2 fusion in lipoblastoma with masked 8q intrachromosomal rearrange-ment. Cancer Genet Cytogenet. 2005;156:183–184. doi: 10.1016/j.cancergencyto.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Al-Momani HM. Recurrent maturing perineal lipoblastoma. Saudi Med J. 2005;26:1815–1817. [PubMed] [Google Scholar]
- 10.Antoniou D, Soutis M, Christopoulos-Geroulanos G, Stefanaki K. A case of maturing perineal lipoblastoma in an infant. Med Princ Pract. 2009;18:335–338. doi: 10.1159/000215735. [DOI] [PubMed] [Google Scholar]
- 11.Ahn KH, Boo YJ, Seol HJ, Park HT, Hong SC, Oh MJ, Kim T, Kim HJ, Kim YT, Kim SH. Prenatally Detected Congenital Perineal Mass Using 3D Ultrasound which was Diagnosed as Lipoblastoma Combined with Anorectal Malformation: Case Report. J Korean Med Sci. 2010;25:1093–1096. doi: 10.3346/jkms.2010.25.7.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagano Y, Uchida K, Inoue M, Ide S, Shimura T, Hashimoto K, Koike Y, Kusunoki M. Mesenteric lipoblastoma presenting as a small intestinal volvulus in an infant: A case report and literature review. Asian J Surg. 2013 doi: 10.1016/j.asjsur.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Chung EB, Enzinger FM. Benign lipoblasto-matosis: an analysis of 35 cases. Cancer. 1973;32:482–492. doi: 10.1002/1097-0142(197308)32:2<482::aid-cncr2820320229>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 14.Eyssartier E, Villemagne T, Maurin L, Machet M, Lardy H. Intrascrotal lipoblastoma: A report of two cases and a review of the literature. J Pediatr Urol. 2013;9:e151–e154. doi: 10.1016/j.jpurol.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Kučera A, Šnajdauf J, Vyhnanek M, Morávek J, Kodet R, Stejskalová E, Dvořáková M. Lipoblastoma in children: an analysis of 5 cases. Acta Chir Belg. 2008;108:580–582. doi: 10.1080/00015458.2008.11680289. [DOI] [PubMed] [Google Scholar]
- 16.Chen CW, Chang WC, Lee HS, Ko KH, Chang CC, Huang GS. MRI features of lipoblastoma: Differentiating from other palpable lipomatous tumor in pediatric patients. Clin Imaging. 2010;34:453–457. doi: 10.1016/j.clinimag.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Del Sordo R, Cavaliere A, Sidoni A, Colella R, Bellezza G. Intrascrotal lipoblastoma: a case report and review of the literature. J Pediatr Surg. 2007;42:e9–11. doi: 10.1016/j.jpedsurg.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 18.Shamookler BM, Enzinger FM. Liposarcoma occurring in children. Cancer. 1983;52:567–74. doi: 10.1002/1097-0142(19830801)52:3<567::aid-cncr2820520332>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 19.Hicks J, Dilley A, Patel D, Barrish J, Zhu SH, Brandt M. Lipoblastoma and lipoblastoma-tosis in infancy and childhood: histopathologic, ultrastructural and cytogenetic features. Ultrastruct Pathol. 2001;25:321–333. doi: 10.1080/019131201753136359. [DOI] [PubMed] [Google Scholar]
- 20.Deen M, Ebrahim S, Schloff D, Mohamed AN. A novel PLAG1-RAD51L1 gene fusion resulting from a t(8;14)(q12;q24) in a case of lipoblastoma. Cancer Genetics. 2013;206:233–237. doi: 10.1016/j.cancergen.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 21.Guidry CA, McGahren ED, Rodgers BM, Kane BJ. Pediatric cervicomediastinal hibernoma: A case report. J Pediatr Surg. 2013;48:258–261. doi: 10.1016/j.jpedsurg.2012.10.059. [DOI] [PubMed] [Google Scholar]
- 22.Seguier-Lipszyc E, Hermann G, Kaplinski C, Lotan G. Fibrous hamartoma of infancy. J Pediatr Surg. 2011;46:753–755. doi: 10.1016/j.jpedsurg.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 23.Kirkham YA, Yarbrough CM, Pippi Salle JL, Allen LM. A rare case of inguinolabial lipoblastoma in a 13-month-old female. J Pediatr Urol. 2013;9:e64–e67. doi: 10.1016/j.jpurol.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Hatanaka K, Tanimoto A, Umekita Y, Yoshioka T, Kanekura T. Unusual anogenital apocrine tumor resembling mammary-like gland adenoma in male perineum: a case report. Diagn Pathol. 2010;5:42. doi: 10.1186/1746-1596-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang XJ, Zhou S, Nie K, Chen DF, Kui GJ, Zhang XH. Dendritic fibromyxolipoma in the right inguinal and perineum regions: a case report and review of the literature. Diagn Pathol. 2013;8:157. doi: 10.1186/1746-1596-8-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Speer AL, Schofield DE, Wang KS, Shin CE, Stein JE, Shaul DB, Mahour GH, Ford HR. Contemporary management of lipoblastoma. J Pediatr Surg. 2008;43:1295–1300. doi: 10.1016/j.jpedsurg.2007.10.068. [DOI] [PubMed] [Google Scholar]


