Abstract
The environmental persistence and bioaccumulation of herbicide atrazine may pose a significant threat to human health. In this experiment, Wistar rats were treated by 5, 25 and 125 mg·kg-1 atrazine respectively for 28 days, and the oxidative stress responses as well as the activations of Nrf2 signaling pathway in ovarian tissues induced by atrazine were observed. The results showed that after be treated by atrazine, the proportion of atretic follicles in the rat ovary were increased, the contents of NO and MDA in the tissue homogenates were increased, the over-expressed Nrf2 transferred into the nuclei and played an antioxidant role by up-regulated the expression of II phase detoxifying enzymes such as HO1 and NQO1 and the expression of antioxidant enzymes such as CAT, SOD and GSH-PX.
Keywords: Atrazine, rat, ovarian tissues, oxidative stress, Nrf2 signal pathway
Introduction
Atrazine (2-chloro-4-(ethylamine)-6-(isopropylamine)-s-triazine, ATR) is an s-triazine herbicide inhibiting photosystem II that has been used extensively worldwide to control pre- and post-emergence broadleaved and grassy weeds in major crops such as maize (Zea mays), sorghum (Sorghum spp.), and sugarcane (Saccharum officinarum) [1]. It is persistent in groundwater and both the herbicide and its main metabolites (deethylatrazine and deisopropylatrazine) are often detected in water resources at concentrations exceeding the EU regulation limit (0.1 μg·l-1). In addition, several studies revealed that atrazine had toxicological impact on non-target species, such as amphibians and could act as an endocrine disruptor. As a result, use of atrazine has recently been banned in the European Union.
Several studies reported that ATR decreases tissue DA levels by interfering with the vesicular storage and/or cellular uptake of DA [2,3]. Perinatal exposure to atrazine could produce subtle functional alterations, which mainly related with neurodevelopmental disorder affecting the social domain and the emotional/affective repertoire [4]. ATR and two of its metabolites, DIP and DE, but not its major mammalian metabolite, DACT, can decrease striatal DA levels by increasing cytosolic DA, which is prone to oxidative breakdown [5]. ATR is capable of inducing splenocytic apoptosis mediated by the Fas/FasL pathway in mice [6]. Findings from in vitro assays indicate that atrazine exposure interfered with the phenotypic and functional maturation of DC at non-cytotoxic concentrations [7]. Besides, ATR exposure appears to be detrimental to the immune system of juvenile mice by decreasing cellularity and affecting lymphocyte distribution [8]. Atrazine significantly decreases the clonogenicity of myeloid cells. In females, the percentage of (colony-forming unit-granulocyte/macrophage, CFU-GM) CFU-GM significantly decreased after atrazine exposure [9]. In vitro indicated that atrazine acted as a competitive inhibitor of cyclic nucleotide phosphodiesterases (PDEs) derived from bovine hearts, leading to diminished conversion of cAMP to 5’-AMP, resulting in the inhibition of Prolactin and thyroid hormones and promotion of endogenous ovarian hormones [10-12]. It has been reported that atrazine exerts an estrogen-like activity in ovarian cancer cells through G protein-coupled receptor 30, and this process requires transactivation of the epidermal growth factor receptor transduction pathway and the involvement of estrogen receptor alpha [13-15]. It has also been suggested that atrazine elicits estrogen action by up-regulating aromatase activity in human adrenocortical carcinoma H295R cells [16]. Atrazine could induce the overproduction of active oxygen. Bhatti reported that oral administration of atrazine and melatonin was given daily for 21 days. A significant increase in the MDA levels and decrease in the GSH was observed in the atrazine treated animals. Also, significant increase in the activities of SOD, CAT, GPx, and GST were observed in atrazine treated group compared to controls [17]. Adesiyan reported that atrazine could induce toxicity in the liver and reproductive system of rats, with the increase of MDA anabolism and the decrease of SOD catabolism in liver and Testis [18].
Nuclear factor-erythroid 2-related factors 2 (Nrf2) plays a vital role in maintaining cellular homeostasis, especially upon the exposure of cells to chemical or oxidative stress, through its ability to regulate the basal and inducible expression of a multitude of antioxidant proteins, detoxification enzymes and xenobiotic transporters [19]. Nrf2 activity may contribute to the maintenance of cellular homeostasis [20]. However, there is no report about Nrf2 regulation in the oxidation of atrazine. Here, to explore the effects and mechanism of Nrf2 in the oxidation of ovary cause by atrazine, we observed the oxidative effect of atrazine to rat’s ovary after 28 days treatment of atrazine in different dosage. Furthermore, we highlight key knowledge gaps in this important field of biology, and suggest how these may be addressed experimentally.
Materials and methods
Chemicals and reagents
Atrazine (2-chloro-4-ethylamino-6-isopropylamino-s-triazine, ATR, 99% purity) and SDS, TEMED, Acrylamide, N,N-Dimethyl-bis-acrylamide, DTT and PMSF were obtained from Sigma Chemical Company (USA). ATR solutions (0.5 mg/ml, 2.5 mg/ml and 12.5 mg/ml) were prepared by dissolving ATR in corn oil. All the solutions were kept at 4°C for a maximum of 1 week. NO, MDA, SOD, CAT and GSH-PX detection kits were purchased from Nanjing Jiancheng Co. RabMab, Nrf2, Keap1, HO1 and NOQ1 Monoclonal antibody were acquired from Proteintech Group USA. Horseradish peroxidase labeled goat anti-rabbit IgG and ECL luminescent kit were production of Promega. RPMI 1640 and fetal calf serum were purchased from Gibco laboratorie (USA).
Animals and treatment
Four-week-old pathogen-free female Wistar rats were purchased from the Experimental Animal Center of Norman Bethune Medical College, Jilin University (Changchun, China), and the animal study was conducted following internationally recognized guidelines and was approved by the Animal Research Committee of Norman Bethune College of Medicine, Jilin University. The animals were housed in a temperature and humidity controlled environment, and were provided with standard laboratory diet and drinking water. Thirty-two animals were randomly divided into four groups by body weight (8/group), and were treated by a daily gavage of 0, 5, 25 and 125 mg·kg-1 atrazine for 28 consecutive days. Animal weights were measured at 4-day intervals. The ovaries were removed from each rat after the last exposure to atrazine.
Pathological examination
Sections of the ovary of sacrificed rat treated with different doses atrazine were fixed in 10% buffered formaldehyde, embedded in paraffin, and then sectioned in 4-μm-thick. The sections were then stained routinely with hematoxylin-eosin for histological assessment.
Detection of contents of NO, MDA and the activity of SOD, CAT, GSH-PX
A 10% homogenate of the ovarian tissue was prepared in 1 ml PBS buffer containing 100 mg tissue, and kept in -20°C. Protein content was estimated by the method of Bradford [21]. The contents of NO, MDA and the activities of SOD, CAT, GSH-PX were determined as described in the detection kits instruction.
Immunohistochemistry
After deparaffinization (target retrieval by autoclaving in citrate buffer) and incubation in 0.3% H2O2 for 30 min and normal serum, ovary sections were incubated at room temperature with primary antibody at a dilution of 1:50 at 4°C for 1 hour, followed by incubation with biotinylated secondary antibody for 20 min and the streptavidin-biotin peroxidase complex (sABC) for 20 min. Subsequently, 3,3’-diaminobenzidine (DAB) was applied as a chromogen. The sections were finally counterstained with hematoxylin and following rinsing in deionized water, and were immersed in ammonia blue for 2 min. The slides were dehydrated and mounted with Permount. In the pictures, the brown particles are Nrf2 protein, and the blue-stained particles are nucleus.
Western blot
Ovaries from the rats were homogenized in ice-cold SET buffer (0.25 M sucrose, 5 mM EDTA, 20 mM Tris base, pH 7.4). Tissue samples were separated by SDS-PAGE (12% polyacrylamide resolving gel) and transferred to a PVDF membrane. Membranes were blocked with 3% BSA, and then were incubated with primary antibody and secondary antibody. All membranes were visualized using ECL and exposure to ECL Hyperfilm. Densitometric analysis of the film was performed using a Model GS-710 imaging densitometer in transmittance mode and analyzed using Bio-Rad Discovery software.
Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) V-10 statistical software. The means and standard deviations (SD) were calculated for each marker. Multiple comparisons between groups were done by means of Two-way ANOVA. P<0.05 was considered statistically.
Results
General state and body weight
All animals survived until the end of the study. There were no overt changes in appearance, diet and behavior. The body weights were measured over 4-day intervals to draw a body weight curve. Significant difference in body weight (Figure 1) of the rats in the 125 mg·kg-1 ATR group was detected from the 8th day until the end of the experiment.
Figure 1.
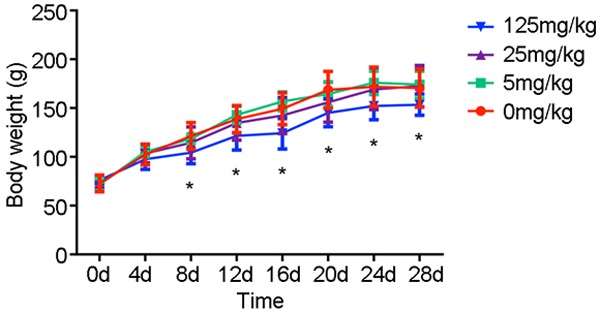
The body weight of rats treated by atrazine. Significant difference in body weight of the rats in the 125 mg·kg-1 ATR group was detected from the 8th day until the end of the experiment. *, p<0.05 vs. control group.
Proportion of ovarian atretic follicles
To evaluate whether exposure to atrazine would elicit changes in the ovarian tissue, the sections of ovarian tissue were stained with hematoxylin-eosin for histological assessment. Proportion of Ovarian atresia was significantly increased in a dose-dependent manner in the atrazine treated animals (Figure 2).
Figure 2.
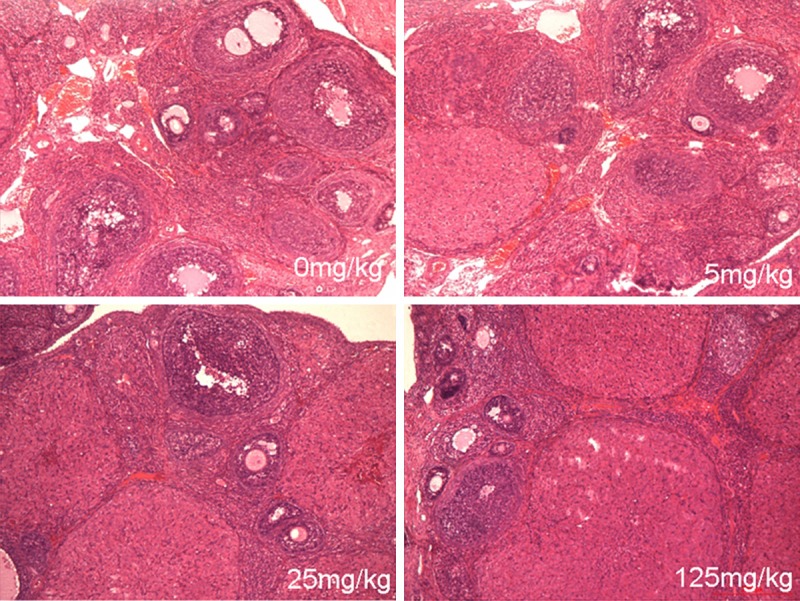
The HE staining of ovarian tissues. Proportion of Ovarian atresia was significantly increased in a dose-dependent manner in the atrazine treated animals.
Contents of NO and MDA
Data from NO and MDA detection in ovarian tissue homogenate are presented in Figure 3. The contents of NO and MDA were significantly increased in ovary homogenate in 25 and 125 mg·kg-1 atrazine treated rats, which indicated that oxidative stress was occurred in the ovarian tissues (p<0.05) (Figure 3).
Figure 3.
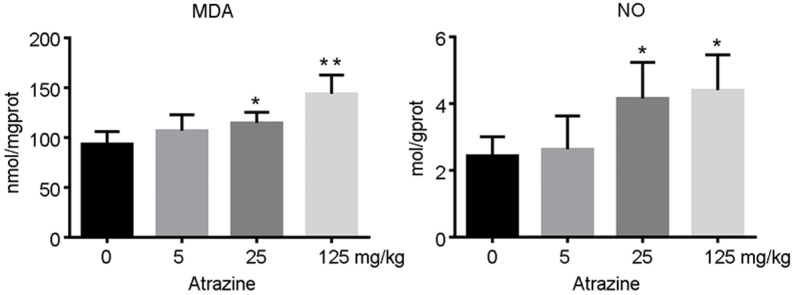
The levels of MDA and NO in ovarian tissues. The contents of NO and MDA were significantly increased in ovary homogenate in 25 and 125 mg·kg-1 atrazine treated rats, which indicated that oxidative stress was occurred in the ovarian tissues (p<0.05). *, p<0.05 vs. control group; **, p<0.01 vs. control group.
Expression of Nrf2 and Keap1
To further explore the effects of oxidant stress on Nrf2 pathway in atrazine treated rats, we estimated the contents of Nrf2 and Keap1 in ovarian tissue by western blot. Our findings showed that the expression of Nrf2 was significantly increased in a dose-dependent manner in all atrazine treated groups (p<0.05, p<0.01). The expression of Keap1 was increased in 5 mg·kg-1 atrazine treated rats, while a dose-related decrease of Keap1 content was presented with the increase of atrazine dose. The contents of Keap1 in 125 mg·kg-1 atrazine treated rats were significantly decreased compared with those of the control group (p<0.05) (Figure 4).
Figure 4.
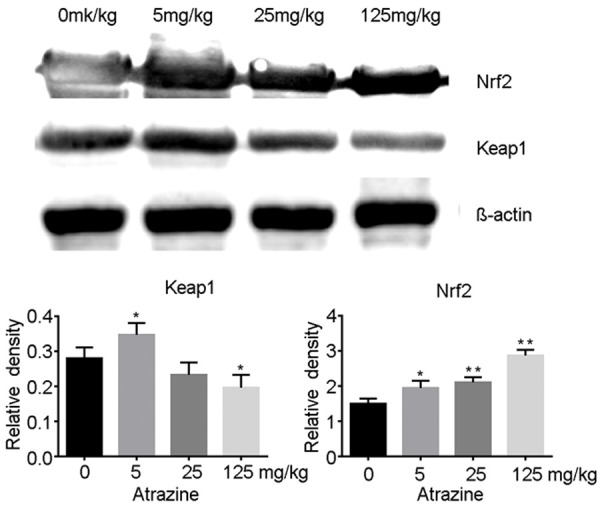
The expressions of Nrf2 and Keap1 in ovarian tissues. The expression of Nrf2 was significantly increased in a dose-dependent manner in all atrazine treated groups (p<0.05, p<0.01). The contents of Keap1 in 5 mg·kg-1 atrazine treated rats were significantly increased, while which in 125 mg·kg-1 atrazine treated rats were significantly decreased compared with those of the control group (p<0.05). *, p<0.05 vs. control group; **, p<0.01 vs. control group.
Translocation of Nrf2 to the nucleus
To investigate whether the up-regulated Nrf2 can translocate to the nucleus and thus exerts its biological function, we used Immunohistochemistry to determine the effect of atrazine on the intercellular localization of Nrf2 in rat. As was shown in Figure 5, there was a significant increase of positive expression of Nrf2 in atrazine treated ovary cell nucleus, which indicated that Nrf2 was activated and transferred into nucleus.
Figure 5.
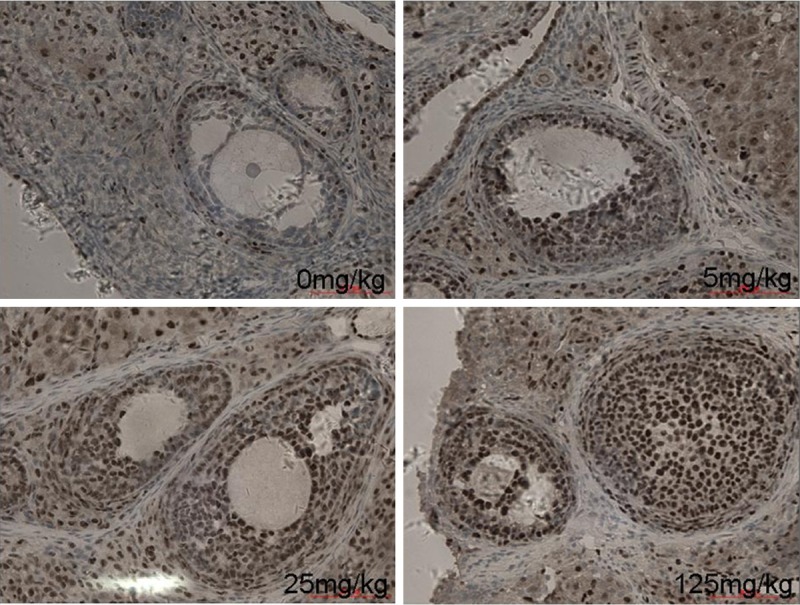
The intracellular location of Nrf2 in ovarian tissues. There was a significant increase of positive expression of Nrf2 in atrazine treated ovary cell nucleus.
Expression of phase II detoxification enzymes
Western blot assays were performed to determine the effect of Nrf2 activation on the expression of Phase II Detoxification enzyme, which include HO1 and NQO1. The expression of HO1 and NQO1 were significantly up-regulated in 5 mg·kg-1 atrazine treated group compared with those of the control. The contents then decreased with the increase of atrazine dose. The expression of HO1 was significantly decreased in 125 mg·kg-1 atrazine treated rats compared with that of the control (Figure 6).
Figure 6.
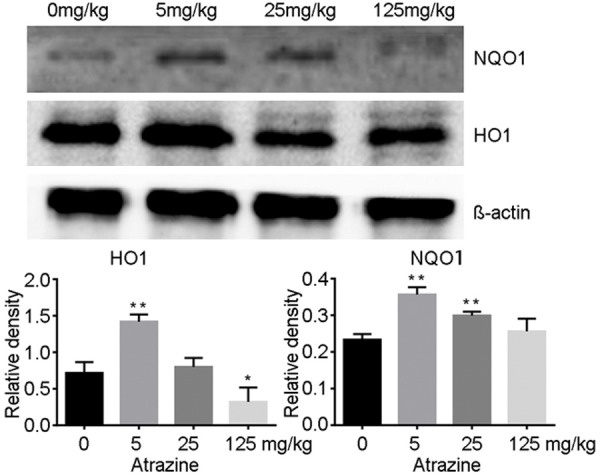
The expression of HO1 and NQO1 in ovarian tissues. The expression of HO1 and NQO1 were significantly up-regulated in 5mg·kg-1 atrazine treated group compared with those of the control. The contents then decreased with the increase of atrazine dose. The expression of HO1 was significantly decreased in 125 mg·kg-1 atrazine treated rats compared with that of the control. *, p<0.05 vs. control group; **, p<0.01 vs. control group.
Activities of SOD, CAT and GSH-PX
To further explore the effect of Nrf2 activation on Antioxidant enzymes, the activities of SOD, CAT and GSH-PX was determined. As is shown in Figure 7, the activities of SOD and GSH-PX of 125 mg·kg-1 group were increased and the activities of CAT was decreased significantly compared with those of control group (p<0.05).
Figure 7.
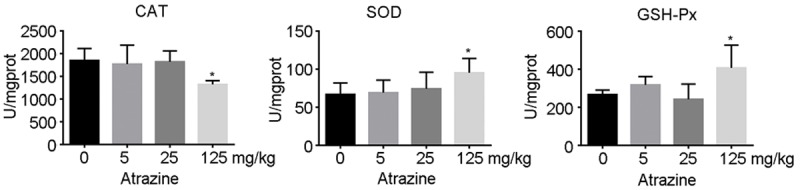
The activity of CAT, SOD and GSH-PX in ovarian tissues. The activities of SOD and GSH-PX of 125 mg·kg-1 group were increased and the activities of CAT was decreased significantly compared with those of control group (p<0.05).
Discussion
Atrazine is one of the most widely used and commonly detected herbicides in the world, its biological toxicity has received a great deal of concern by academic investigators. In this study, 4-week Wistar rats were given atrazine by gavage for 28 days, at daily dosages of 0 (control), 5, 25 and 125 mg·kg-1. The body weights were significantly slow down at 125 mg·kg-1 from the 8th day to the end compared with the control. Ovaries were fixed and stained with hematoxylin and eosin for histological examination on the 29th day. The results showed that the proportion of primary follicles was significantly reduced, while the proportion of ovarian atresia was significantly increased in a dose-dependent manner in the atrazine treated animals.
Oxidative tissue damage was reported as one of the early mechanism of adverse effects of exogenous compounds. The accumulation of reactive oxygen species (ROS), including O2-, H2O2, OH, NO and ONOO-, are the evidence that body is in oxidative stress status [22]. Malondialdehyde (MDA) is considered a presumptive biomarker of the involvement of free radical damage in living organisms. Determination of MDA levels is still the most commonly applied assay for lipid peroxidation in biomedical sciences [23]. To further investigate the effect of atrazine on ovary, our study detected the NO and MDA in ovary homogenous and found that level of MDA and NO were upregulated in ovarian tissue, which indicated that atrazine could induce the overproduction of active oxygen in ovarian tissue.
Oxidative stress depicts the existence of free radicals and reactive oxygen species, which are formed in normal physiology but become deleterious when not being quenched by a cascade of antioxidants systems. This can result in an imbalance between the generation of ROS and the antioxidant defense capacity of the body. ROS oxidize various types of biomolecules, finally leading to cellular lesions by damaging DNA or stimulating apoptosis for cell death. Oxidative stress as intracellular signaling molecules also activate several signaling pathways to regulate cell responses.
Nrf2 is a redox-sensitive transcription factor that regulates the expression of phase II anti oxidant genes and confers cytoprotection against oxidative stress [19]. In unstressed cells Nrf2 is sequestered by its inhibitor, Keap1, that promotes rapid proteasome-mediated degradation via a Cul3 based E3 ubiquitin ligase complex [24-27]. The half-period is about 10~40 min [28,29]. However, in response to oxidative stress, Nrf2 is stabilized by dissociating from Keap1, and binds to cis-elements called antioxidant response elements (ARE) as a heterodimer with other members of the basic leucine zipper protein family, such as Maf or Jun [30]. Our study estimated the contents of Nrf2 and Keap1 in ovarian tissue by western blot, and found that the expression of Nrf2 was significantly increased in a dose-dependent manner in all atrazine treated groups (p<0.05, p<0.01). The expression of Keap1 was increased in 5 mg·kg-1 atrazine treated rats, while a dose-related decrease of Keap1 content was presented with the increase of atrazine dose. The contents of Keap1 in 125 mg·kg-1 atrazine treated rats were significantly decreased compared with those of the control group. The data suggested that low dose atrazine could induce excessive pro-oxidant substances in ovarian tissue. The expression of Nrf2 was upregulated to exert the cytoprotective effect against oxidative stress. The expression of Keap1 was also increased to combine with increased Nrf2 in the tissue. However, with the increase of pro-oxidant substances in the tissue, the expression of Nrf2 was further upregulated whereas the expression of Keap1 was down-regulated, thus the Nrf2 could translocated into nucleus to exert its transcription function. To further verify this hypothesis, we detected the location of Nrf2 in ovarian tissue by immunohistochemistry, and found that 25 mg·kg-1 and 125 mg·kg-1 atrazine could promote the translocation of Nrf2 into nucleus. These results indicated that Nrf2 might play an important role in the cytoprotective mechanism against oxidative damage induced by atrazine.
As with other members of the cap ‘n’ collar (CNC) family of transcription factors, Nrf2 contains a C-terminal basic leucine zipper (bZip) structure that facilitates dimerization and DNA binding [31]. During oxidative stress, Keap1 is inactivated by modification of its highly reactive cyestein residues and disassociated with Nrf2, which then undergoes nuclear translocation, binds in heterodimeric combinations with members of the small Maf family of nuclear factors, to the 5’-upstream AREs [32,33], and detoxify genes, such as Glutathione S-transferase (GST), NAD(P)H: quinone oxidoreductase l (NQOl), hemeoxygenase 1 (HO1), Catalase (CAT), Superoxide Dismutase (SOD), Sulfiredoxin (SRX), Glutathione peroxidase (GSH-PX) and γ-glutamylcysteine synthetase (γ-GCS), and thus modulates their expressions [34,35].
HO1, formerly known as phase II detoxifying antioxidant enzyme, is the rate-limiting enzyme that catalyzes the degradation of heme to produce biliverdin, iron, and carbon monoxide [36]. NQO1, prevalent in most eukaryotic cells, is a flavin protease which catalyzes quinone two-electron reduction reaction, thereby preventing the oxidation-reduction reaction and the generation of ROS. It also catalyzes α-tocopher olquinone to generate efficient antioxidants α-tocopher-olhydroquinone [37,38]. In this study, the upregulation of HO1 and NQO1 in 5 mg·kg-1 atrazine treated rats indicated that Nrf2 combined with ARE and bZIP protein after translocation to the nucleus, and triggered the expression of phase II detoxifying enzymes. While with the increase of atrazine administration, the accumulation of Pro-oxidant substances consumed more phase II detoxifying enzymes simultaneously, thus the detectable contents of HO1 and NQO1 decreased on the contrary. Especially the HO1 in 125 mg·kg-1 atrazine treated group was significantly lower than that of control group.
Intracellular antioxidant enzyme system plays a key role in fighting against oxidative stress. SOD is the first defense which catalyzes the dismutation of superoxide anion into O2 and hydrogen peroxide (H2O2). H2O2 is then reduced to H2O by glutathione peroxidase (GSH-Px) in the cytosol, or by catalase (CAT) in the peroxisomes. In our study, compared with those of the control group, the activities of SOD and GSH-PX increased significantly in 125 mg·kg-1 atrazine group compared with those of the control group, while the activity of CAT decreased. These changes could be due to the activation of Nrf2, which promote the expression of antioxidant enzymes. Meanwhile, the pro-oxidant substances can consume antioxidant enzymes. The excessive intracellular H2O2 induced by atrazine consumed much CAT, and finally lead to the decrease of CAT activity.
In summary, atrazine could induce oxidative stress response in rats ovary. Nrf2 has an important role in the defence against oxidative stress by regulating the expression of phase II detoxifying and antioxidative enzymes. Continued advances on the effect of atrazine exposure to Nrf2 signaling pathway will contribute to understanding the mechanism of atrazine-induced ovary damage, and increase the likelihood of the transcription factor being targeted for therapeutic benefit in the near future.
In conclusion, the present study shows that atrazine could induce oxidative stress response in rats’ ovary after 28 days administration. Nrf2 protects cells from oxidative stress by a mechanism that regulates ARE related genes including phase II detoxifying and antioxidant enzymes.
Disclosure of conflict of interest
None.
References
- 1.Lasserre JP, Fack F, Revets D, Planchon S, Renaut J, Hoffmann L, Gutleb AC, Muller CP, Bohn T. Effects of the endocrine disruptors atrazine and PCB 153 on the protein expression of MCF-7 human cells. J Proteome Res. 2009;8:5485–5496. doi: 10.1021/pr900480f. [DOI] [PubMed] [Google Scholar]
- 2.Greenlee AR, Ellis TM, Berg RL. Low-dose agrochemicals and lawn-care pesticides induce developmental toxicity in murine preimplantation embryos. Environ Health Perspect. 2004;112:703–709. doi: 10.1289/ehp.6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filipov NM, Stewart MA, Carr RL, Sistrunk SC. Dopaminergic toxicity of the herbicide atrazine in rat striatal slices. Toxicology. 2007;232:68–78. doi: 10.1016/j.tox.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belloni V, Dessi-Fulgheri F, Zaccaroni M, Di Consiglio E, De Angelis G, Testai E, Santochirico M, Alleva E, Santucci D. Early exposure to low doses of atrazine affects behavior in juvenile and adult CD1 mice. Toxicology. 2011;279:19–26. doi: 10.1016/j.tox.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Hossain MM, Filipov NM. Alteration of dopamine uptake into rat striatal vesicles and synaptosomes caused by an in vitro exposure to atrazine and some of its metabolites. Toxicology. 2008;248:52–58. doi: 10.1016/j.tox.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Wang M, Gao S, Ren R, Zheng J, Zhang Y. Atrazine-induced apoptosis of splenocytes in BALB/C mice. BMC Med. 2011;9:117. doi: 10.1186/1741-7015-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinchuk LM, Lee SR, Filipov NM. In vitro atrazine exposure affects the phenotypic and functional maturation of dendritic cells. Toxicol Appl Pharmacol. 2007;223:206–217. doi: 10.1016/j.taap.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filipov NM, Pinchuk LM, Boyd BL, Crittenden PL. Immunotoxic effects of short-term atrazine exposure in young male C57BL/6 mice. Toxicol Sci. 2005;86:324–332. doi: 10.1093/toxsci/kfi188. [DOI] [PubMed] [Google Scholar]
- 9.Cimino-Reale G, Ferrario D, Casati B, Brustio R, Diodovich C, Collotta A, Vahter M, Gribaldo L. Combined in utero and juvenile exposure of mice to arsenate and atrazine in drinking water modulates gene expression and clonogenicity of myeloid progenitors. Toxicol Lett. 2008;180:59–66. doi: 10.1016/j.toxlet.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Carreon JI, Dargent C, Merhi M, Fattel-Fazenda S, Arce-Popoca E, Villa-Trevino S, Rouimi P. Tumor promoting and co-carcinogenic effects in medium-term rat hepatocarcinogenesis are not modified by co-administration of 12 pesticides in mixture at acceptable daily intake. Food Chem Toxicol. 2009;47:540–546. doi: 10.1016/j.fct.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Tsuda H, Fukamachi K, Ohshima Y, Ueda S, Matsuoka Y, Hamaguchi T, Ohnishi T, Takasuka N, Naito A. High susceptibility of human c-Ha-ras proto-oncogene transgenic rats to carcinogenesis: a cancer-prone animal model. Cancer Sci. 2005;96:309–316. doi: 10.1111/j.1349-7006.2005.00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rooney AA, Matulka RA, Luebke RW. Developmental atrazine exposure suppresses immune function in male, but not female Sprague-Dawley rats. Toxicol Sci. 2003;76:366–375. doi: 10.1093/toxsci/kfg250. [DOI] [PubMed] [Google Scholar]
- 13.Simpkins JW, Swenberg JA, Weiss N, Brusick D, Eldridge JC, Stevens JT, Handa RJ, Hovey RC, Plant TM, Pastoor TP, Breckenridge CB. Atrazine and breast cancer: a framework assessment of the toxicological and epidemiological evidence. Toxicol Sci. 2011;123:441–459. doi: 10.1093/toxsci/kfr176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holloway AC, Anger DA, Crankshaw DJ, Wu M, Foster WG. Atrazine-induced changes in aromatase activity in estrogen sensitive target tissues. J Appl Toxicol. 2008;28:260–270. doi: 10.1002/jat.1275. [DOI] [PubMed] [Google Scholar]
- 15.Rivest P, Devine PJ, Sanderson JT. Evaluation of a bioluminescent mouse model expressing aromatase PII-promoter-controlled luciferase as a tool for the study of endocrine disrupting chemicals. Toxicol Appl Pharmacol. 2010;249:33–40. doi: 10.1016/j.taap.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Albanito L, Lappano R, Madeo A, Chimento A, Prossnitz ER, Cappello AR, Dolce V, Abonante S, Pezzi V, Maggiolini M. G-protein-coupled receptor 30 and estrogen receptor-alpha are involved in the proliferative effects induced by atrazine in ovarian cancer cells. Environ Health Perspect. 2008;116:1648–1655. doi: 10.1289/ehp.11297. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Bhatti JS, Sidhu IP, Bhatti GK. Ameliorative action of melatonin on oxidative damage induced by atrazine toxicity in rat erythrocytes. Mol Cell Biochem. 2011;353:139–149. doi: 10.1007/s11010-011-0780-y. [DOI] [PubMed] [Google Scholar]
- 18.Adesiyan AC, Oyejola TO, Abarikwu SO, Oyeyemi MO, Farombi EO. Selenium provides protection to the liver but not the reproductive organs in an atrazine-model of experimental toxicity. Exp Toxicol Pathol. 2011;63:201–207. doi: 10.1016/j.etp.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi A, Ohta T, Yamamoto M. Unique function of the Nrf2-Keap1 pathway in the inducible expression of antioxidant and detoxifying enzymes. Methods Enzymol. 2004;378:273–286. doi: 10.1016/S0076-6879(04)78021-0. [DOI] [PubMed] [Google Scholar]
- 20.Bryan HK, Olayanju A, Goldring CE, Park BK. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem Pharmacol. 2013;85:705–717. doi: 10.1016/j.bcp.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 22.Fridovich I. The biology of oxygen radicals. Science. 1978;201:875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- 23.Valls-Belles V, Torres Mdel C, Boix L, Muniz P, Gonzalez-Sanjose ML, Codoner-Franch P. alpha-Tocopherol, MDA-HNE and 8-OHdG levels in liver and heart mitochondria of adriamycin-treated rats fed with alcohol-free beer. Toxicology. 2008;249:97–101. doi: 10.1016/j.tox.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Kang MI, Kobayashi A, Wakabayashi N, Kim SG, Yamamoto M. Scaffolding of Keap1 to the actin cytoskeleton controls the function of Nrf2 as key regulator of cytoprotective phase 2 genes. Proc Natl Acad Sci U S A. 2004;101:2046–2051. doi: 10.1073/pnas.0308347100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furukawa M, Xiong Y. BTB protein Keap1 targets antioxidant transcription factor Nrf2 for ubiquitination by the Cullin 3-Roc1 ligase. Mol Cell Biol. 2005;25:162–171. doi: 10.1128/MCB.25.1.162-171.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cullinan SB, Gordan JD, Jin J, Harper JW, Diehl JA. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol Cell Biol. 2004;24:8477–8486. doi: 10.1128/MCB.24.19.8477-8486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart D, Killeen E, Naquin R, Alam S, Alam J. Degradation of transcription factor Nrf2 via the ubiquitin-proteasome pathway and stabilization by cadmium. J Biol Chem. 2003;278:2396–2402. doi: 10.1074/jbc.M209195200. [DOI] [PubMed] [Google Scholar]
- 29.Tong KI, Katoh Y, Kusunoki H, Itoh K, Tanaka T, Yamamoto M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model. Mol Cell Biol. 2006;26:2887–2900. doi: 10.1128/MCB.26.8.2887-2900.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wakabayashi N, Dinkova-Kostova AT, Holtzclaw WD, Kang MI, Kobayashi A, Yamamoto M, Kensler TW, Talalay P. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc Natl Acad Sci U S A. 2004;101:2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Horssen J, Schreibelt G, Drexhage J, Hazes T, Dijkstra CD, van der Valk P, de Vries HE. Severe oxidative damage in multiple sclerosis lesions coincides with enhanced antioxidant enzyme expression. Free Radic Biol Med. 2008;45:1729–1737. doi: 10.1016/j.freeradbiomed.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi A, Ito E, Toki T, Kogame K, Takahashi S, Igarashi K, Hayashi N, Yamamoto M. Molecular cloning and functional characterization of a new Cap’n’ collar family transcription factor Nrf3. J Biol Chem. 1999;274:6443–6452. doi: 10.1074/jbc.274.10.6443. [DOI] [PubMed] [Google Scholar]
- 33.Johnsen O, Skammelsrud N, Luna L, Nishizawa M, Prydz H, Kolsto AB. Small Maf proteins interact with the human transcription factor TCF11/Nrf1/LCR-F1. Nucleic Acids Res. 1996;24:4289–4297. doi: 10.1093/nar/24.21.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talalay P, Fahey JW. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J Nutr. 2001;131:3027S–3033S. doi: 10.1093/jn/131.11.3027S. [DOI] [PubMed] [Google Scholar]
- 35.Rushmore TH, Kong AN. Pharmacogenomics, regulation and signaling pathways of phase I and II drug metabolizing enzymes. Curr Drug Metab. 2002;3:481–490. doi: 10.2174/1389200023337171. [DOI] [PubMed] [Google Scholar]
- 36.Dore S, Takahashi M, Ferris CD, Zakhary R, Hester LD, Guastella D, Snyder SH. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc Natl Acad Sci U S A. 1999;96:2445–2450. doi: 10.1073/pnas.96.5.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Gordon GB. A strategy for cancer prevention: stimulation of the Nrf2-ARE signaling pathway. Mol Cancer Ther. 2004;3:885–893. [PubMed] [Google Scholar]
- 38.Dinkova-Kostova AT, Talalay P. Persuasive evidence that quinone reductase type 1 (DT diaphorase) protects cells against the toxicity of electrophiles and reactive forms of oxygen. Free Radic Biol Med. 2000;29:231–240. doi: 10.1016/s0891-5849(00)00300-2. [DOI] [PubMed] [Google Scholar]


