Abstract
Infantile hemangioma, a common benign tumor of infancy, grows quickly in the first year of life, and then regresses slowly to fibrofatty tissue in childhood. The accumulation of fibrofatty tissue in hemangioma involution indicates adipogenesis during this period. Perivascular cells (PCs) from multiple organs display multi-lineage differentiation, including adipogenesis. So we supposed that PCs in hemangioma may contribute to the adipogenesis in the involution. In this study, PDGFR-β (+) PCs was isolated from hemangioma tissue (hemangioma-derived perivascular cells, Hem-PCs) by fluorescence-activated cell sorter. In vitro, Hem-PCs showed fibroblast-like morphology. Immunofluorescence staining and flow cytometry showed Hem-PCs expressed MSCs markers CD105, CD90, CD29 and vimentin, pericyte markers α-SMA and PDGFR-β, stem cell marker CD133, and the adipogenic transcription factor PPAR-γ, but not hematopoietic/endothelial markers CD45, CD34, CD31, and flt-1. In vitro inductions confirmed multi-lineage differentiation of Hem-PCs, especially strong adipogenic potential. Then a murine model was established to observe in vivo differentiation of Hem-PCs by subcutaneous injection of cells/Matrigel compound into nude mice. The results showed Hem-PCs differentiated into adipocytes in vivo. To the best of our knowledge, this is the first study reporting the isolation of multipotential PDGFR-β (+) PCs from hemangioma, and observing their adipogenic differentiation in vivo. PCs may be the cellular basis of adipogenesis in hemangioma involution, and may be the target cells of adipogenic induction to promote hemangioma involution.
Keywords: Hemangioma, perivascular cells, mesenchymal stem cells, adipogenesis
Introduction
Infantile hemangioma (IH), a common benign tumor of infancy with higher prevalence in females, grows quickly in the first year of life, and then regresses slowly to fibrofatty tissue in childhood [1-3]. Although most IHs tends to regress spontaneously, rapid growth of the proliferating hemangioma may lead to hypertrophy, hemorrhage, ulceration or even abnormal hemodynamics and subsequent congestive heart failure. So, how to prevent the proliferation of hemangioma and accelerate its involution still remains a clinical challenge.
A unique character of hemangioma in involution is the accumulation of fibrofatty tissue, which indicates adipogenesis during this period [1]. The mechanism of this phenomenon isn’t totally clear till now. Mesenchymal stem cells isolated from hemangioma (hemangioma-derived mesenchymal stem cells, Hem-MSCs) [4] had the potential of adipogenesis in vitro. CD133 (+) multipotential stem cells (Hem-SCs) have the features of MSCs and recapitulate IH’s evolution in an immunodeficient mouse model [5]. The latest studies show that perivascular cells (PCs) are the source of MSCs in multiple organs, including the skeletal muscle, pancreas, adipose tissue, placenta, umbilical cord, brain, and dermis [6-10]. In the former study [11], we observed that MSCs in hemangioma also resided in the perivascular region, and most of the cells expressed PDGFR-β, a marker antigen of pericytes.
In this study, we isolated PDGFR-β (+) PCs from both proliferating and involuting hemangiomas (hemangioma-derived PCs, Hem-PCs) by fluorescence-activated cell sorter (FACS). The antigen profile of Hem-PCs was examined and the multipotency was tested in vitro and in vivo. The results suggested that PCs from hemangioma displayed the features of MSCs and showed stronger adipogenic potential in vitro and in vivo. They may be the cellular basis of adipogenesis in hemangioma involution.
Materials and methods
Isolation and culture of PCs from hemangioma
Six fresh IH samples in the proliferating phase and three in the involuting phase were obtained from Children’s Hospital and Jinling Hospital in Nanjing, China under a human subject’s protocol approved by the Committee on Clinical Investigation. Informed consent was obtained according to the Declaration of Helsinki. Samples were rinsed in phosphate-buffered saline (PBS), minced, and digested with 0.2% collagenase A (Sigma-Aldrich) at 37°C for one and a half hours. The tissue was homogenized and filtered through 70-μm cell strainers (BD FalconTM) to get a single-cell suspension. Red blood cells were lysed in ammonium chloride (Promega Corp.), labeled by FITC-conjugated mouse anti-human PDGFR-β antibody (LS-C38281, Lifespan), incubated at 4°C for 20 min, washed, and resuspended by L-DMEM/10%FBS. Then, PDGFR-β (+) PCs were selected by FACS. The selected cells were cultured in DMEM-L/10%FBS supplemented with 1 × PG (100 U/ml penicillin, 100 μg/ml gentamycin).
MSCs from bone marrow and fibroblasts from children’s foreskin were obtained according to the literature [12,13]. Human umbilical vein endothelial cells (HUVECs) were obtained commercially (ATCC). They were cultured by DMEM-L/10%FBS supplemented with 1 × PG.
Immunofluorescence staining
Cells at passage 3~5 were digested by 0.25% trypsin solution and plated on cell culture coverslips (Cosmobrand). On the second day, cells were fixed by 4% paraformalde, washed by PBS, incubated with the first antibodies mouse anti-human CD105 (MS-1290, Thermo Scientific), α-SMA (Ab7817, Abcam), vimentin (MS-129, Thermo Scientific), CD31 (M0823, Dako), CD34 (MS-363, Thermo Scientific), CD45 (MS-1846, Thermo Scientific), desmin (MS-376, Thermo Scientific) antibodies, and rabbit anti-human CD133 (ab19898, Abcam), PPAR-γ (BS1587, Bioworld Technology), flt-1 (RB-1527, Thermo Scientific) antibodies, followed by the second antibodies Alexa Fluor® 555 donkey anti-mouse IgG antibody (A-31570, Invitrogen) or Alexa Fluor® 488 donkey anti-rabbit IgG antibody (A-21206, Invitrogen), and counterstained with DAPI (D-21490, Invitrogen). Fluorescent images were taken with a fluorescence microscope (BX51, OLYMPUS), equipped with a digital microscope camera (ProgRes MFcool, JENOPTIK) and image analysis system (IMSTAR KARYO, IMSTAR).
Flow cytometry
Cells at passage 3~5 were labeled with FITC-conjugated mouse anti-human PDGFR-β (LS-C38281, Lifespan), CD31 (ab27333, Abcam) antibodies and PE--conjugated mouse anti-human CD90 (12-0909, eBioscience), CD29 (12-0299, eBioscience) antibodies. The isotype controls included FITC-conjugated mouse IgG1 (11-4714, eBioscience) and PE-conjugated mouse IgG1 (12-4714, eBioscience). Flow cytometry was performed on a BD FACScan. Data were analyzed by FlowJo software.
Reverse transcriptional PCR (RT-PCR)
Total RNA was extracted from Hem-PCs, hBM-MSCs, and fibroblasts using Trizol total RNAisolation kit (KGA1203, Keygen, Nanjing, China). Purity of the RNA samples was assessed by determining the optical density (OD) at 260: 280 nm. cDNA synthesis and PCR reaction were performed using RT-PCR kit (KGA1303, Keygen, Nanjing, China). The primers for PPAR-γ2 gene included a forward primer (5’-CAGGAGCAGAGCAAAGAGGT-3’) and a reverse primer (5’-TCAATGGGCTTCACATTCAG-3’). The primers for OCT-4 gene included a forward primer (5’-GTGGAGGAAGCTGACAACAATG-3’) and a reverse primer (5’-GGGCCAGAGGAAAGGACACT-3’). The primers for internal control β-actin gene included a forward primer (5’-GTCACCAACTGGGACGACATG-3’) and a reverse primer (5’-GCCGTCAGGCAGCTCGTAGC-3’). All reactions were performed for 30 cycles with the following temperature profiles: 95°C for 5 min (initiation; 45 s/cycle thereafter); 56°C for 30 s (annealing); and 72°C for 30 s (extension). Size of the PCR products was examined by running 5 μl products on 3% agarose gel.
Multi-lineage differentiation of Hem-PCs
For the induction of adipogenesis and osteogenesis, cells were plated on cell culture coverslips (1101-020, Cosmobrand) and induced by adipogenic media (DMEM/10%FBS, 5 μg/ml insulin, 1 μM dexamethasone, 0.5 mM isobutylmethylxanthine, 60 μM indomethacin, and 1 × PG) and osteogenic media (DMEM/10%FBS, 10 mmol/L β-glycerophosphate, 1 μM dexamethasone, 100 umol/L antiscorbic acid, and 1 × PG). The medium was changed every 3 days. After induction for 14 days, cells were stained with Oil Red O (O0625, Sigma-Aldrich) and Alizarin red (A5533, Sigma-Aldrich) to detect adipocytes and osteoblasts, respectively. Chondrogenesis was induced in a “pellet” culture system with revision [17]. In summary, the freshly trypsinized cells were placed into 15 ml polypropylene tubes, centrifuged to form the “pellets”, and cultured in the chondrogenic media (DMEM/10%FBS, 1 × ITS-A, 1 μM dexamethasone, 100 umol/L antiscorbic acid, 10 ng/ml TGF-β1, and 1 × PG) for 21 days. Pellets were harvested, fixed by 10% buffered formaldehyde, paraffin imbedded, sliced into 5 μm sections, and stained by Alcian blue dye (A9186, Sigma-Aldrich).
Comparison of adipogenesis between Hem-PCs and hBM-MSCs
Hem-PCs and hBM-MSCs were plated on the culture coverslips in six-well culture dishes at 1 × 104 cells/well. When cells reached 80% confluence, adipogenic induction was initiated by the medium above. On the day 7 and 14 after induction, the coverslips were taken out, and Oil Red “O” staining was performed to observe the cytoplasmic lipid in cells. A bright-field microscope equipped with a digital camera (DS-L1-5M, Nikon, Japan) was used to observe the staining, and 10 pictures were taken randomly for each coverslip. The software “Image Proplus 6.0” was used to analyze the area of positive staining in each picture. The cells before induction were used as the control.
In vivo murine model of adipogenesis
The animal model was established as described previously with minor modifications [5]. All experiments were carried out with 1.5 × 106 cells/150 μL Matrigel per animal. Hem-PCs were trypsinized, counted, and resuspended in phenol red-free Matrigel (BD Biosciences). The cells/Matrigel mixture was injected subcutaneously into the back of 6-week-old, male athymic nu/nu mice (n = 4/group; Jinling Hospital, Nanjing, Jiangsu, China). Hem-PCs/Matrigel plugs were harvested at 1, 2, 4, and 8 weeks, fixed in 4% formaldehyde, paraffin embedded, and H-E stained to observe the structure of the plug. Immunohistochemistry staining was carried out to detect the expression of perilipin A, and human nuclear antigen in the plugs. The first antibodies included goat anti-human perilipin A antibody (ab61682, Abcam), and mouse anti-human nuclear antibody (MAB1281, 235-1, Millipore). MaxvisionTM HRP-polymer anti-mouse IHC kit and DAB kit (Maixin Biotechnology, China) were used according to the manufacturer’s instructions. hBM-MSCs/Matrigel compound was used as the control in the parallel study.
Statistics
Statistics was performed with Microsoft® Office Excel. Data were expressed as mean ± SD and analyzed by Student’s two-tailed t test where appropriate. Differences were considered significant at P < 0.05.
Results
Culture of PCs from hemangioma
PDGFR-β (+) PCs were isolated from both proliferating and involuting hemangiomas. Hem-PCs were morphologically fibroblast-like, grew to confluence in1-2 weeks (Figure 1A), and were trypsin passaged at the ratio 1:3. After passage, the cells grew quickly. The growth curve showed that the doubling time of the cells was about 2~3 days, which was similar to that of HUVECs, but shorter than that of hBM-MSCs and fibroblasts (Figure 1B). The cells remained their morphology at the 13th passage.
Figure 1.
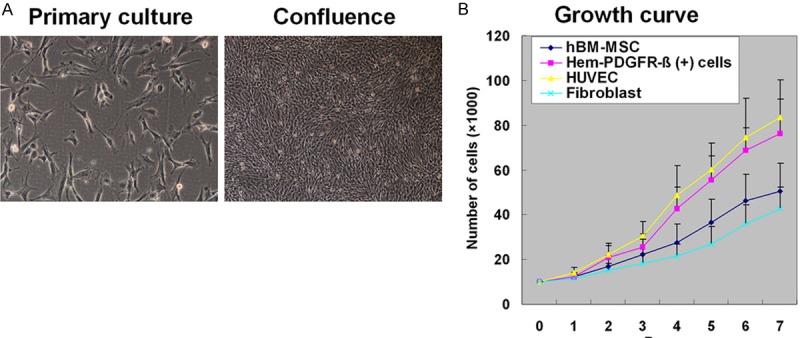
Culture of perivascular cells (PCs) from hemangioma. A: PDGFR-β (+) PCs selected by FACS are fibroblast-like and grow confluent in 1-2 weeks. B: The growth curve shows that the doubling time of PCs is 2~3 days, similar to that of HUVECs, shorter than that of hBM-MSCs and fibroblasts.
Antigenic marker profiles of Hem-PCs
Flow cytometry (Figure 2A) and immunofluorescence staining (Figure 2B) showed that Hem-PCs and hBM-MSCs uniformly expressed the MSC markers CD105, CD90, CD29, and vimentin, not the hematopoietic/endothelial markers CD45, CD34, CD31 and flt-1. The expression of pericyte marker PDGFR-β and α-SMA was observed in most Hem-PCs, and very low in hBM-MSCs. The expression of stem/progenitor cell marker CD133 was observed on the membrane of both Hem-PCs and hBM-MSCs, and the adipogenic transcription factor PPAR-γ in the nuclei. Only vimentin was expressed in fibroblasts. PCs from proliferating hemangioma had similar antigenic marker profiles with thatfrom involuting hemangioma. The antigenic marker profiles of the three kinds of cells are summarized in Table 1.
Figure 2.
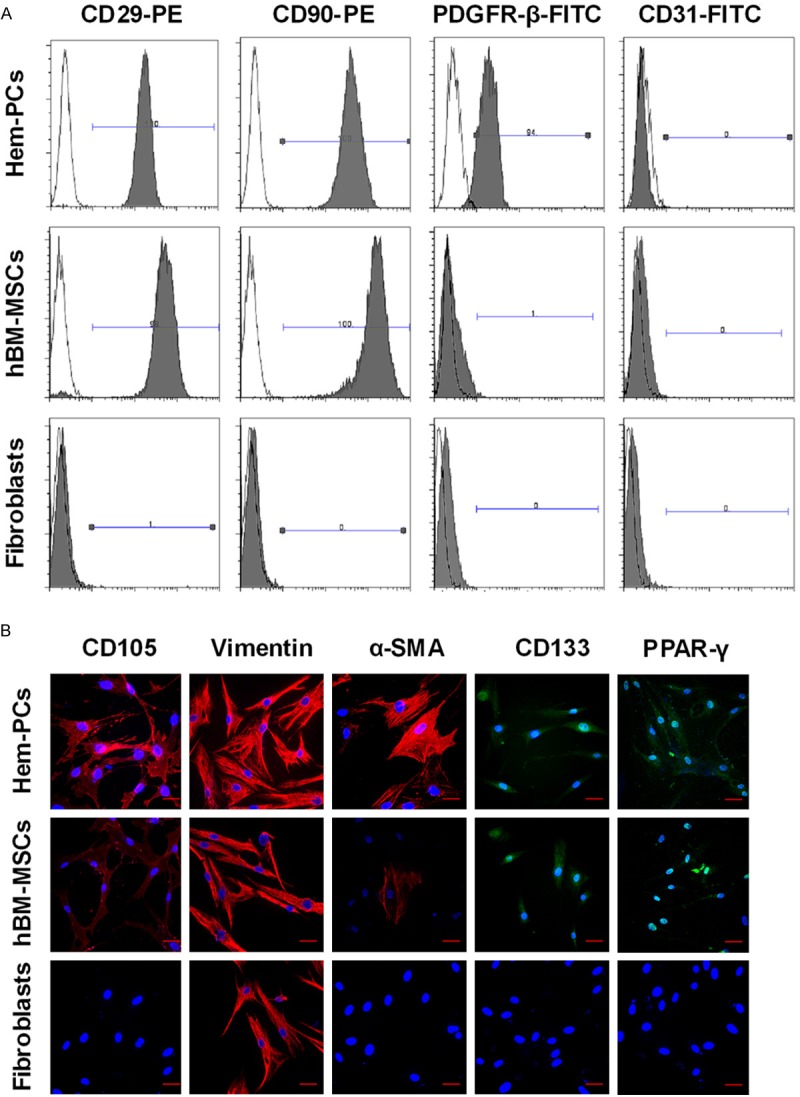
Antigenic marker profile of Hem-PCs. Flow cytometry (A) and immunofluorescence staining (B) show that Hem-PCs and hBM-MSCs uniformly express CD105, CD90, CD29, and vimentin, not CD31, CD45, CD34 and flt-1 (data not showed). PDGFR-β and α-SMA are expressed in most Hem-PCs, rarely seen in hBM-MSCs. Both Hem-PCs and BM-MSCs express CD133 on the membrane and PPAR-γ in the nucleus. Only vimentin expression is seen in fibroblasts. Scale bar: 50 μm.
Table 1.
Antigen profile of Hem-PCs, hBM-MSCs, and fibroblast
| Marker | Hem-PCs | hBM-MSCs | Fibroblasts |
|---|---|---|---|
| CD105 | + | + | - |
| CD90 | + | + | - |
| CD29 | + | + | - |
| Vimentin | + | + | + |
| PPAR-γ | + | + | - |
| CD133 | + | + | - |
| α-SMA | + | ± | - |
| PDFGR-β | + | - | - |
| Desmin | - | - | - |
| CD45 | - | - | - |
| CD34 | - | - | - |
| CD31 | - | - | - |
| flt-1 | - | - | - |
Expression of PPAR-γ2 and OCT-4 gene
PPAR-γ is an important transcription factor in promoting adipogenesis and plays a key role in the adipogenic differentiation of preadipocytes and mesenchymal stem cells. OCT-4 is a member of the POU transcription factor family, which is important for stem cells to maintain their pluripotency and self-renewal ability. In this study, RT-PCR showed that PPAR-γ2 gene was expressed by both Hem-PCs and hBM-MSCs, not by fibroblasts. OCT-4 gene was expressed by Hem-PCs, weakly expressed by hBM-MSCs, not by fibroblasts (Figure 3A). Semi-quantitatively analysis by “Image J” indicated that Hem-PCs expressed PPAR-γ2 gene and OCT-4 gene more strongly than hBM-MSCs (Figure 3B). PCs from involuting hemangioma had stronger expression of PPAR-γ2 gene and similar expression of OCT-4 gene than that from proliferating hemangioma.
Figure 3.
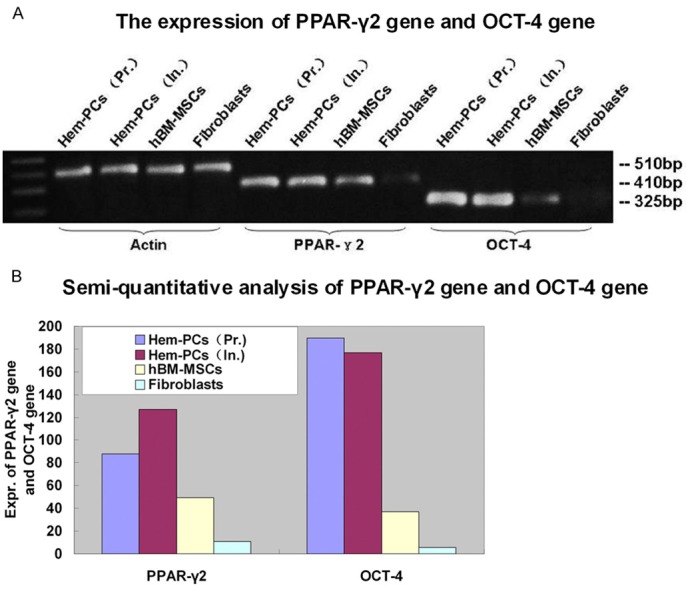
Expression of PPAR-γ2 and OCT-4 gene. A: RT-PCR shows that PPAR-γ2 gene expression is seen in both Hem-PCs and hBM-MSCs, not in fibroblasts. OCT-4 gene is expressed in Hem-PCs, weakly in hBM-MSCs, not in fibroblasts. B: Semi-quantitatively analysis by “Image J” indicates that Hem-PCs express PPAR-γ2 gene and OCT-4 gene more strongly than hBM-MSCs. PCs from involuting hemangioma had stronger expression of PPAR-γ2 gene and similar expression of OCT-4 gene compared with that from proliferating hemangioma.
Multilineage differentiation of Hem-PCs
As expected, our in vitro induction tests confirmed the multipotency of Hem-PCs. In adipogenic differentiation, the expression of oil red O-stained cytoplasmic lipid was observed on day 7 and increased on day 14. Alizarin red staining showed mineralization in the extracellular matrix, suggesting the osteogeic differentiation (Figure 4A). Hem-PCs also differentiated into chondroblasts. Alcian blue staining showed glycoprotein in the extracellular matrix in the pellets of Hem-PCs on day 21 (Figure 4A). hBM-MSCs also showed multi-lineage differentiation (Figure 4A). Fibroblasts did not display any of the differentiations tested.
Figure 4.
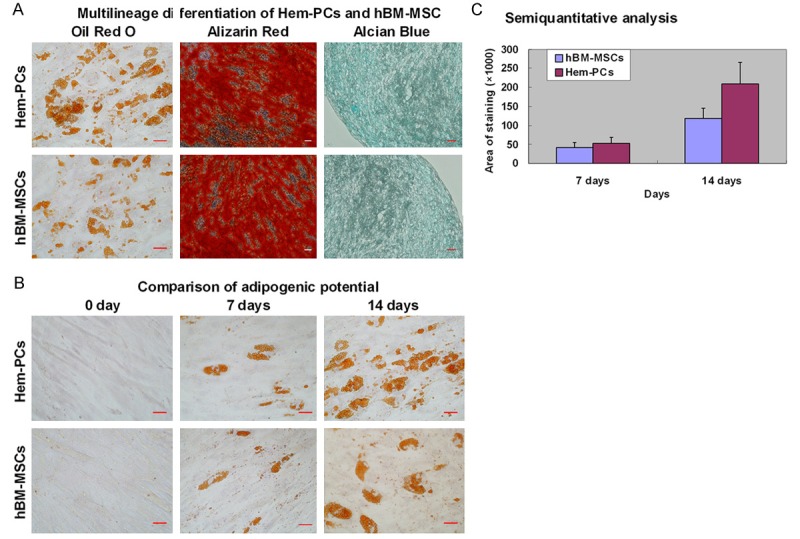
Multilineage differentiation of Hem-PCs. A: An in vitro induction confirm the multilineage differentiation of Hem-PCs and hBM-MSCs, including adipocytes (Oil Red O staining), osteoblasts (Alizarin red staining) and chondroblasts (Alcian Blue staining). B: Both Hem-PCs and hBM-MSCs differentiate into adipocytes in the adipogenic medium. C: Analysis of “Image Proplus 6.0” shows that the staining area of Hem-PCs on day 7 is larger than that of hBM-MSCs without statistically significant difference. And the staining area of Hem-PCs on day 14 is significantly larger than that of hBM-MSCs, suggesting stronger adipogenic potential of Hem-PCs than that of hBM-MSCs. Scale bar = 50 μm.
Hem-PCs have stronger adipogenic potential than hBM-MSCs
Both Hem-PCs and hBM-MSCs had multipotency in vitro. Stronger expression of PPAR-γ2 gene of Hem-PCs than that of hBM-MSCs suggested that Hem-PCs might have stronger adipogenic potential than hBM-MSCs. To confirm this, Hem-PCs and hBM-MSCs were induced by adipogenic medium for 7 and 14 days, stained with oil red “O” to detect cytoplasmic lipid (Figure 4A), and analyzed the area of red staining with “Image Proplus 6.0” Software. The results showed that the staining area of Hem-PCs on day 7 was larger than that of hBM-MSCs, though the difference was not statistically significant. On day 14, Hem-PCs showed significantly stronger staining than hBM-MSCs (Figure 4B, 4C).
Hem-PCs differentiated into adipocytes in vivo
To observe the in vivo differentiation of Hem-PCs, cells were mixed with Matrigel and subcutaneously injected into the back of immunodeficient mice. Hem-PCs/Matrigel plugs were removed 1-8 weeks after transplantation (Figure 5A). H-E staining showed a number of adipocytes at week 1, with abundant extracellular matrix; at week 2, there were more adipocytes, with reduced extracellular matrix; and at weeks 4 and 8, mature fat tissue developed (Figure 5B). To observe adipogenesis in Hem-PCs/Matrigel plugs, immunohistochemistry staining of perilipin A (one of adipose differentiation related proteins) were performed. The results showed positive staining in all Hem-PCs/Matrigel plugs. The most intensive expression of perilipin A was detected in the plug at week 2, suggesting active adipogenesis during this period (Figure 5B).
Figure 5.
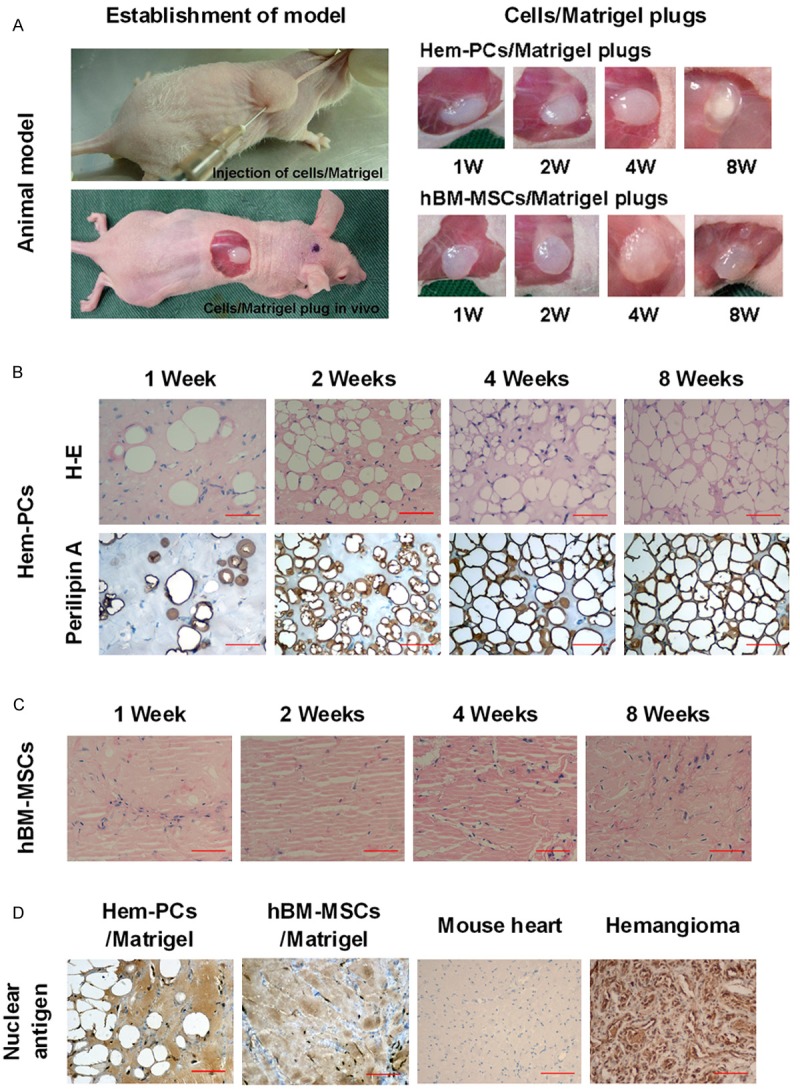
In vivo murine model of adipogenesis of Hem-PCs. A: Establishment of the animal model. B: H&E staining shows adipogenesis in the Hem-PCs/Matrigel plugs. Immunohistochemistry staining of perilipin A shows positive staining in all Hem-PCs/Matrigel plugs. The most intensive expression of perilipin A is seen in the plug at week 2. C: hBM-MSCs/Matrigel plugs did not display adipogenesis, and finally developed into fibro-connective-like tissue. D: Human nuclear antigen is expressed in adipocytes of the Hem-PCs/Matrigel plugs, fibroblasts of hBM-MSCs/Matrigel plugs, and hemangioma tissue, but not in the mouse heart. Scale bar: 100 μm.
In the parallel study, hBM-MSCs/Matrigel was implanted into the immunodeficient mice. No adipocyte was observed in any of the hBM-MSCs/Matrigel plugs. Instead, they finally developed into fibro-connective-like tissue (Figure 5C).
To identify the origin of the cells in plugs, human nuclear antigen in the Hem-PCs/Matrigel plugs and hBM-MSCs/Matrigel plugs was stained, using the proliferating hemangioma as the positive control and the mouse heart as the negative control. The expression of human nuclear antigen was observed in the adipocytes of Hem-PCs/Matrigel plugs, the fibroblasts of hBM-MSCs/Matrigel plugs, and hemangioma tissue, but not in the mouse heart, confirming that the cells in plugs were human-derived, not host-derived (Figure 5D).
Discussion
In this study, we isolated PDGFR-β (+) PCs from proliferating and involuting hemangiomas by FACS. Hem-PCs expressed both MSCs and pericytes markers, displayed multilineage differentiation in vitro, especially the strong adipogenic potential. In the immunodeficient mouse model, Hem-PCs differentiated into adipocytes.
There are a number of stem/progenitor cells isolated from hemangioma, including endothelial progenitor cells (Hem-EPCs) [14], mesenchymal stem cells (Hem-MSCs) [4], and CD133 (+) stem cells (Hem-SCs) [5]. Among them, Hem-SCs have been widely reported [5,15-20]. Hem-SCs displayed multi-lineage differentiation in vitro [5]. In an immunodeficient mouse model, microvessels appeared in Hem-SCs/Matrigel plugs, and finally regressed into adipose tissue [5]. Hem-SCs displayed both vasculogenesis/angiogenesis and adipogenesis in the animal model, indicating that they could differentiate into several subpopulations of cells, which initiate the vasculogenesis/angiogenesis firstly, and transfer to adipogenesis secondly. As expected, further study confirmed that Hem-SCs differentiated into GLUT (+) endothelial cells [18] and pericytes/smooth muscle cells [17] in vitro and in vivo.
PDGFR-β (+) Hem-PCs in our study also expressed CD133 and showed multilineage differentiation in vitro. But in the animal model, Hem-PCs displayed only adipogenesis, no vasculogenesis/angiogenesis. So we think that CD133 (+) PDGFR-β (+) Hem-PCs are a subpopulation of CD133 (+) Hem-SCs. They may be the intermediate cells between Hem-SCs and mature pericytes, such as the progenitor of pericytes or immature pericytes. Another subpopulation of Hem-SCs was CD133 (+) PDGFR-β (-) SCs. They may differentiate into EPCs and ECs. In early hemangioma, pericytes originating from CD133 (+) PDGFR-β (+) PCs and EPCs/ECs originating from CD133 (+) PDGFR-β (-) SCs initiate vasculogenesis/angiogenesis and lead to the rapid growth of tumor [21]. The abnormality of VEGF signaling pathway may be related to the abnormal active angiogenesis [22,23]. In hemangioma involution, EPCs/ECs become apoptotic and disappear [24-26], whereas PCs/pericytes remain in situ and differentiate into adipocytes, so that the microvessels in hemangioma are ultimately replaced by fibrofatty tissue (Figure 6).
Figure 6.
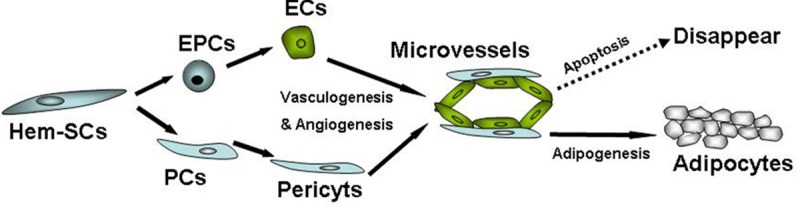
Possible evolution of hemangioma cells. Hem-SCs may contain two subpopulations, the CD133 (+) PDGFR-β (-) subpopulation and the CD133 (+) PDGFR-β (+) subpopulation (PCs). CD133 (+) PDGFR-β (-) cells may differentiate into EPCs/ECs, and initiate vasculogenesis/angiogenesis with pericytes originating from CD133 (+) PDGFR-β (+) PCs. In early hemangioma, active vasculogenesis/angiogenesis lead to rapid tumor growth. In hemangioma involution, EPCs/ECs become apoptotic and disappear, whereas PCs/pericytes differentiate into adipocytes so the microvessels in hemangioma are ultimately replaced by fibrofatty tissue.
PPAR-γ is an important transcription factor in promoting adipogenesis and plays a key role in the adipogenic differentiation of preadipocytes and mesenchymal stem cells [27-30]. The study of Tang [31] found that white fat progenitors (PPAR-γ-expressing cells) resided in the mural cell (PDGFR-β-expressing cell) compartment of the adipose vasculature. They found that PDGFR-β (+) cells from adipose tissue differentiated adipocytes in nude mice and the adipogenesis was stimulated by thiazolidinediones (TZDs), the agonist of PPAR-γ. In contrast, PDGFR-β (+) cells isolated from other organs, such as kidney, did not display such potential and were unresponsive to TZDs. Their results suggest that not all PDGFR-β (+) cells from different tissues had the adipogenic potential [31]. Our study observed that PDGFR-β (+) PCs from hemangioma strongly expressed PPAR-γ, and differentiated into adipocytes in vitro and in vivo. We suppose that TZDs, such as rosiglitazone, may be potential drugs for IH, which may promote the adipogenesis of PCs and accelerate hemangioma’s involution.
In conclusion, PDGFR-β (+) Hem-PCs expressed both MSCs and pericytes markers, displayed multilineage differentiation in vitro, especially strong adipogenesis, and differentiated into adipocytes in an immunodeficient mouse model. Our results indicated that PCs in hemangioma were specific MSCs with strong adipogenic potential, which might be the cellular basis of adipogenesis in hemangioma involution.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81272989). We thank Dr. Zhi-Jian Hong and Dr. Hui-Qing Jiang for their help in sample collection, and Bo Yu and Ru-Song Zhang for their technical assistance.
Disclosure of conflict of interest
None.
References
- 1.Greenberger S, Bischoff J. Pathogenesis of infantile haemangioma. Br J Dermatol. 2013;169:12–9. doi: 10.1111/bjd.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng JW, Zhang L, Zhou Q, Mai HM, Wang YA, Fan XD, Qin ZP, Wang XK, Zhao YF. A practical guide to treatment of infantile hemangiomas of the head and neck. Int J Clin Exp Med. 2013;6:851–60. [PMC free article] [PubMed] [Google Scholar]
- 3.Chiller KG, Passaro D, Frieden IJ. Hemangiomas of infancy: clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, sex. Arch Dermatol. 2002;138:1567–1576. doi: 10.1001/archderm.138.12.1567. [DOI] [PubMed] [Google Scholar]
- 4.Yu Y, Fuhr J, Boye E, Gyorffy S, Soker S, Atala A, Mulliken JB, Bischoff J. Mesenchymal Stem Cells and Adipogenesis in Hemangioma Involution. Stem Cells. 2006;24:1605–1612. doi: 10.1634/stemcells.2005-0298. [DOI] [PubMed] [Google Scholar]
- 5.Khan ZA, Boscolo E, Picard A, Psutka S, Melero-Martin JM, Bartch TC, Mulliken JB, Bischoff J. Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice. J Clin Invest. 2008;118:2592–2599. doi: 10.1172/JCI33493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Zebardast N, Lickorish D, Davies JE. Human umbilical cord perivascular cells (HUCPVC): A mesenchymal cell source for dermal wound healing. Organogenesis. 2010;6:197–203. doi: 10.4161/org.6.4.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paul G, Özen I, Christophersen NS, Reinbothe T, Bengzon J, Visse E, Jansson K, Dannaeus K, Henriques-Oliveira C, Roybon L, Anisimov SV, Renström E, Svensson M, Haegerstrand A, Brundin P. The adult human brain harbors multipotent perivascular mesenchymal stem cells. PLoS One. 2012;7:e35577. doi: 10.1371/journal.pone.0035577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feisst V, Brooks AE, Chen CJ, Dunbar PR. Characterization of mesenchymal progenitor cell populations directly derived from human dermis. Stem Cells Dev. 2014;23:631–42. doi: 10.1089/scd.2013.0207. [DOI] [PubMed] [Google Scholar]
- 10.Crisan M, Corselli M, Chen WC, Péault B. Perivascular cells for regenerative medicine. J Cell Mol Med. 2012;16:2851–60. doi: 10.1111/j.1582-4934.2012.01617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan SM, Chen RL, Shen WM, Chen HN, Zhou XJ. Mesenchymal stem cells in infantile hemangioma reside in the perivascular region. Pediatr Dev Pathol. 2012;15:5–12. doi: 10.2350/11-01-0959-OA.1. [DOI] [PubMed] [Google Scholar]
- 12.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 13.St Geme JW Jr, Horrigan DS. Comparative production of interferon by foreskin fibroblasts from newborn infants and children. J Lab Clin Med. 1969;74:946–949. [PubMed] [Google Scholar]
- 14.Yu Y, Flint AF, Mulliken JB, Wu JK, Bischoff J. Endothelial progenitor cells in infantile hemangioma. Blood. 2004;103:1373–5. doi: 10.1182/blood-2003-08-2859. [DOI] [PubMed] [Google Scholar]
- 15.Greenberger S, Boscolo E, Adini I, Mulliken JB, Bischoff J. Corticosteroid suppression of VEGF-A in infantile hemangioma-derived stem cells. N Engl J Med. 2010;362:1005–1013. doi: 10.1056/NEJMoa0903036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenberger S, Yuan S, Walsh LA, Boscolo E, Kang KT, Matthews B, Mulliken JB, Bischoff J. Rapamycin suppresses self-renewal and vasculogenic potential of stem cells isolated from infantile hemangioma. J Invest Dermatol. 2011;131:2467–76. doi: 10.1038/jid.2011.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boscolo E, Stewart CL, Greenberger S, Wu JK, Durham JT, Herman IM, Mulliken JB, Kitajewski J, Bischoff J. JAGGED1 signaling regulates hemangioma stem cell-to-pericyte/vascular smooth muscle cell differentiation. Arterioscler Thromb Vasc Biol. 2011;31:2181–92. doi: 10.1161/ATVBAHA.111.232934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boscolo E, Mulliken JB, Bischoff J. VEGFR-1 mediates endothelial differentiation and formation of blood vessels in a murine model of infantile hemangioma. Am J Pathol. 2011;179:2266–77. doi: 10.1016/j.ajpath.2011.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smadja DM, Guerin CL, Boscolo E, Bieche I, Mulliken JB, Bischoff J. α6-Integrin Is Required for the Adhesion and Vasculogenic Potential of Hemangioma Stem Cells. Stem Cells. 2014;32:684–93. doi: 10.1002/stem.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Mai HM, Zheng J, Zheng JW, Wang YA, Qin ZP, Li KL. Propranolol inhibits angiogenesis via down-regulating the expression of vascular endothelial growth factor in hemangioma derived stem cell. Int J Clin Exp Pathol. 2013;7:48–55. [PMC free article] [PubMed] [Google Scholar]
- 21.Boscolo E, Bischoff J. Vasculogenesis in infantile hemangioma. Angiogenesis. 2009;12:197–207. doi: 10.1007/s10456-009-9148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acevedo LM, Cheresh DA. Suppressing NFAT increases VEGF signaling in hemangiomas. Cancer Cell. 2008;14:429–430. doi: 10.1016/j.ccr.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Jinnin M, Medici D, Park L, Limaye N, Liu Y, Boscolo E, Bischoff J, Vikkula M, Boye E, Olsen BR. Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangioma. Nat Med. 2008;14:1236–1246. doi: 10.1038/nm.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Razon MJ, Kraling BM, Mulliken JB, Bischoff J. Increased apoptosis coincides with onset of involution in infantile hemangioma. Mircocirculation. 1998;5:189–195. [PubMed] [Google Scholar]
- 25.Hasan Q, Rüger BM, Tan ST, Gush J, Davis PF. Clusterin/apoJ expression during the development of hemangioma. Hum Pathol. 2000;31:691–697. doi: 10.1053/hupa.2000.7638. [DOI] [PubMed] [Google Scholar]
- 26.Mancini AJ, Smoller BR. Proliferation and apoptosis within juvenile capillary hemangiomas. Am J Dermatopathol. 1996;18:505–514. doi: 10.1097/00000372-199610000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Qian SW, Li X, Zhang YY, Huang HY, Liu Y, Sun X, Tang QQ. Characterization of adipocyte differentiation from human mesenchymal stem cells in bone marrow. BMC Dev Biol. 2010;10:47. doi: 10.1186/1471-213X-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott MA, Nguyen VT, Levi B, James AW. Current methods of adipogenic differentiation of mesenchymal stem cells. Stem Cells Dev. 2011;20:1793–804. doi: 10.1089/scd.2011.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu WH, Li FG, Chen XY, Li JT, Wu YH, Huang LH, Wang Z, Li P, Wang T, Lahn BT, Xiang AP. PPARγ suppression inhibits adipogenesis but does not promote osteogenesis of human mesenchymal stem cells. Int J Biochem Cell Biol. 2012;44:377–84. doi: 10.1016/j.biocel.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 30.Kim J, Kwak HJ, Cha JY, Jeong YS, Rhee SD, Cheon HG. The role of prolyl hydroxylase domain protein (PHD) during rosiglitazone-induced adipocyte differentiation. J Biol Chem. 2014;289:2755–64. doi: 10.1074/jbc.M113.493650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitors reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]


