Abstract
This study aimed to assess BA impact on inflammation markers and repair of intestinal mucosa. Forty-eight rats were randomly divided into stress (n = 24) and BA (n = 24) groups. Stress was induced by fettering in all animals, fed enterally with 125.4 kJ/kg/d and 0.2 g/kg/d nitrogen. Then, rats were treated for 8 days with 5 mg/kg/d BA (BA group) or 5 mg/kg/d saline (Stress group). Levels of NF-κB, IL-10, TNF-α, and IFN-γ were measured at different time points, in plasma and intestinal mucosa samples. Changes in intestinal mucosa morphology were observed by electron microscopy. Plasma and/or mucosal levels of NF-κB, TNF-α, and IFN-γ were significantly higher in both groups after stress induction (P < 0.05). These high levels persisted in control animals throughout the experiment, and were significantly reduced in the BA group, 3 and 8 days after stress induction (P < 0.05). Interestingly, IL-10 levels were increased after BA treatment (P < 0.05). At day 8, ileal mucosal villi and crypt structure were significantly restored in the BA group. Bifidobacterial adhesin plays a role in repairing intestinal mucosa injury after stress by regulating the release of inflammatory mediators in the intestinal mucosa.
Keywords: Bifidobacterial adhesion, restrain stress, intestinal mucosa, NF-κB, cytokines
Introduction
Stress is a constellation of events, which begins with a stimulus (stressor), which precipitates a reaction in the brain (stress perception), which subsequently results in the activation of certain physiologic systems in the body (stress response) [1]. Acute stressors are the briefest and often involve a tangible threat that is readily identified as a stressor, in contrast to chronic stressors of longer duration [2]. Stress conditions induce intestinal mucosa damage, release of enterogenous inflammatory mediators and cytokines, causing inflammatory reaction syndrome [3,4]. This will in turn result in easy endotoxin uptake through damaged mucosal layers of the intestine and further inflammation, causing multiple organ dysfunction [5,6].
Interestingly, probiotics can act as biological immunomodulators in healthy people, increasing both intestinal and systemic immune responses and their use in stress situations may aid in reinforcing the immune system [7]. For instance, Bifidobacteria, the most important probiotics in the human intestine, have an important role in maintaining the balance of intestinal microflora and intestinal mucosal barrier function [8]. Bifidobacterium adhesin (BA) is a protein with multistructure and multifunction [9-11], short peptides, glycoproteins, glycolipids and polysaccharides [12-15]. BA is located in cell wall, flagella, capsules, and small filaments of Bifidobacteria [16,17]. It is known that BA mediates bacterial adhesion to target cells and stimulates cell signal transduction during adhesion [18]. In addition, this adhesin inhibits colonization intestinal mucosa by pathogenic bacteria, competing for pathogen specific binding sites on the surface of intestinal mucosa [19,20]. However, the effect of BA on stress is largely unknown. Therefore, this study aimed to assess the effects of BA on intestinal barrier function under stress conditions and unveil the mechanisms underlying such effects.
We found that treatment of stressed rats with BA resulted in significant repair of the ileal mucosa. In addition, plasma and/or tissue levels of pro-inflammatory cytokines (NF-κB, TNF-α, and IFN-γ) were significantly lower in the BA group, concomitantly with reduced IL-10 levels, compared with saline treated control animals.
These findings suggest that BA plays a role in repairing intestinal mucosa injury after stress by regulating the release of inflammatory mediators in the intestinal mucosa.
Materials and methods
Animal stress model
All experimental procedures were approved by the Animal Use Committee of East Hospital, Tong Ji University School of Medicine.
A total of 48 male Sprague-Dawley rats (155 ± 17 g) were purchased from the experimental animal center of TongJi University and randomly divided into stress (control) and stress + bifidobacterium adhesin (BA) groups (n = 24) after three days of adaptive feeding. Control and BA rats were placed in 3~6 cm radius iron barrel-shaped small cabins, fed in single cages with restricted activity and restraint stress 6 h every day for 21 days, resulting in rat stress model.
Animal treatment
Stressed rats were randomly divided into control and BA groups (n = 24). Rats in each group were housed singly in a clean animal room, in stainless steel cages. Rats were enterally fed Nutrison (Nutricia, China) with the same energy (125.4 kJ/kg/d) and nitrogen (0.2 g/kg/d). BA animals were orally administered 5 mg/kg/d adhesin while control rats received 5 mg/kg/d saline. Food intake and weight changes were recorded. Before modeling (normal parameters), after modeling (after stress was established), and at 3 and 8 days of treatment (with BA or saline), 6 rats were sacrificed and 5 ml femoral artery blood collected from each animal. Serum was obtained by centrifugation of blood samples for 10 min at 3000 rpm and stored at -80°C until use. Intestinal tissues were extracted and the small intestine 10 cm away from the ileocecal valve was used for pathological analysis.
Extraction and purification of bifidobacterium adhesion
Bifidobacterium adolescent 1027 was inoculated in thioglycollate broth and incubated anaerobically at 37°C for 48 h. In liquid cultures, Bifidobacterium adhesin (BA) is a secreted protein. To obtain BA, culture supernatants were by centrifugation and precipitated with 300 g/L ammonium sulfate. Then, the precipitate was collected by centrifugation at 4°C at 8000 rpm for 30 min. For purification of BA, 16 kDa, supernatant constituents were separated on a Superdex75 column gel filtration column and the fraction containing the protein subsequently submitted to ion exchange chromatography using Q-SepharoseFF.
Immunohistochemical detection NF-κB in intestinal tissues
Tissue sections were prepared from ileum samples of SD rats and stained with H&E. Histological changes in the intestinal villi and crypt structure etc. were observed under the electron microscopy.
Tissue immunochemistry staining was carried out according to previous reports [43]. Rabbit anti-human NF-κB polyclonal antibodies were purchased from Bioworld (USA). A broad spectrum immunohistochemical SP method kit SP-9000 and a DAB chromogenic kit were purchased from Beijing Zhongshan Golden Bridge Company (China). A known positive biopsy was used as a positive control and phosphate buffered saline (PBS) as a negative control instead of primary antibodies. Assessment was done by the semi-quantitative method, based on the scope of cell staining: positive cells < 5%, 5 to 25%, 26~50%, > 50% were denoted as 0, 1, 2, 3, respectively. The staining intensity of positive cells on each slice by uncolored, yellow, brown and tan were labeled 0, 1, 2, 3. In accordance with the sum of the two ratings samples were negative if value of < 3 was obtained and positive value ≥ 3.
Assessment of plasma and intestinal mucosal IL-10, TNF-α and IFN-γ
Two cm small intestine was dissected near the jejunum proximal and washed with saline. 200~300 mg intestinal mucosa was scraped with a scalpel and homogenized. The resulting homogenates were diluted with a 9-fold volume of saline, centrifuged at 800 × g for 10 minutes, and supernatants collected. IL-10, IFN-γ and TNF-α contents were evaluated by ELISA using specific kits purchased from R&D (USA). Plasma concentrations of cytokines were expressed in ng/L, and the intestinal mucosa content expressed in pg/g (wet weight).
Statistical analysis
The SPSS (version 13.0) software program (SPSS Company, Chicago, Illinois, USA) was used for statistical analyses. Measurement data were expressed as mean ± standard deviation. Means in each group were compared by paired t-test. The χ 2 test was used in counted values. P < 0.05 was considered statistically significant.
Results
Assessment of morphological changes in the intestinal mucosa
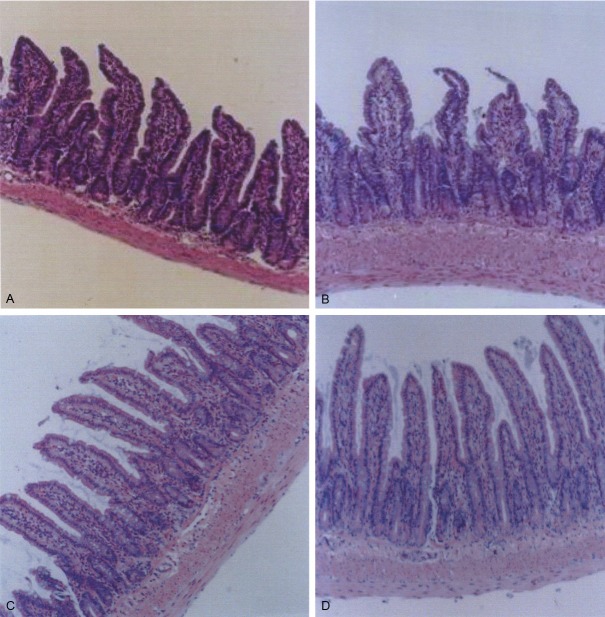
The structure of rat intestinal mucosa villi and crypts before modeling was normal as analyzed by electron microscopy (data not shown). In addition, no inflammatory cells and lymphocytes were found in the lamina propria (Figure 1A). After modeling villi and crypt structures were damaged to some extent: the hair became thin and shorter; a heavy inflammatory cell infiltration in the intrinsic membrane was observed; lymphatic dilatation and edema were present (Figure 1B). However, 8 days after treatment with BA, intestinal villi and crypt structure recovered significantly compared with the control group and rats after stress induction, showing organ structure similar to that observed before modeling. However, a small amount of inflammatory cells were observed in the intrinsic membrane (Figure 1C). Compared with rats after modeling, there was no obvious recovery of intestinal villi and crypt architecture in the control group: marked inflammatory cell infiltration in the intrinsic membrane (Figure 1D).
Figure 1.
Changes in the morphology of ileum intestinal mucosal in rats before modeling (A), after modeling (B), and after 8 days treatment of stressed rats with BA (C) and saline (D) H&E staining, × 100.
Immunohistochemical detection of NF-κB in rat intestinal tissues
Positive NF-κB cells in the control group were 0.0, 79.0, 63.5, and 60.6%, before modeling, after modeling, and 3 and 8 days of treatment, respectively. In the BA group, 0.0, 72.4, 55.7, and 40.2% were obtained before modeling, after modeling, and 3 and 8 days of treatment, respectively. These data suggested that more cells in the intestinal mucosa produced NF-κB after stress induction, regardless to animal groups. In addition, fewer intestinal cells expressed NF-κB after 3 and 8 days after treatment with BA, a statistically significant difference compared with control rats (χ2 = 29.73, 32.54, P = 0.000), although similar levels of NF-κB cells were obtained after stress induction (79.0 vs. 72.4% in control and BA groups, respectively, P > 0.05). (Table 1).
Table 1.
Expression of NF-κB examined by immunohistochemistry in intestinal mucosal (n%)
| Group | n | Before | After | 3rd day | 8th day | P1 | P2 | P3 |
|---|---|---|---|---|---|---|---|---|
| Control | 24 | 0 (0.0) | 19 (79.2) | 15 (63.5) | 16 (66.7) | 0.000 | 0.000 | 0.000 |
| Adhesion | 24 | 0 (0.0) | 17 (68.4) | 14 (55.7) | 11 (45.8)a,b | 0.000 | 0.000 | 0.000 |
P4 = 0.015;
P5 = 0.021.
P values were represented comparisons between animals before modeling and after modeling (P1), before modeling and 3 days of treatment (P2), before modeling and 8 days of treatment (P3), BA group and control groups after 8 days treatment (P4), and BA group and before modeling (P5).
Intestinal mucosa and plasma levels of IL-10, TNF-α and IFN-γ
Tissue and plasma TNF-α and IFN-γ levels were markedly increased after stress induction in both groups, statistically significant difference at P = 0.000 (Table 2). However, IL-10 levels were similar before and after stress induction (P > 0.05). In the control group, intestinal mucosa and plasma levels of IFN-γ and TNF-α significantly increased in comparison with those obtained before modeling both 3 and 8 days after stress induction (P = 0.000); the difference in plasma IL-10 levels was not statistically significant (P > 0.05) in control animals. Interestingly, rats treated with BA showed tissue and plasma levels of IL-10, TNF-α and IFN-γ similar to those of rats before stress induction at P > 0.05. Unsurprisingly, TNF-α and IFN-γ levels were significantly lower in tissue and plasma samples of BA rats, in comparison with control animals, both at days 3 and 8 (P < 0.05). This BA induced decrease in proinflammatory cytokines was accompanied by a marked decrease in IL-10 levels (P < 0.05), as shown in Table 2.
Table 2.
Changes in TNF-α, IFN-γ and IL-10 levels in rat plasma and intestinal mucosa
| Group | n | Intestinal mucosa (pg/g) | Plasma (ng/L) | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| TNF-α | IFN-γ | IL-10 | TNF-α | IFN-γ | IL-10 | ||
| Stress group | 24 | ||||||
| Before modeling | 6 | 154.63 ± 17.52 | 39.47 ± 5.76 | 56.82 ± 7.33 | 83.31 ± 9.78 | 17.35 ± 2.62 | 38.17 ± 4.3 |
| After modeling | 6 | 198.72 ± 26.59 | 55.32 ± 5.93 | 59.31 ± 7.26 | 117.64 ± 15.37 | 28.73 ± 4.17 | 37.26 ± 3.92 |
| 3rd day | 6 | 215.76 ± 31.54 | 58.16 ± 7.38 | 56.78 ± 7.02 | 125.71 ± 17.38 | 29.35 ± 4.76 | 37.88 ± 4.02 |
| 8th day | 6 | 211.83 ± 33.61 | 56.37 ± 7.29 | 59.35 ± 7.51 | 141.26 ± 19.65 | 30.25 ± 3.67 | 38.35 ± 4.03 |
| P1 | 0.035 | 0.039 | 0.086 | 0.028 | 0.018 | 0.073 | |
| P2 | 0.026 | 0.032 | 0.072 | 0.031 | 0.020 | 0.077 | |
| P3 | 0.028 | 0.037 | 0.091 | 0.025 | 0.016 | 0.083 | |
| Adhesion group | 24 | ||||||
| Before modeling | 6 | 154.63 ± 17.52 | 39.47 ± 5.76 | 56.82 ± 7.33 | 83.31 ± 9.78 | 17.35 ± 2.62 | 38.17 ± 4.3 |
| After modeling | 6 | 201.45 ± 28.16 | 60.75 ± 7.68 | 57.37 ± 6.46 | 114.82 ± 13.78 | 30.56 ± 4.85 | 38.18 ± 4.17 |
| 3rd day | 6 | 165.43 ± 24.58 | 42.35 ± 4.92 | 62.82 ± 8.34 | 103.96 ± 13.68 | 20.78 ± 2.84 | 43.32 ± 5.28 |
| 8th day | 6 | 171.57 ± 26.87 | 40.58 ± 4.65 | 75.16 ± 9.65 | 94.53 ± 12.66 | 19.65 ± 2.45 | 55.64 ± 6.87 |
| P1 | 0.037 | 0.016 | 0.076 | 0.032 | 0.036 | 0.085 | |
| P2 | 0.065 | 0.075 | 0.058 | 0.038 | 0.064 | 0.083 | |
| P4 | 0.045 | 0.063 | 0.035 | 0.052 | 0.076 | 0.037 | |
| P5 | 0.021 | 0.033 | 0.039 | 0.027 | 0.026 | 0.036 | |
Note: TNF-α, tumor necrosis factor alpha; IFN-γ, interferon-gamma; IL-10, interleukin-10.
P values were represented comparisons between animals before modeling and after modeling (P1), before modeling and 3 days of treatment (P2), before modeling and 8 days of treatment (P3), BA group and after modeling BA group (P4) and control groups after 8 days treatment (P5).
Discussion
The intestinal mucosal barrier plays an important role by protecting the body from microbial damage and maintaining homeostasis [20]. Under traumatic stress, the intestinal mucosa tissue presents villus epithelial cell necrosis, shorter villi, interstitial edema, inflammatory cell infiltration, and mucosa barrier damage [21-26]. As described above, normal fossae structure, and lamina propria with no inflammatory cell infiltration were observed in animals before stress induction. However, upon stress, there was significant damage characterized by sparse and short hairs, inflammatory cells infiltration, lymphatic outspread and edema. Interestingly, after 8 days treatment with BA, a significant improvement was observed in comparison with control animals submitted to stress. Nevertheless, the recovery was not complete as a certain degree of damage was observed at 8 days of BA treatment: the nap was a little sparse; intrinsic membrane oedema was present; some membrane inflammatory cells infiltration was observed.
In stress conditions, levels of intestinal inflammatory mediators and cytokines increase and they are transported by blood to impact local or distal tissues, further exacerbating inflammatory response and intestinal mucosa damage [4,27]. TNF-α and IFN-γ are cytokines produced by mononuclear macrophages and endothelial cells [28-30], which are released massively in stress conditions [27,31]. Studies have shown that Bifidobacterium can repair the impaired intestinal barrier function and lessen the secretion of TNF-α and IFN-γ in the intestinal mucosa [32,33]. Our data showed that intestinal mucosa and plasma levels of TNF-α and IFN-γ were significantly higher in stressed animals compared with animals not submitted to stress. Interestingly, the levels of these cytokines were significantly lower after treatment with BA, suggesting that the adhesin significantly alleviated the inflammatory response induced by stress. It is possible that BA interfered with adhesion of the microbes in the gut, therefore repressing the production of pro-inflammatory cytokines. This is in agreement with the increase in L-10 observed after BA treatment. IL-10 is an anti-inflammatory interleukin, which inhibits T cells production and prevent intestinal secretion of pro-inflammatory substances such as TNF-α and IFN-γ [34,35]. Bifidobacteria stimulate dendritic cells to increase intestinal cells to produce IL-10, achieving immune regulation [36,37].
NF-κB is a transcription factor that plays a modulatory role in inflammation [38,39]. Under normal conditions, NF-κB and its inhibitor factor I-κB form an inactive cytoplasmic complex [40]. Stimulation triggers the release of NF-κB from IkB, and NF-κB is translocated to the nucleus, where it binds to DNA, rapidly inducing a variety of genes encoding signaling proteins including TNF alpha and Il-6, promoting the inflammatory reaction [41,42]. Our data showed that after stress induction, levels of intestinal mucosa NF-κB significantly increased, in parallel to pro-inflammatory cytokines TNF alpha and IFN-γ, indicating that stress can increase NF-κB mediated inflammatory response. Interestingly, 8 days after adhesin treatment, NF-κB expression significantly decreased, almost to the levels of control animals not submitted to stress. Similar results were obtained for TNF alpha and IFN-γ, which decreased as a result of BA treatment, indicating that BA can reduce NF-κB induced inflammatory reaction. However, the specific mechanisms by which NF-κB activation is repressed is unclear.
Generally speaking, under stress conditions, Bifidobacterium adhesins can influence release of inflammatory mediators and cytokines by decreasing proinflammatory substances TNF-α and IFN-γ and increasing IL-10 levels to reduce inflammation, and repair damaged intestinal mucosa structure, thereby protect the function of intestinal mucosal barrier.
Acknowledgements
This work was funded by the Science and technology support plan (2012BAI35B03).
Disclosure of conflict of interest
None.
References
- 1.Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- 2.Dienstbier RA. Arousal and physiological toughness: implications for mental and physical health. Psychol Rev. 1989;96:84–100. doi: 10.1037/0033-295x.96.1.84. [DOI] [PubMed] [Google Scholar]
- 3.Lee CY. Chronic restraint stress induces intestinal inflammation and alters the expression of hexose and lipid transporters. Clin Exp Pharmacol Physiol. 2013;40:385–391. doi: 10.1111/1440-1681.12096. [DOI] [PubMed] [Google Scholar]
- 4.Mura M, Andrade CF, Han B, Seth R, Zhang Y, Bai XH, Waddell TK, Hwang D, Keshavjee S, Liu M. Intestinal ischemia-reperfusion-induced acute lung injury and oncotic cell death in multiple organs. Shock. 2007;28:227–238. doi: 10.1097/01.shk.0000278497.47041.e3. [DOI] [PubMed] [Google Scholar]
- 5.Gustot T. Multiple organ failure in sepsis: prognosis and role of systemic inflammatory reresponse. Curr Opin Crit Care. 2011;17:153–159. doi: 10.1097/MCC.0b013e328344b446. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu K, Ogura H, Hamasaki T, Goto M, Tasaki O, Asahara T, Nomoto K, Morotomi M, Matsushima A, Kuwagata Y. Altered gut flora are associated with septic complications and death in critically ill patients with systemic inflammatory response syndrome. Dig Dis Sci. 2011;56:1171–1177. doi: 10.1007/s10620-010-1418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palomar MM, Maldonado Galdeano C, Perdigon G. Influence of a probiotic lactobacillus strain on the intestinal ecosystem in a stress model mouse. Brain Behav Immun. 2014;35:77–85. doi: 10.1016/j.bbi.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Fujimura KE, Slusher NA, Cabana MD, Lynch SV. Role of the gut microbiota in defining human health. Expert Rev Anti Infect Ther. 2010;8:435–454. doi: 10.1586/eri.10.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu H, He J, Wu Z, Xu W, Zhang H, Ye P, Yang J, Zhen S, Li L. Assessment of microbiome variation during the perioperative period in liver transplant patients: a retrospective analysis. Microb Ecol. 2013;65:781–791. doi: 10.1007/s00248-013-0211-6. [DOI] [PubMed] [Google Scholar]
- 10.Lopez P, Gonzalez-Rodriguez I, Sanchez B, Ruas-Madiedo P, Suarez A, Margolles A, Gueimonde M. Interaction of Bifidobacterium bifidum LMG13195 with HT29 cells influences regulatory-T-cell associated chemokine receptor expression. Appl Environ Microbiol. 2012;78:2850–2857. doi: 10.1128/AEM.07581-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumoto M, Kurihara S, Kibe R, Ashida H, Benno Y. Longevity in mice is promoted by probiotic-induced suppression of colonic senescence dependent on upregulation of gut bacterial polyamine production. PLoS One. 2011;6:e23652. doi: 10.1371/journal.pone.0023652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bermudez-Brito M, Plaza-Diaz J, Munoz-Quezada S, Gomez-Llorente C, Gil A. Probiotic mechanisms of action. Ann Nutrition Metab. 2012;61:160–174. doi: 10.1159/000342079. [DOI] [PubMed] [Google Scholar]
- 13.Guo CF, Zhang LW, Han X, Yi HX, Li JY, Tuo YF, Zhang YC, Du M, Shan YJ, Yang L. Screening for cholesterol-lowering probiotic based on deoxycholic acid removal pathway and studying its functional mechanisms in vitro. Anaerobe. 2012;18:516–522. doi: 10.1016/j.anaerobe.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Rodriguez I, Sanchez B, Ruiz L, Turroni F, Ventura M, Ruas-Madiedo P, Gueimonde M, Margolles A. Role of extracellular transaldolase from Bifidobacterium bifidum in mucin adhesion and aggregation. Appl Environ Microbiol. 2012;78:3992–3998. doi: 10.1128/AEM.08024-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei YX, Zhang ZY, Liu C, Malakar PK, Guo XK. Safety assessment of Bifidobacterium longum JDM301 based on complete genome sequences. World J Gastroenterol. 2012;18:479–488. doi: 10.3748/wjg.v18.i5.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valueva OA, Shashkov AS, Zdorovenko EL, Chizhov AO, Kiseleva E, Novik G, Knirel YA. Structures of cell-wall phosphate-containing glycopolymers of Bifidobacterium longum BIM B-476-D. Carbohydr Res. 2013;373:22–27. doi: 10.1016/j.carres.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Nybom SM, Dziga D, Heikkila JE, Kull TP, Salminen SJ, Meriluoto JA. Characterization of microcystin-LR removal process in the presence of probiotic bacteria. Toxicon. 2012;59:171–181. doi: 10.1016/j.toxicon.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Pellon-Cardenas O, Clancy J, Uwimpuhwe H, D’Souza-Schorey C. ARF6-regulated endocytosis of growth factor receptors links cadherin-based adhesion to canonical Wnt signaling in epithelia. Mol Cell Biol. 2013;33:2963–2975. doi: 10.1128/MCB.01698-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun J, Le GW, Shi YH, Su GW. Factors involved in binding of Lactobacillus plantarum Lp6 to rat small intestinal mucus. Lett Appl Microbiol. 2007;44:79–85. doi: 10.1111/j.1472-765X.2006.02031.x. [DOI] [PubMed] [Google Scholar]
- 20.Kinoshita H, Wakahara N, Watanabe M, Kawasaki T, Matsuo H, Kawai Y, Kitazawa H, Ohnuma S, Miura K, Horii A, Saito T. Cell surface glyceraldehyde-3-phosphate dehydrogenase (GAPDH) of Lactobacillus plantarum LA 318 recognizes human A and B blood group antigens. Res Microbiol. 2008;159:685–691. doi: 10.1016/j.resmic.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Cheng YT, Wu CH, Ho CY, Yen GC. Catechin protects against ketoprofen-induced oxidative damage of the gastric mucosa by up-regulating Nrf2 in vitro and in vivo. J Nutr Biochem. 2013;24:475–483. doi: 10.1016/j.jnutbio.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Bhattacharjee S, Ershov D, Fytianos K, van der Gucht J, Alink GM, Rietjens IM, Marcelis AT, Zuilhof H. Cytotoxicity and cellular uptake of tri-block copolymer nanoparticles with different size and surface characteristics. Part Fibre Toxicol. 2012;9:11. doi: 10.1186/1743-8977-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Proskuryakov SY, Konoplyannikov A, Verkhovskii YG, Ulyanova L, Tsyb A. Where intestinal epithelial stem cells are localized? About molecular markers. Biomed Khim. 2011;5:1–9. [PubMed] [Google Scholar]
- 24.Croix JA, Carbonero F, Nava GM, Russell M, Greenberg E, Gaskins HR. On the relationship between sialomucin and sulfomucin expression and hydrogenotrophic microbes in the human colonic mucosa. PLoS One. 2011;6:e24447. doi: 10.1371/journal.pone.0024447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivanova EA. Individual peculiarities in the reaction of jejunum lymphoid structures in rats exposed to stress. Morfologiia. 2011;139:45–48. [PubMed] [Google Scholar]
- 26.van der Meulen J, Koopmans SJ, Dekker RA, Hoogendoorn A. Increasing weaning age of piglets from 4 to 7 weeks reduces stress, increases post-weaning feed intake but does not improve intestinal functionality. Animal. 2010;4:1653–1661. doi: 10.1017/S1751731110001011. [DOI] [PubMed] [Google Scholar]
- 27.Li Q, Zhang Q, Wang M, Zhao S, Ma J, Luo N, Li N, Li Y, Xu G, Li J. Interferon-gamma and tumor necrosis factor-alpha disrupt epithelial barrier function by altering lipid composition in membrane microdomains of tight junction. Clin Immunol. 2008;126:67–80. doi: 10.1016/j.clim.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 28.Pelikan Z. Delayed type of asthmatic response to allergen challenge and cytokines in the peripheral blood. Respiration. 2012;84:385–395. doi: 10.1159/000335258. [DOI] [PubMed] [Google Scholar]
- 29.Freytes DO, Kang JW, Marcos-Campos I, Vunjak-Novakovic G. Macrophages modulate the viability and growth of human mesenchymal stem cells. J Cell Biochem. 2013;114:220–229. doi: 10.1002/jcb.24357. [DOI] [PubMed] [Google Scholar]
- 30.Arseneault-Breard J, Rondeau I, Gilbert K, Girard SA, Tompkins TA, Godbout R, Rousseau G. Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model. Br J Nutr. 2012;107:1793–1799. doi: 10.1017/S0007114511005137. [DOI] [PubMed] [Google Scholar]
- 31.Kusnierz-Cabala B, Gurda-Duda A, Solnica B, Fedak D, Dumnicka P, Panek J, Kulig J. Serum matrix Gla protein concentrations in patients with mild and severe acute pancreatitis. Clin Lab. 2011;57:999–1006. [PubMed] [Google Scholar]
- 32.Pozo-Rubio T, Mujico JR, Marcos A, Puertollano E, Nadal I, Sanz Y, Nova E. Immunostimulatory effect of faecal Bifidobacterium species of breast-fed and formula-fed infants in a peripheral blood mononuclear cell/Caco-2 co-culture system. Br J Nutr. 2011;106:1216–1223. doi: 10.1017/S0007114511001656. [DOI] [PubMed] [Google Scholar]
- 33.Henderson MA, Yong CS, Duong CP, Davenport AJ, John LB, Devaud C, Neeson P, Westwood JA, Darcy PK, Kershaw MH. Chimeric antigen receptor-redirected T cells display multifunctional capacity and enhanced tumor-specific cytokine secretion upon secondary ligation of chimeric receptor. Immunotherapy. 2013;5:577–590. doi: 10.2217/imt.13.37. [DOI] [PubMed] [Google Scholar]
- 34.Leonel AJ, Teixeira LG, Oliveira RP, Santiago AF, Batista NV, Ferreira TR, Santos RC, Cardoso VN, Cara DC, Faria AM, Alvarez-Leite J. Antioxidative and immunomodulatory effects of tributyrin supplementation on experimental colitis. Br J Nutr. 2013;109:1396–1407. doi: 10.1017/S000711451200342X. [DOI] [PubMed] [Google Scholar]
- 35.de Moreno de LeBlanc A, Chaves S, Carmuega E, Weill R, Antoine J, Perdigon G. Effect of long-term continuous consumption of fermented milk containing probiotic bacteria on mucosal immunity and the activity of peritoneal macrophages. Immunobiology. 2008;213:97–108. doi: 10.1016/j.imbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Kubo M, Motomura Y. Transcriptional regulation of the anti-inflammatory cytokine IL-10 in acquired immune cells. Front Immunol. 2012;3:275. doi: 10.3389/fimmu.2012.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han W, Li H, Cai J, Gleaves LA, Polosukhin VV, Segal BH, Yull FE, Blackwell TS. NADPH oxidase limits lipopolysaccharide-induced lung inflammation and injury in mice through reduction-oxidation regulation of NF-kappaB activity. J Immunol. 2013;190:4786–4794. doi: 10.4049/jimmunol.1201809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Indra MR, Karyono S, Ratnawati R, Malik SG. Quercetin suppresses inflammation by reducing ERK1/2 phosphorylation and NF kappa B activation in Leptin-induced Human Umbilical Vein Endothelial Cells (HUVECs) BMC Res Notes. 2013;6:275. doi: 10.1186/1756-0500-6-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hiramatsu Y, Satho T, Irie K, Shiimura S, Okuno T, Sharmin T, Uyeda S, Fukumitsu Y, Nakashima Y, Miake F, Kashige N. Differences in TLR9-dependent inhibitory effects of H(2)O(2)-induced IL-8 secretion and NF-kappa B/I kappa B-alpha system activation by genomic DNA from five Lactobacillus species. Microbes Infect. 2013;15:96–104. doi: 10.1016/j.micinf.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Kang J, Tae N, Min BS, Choe J, Lee JH. Malabaricone C suppresses lipopolysaccharide-induced inflammatory responses via inhibiting ROS-mediated Akt/IKK/NF-kappaB signaling in murine macrophages. Int Immunopharmacol. 2012;14:302–310. doi: 10.1016/j.intimp.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Chung S, Sundar IK, Hwang JW, Yull FE, Blackwell TS, Kinnula VL, Bulger M, Yao H, Rahman I. NF-kappaB inducing kinase, NIK mediates cigarette smoke/TNFalpha-induced histone acetylation and inflammation through differential activation of IKKs. PLoS One. 2011;6:e23488. doi: 10.1371/journal.pone.0023488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koplik EV, Ivanova EA. Morphological features of the lymphoepithelial structures of the jejunum after the stress in rats. Bull Exp Biol Med. 2012;153:791–794. doi: 10.1007/s10517-012-1828-z. [DOI] [PubMed] [Google Scholar]
- 43.Bubendorf L, Nocito A, Moch H, Sauter G. Tissue microarray (TMA) technology: miniaturized pathology archives for high-throughput in situ studies. J Pathol. 2001;195:72–79. doi: 10.1002/path.893. [DOI] [PubMed] [Google Scholar]