Abstract
Matrix metalloproteinase (MMP)-2 and MMP-9, two important members of the matrix metalloproteinase family, have been shown critical contributions in intra-tumor angiogenesis and invasion of tumor progression, and they might also play important roles in the angiogenesis as well as the pannus formation of rheumatoid arthritis (RA). In the present study, we used the immunohistochemistry, the immunofluorescence staining and the con-focal scanning methods to characterize the immunolocalization of MMP-2 and MMP-9 in RA synovium tissues. Our results showed that both MMP-2 and MMP-9 immunostaining could be found in synoviocytes and vascular endothelial cells. Moreover, our con-focal scanning also showed that MMP-2 could be found in infiltrating CD14+ monocytes and CD68+ macrophages, and MMP-9 could be found in infiltrating CD68+ macrophages in RA synovium tissues, while weak or negative staining of these two MMPs could be found in infiltrating CD20+B cells and CD3+T cells in RA synovium. Thus, our finding suggests that both MMP-2 and MMP-9 expressed by synoviocytes as well as certain infiltrating immune cells role importantly in the angiogenesis in RA progression.
Keywords: Matrix metalloproteinase-2, matrix metalloproteinase-9, confocal scanning, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA), which is a chronic inflammatory autoimmune disease, affects approximately 1% of the population worldwide [1,2]. RA usually clinicopathologically represents as progressive impairment of many tissues and organs, especially attacks the peripheral joints and leads to cartilage and bone destruction [3,4]. The angiogenesis involves in perpetuating inflammatory and immune responses, as well as supporting pannus growth and development in RA [5]. The pannus with excessive angiogenesis in RA could also be viewed as non-malignant hyper-proliferative disease, pushing the progression of RA by cartilage and bone erosion, and leading to irreversible destruction of joint structure and function [6].
Matrix metalloproteinases (MMPs), a family of zinc-dependent endopeptidases, can degrade the basement membranes as well as other extracellular matrix components [7]. According to their structures and substrate specificities, MMPs are predominantly divided into five subgroups: collagenases, gelatinases, stromelysins, membrane-type MMPs and other MMPs [8,9]. MMPs have been shown to be closely related with tumor invasion, metastasis and intratumoral angiogenesis. Moreover, MMPs could also process several bio-active factors, apoptotic chemokines, and cell signaling factors, which affect immune responses [10]. MMPs also role important roles in RA, and the expression levels of certain members of the MMPs, such as MMP-3, MMP-8 and MMP-9, significantly associated the disease progression [11-15].
MMP-2 (also known as gelatine-A) and MMP-9 (also known as gelatinase-B), are the two key members of MMPs family, are capable of cleaving gelatine, type I, IV and V collagens, elastin and vitronectin [16]. The role of MMP-9 and MMP-2 in tumor angiogenesis has been supported by in vivo and in vitro evidences. As we know, the pannus with excessive angiogenesis behave in tumor-like fashion, therefore we herein suspected that MMP-2 and MMP-9, might also be involved in angiogenesis process and pannus formation in RA. In the present study, we used the immunohistochemistry, the immunofluorescence staining and the con-focal scanning methods to characterize the immunolocalization of MMP-2 and MMP-9 in RA synovium tissues, in order to investigate their roles in angiogenesis process and pannus formation of RA.
Methods and materials
Patient and tissue samples
The synovium tissues of the knee joint from one patient with RA at active stage were collected by using arthroscopic biopsy in the Department of Orthopedics of our hospital. The patient met the American College of Rheumatology criteria for RA, and the diagnosis of pathological changes was confirmed by using H&E staining. Laboratory assessment including the measurement of serum levels of C reactive protein (CRP), erythrocyte sedimentation rate (ESR), anti-CCP antibody, rheumatoid factors (RF), and blood routine examination were performed. The protocol for the present study was approved by the Ethics Committee of our hospital.
Antibodies and reagents
Rabbit anti-human MMP-2 monoclonal antibody, rabbit anti-human MMP-9 monoclonal antibody and mouse anti-human CD14 monoclonal antibody were purchased from Novus Biologicals (Littleton, CO, USA). Mouse anti-human CD3 monoclonal antibody (ready to use) was purchased from Beijing Zhongshan Golden Bridge Biology (Beijing, China). Mouse anti-human CD20 monoclonal antibody (ready to use), mouse anti-human CD68 monoclonal antibody (ready to use), mouse anti-human CD34 monoclonal antibody (ready to use), and goat serum used in blockade were purchased from Fuzhou Maxin Biotechnology (Fuzhou, China). Alexa Fluor® 488 goat anti-rabbit IgG (H+L) and Alexa Fluor® 555 goat anti-mouse IgG (H+L) were purchased from Invitrogen (Grand Island, NY, USA). Rabbit IgG was purchased from Southern Biotech (Birmingham, AL, USA), and mouse IgG1 was purchased from eBioscience (San Diego, CA, USA). 4,6-Diamino-2-phenyl indole (DAPI) used in fluorescent counterstaining of cell nuclei was purchased from Beyotime (Shanghai, China).
Immunofluorescence staining and confocal laser scanning
Formalin-fixed, paraffin-embedded rheumatoid synovium tissues were cut into consecutive 3-μm-thick sections. After the sections were dewaxed in xylene, rehydrated via graded ethanol solutions, antigen retrieval was performed by heating the sections for 30 min at 100°C in citrate solution or in EDTA solution when needed. Then the sections were cooled and rinsed in PBS for 5 min, and subsequently blocked using 3% bovine serum albumin. The sections were then incubated with primary antibody against MMP2 (diluted in 1:300) or anti-MMP-9 (diluted in 1:200) in combination with anti-CD3, anti-CD14 (diluted in 1:500), anti-CD20, anti-CD34 and anti-CD68, respectively, at 4°C overnight. The section incubated with rabbit IgG in combination with mouse IgG instead of the primary antibodies was performed as a negative control. After washing three times in PBS (5 min per wash), the sections were incubated with mixed Alexa Fluor® 488 goat anti-rabbit IgG (diluted in 1:100) and Alexa Fluor® 555 goat anti-mouse IgG (diluted in 1:100) for one hour at 37°C. Then the sections were rinsed in PBS for 5 min, and incubated with DAPI for 10 min to stain the nuclei and mounted by using anti-fade mounting medium. Finally, the sections were examined by using a Leica SP5 laser scanning confocal microscope (Leica Microsystems, Wetzlar, Germany).
Results
Immunolocalization of MMP-2 and MMP-9 in synoviocytes and vascular endothelial cells in RA synovium tissue
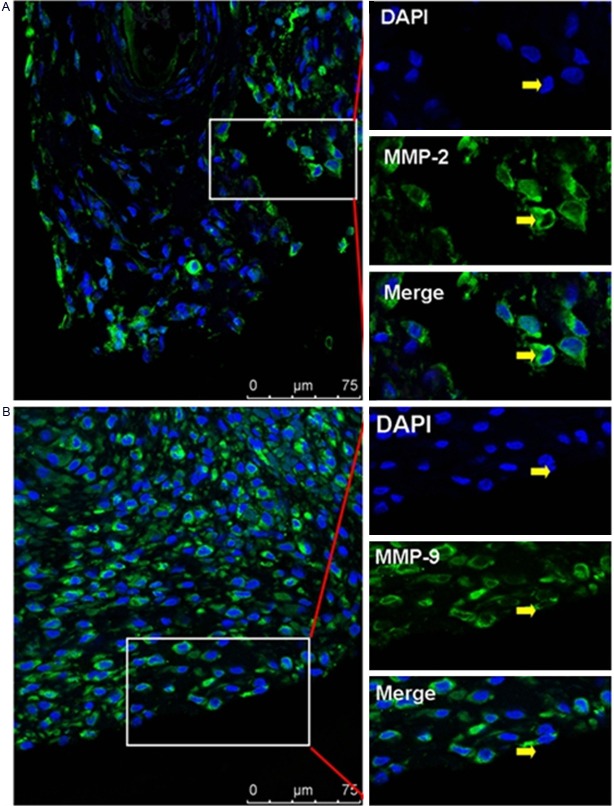
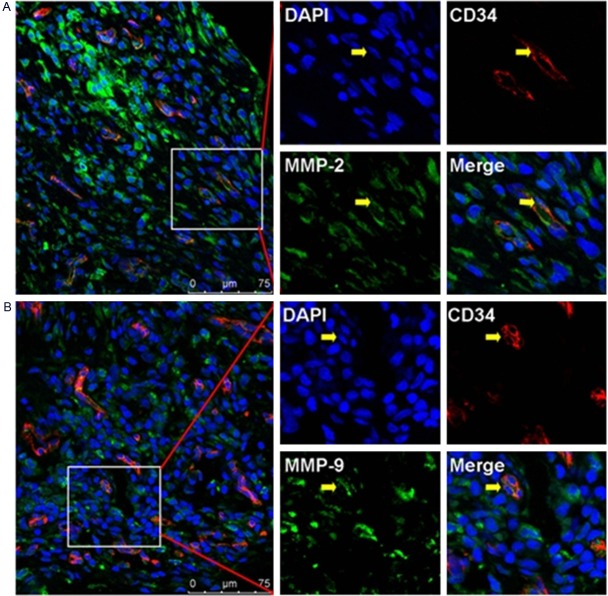
Our con-focal scanning results showed that the immunostaining of MMP-2 and MMP-9 could be found in the synoviocytes of synovium tissue from RA patient (Figure 1). Neo-vessels labeled by using CD34+ vascular endothelial cells could also be found in synovium tissue from RA patient, which implicated that rich blood vessel formation as well as pannus formation was involved in the progression of RA disease. And we also found that MMP-2+CD34+ or MMP-9+CD34+ vascular endothelial cells (Figure 2) involved in the pannus formation in synovium tissue of RA patient.
Figure 1.
A: Positive staining of MMP-2 in synoviocytes of rheumatoid synovium. B: Positive staining of MMP-9 in synoviocytes of rheumatoid synovium. Scale bar = 75 μm.
Figure 2.
Immunolocalization of MMP-2 and MMP-9 in vascular epithelial cells in RA synovium tissues. A: Positive staining of MMP-2 in CD34+ vascular epithelial cells. B: Positive staining of MMP-9 in CD34+ vascular epithelial cells. Scale bar = 75 μm.
Immunolocalization of MMP-2 and MMP-9 in infiltrating immune cells in RA synovium tissue
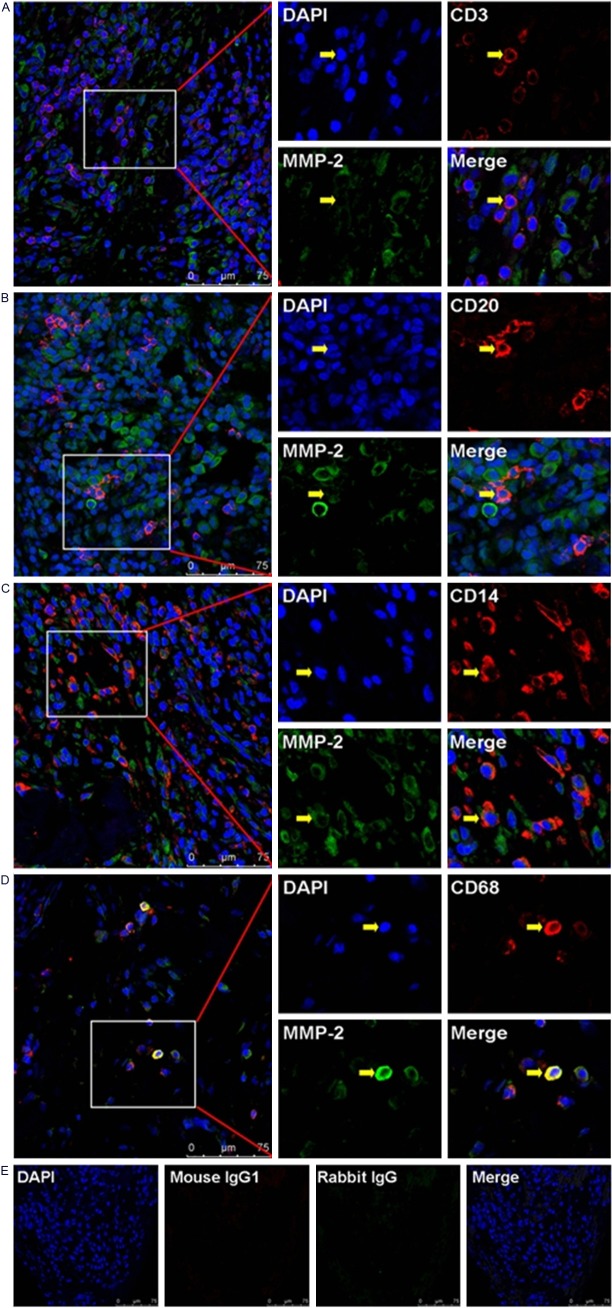
In the hyperplastic rheumatoid synovium tissue, we could found varieties of infiltrating immune cells, such as monocytes and lymphocytes. In order to investigate the immunolocalization of MMP-2 and MMP-9 in subsets of those immune cells, we used immunofluorescence double-staining and confocal scanning in the present study. Our data showed that the negative or weak immunostaining of MMP-2 could be found in infiltrating CD3+T cells and CD20+B cells, while the positive immunostaining of MMP-2 could be found in infiltrating CD68+ macrophages and CD14+ monocytos (Figure 3). Meanwhile, our data also showed that the negative or weak immunostaining of MMP-9 could be found in infiltrating CD3+T cells, CD20+B cells and CD14+ monocytos, while the positive immunostaining of MMP-9 could be found in infiltrating CD68+ macrophages (Figure 4).
Figure 3.
Immunolocalization of MMP-2 in infiltrating immune cells in RA synovium tissues. A: Negative staining of MMP-2 in infiltrating CD3+T cells. B: Negative staining of MMP-2 in infiltrating CD20+B cells. C: Positive staining of MMP-2 in infiltrating CD14+ monocytes. D: Positive staining of MMP-2 in infiltrating CD68+ macrophages. E: Negative controls. Scale bar = 75 μm.
Figure 4.
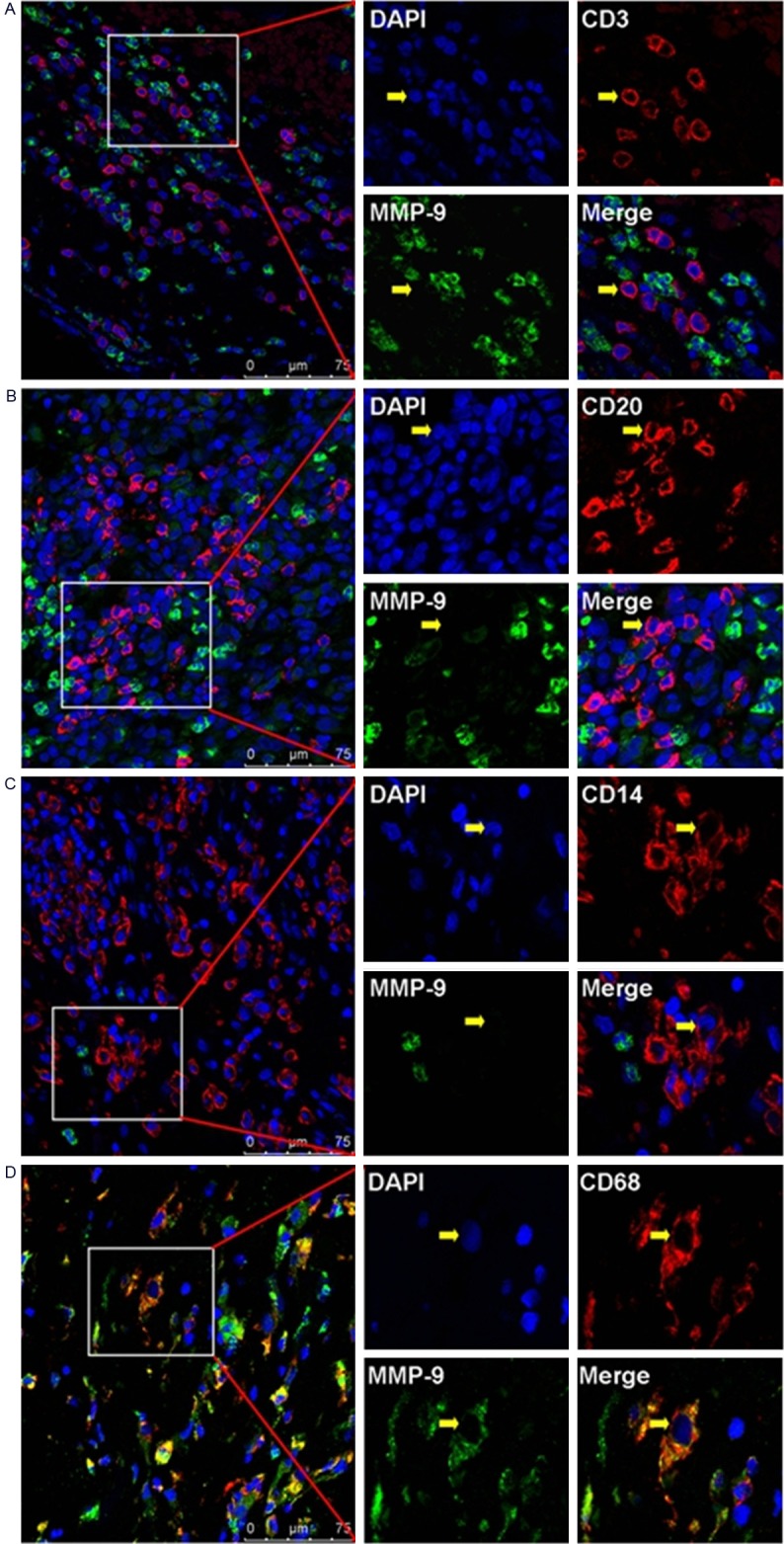
Immunolocalization of MMP-9 in infiltrating immune cells in RA synovium tissues. A: Negative staining of MMP-9 in infiltrating CD3+T cells. B: Negative staining of MMP-9 in infiltrating CD20+B cells. C: Negative staining of MMP-9 in infiltrating CD14+ monocytes. D: Positive staining of MMP-9 in infiltrating CD68+ macrophages. Scale bar = 75 μm.
Discussion
The pannus formation, which is defined as the microscopic invasive granulation tissue covering the articular cartilage and synovium in the progression of RA, is involved in the cartilage destruction [17]. The angiogenesis, a critical step in the pannus formation, provides oxygen and nutrients to the hypertrophic granulation tissue, and also provides the means for recruitment of inflammatory cells forward to the articular synovium, and finally leads to the irreversible destruction of joint structure and function [2,6]. As we all know, the healthy joints usually maintain a balance between the synthesis of extracellular matrix molecules and the proteolytic degradation of damaged ones, and this balance is shifted toward matrix destruction in the progression of RA due to the increased production of cleavage enzymes [18,19]. MMP-2 and MMP-9 are the two major components of matrix metalloproteinases family. It has been demonstrated that the expression levels of MMP-2 and MMP-9 in peripheral blood or in synovial fluid of patients with rheumatoid arthritis [20,21]. However, the detailed localization and the potential function of these matrix metalloproteinases in rheumatoid synovium tissues are still remains elusive.
In the present study, we found that the immunostaining of MMP-2 and MMP-9 could be found in the synoviocytes and CD34+ vascular endothelial cells of synovium tissue from RA patient, suggesting that MMP-2 and MMP-9 involved in the progression of RA, especially in the physio-pathological process of the angiogenesis and the pannus formation. Moreover, the positive immunostaining of MMP-2 could be found in infiltrating CD68+ macrophages and CD14+ monocytos, and the positive immunostaining of MMP-9 could be found in infiltrating CD68+ macrophages in rheumatoid synovium tissues.
MMP-2 could modulate the vascular endothelial growth factor (VEGF) availability and played an important role in angiogenesis, and enhanced capability of growth and invasion in human tumors [22,23]. And MMP-9 has been described to release the biologically active form of VEGF and has direct proteolytic degradation of vascular basement membrane proteins [24]. It was also reported a long-term role for MMP-9 in vascular remodeling after islet transplantation and re-vascularization which was indicated by the impaired revascularization of islets transplanted to MMP-9 deficient mice [25]. In according to the results of several reports suggesting that MMP-2 and MMP-9 are important for angiogenesis. Kim et al. [21] reported that the high levels of MMP-2 and MMP-9 in synovial fluid of RA which were greatly higher than that in OA, and found that the level of MMP-9 was associated with the level of VEGF in both RA and OA. So those two gelatinases may also taking parts in neo-vascularization in RA progression. In this study, we investigated the immunolocalization of MMP-2 and MMP-9 in the rheumatoid synovium, and the data showed that both MMP-2 and MMP-9 could be expressed by synoviocytes, CD34+ endothelial cells, which was consistent with the report above and could also support the notion that these two matrix metalloproteinases expressed by synoviocytes may be involved in the pannus formation in RA progression.
The immune cell subsets are important components of the RA pannus, and such inflammatory process is characterized by the infiltration of inflammatory cells such as macrophages, T cells, B cells, monocytes and dendritic cells into the joints in RA [6]. The gelatinases MMP-2 and MMP-9 have been found in tumor infiltrating CD3+ lymphocytes, suggesting the increased expression in tumor infiltrating lymphocytes might also be involved in the intratumoral angiogenesis [26]. And it also has been reported that various immune cells, such as macrophages, neutrophils and mast cells, initiate tumor angiogenesis by induced the expression of proteases such as MMP-9 [27]. Thus the information of the detailed immunolocalization of those gelatinases in those immune cell subsets in rheumatoid synovium still remains elusive. Therefore, we herein studied the immunolocalization of MMP-2 and MMP-9 in some major subsets of infiltrating immune cells in rheumatoid synovium tissue. And our data showed that the positive immunostaining of both MMP-2 and MMP-9 could be found in infiltrating CD68+ macrophages, and MMP-2 could also be found in infiltrating CD14+ monocytes, whereas negative expression of both MMP-2 and MMP-9 can be found on infiltrating CD3+T cells as well as CD20+B cells. Our data suggested that the macrophages and the monocytes were major component of RA pannus and might contribute to locally cartilage destruction and blood vessel growth by the mean of releasing collagenolytic enzymes like MMP-2 and MMP-9.
In conclusion, our present study demonstrated that that the expression of MMP-2 and MMP-9 can be found on synoviocytes, endothelial cells, monocytes and macrophages in rheumatoid synovium, which indicate an important role of these two molecules in the pannus formation and invasion in RA progression, and the detailed mechanism merits further research.
Acknowledgements
This work was supported in part by grants from the National Natural Science Foundation of China (81301960), the Changzhou Health Bureau (ZD201207), the Bureau of Science and Technology of Changzhou (CJ20122008), and the Innovative Talents Training Project of Changzhou Health Bureau.
Disclosure of conflict of interest
None.
References
- 1.Harris ED Jr. Rheumatoid arthritis. Pathophysiology and implications for therapy. New Engl J Med. 1990;322:1277–89. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- 2.Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358:903–11. doi: 10.1016/S0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- 3.Schett G, Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol. 2012;8:656–64. doi: 10.1038/nrrheum.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crotti TN, Dharmapatni AA, Alias E, Zannettino AC, Smith MD, Haynes DR. The immunoreceptor tyrosine-based activation motif (ITAM) -related factors are increased in synovial tissue and vasculature of rheumatoid arthritic joints. Arthritis Res Ther. 2012;14:R245. doi: 10.1186/ar4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kong X, Zhang Y, Liu C, Guo W, Li X, Su X, Wan H, Sun Y, Lin N. Anti-angiogenic effect of triptolide in rheumatoid arthritis by targeting angiogenic cascade. PLoS One. 2013;8:e77513. doi: 10.1371/journal.pone.0077513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Lu Y, Chu Y, Xie J, Ding W, Wang F. Tissue factor expression in rheumatoid synovium: a potential role in pannus invasion of rheumatoid arthritis. Acta Histochem. 2013;115:692–7. doi: 10.1016/j.acthis.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Murphy G, Knauper V, Atkinson S, Butler G, English W, Hutton M, Stracke J, Clark I. Matrix metalloproteinases in arthritic disease. Arthritis Res. 2002;4(Suppl 3):S39–49. doi: 10.1186/ar572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kerkela E, Ala-Aho R, Jeskanen L, Rechardt O, Grenman R, Shapiro SD, Kahari VM, Saarialho-Kere U. Expression of human macrophage metalloelastase (MMP-12) by tumor cells in skin cancer. J Invest Dermatol. 2000;114:1113–9. doi: 10.1046/j.1523-1747.2000.00993.x. [DOI] [PubMed] [Google Scholar]
- 9.Chen L, Di D, Luo G, Zheng L, Tan Y, Zhang X, Xu N. Immunochemical staining of MT2-MMP correlates positively to angiogenesis of human esophageal cancer. Anticancer Res. 2010;30:4363–8. [PubMed] [Google Scholar]
- 10.Korpi JT, Hagstrom J, Lehtonen N, Parkkinen J, Sorsa T, Salo T, Laitinen M. Expression of matrix metalloproteinases-2, -8, -13, -26, and tissue inhibitors of metalloproteinase-1 in human osteosarcoma. Surg Oncol. 2011;20:e18–22. doi: 10.1016/j.suronc.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 11.de Rooy DP, Zhernakova A, Tsonaka R, Willemze A, Kurreeman BA, Trynka G, van Toorn L, Toes RE, Huizinga TW, Houwing-Duistermaat JJ, Gregersen PK, van der Helm-van Mil AH. A genetic variant in the region of MMP-9 is associated with serum levels and progression of joint damage in rheumatoid arthritis. Ann Rheum Dis. 2014;73:1163–9. doi: 10.1136/annrheumdis-2013-203375. [DOI] [PubMed] [Google Scholar]
- 12.Houseman M, Potter C, Marshall N, Lakey R, Cawston T, Griffiths I, Young-Min S, Isaacs JD. Baseline serum MMP-3 levels in patients with Rheumatoid Arthritis are still independently predictive of radiographic progression in a longitudinal observational cohort at 8 years follow up. Arthritis Res Ther. 2012;14:R30. doi: 10.1186/ar3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahmoud RK, El-Ansary AK, El-Eishi HH, Kamal HM, El-Saeed NH. Matrix metalloproteinases MMP-3 and MMP-1 levels in sera and synovial fluids in patients with rheumatoid arthritis and osteoarthritis. Ital J Biochem. 2005;54:248–57. [PubMed] [Google Scholar]
- 14.Zeisel MB, Druet VA, Wachsmann D, Sibilia J. MMP-3 expression and release by rheumatoid arthritis fibroblast-like synoviocytes induced with a bacterial ligand of integrin alpha5beta1. Arthritis Res Ther. 2005;7:R118–26. doi: 10.1186/ar1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mattey DL, Nixon NB, Dawes PT. Association of circulating levels of MMP-8 with mortality from respiratory disease in patients with rheumatoid arthritis. Arthritis Res Ther. 2012;14:R204. doi: 10.1186/ar4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakrabarti S, Patel KD. Matrix metalloproteinase-2 (MMP-2) and MMP-9 in pulmonary pathology. Exp Lung Res. 2005;31:599–621. doi: 10.1080/019021490944232. [DOI] [PubMed] [Google Scholar]
- 17.Furuzawa-Carballeda J, Macip-Rodriguez PM, Cabral AR. Osteoarthritis and rheumatoid arthritis pannus have similar qualitative metabolic characteristics and pro-inflammatory cytokine response. Clin Exp Rheumatol. 2008;26:554–60. [PubMed] [Google Scholar]
- 18.Lee A, Park K, Choi SJ, Seo DH, Kim K, Kim HS, Kwon IC, Choi K, Youn SY, Youn I. Prediction of anti-arthritic drug efficacies by monitoring active matrix metalloproteinase-3 (MMP-3) levels in collagen-induced arthritic mice using the MMP-3 probe. Mol Pharm. 2014;11:1450–8. doi: 10.1021/mp400622q. [DOI] [PubMed] [Google Scholar]
- 19.Antipova O, Orgel JP. Non-enzymatic decomposition of collagen fibers by a biglycan antibody and a plausible mechanism for rheumatoid arthritis. PLoS One. 2012;7:e32241. doi: 10.1371/journal.pone.0032241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang YH, Lin IL, Tsay GJ, Yang SC, Yang TP, Ho KT, Hsu TC, Shiau MY. Elevated circulatory MMP-2 and MMP-9 levels and activities in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Biochem. 2008;41:955–9. doi: 10.1016/j.clinbiochem.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Kim KS, Choi HM, Lee YA, Choi IA, Lee SH, Hong SJ, Yang HI, Yoo MC. Expression levels and association of gelatinases MMP-2 and MMP-9 and collagenases MMP-1 and MMP-13 with VEGF in synovial fluid of patients with arthritis. Rheumatol Int. 2011;31:543–7. doi: 10.1007/s00296-010-1592-1. [DOI] [PubMed] [Google Scholar]
- 22.Rojiani MV, Alidina J, Esposito NandRojiani AM. Expression of MMP-2 correlates with increased angiogenesis in CNS metastasis of lung carcinoma. Int J Clin Exp Pathol. 2010;3:775–81. [PMC free article] [PubMed] [Google Scholar]
- 23.Badiga AV, Chetty C, Kesanakurti D, Are D, Gujrati M, Klopfenstein JD, Dinh DH, Rao JS. MMP-2 siRNA inhibits radiation-enhanced invasiveness in glioma cells. PLoS One. 2011;6:e20614. doi: 10.1371/journal.pone.0020614. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids. 2011;41:271–90. doi: 10.1007/s00726-010-0689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christoffersson G, Vagesjo E, Vandooren J, Liden M, Massena S, Reinert RB, Brissova M, Powers AC, Opdenakker G, Phillipson M. VEGF-A recruits a proangiogenic MMP-9-delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue. Blood. 2012;120:4653–62. doi: 10.1182/blood-2012-04-421040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jedryka M, Chrobak A, Chelmonska-Soyta A, Gawron D, Halbersztadt A, Wojnar A, Kornafel J. Matrix metalloproteinase (MMP)-2 and MMP-9 expression in tumor infiltrating CD3 lymphocytes from women with endometrial cancer. Int J Gynecol Cancer. 2012;22:1303–9. doi: 10.1097/IGC.0b013e318269e27b. [DOI] [PubMed] [Google Scholar]
- 27.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]