Abstract
Accumulating evidences demonstrated that many long non-coding RNAs (lncRNAs) can cooperate with the adjacent coding genes, forming into “lncRNA-mRNA gene pairs” in multiple biological cellular processes. Here, we showed that a novel long non-coding RNA FOXCUT (FOXC1 promoter upstream transcript) and its neighboring gene FOXC1 played a similar important role in the oncogenesis and progression of esophageal squamous cell carcinoma (ESCC). In this study, the expression of FOXCUT/FOXC1 was measured in 82 ESCC tissues and adjacent noncancerous tissues by real-time quantitative PCR (qPCR). The prognostic significance of the lncRNA-mRNA gene pair was evaluated using Kaplan-Meier survival analysis and log-rank test. Cell biological experiments were performed in ESCC cell lines to explore their functions in tumor progression. Notably elevated FOXCUT and FOXC1 expression levels were observed in cancerous tissues compared to adjacent noncancerous tissues (86.6% and 84.1%, respectively; P < 0.01), showing strong correlations with poor differentiation, advanced lymph node classification and metastasis (P < 0.05). Moreover, patients with upregulated FOXCUT or FOXC1 experienced a significantly worse prognosis than those with downregulated FOXCUT or FOXC1 (P < 0.001 and P = 0.014, respectively). In addition, the expression of FOXCUT was positively correlated with expression of FOXC1 in ESCC specimens. And the expression of FOXC1 was also decreased as the FOXCUT expression was silenced by siRNA. Assays in vitro demonstrated that knockdown of either FOXCUT or FOXC1 remarkably inhibited cell proliferation, colony formation, migration, invasion in ESCC cells. In conclusion, FOXCUT may be functionally involved in the tumor progression and survival of ESCC patients, at least in part, by modulating FOXC1. FOXCUT and FOXC1 may function as a lncRNA-mRNA gene pair, which may represent a potential prognostic biomarker and therapeutic target for ESCC patients.
Keywords: ESCC, lncRNA, FOXC1, FOXCUT, progression, prognosis
Introduction
Esophageal cancer (EC) is one of the most aggressive malignant tumors, ranking eighth by global morbidity and sixth by global mortality rate among all types of cancers [1,2]. Histologically, the two main types of esophageal cancer, esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC), exhibit different etiologic and pathologic characteristics. In China, ESCC is the predominant subtype and contributes to nearly 90% of all ECs [3,4]. Despite recent considerable advances in diagnosis and treatment, the overall prognosis for ESCC remains still poor, with 5-year survival rate of 5-45% [5-8]. The treatment failure can be attributed to the extensive local invasion and regional lymph node metastasis [9]. Therefore, to identify more accurate biomarkers for early diagnosis, therapeutic strategies and prognosis of ESCC is urgently needed.
Recently, accumulating evidences demonstrated that long non-coding RNAs (lncRNAs), the largest transcript class in human genome, may play an important role in the tumorigenesis and tumor progression [10-13]. LncRNAs are defined as transcribed RNA molecules that are longer than 200 nucleotides, possessing no potential protein-coding capacity [14,15]. With advances in technologies, lncRNAs are found to be as important regulators involved in various molecular mechanisms in gene networks [16,17]. Studies showed that a large number of lncRNAs can functionally contact with their adjacent mRNAs developing into a new form of “lncRNA-mRNA pairs” in the regulatory networks. This new model indicated that transcription of lncRNAs may often be co-regulated with the adjacent protein-coding gene [18,19].
Here, with bioinformatics analysis, we found a novel lncRNA TCONS_00011636 (http://genome.ucsc.edu/), which is situated at chromosome 6p25 and transcribed from the upstream side of FOXC1 promoter. So, we denominated it as “FOXC1 promoter upstream transcript, FOXCUT”. And the lncRNA-mRNA pair (FOXCUT and FOXC1) may be a new functional form. FOXC1, a member of the Forkhead Box, is featured as a conserved 110 amino-acid DNA-binding domain. FOXC1 proteins are key regulators of diverse biological processes including the development of many organ systems [20], embryogenesis, tumorigenesis, and tumor progression [21-23]. Recent studies have demonstrated that FOXC1 was overexpressed in many kinds of cancer tissues and the high expression level of FOXC1 had a strong association with the poor prognosis in patients of multiple malignant cancers, such as gastric cancer, breast cancer, hepatocellular carcinoma, non-small cell lung cancer, pancreatic ductal adenocarcinoma [22,24-27]. However, it hasn’t been reported in ESCC yet. Considering the extensive clinical value of FOXC1, we speculated FOXCUT, the other part of lncRNA-mRNA pair, may be functionally involved in the tumor progression and survival of ESCC patients with FOXC1 together.
In the present study, we reported the expression patterns of FOXC1 and FOXCUT in ESCC tissues and adjacent non-cancerous tissues and analyzed the correlation between FOXCUT/FOXC1 and clinicopathological characteristics for the first time. Then, we explored their functional role in ESCC cells. In all, this study was to offer a new functional lncRNA-mRNA pair in ESCC and to evaluate the lncRNA-mRNA pair as a new biomarker that predicts poor prognosis in ESCC patients.
Materials and methods
Patient samples
A total of 82 fresh ESCC tissue samples and paired adjacent noncancerous tissue samples (> 1.5 cm away from cancer) were collected from patients who underwent surgery at Chinese PLA General Hospital (Beijing, China) between 2007 and 2012. The ESCC diagnosis was histopathologically confirmed. None of the patients received preoperative therapy such as radiotherapy or chemotherapy before surgical resection. All tissue specimens were immediately frozen and stored in liquid nitrogen after surgery until the extraction of total RNA. The data from all subjects were obtained from medical records, pathology reports and personal interviews. The acquired clinical information for all of the samples is summarized in Table 1. Follow-up periods ranged from 1 month to 72 months, and the result of patients who were lost to follow-up or died from other etiology instead of ESCC were regarded as censored data. The research was approved by the ethical committee of PLA General Hospital. Written informed consent was signed by all participants.
Table 1.
Correlations of FOXCUT and FOXC1 expression with clinicopathological characteristics in ESCC patients
| Characteristics | FOXCUT expression | P value | FOXC1 expression | P value | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Low expression | High expression | Low expression | High expression | |||
| Age (mean = 59.48) | 0.022* | 0.246 | ||||
| < 60 | 25 | 19 | 23 | 21 | ||
| ≥ 60 | 12 | 26 | 15 | 23 | ||
| Gender | 0.864 | 0.158 | ||||
| Male | 24 | 30 | 22 | 32 | ||
| Female | 13 | 15 | 16 | 12 | ||
| Tumor location | 0.164 | 0.23 | ||||
| Upper | 0 | 2 | 0 | 2 | ||
| Middle | 10 | 18 | 11 | 17 | ||
| Lower | 27 | 25 | 27 | 25 | ||
| Differentiation | 0.001* | 0.001* | ||||
| Well | 2 | 23 | 3 | 22 | ||
| Moderate | 19 | 19 | 21 | 17 | ||
| Poor | 16 | 3 | 14 | 5 | ||
| T classification | 0.259 | 0.839 | ||||
| T1-2 | 27 | 26 | 25 | 28 | ||
| T3-4 | 11 | 18 | 13 | 16 | ||
| N classification | 0.007* | 0.045* | ||||
| N0 | 12 | 4 | 11 | 5 | ||
| N1-2 | 25 | 41 | 27 | 39 | ||
| Metastasis | 0.001* | 0.001* | ||||
| M0 | 31 | 15 | 30 | 16 | ||
| M1 | 6 | 30 | 8 | 28 | ||
| Clinical Stage | 0.120 | 0.376 | ||||
| I-II | 22 | 19 | 21 | 20 | ||
| III-IV | 15 | 26 | 17 | 24 | ||
P-values were two-tailed and based on the Pearson χ2 test;
Statistically significant.
Cell line and cell culture
Human ESCC cell lines (KYSE30, KYSE70, KYSE140, KYSE150, and KYSE180) were cultured at 37°C with 5% CO2 in RPMI 1640 medium (Gibco, USA) supplemented with 10% fetal bovine serum, 100 units/ml penicillin and 100 μg/ml streptomycin (Hyclone, Logan, UT). One normal esophageal cell line Het-1A was cultured in LHC-9 medium supplemented with 2% fetal bovine serum (Hyclone, Logan, UT).
RNA extraction and real-time quantitative PCR
Total RNA was extracted from ESCC cancerous, matched adjacent noncancerous specimens and ESCC cells using the Trizol Total RNA Reagent (Invitrogen, Carlsbad CA). The concentration and A260/280 ratio were measured by NanoVue Plus (GE Healthcare, USA). The quality assessment of RNA was evaluated in the 28S and 18 S bands using agarose gel electrophoresis. 1 μg RNA was reversely transcribed into cDNAs using the PrimeScript® RT reagent Kit (TAKARA, Dalian, China), according to the manufacturer’s protocol. Real-time quantitative PCR (qPCR) was performed using the SYBR® Premix Ex TaqTM (Takara, Dalian, China), in an Applied Biosystems 7500 Fluorescent Quantitative PCR System (Applied Biosystems, Foster City, CA). The reaction mixtures were incubated at 95°C for 30 s, followed by 40 amplification cycles of 95°C for 5 s and 60°C for 34 s. The comparative CT method was applied to quantify relative expression of mRNA and lncRNA. Results were normalized to the expression of house-keeping gene GAPDH. The primers used in this study were as follows: FOXC1 (Forward) 5′- GGCGAGCAGAGCTACTACC -3′, (Reverse) 5′- TGCGAGTACACGCTCATGG -3′; FOXCUT (Forward) 5′- GTCGCACCGATGACTAACG -3′, (Reverse) 5′- GCCCTGAAAGCCGAACTG -3′.
Transfection of siRNA
The siRNA sequences were designed by us and synthesised by GenePharma (Shanghai, China), including one negative control siRNA (NC siRNA) sequence, two FOXCUT siRNA sequences and two FOXC1 sequence. The sequences were as follows: si-FOXC1-1 sense strand 5’rCrCrArGrArUrArArCrArCrGrUrArArGrUrUrUrCrUrUrCTT, antisense strand 5’rArArGrArArGrArArArCrUrUrArCrGrUrGrUrUrArUrCrUrGrGrArG; si-FOXC1-2 sense strand 5’rCrGrUrUrArArArUrUrGrCrCrUrGrArArArCrUrUrUrArAAT, antisense strand 5’rArUrUrUrArArArGrUrUrUrCrArGrGrCrArArUrUrUrArArCrGrUrC. si-FOXCUT-1 sense strand 5’rGrArArUrGrGrArGrArArCrUrArArGrArCrArArUrUrArUCT, antisense strand 5’rArGrArUrArArUrUrGrUrCrUrUrArGrUrUrCrUrCrCrArUrUrCrGrG; si-FOXCUT-2 sense strand 5’rCrArGrCrCrUrCrCrCrUrCrCrUrGrUrGrUrGrUrGrCrArGAG, antisense strand 5’rCrUrCrUrGrCrArCrArCrArCrArGrGrArGrGrGrArGrGrCrUrGrCrA. Transfection of siRNA was conducted by X-tremeGENE transfection reagent (Roche) according to the manufacturer’s instructions. After transfection, total cells were collected for RNA isolation, cell proliferation assay, colony formation assay and scratch wound healing assay.
Cell proliferation assay
After 24 hours (h) of transfection, cell proliferation was measured by MTS assay (Promega) following the manufacturer’s protocol. KYSE30 (1,000 cells per well) were seeded in 96-well plates. The cells were incubated for 0, 1, 2, 3 or 4 days, respectively. And 20 μl of the MTS reagent was added to each well containing 100 μl culture medium. The plate was incubated for 2 h at 37°C in a humid, 5% CO2 atmosphere. The absorbance values of each well were detected with a universal microplate reader at the wavelength of 490 nm.
Colony formation assay
After 24 h of transfection, the cells (KYSE30) were reseeded into 6-well plates at 1000 cells per well. The culture medium was replaced every 5 days. Cells were stopped after 10 days’ incubation at 37°C, and were washed twice with PBS, fixed and stained with 0.5% crystal violet. Colonies were counted by under an optical microscope.
Scratch wound healing assay
KYSE30 were seeded on plastic 6-well plates. When cell confluence reached approximately 90% at 24 h of transfection, wounds were made in confluent cells using a 10 μl pipette tip. Wound healing was observed at 0 h and 48 h respectively under optical microscope. Duplicate wells for each condition were examined, and each experiment was repeated in triplicate.
Migration and invasion assay
The cell migration assay was carried out by using Transwell® Permeable Supports with 8 mm pores in 24-well tissue culture plates (Corning, USA). A cell invasion assay was performed using modified BD BioCoat™ Matrigel™ Invasion Chamber with 8 mm pores in 24-well tissue culture plates (BD, USA). 1 x 105 cells in 200 μl serum-free RPMI 1640 medium were added to the upper chambers of the inserts of a 24-well culture plate. In contrast, culture medium containing 20% fetal bovine serum in the lower chamber served as the chemoattractant. The cells that had migrated through the filter to the lower sides of the chambers were stained with crystal violet, air-dried, photographed and counted.
Statistical analysis
All statistical analyses were performed by using SPSS version 18.0 (SPSS, Chicago, IL). Differences between groups were analyzed using Student’s t test, one-way ANOVA, chi-square test. Correlation between gene expressions was analyzed by using Pearson’s correlation coefficient. Oveall survival probability was calculated by the Kaplan-Meier methods and was evaluated by log-rank test. For all statistical analyses, P < 0.05 was considered statistically significant.
Results
FOXC1 and FOXCUT were co-overexpressed in ESCC tissue specimens and ESCC cell lines
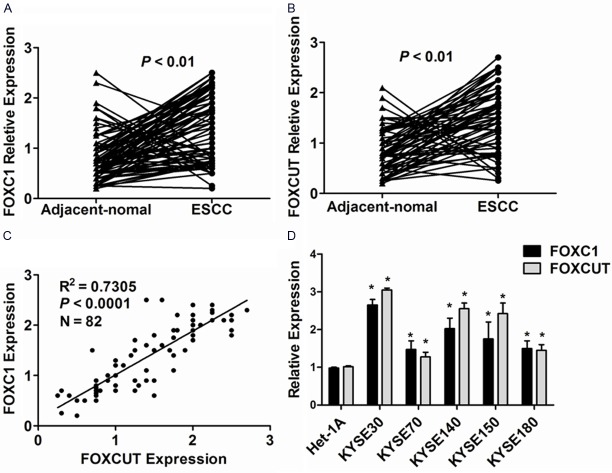
The FOXC1 mRNA and FOXCUT lncRNA expression levels were detected in a total of 82 paired ESCC cancerous and adjacent noncancerous tissues from ESCC patients by qPCR. Using GAPDH as the normalization control, 69 of the 82 ESCC patients (84.1%, P < 0.01) exhibited remarkably higher expression of FOXC1 mRNA in cancerous tissues than in noncancerous tissues (Figure 1A) and 71 of the 82 ESCC patients (86.6%, P < 0.01) showed significantly higher expression FOXCUT lncRNA in cancerous tissues compared to the levels in noncancerous tissues (Figure 1B). In particular, the relative expression of FOXC1 was positively correlated with that of FOXCUT in ESCC tissue specimens (R2 = 0.7305, P < 0.0001, Figure 1C). Then, the expression of FOXC1 and FOXCUT were assessed in ESCC cell lines, including KYSE30, KYSE70, KYSE140, KYSE150, and KYSE180 and in the normal esophageal cell line Het-1A. The expression of FOXC1 and FOXCUT were remarkably higher in these ESCC cell lines than Het-1A. Of the five ESCC cell lines, KYSE30 cell lines expressed the highest levels of FOXC1 and FOXCUT (P < 0.05, Figure 1D).
Figure 1.
FOXC1 and lncRNA-FOXCUT expression levels were analyzed by qPCR in 82 ESCC tissue samples and ESCC cell lines. A: The expression level of FOXC1 in ESCC cancerous tissues was remarkably higher than those in adjacent noncancerous tissues (P < 0.01). B: The expression level of lncRNA-FOXCUT in ESCC cancerous tissues was also significantly higher than those in adjacent noncancerous tissues (P < 0.01). C: Linear Regression analysis was performed on FOXC1 and lncRNA-FOXCUT expression levels in 82 ESCC tissue samples (R2 = 0.7305, P < 0.0001). D: The FOXC1 and lncRNA-FOXCUT expression levels were evidently higher in ESCC cell lines compared to normal esophageal cell line Het-1A (P < 0.05, the symbol * indicates statistically significant).
FOXC1 and FOXCUT were correlated respectively with clinicopathological characteristics in ESCC
According to the mean value of relative FOXC1 and FOXCUT expression (1.438 and 1.488, respectively) in tumor tissues, the 82 ESCC patients were divided into two groups including the high expression of FOXC1 (n = 44)/FOXCUT (n = 45) and the low expression of FOXC1 (n = 38)/FOXCUT (n = 37). We then evaluated the correlation of FOXC1 and FOXCUT expression levels with clinicopathological characteristics in ESCC patients (Table 1). FOXC1 upregulation was correlated with poor differentiation (P = 0.001, Table 1), advanced lympth node classification (P = 0.045, Table 1) and metastasis (P = 0.001, Table 1), however, statistical analyses showed no correlation of FOXC1 with age, gender, tumor location, tumor size, and clinical stage. Similarly, high expression of FOXCUT was correlated with age (P = 0.022), poor differentiation (P = 0.001), advanced lymph node classification (P = 0.007) and metastasis (P = 0.001) and has no association with gender, tumor location, tumor classification, and clinical stage. Furthermore, we discovered that FOXC1 and FOXCUT expression levels were remarkably higher in metastatic ESCC tumor tissues (n = 36) than in non-metastatic ESCC tumor tissues (n = 46) (P < 0.01, Figure 2A, 2B). And FOXC1 and FOXCUT expression levels were significantly elevated in poorly differentiated tumor tissues (P < 0.01, Figure 2C, 2D). Combined with all these above results, it showed that both of the elevated expression levels of FOXC1 and FOXCUT were related to the progression of ESCC respectively.
Figure 2.
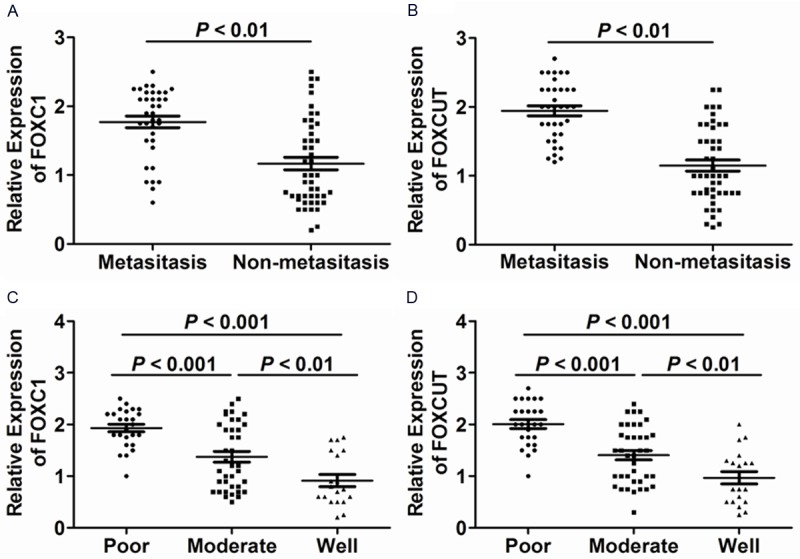
FOXC1 and lncRNA-FOXCUT expression levels were elevated in metastatic ESCC and poorly differentiated tumor tissues. (A) The expression levels of FOXC1 and (B) lncRNA-FOXCUT were higher in metastatic tumor tissues (n = 36) compared to non-metastatic tumor tissues (n = 46) (P < 0.01). (C) The expression levels of FOXC1 and (D) lncRNA-FOXCUT were highest in poorly differentiated tumor tissues (n = 25, P < 0.001) and were elevated in moderately differentiated tumor tissues (n = 38) compared to well differentiated tumor tissues (n = 19) (P < 0.01).
Upregulation of FOXC1 and FOXCUT were correlated with poor prognosis in ESCC patients
Kaplan-Meier survival analysis and log-rank tests were conducted to further evaluate the relationship between FOXC1/FOXCUT and prognosis of ESCC patients. From the Kaplan-Meier survival curve, we found that the median survival time of patients with high and low expression levels of FOXC1 were 20 months and 32 months, respectively. The five-year survival rate of high expression group (15.2%) was remarkably lower than that of low expression group (33.3%). The patients with upregulation of FOXC1 (n = 44) had significantly shorter survival time than those with downregulation of FOXC1 (P = 0.014, Figure 3A). Similarly, the median survival time of patients with high and low expression levels of FOXCUT were 20 months and 48 months, respectively. The five-year survival rate of high expression group (11.10%) was significantly lower than that of low expression group (39.0%). The patients with upregulation of FOXCUT (n = 45) had remarkably shorter survival time than those with downregulation of FOXCUT (P < 0.001, Figure 3B). These findings supported that upregulation of FOXC1 and FOXCUT were correlated with poor prognosis in ESCC patients.
Figure 3.
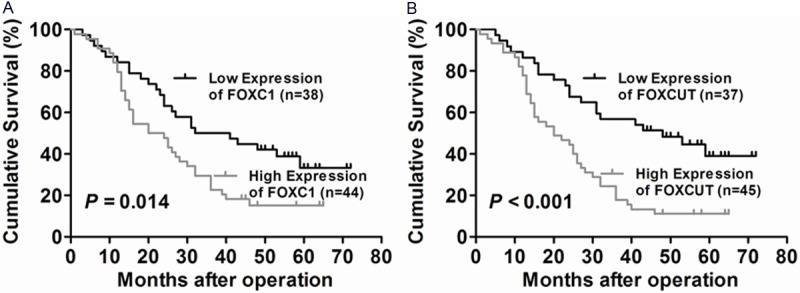
Kaplan-Meier overall survival curve for ESCC patients (n = 82) with different FOXC1 and FOXCUT expression levels. A: Difference in overall survival for ESCC patients with high expression and low expression of FOXC1 was analyzed by log-rank test (P = 0.014). Patients with high expression of FOXC1 had a significantly worse prognosis than those with low expression of FOXC1. B: Difference in overall survival for ESCC patients with high expression and low expression of FOXCUT was analyzed by log-rank test (P < 0.001). Patients with high expression of FOXCUT had a remarkably worse prognosis than those with low expression of FOXCUT.
FOXC1 expression in KYSE30 cells was suppressed by FOXC1 siRNA and FOXCUT siRNA
In ESCC cell line KYSE30, RNAi technique was executed to further demonstrate the correlation between the expression of lncRNA-FOXCUT and mRNA FOXC1. The results proved that the FOXC1 expression level was apparently knocked down by two kinds of FOXC1 siRNAs (P < 0.05, Figure 4A). And si-FOXC1-2 played more significant effect than si-FOXC1-1 (Figure 4A). Moreover, the FOXC1 expression level was also down-regulated in both FOXCUT siRNAs compared with the control siRNA (Figure 4B). Particularly, as the lncRNA-FOXCUT expression was knocked down up to 86% by si-FOXCUT-1, the FOXC1 mRNA expression was co-suppressed by 64% (Figure 4B). Whereas, the FOXCUT expression levels did not decrease together with the FOXC1 downregulation caused by FOXC1 siRNAs (Figure 4A). Considering these findings, it indicated that the expression of mRNA FOXC1 might be regulated by lncRNA FOXCUT.
Figure 4.
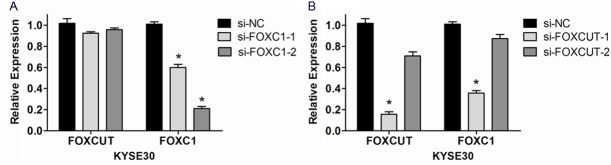
The expression levels of FOXC1 mRNA and FOXCUT lncRNA in KYSE30 cells after siRNA transfection. A: The expression level of FOXC1 in si-FOXC1-2 group was significantly knocked down in KYSE30 cells (P < 0.05, the symbol * indicates statistically significant). B: The expression levels of both FOXCUT and FOXC1 were significantly knocked down in si-FOXCUT-1 group (P < 0.05, the symbol * indicates statistically significant).
Knockdown of FOXC1 inhibited cell proliferation, migration, invasion abilities in KYSE30
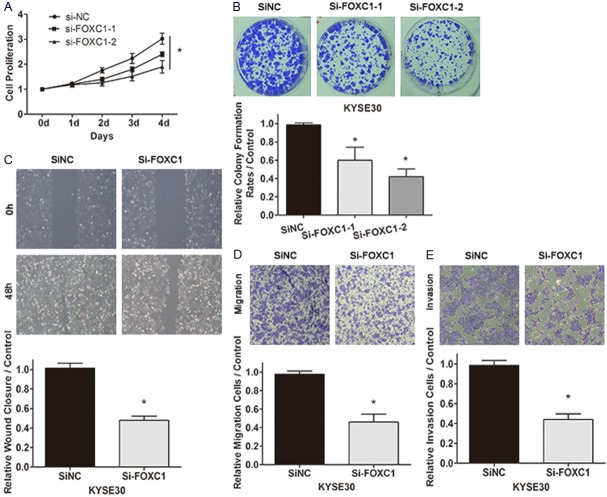
Cell proliferation, migration, invasion abilities were important aspects of cancer progression. To clarify whether FOXC1 has a functional role in facilitating ESCC cell progression, a series of cell function experiments were conducted in KYSE30 after siRNA transfection. MTS assay and colony formation assay showed that suppression of FOXC1 notably repressed the cell proliferation of KYSE30 in contrast with the negative control (Figure 5A) and the number of cell colonies in the knockdown of FOXC1 groups was also significantly reduced compared with the NC siRNA group (Figure 5B). And si-FOXC1-2 played more significant value than si-FOXC1-1 in KYSE30 which was in accordance with downregulation of FOXC1 by the two FOXC1 siRNAs (Figures 4A, 5A and 5B). Moreover, both scratch wound healing assay and migration assay proved that knockdown of FOXC1 inhibited cell migration by 52% and 54% respectively (Figure 5C, 5D). In addition, the matrigel invasion assay similarly indicated the silence of FOXC1 in KYSE30 cells declined cell invasion in the Matrigel substrate by 56% (Figure 5E). These data demonstrated that FOXC1 promoted cell proliferation, enhanced cell migration, invasion abilities in KYSE30.
Figure 5.
Knockdown of FOXC1 inhibited cell proliferation, migration, invasion abilities in KYSE30. A: Si-FOXC1-2 remarkably inhibited the KYSE30 cell proliferation compared with siNC in MTS assay (P < 0.05). B: The colony formation rate of si-FOXC1-2 was significantly decreased compared with siNC (P < 0.05). C, D: Both scratch wound healing assay and migration assay proved that knockdown of FOXC1 inhibited cell migration by 52% and 54% respectively. E: The silence of FOXC1 evidently impaired the capacity of cell invasion in the Matrigel substrate (P < 0.05).
Knockdown of FOXCUT inhibited cell proliferation, migration and invasion abilities in KYSE30
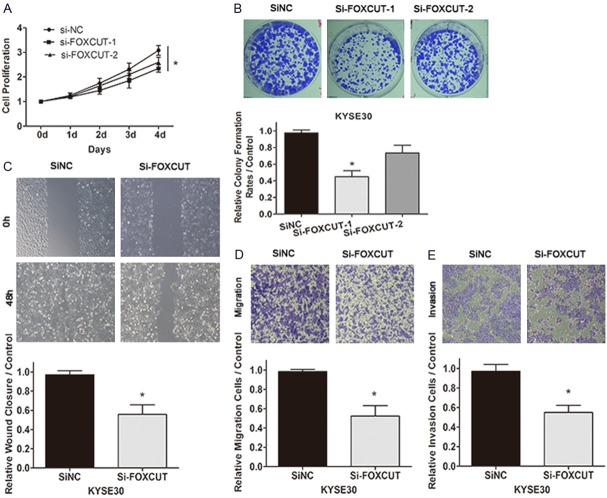
To further testify the function of FOXCUT on the growth characteristics of the ESCC cell lines, the same series of cell function experiments were performed in KYSE30 after siRNA transfection. The results showed that cell proliferation ability of KYSE30 was also efficiently suppressed by si-FOXCUT (Figure 6A) and the number of cell colonies was apparently decreased through knockdown of FOXCUT compared with the NC siRNA (Figure 6B). Similarly, si-FOXCUT-1 played more vital part than si-FOXCUT-2 in KYSE30 corresponding to downregulation of FOXCUT by the two FOXCUT siRNAs (Figures 4B, 6A and 6B). Furthermore, silence of FOXCUT inhibited cell migration by 44% and 48% respectively (Figure 6C, 6D) by scratch wound healing assay and migration assay. Besides, the matrigel invasion assay similarly proved the downregulation of FOXCUT in KYSE30 cells retarded cell invasion significantly in the Matrigel substrate by 43% (Figure 6E). Taking these remarkable results into account, FOXCUT may promote cell proliferation, enhance cell migration, invasion abilities in KYSE30.
Figure 6.
Knockdown of FOXCUT inhibited cell proliferation, migration, invasion abilities in KYSE30. A: Si-FOXCUT-1 remarkably inhibited the KYSE30 cell proliferation compared with siNC in MTS assay (P < 0.05). B: The colony formation rate of si-FOXCUT-1 was significantly decreased compared with siNC (P < 0.05). C, D: Both scratch wound healing assay and migration assay proved that knockdown of FOXCUT-1 inhibited cell migration by 44% and 48% respectively. E: The silence of FOXCUT-1 evidently impaired the capacity of cell invasion in the Matrigel substrate (P < 0.05).
Discussion
In the present study, we first identified a new lncRNA-mRNA pair, lncRNAFOXCUT and its adjacent mRNA FOXC1, as a new form of cancer-related gene compound correlated clinically with aggressive biological behaviors and poor survival in ESCC.
ESCC is a kind of tumor involved in complex dynamic biological processes and it initiates from multiple steps of genetic and epigenetic alterations [28]. Previous studies about ESCC related genes mainly focused on protein-coding genes. Recently, advances in high-throughput sequencing has helped revealing great functions of lncRNAs in cancers [18]. Numerous new lncRNA molecules have been proved to play significant roles in the tumorigenesis, progression of various malignant tumors [29-31]. In ESCC, several lncRNAs have been identified as new biomarkers and therapeutic targets such as PlncRNA-1, HOTAIR [32,33]. However, the expression and functional roles of most lncRNAs are still unknown in ESCC.
Emerging evidences have demonstrated that FOXC1 was overexpressed in a wide variety of malignant cancers and it can promote tumorigenesis, and tumor progression [21-23]. In our current study, we found a new lncRNA FOXCUT transcribed from the upstream side of FOXC1 promoter through bioinformatics analysis. FOXCUT, an adjacent lncRNA of FOXC1, belongs to promoter upstream transcripts (PROMPTs) [34,35]. The PROMPTs are often associated with the adjacent protein coding transcripts in their functions [19]. So, we speculated that lncRNA FOXCUT and mRNA FOXC1 may constitute into a new functional lncRNA-mRNA gene pair.
In our study, it is the first time to show that the expression of FOXCUT and FOXC1 (lncRNA-mRNA gene pair) were both identified to be upregulated in 82 ESCC tissues compared with adjacent noncancerous tissues. Linear regression analysis revealed that FOXCUT and FOXC1 had a positive correlation with each other in ESCC. Furthermore, the expression of FOXC1 was also decreased as the FOXCUT expression was downregulated by siRNA. Whereas, the silence of FOXC1 did not influence the expression of FOXCUT. It indicates that lncRNA FOXCUT may regulate the expression of FOXC1 by some specific mechanisms, which needs further researches to be elucidated completely.
Additionally, our study demonstrated that FOXC1 and FOXCUT expression levels were significantly elevated in tumor samples from patients with the presence of metastasis and poor differentiation. Upregulation of FOXC1 and FOXCUT were both correlated with aggressive clinicopathological characteristics, such as poor differentiation, advanced lymph node classification, metastasis. And, patients with high expression of FOXCUT or FOXC1 had a significantly poor prognosis. These data suggests that high expression of FOXCUT or FOXC1 might play an important role in the tumorigenesis, development, progression of ESCC, and both of them may serve as prognostic biomarkers for ESCC patients.
To further explore the functional role of this new lncRNA-mRNA gene pair in ESCC cells, we designed siRNAs to knock down the expression of FOXC1 or FOXCUT. Functional assays in vitro demonstrated that knockdown of FOXCUT or FOXC1 remarkably impeded cell proliferation, migration and invasion abilities in ESCC cells which were consistent with the reported functions of FOXC1 in other cancers [22]. The results indicate that this new lncRNA-mRNA gene pair is an important compound functioning in the ESCC cell aggressive biological behaviours, similar to the well-know lncRNA, HOTAIR in ESCC [6,32].
In conclusion, FOXCUT and FOXC1, the new lncRNA-mRNA gene pair, are both novel upregulated functional molecules in ESCC. Upregulation of the lncRNA-mRNA pair may be a prognostic factor for ESCC patients, indicating short survival and high risk for metastasis and poor differentiation. FOXCUT may inhibit ESCC cell proliferation, migration and invasion abilities, partially by the regulation of FOXC1 expression. And both FOXCUT and FOXC1 may serve as potential diagnostic markers and therapeutic targets for future ESCC treatment.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81301781). We would like to thank the Key Laboratory of Oncology, Cancer Center, Division of Internal Medicine, Chinese PLA General Hospital & Chinese PLA Medical School.
Disclosure of conflict of interest
None.
References
- 1.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 2.Kano M, Seki N, Kikkawa N, Fujimura L, Hoshino I, Akutsu Y, Chiyomaru T, Enokida H, Nakagawa M, Matsubara H. miR-145, miR-133a and miR-133b: Tumor-suppressive miRNAs target FSCN1 in esophageal squamous cell carcinoma. Int J Cancer. 2010;127:2804–2814. doi: 10.1002/ijc.25284. [DOI] [PubMed] [Google Scholar]
- 3.Hiyama T, Yoshihara M, Tanaka S, Chayama K. Genetic polymorphisms and esophageal cancer risk. Int J Cancer. 2007;121:1643–1658. doi: 10.1002/ijc.23044. [DOI] [PubMed] [Google Scholar]
- 4.Li T, Lu ZM, Chen KN, Guo M, Xing HP, Mei Q, Yang HH, Lechner JF, Ke Y. Human papillomavirus type 16 is an important infectious factor in the high incidence of esophageal cancer in Anyang area of China. Carcinogenesis. 2001;22:929–934. doi: 10.1093/carcin/22.6.929. [DOI] [PubMed] [Google Scholar]
- 5.Sato F, Shimada Y, Watanabe G, Uchida S, Makino T, Imamura M. Expression of vascular endothelial growth factor, matrix metalloproteinase-9 and E-cadherin in the process of lymph node metastasis in oesophageal cancer. Br J Cancer. 1999;80:1366–1372. doi: 10.1038/sj.bjc.6690530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lv XB, Lian GY, Wang HR, Song E, Yao H, Wang MH. Long noncoding RNA HOTAIR is a prognostic marker for esophageal squamous cell carcinoma progression and survival. PLoS One. 2013;8:e63516. doi: 10.1371/journal.pone.0063516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roder JD, Busch R, Stein HJ, Fink U, Siewert JR. Ratio of invaded to removed lymph nodes as a predictor of survival in squamous cell carcinoma of the oesophagus. Br J Surg. 1994;81:410–413. doi: 10.1002/bjs.1800810330. [DOI] [PubMed] [Google Scholar]
- 8.Thompson SK, Ruszkiewicz AR, Jamieson GG, Esterman A, Watson DI, Wijnhoven BP, Lamb PJ, Devitt PG. Improving the accuracy of TNM staging in esophageal cancer: a pathological review of resected specimens. Ann Surg Oncol. 2008;15:3447–3458. doi: 10.1245/s10434-008-0155-0. [DOI] [PubMed] [Google Scholar]
- 9.Wang LS, Chow KC, Chi KH, Liu CC, Li WY, Chiu JH, Huang MH. Prognosis of esophageal squamous cell carcinoma: analysis of clinicopathological and biological factors. Am J Gastroenterol. 1999;94:1933–1940. doi: 10.1111/j.1572-0241.1999.01233.x. [DOI] [PubMed] [Google Scholar]
- 10.Huarte M, Rinn JL. Large non-coding RNAs: missing links in cancer? Hum Mol Genet. 2010;19:R152–161. doi: 10.1093/hmg/ddq353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1:391–407. doi: 10.1158/2159-8290.CD-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai MC, Spitale RC, Chang HY. Long intergenic noncoding RNAs: new links in cancer progression. Cancer Res. 2011;71:3–7. doi: 10.1158/0008-5472.CAN-10-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Y, Zhang X, Klibanski A. MEG3 noncoding RNA: a tumor suppressor. J Mol Endocrinol. 2012;48:R45–53. doi: 10.1530/JME-12-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qi P, Du X. The long non-coding RNAs, a new cancer diagnostic and therapeutic gold mine. Mod Pathol. 2013;26:155–165. doi: 10.1038/modpathol.2012.160. [DOI] [PubMed] [Google Scholar]
- 15.Ma L, Bajic VB, Zhang Z. On the classification of long non-coding RNAs. RNA Biol. 2013;10:925–933. doi: 10.4161/rna.24604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, Cabili MN, Jaenisch R, Mikkelsen TS, Jacks T, Hacohen N, Bernstein BE, Kellis M, Regev A, Rinn JL, Lander ES. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez DS, Hoage TR, Pritchett JR, Ducharme-Smith AL, Halling ML, Ganapathiraju SC, Streng PS, Smith DI. Long, abundantly expressed non-coding transcripts are altered in cancer. Hum Mol Genet. 2008;17:642–655. doi: 10.1093/hmg/ddm336. [DOI] [PubMed] [Google Scholar]
- 18.Cao W, Wu W, Shi F, Chen X, Wu L, Yang K, Tian F, Zhu M, Chen G, Wang W, Biddle FG, Gu J. Integrated analysis of long noncoding RNA and coding RNA expression in esophageal squamous cell carcinoma. Int J Genomics. 2013;2013:480534. doi: 10.1155/2013/480534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sigova AA, Mullen AC, Molinie B, Gupta S, Orlando DA, Guenther MG, Almada AE, Lin C, Sharp PA, Giallourakis CC, Young RA. Divergent transcription of long noncoding RNA/mRNA gene pairs in embryonic stem cells. Proc Natl Acad Sci U S A. 2013;110:2876–2881. doi: 10.1073/pnas.1221904110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hannenhalli S, Kaestner KH. The evolution of Fox genes and their role in development and disease. Nat Rev Genet. 2009;10:233–240. doi: 10.1038/nrg2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–859. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 22.Xia L, Huang W, Tian D, Zhu H, Qi X, Chen Z, Zhang Y, Hu H, Fan D, Nie Y, Wu K. Overexpression of forkhead box C1 promotes tumor metastasis and indicates poor prognosis in hepatocellular carcinoma. Hepatology. 2013;57:610–624. doi: 10.1002/hep.26029. [DOI] [PubMed] [Google Scholar]
- 23.Xu ZY, Ding SM, Zhou L, Xie HY, Chen KJ, Zhang W, Xing CY, Guo HJ, Zheng SS. FOXC1 contributes to microvascular invasion in primary hepatocellular carcinoma via regulating epithelial-mesenchymal transition. Int J Biol Sci. 2012;8:1130–1141. doi: 10.7150/ijbs.4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ray PS, Wang J, Qu Y, Sim MS, Shamonki J, Bagaria SP, Ye X, Liu B, Elashoff D, Hoon DS, Walter MA, Martens JW, Richardson AL, Giuliano AE, Cui X. FOXC1 is a potential prognostic biomarker with functional significance in basal-like breast cancer. Cancer Res. 2010;70:3870–3876. doi: 10.1158/0008-5472.CAN-09-4120. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Gu F, Liu CY, Wang RJ, Li J, Xu JY. High level of FOXC1 expression is associated with poor prognosis in pancreatic ductal adenocarcinoma. Tumour Biol. 2013;34:853–858. doi: 10.1007/s13277-012-0617-7. [DOI] [PubMed] [Google Scholar]
- 26.Wei LX, Zhou RS, Xu HF, Wang JY, Yuan MH. High expression of FOXC1 is associated with poor clinical outcome in non-small cell lung cancer patients. Tumour Biol. 2013;34:941–946. doi: 10.1007/s13277-012-0629-3. [DOI] [PubMed] [Google Scholar]
- 27.Xu Y, Shao QS, Yao HB, Jin Y, Ma YY, Jia LH. Overexpression of FOXC1 correlates with poor prognosis in gastric cancer patients. Histopathology. 2014;64:963–70. doi: 10.1111/his.12347. [DOI] [PubMed] [Google Scholar]
- 28.Hao JJ, Gong T, Zhang Y, Shi ZZ, Xu X, Dong JT, Zhan QM, Fu SB, Wang MR. Characterization of gene rearrangements resulted from genomic structural aberrations in human esophageal squamous cell carcinoma KYSE150 cells. Gene. 2013;513:196–201. doi: 10.1016/j.gene.2012.09.091. [DOI] [PubMed] [Google Scholar]
- 29.Kim K, Jutooru I, Chadalapaka G, Johnson G, Frank J, Burghardt R, Kim S, Safe S. HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene. 2013;32:1616–1625. doi: 10.1038/onc.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakagawa T, Endo H, Yokoyama M, Abe J, Tamai K, Tanaka N, Sato I, Takahashi S, Kondo T, Satoh K. Large noncoding RNA HOTAIR enhances aggressive biological behavior and is associated with short disease-free survival in human non-small cell lung cancer. Biochem Biophys Res Commun. 2013;436:319–324. doi: 10.1016/j.bbrc.2013.05.101. [DOI] [PubMed] [Google Scholar]
- 31.Spizzo R, Almeida MI, Colombatti A, Calin GA. Long non-coding RNAs and cancer: a new frontier of translational research? Oncogene. 2012;31:4577–4587. doi: 10.1038/onc.2011.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Wu Z, Mei Q, Guo M, Fu X, Han W. Long non-coding RNA HOTAIR, a driver of malignancy, predicts negative prognosis and exhibits oncogenic activity in oesophageal squamous cell carcinoma. Br J Cancer. 2013;109:2266–2278. doi: 10.1038/bjc.2013.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang CM, Wu QQ, Li SQ, Chen FJ, Tuo L, Xie HW, Tong YS, Ji L, Zhou GZ, Cao G, Wu M, Lv J, Shi WH, Cao XF. Upregulation of the Long Non-coding RNA PlncRNA-1 Promotes Esophageal Squamous Carcinoma Cell Proliferation and Correlates with Advanced Clinical Stage. Dig Dis Sci. 2014;59:591–597. doi: 10.1007/s10620-013-2956-7. [DOI] [PubMed] [Google Scholar]
- 34.Preker P, Almvig K, Christensen MS, Valen E, Mapendano CK, Sandelin A, Jensen TH. PROMoter uPstream Transcripts share characteristics with mRNAs and are produced upstream of all three major types of mammalian promoters. Nucleic Acids Res. 2011;39:7179–7193. doi: 10.1093/nar/gkr370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Preker P, Nielsen J, Kammler S, Lykke-Andersen S, Christensen MS, Mapendano CK, Schierup MH, Jensen TH. RNA exosome depletion reveals transcription upstream of active human promoters. Science. 2008;322:1851–1854. doi: 10.1126/science.1164096. [DOI] [PubMed] [Google Scholar]