Abstract
Natural killer (NK) cells are important effector cells for the first line of defense against tumor. Distant MHC class I homolog MICA has been identified as human ligand for NK cell activating receptor NKG2D. Engagement of MICA triggers NK cells and augments antigen-specific CTL anti-tumor immunity. However, the expression level of MICA and its clinical significance in hepatocellular carcinoma remains to be elucidated. In the present study, a hospital-based study cohort of 143 HCC patients was involved. MICA expression levels were determined by immunohistochemical staining. The association of MICA expression with tumor clinicopathologic features, disease-free survival, and overall survival of HCC patients were analyzed. Significantly decreased expression of MICA was detected in tumor specimens. MICA expression was significantly associated with AFP level (P < 0.001) and tumor node metastasis stage (P = 0.003). Patients with reduced level of MICA had a statistically significantly shorter disease-free survival and overall survival duration than patients with preserved expression of MICA. However, in multivariate analysis, MICA expression level was found not to be an independent prognostic factor for both disease-free survival and overall survival of HCC patients. Our findings suggest that decreased MICA expression may play an important role in HCC tumor evasion of host immunity, which warrants further investigation in future studies.
Keywords: MHC class I-related chain, hepatocellular carcinoma, immunohistochemistry
Introduction
Hepatocellular carcinoma (HCC) ranks as the fifth most common malignancies and the third leading cause of cancer-related mortality worldwide, being responsible for 80% of primary malignant liver tumors in adults [1,2]. HCC has a 5-year relative survival rate of approximately 7% and causes more than 600,000 deaths annually worldwide [3]. Although its prevalence is highest in Africa and Asia, its incidence in western countries is rising mainly due to increasing rates of alcoholic liver diseases and hepatitis C infection [4]. Currently, there is no effective treatment of HCC except surgical resection and liver transplantation for early-stage cancer. However, fewer than 15% of patients undergo surgery because of late clinical presentation and diagnosis [5]. Therefore, the development of new therapeutic targets and biomarkers is urgently needed for early detection of HCC and thus the individualized treatment decision-making.
Although multiple major risk factors have been identified, such as genetic factors, environmental toxins, alcohol abuse, obesity, and metabolic disorders [6], infection with hepatitis virus B (HBV) or C (HCV) remains to be the major etiological factor for HCC [2]. Recent studies have demonstrated that NK cells are a major component of innate lymphocytes that responses to eradicate viral infection from the infected liver [7], and play a critical role in innate resistance against tumors [8,9]. In addition, previous studies have revealed that NK cells can modulate the functions of dendritic cells (DC), the major sentinel of innate and adaptive immunity [10,11]. Therefore, NK cells may also affect the magnitude and direction of adaptive immune responses against tumors. NK cell functions are regulated by a balance of negative and positive signals, which are mediated by inhibitory and activating receptors; the former includes killer cell immunoglobulin-like receptors (KIRs) and C-type lectin-like molecules, such as CD94 and NKG2A/E, and the latter includes the NKG2D activating receptor [12,13].
A stress-inducible MHC class I-related chain A (MICA) has been identified as a human ligand of NKG2D [14] expressed on the membrane of viral-infected cells and many carcinoma cells such as in lung, breast, ovary, prostate, colon cancer, but is usually absent from normal tissue [15,16]. MICA share structural homology with MHC class I molecules but have no role in antigen presentation [17]. It acts as a ligand for NKG2D to activate the antitumor effects of NK cells and CD8+ T cells [14,18,19]. This NKG2D-mediated tumor elimination is considered to be effective in the early stages of tumor growth [20-22]. Thus, the expression level of MICA on the tumor cell surface may determine the antitumor efficacy. On the other hand, MICA is also secreted into the serum by cleavage at the transmembrane domain with matrix metalloproteinases [23,24] and inhibits the anti-tumor effect of natural killer cells and CD8+ T cells by blocking their action [19,25,26]. Thus the levels of shedding MICA in serum may act as a decoy of NKG2D to avoid tumor rejection.
Previous works have demonstrated that a tumor-specific expression pattern of MICA has been observed in a broad range of epithelial tumor cells, such as melanoma, colon, breast, lung, ovary and renal [15,27]. However, the expression and biological functions of MICA in HCC cells has not been investigated. In this study, we sought to extensively explore the association of MICA with human HCC by determining their expression levels in a larger cohort of primary hepatocellular carcinoma tissues. In addition, we analyzed its association with clinicopathological parameters and prognosis in HCC patients.
Materials and methods
Study population
A total of 143 HCC patients were enrolled at the Eastern Hepatobiliary Surgery Hospital, Second Military Medical University in Shanghai, China. Patients who met all the following eligibility criteria were included in our study: (1) diagnosis of primary HCC identified by histopathological examination; (2) treatment with radical resection; (3) availability of complete follow-up data; (4) no preoperative anticancer treatment, such as chemotherapy and radiotherapy; (5) no history of familial malignancy or other synchronous malignancy, and (6) no death within 3 months after operation. The histopathological type and grade were determined using the criteria of the World Health Organization (WHO) classification. All patients were staged according to the seventh-edition TNM staging system of the Union for International Cancer Control (UICC) and American Joint Committee on Cancer (AJCC). Postoperative follow-up, including physical and laboratory examinations, was performed at the outpatient department every three months for the first two years, every six months for the third to fifth years and annually thereafter until at least five years after the operation or until the patient died, whichever came first. The last follow-up date was August, 2013. Informed consent was obtained from each patient. This study was approved by the Ethical Committee of Second Military Medical University and performed in accordance with the ethical standards of the Helsinki Declaration.
Tissue samples
For immunohistochemical staining, formalin-fixed, paraffin-embedded primary HCC samples were collected from 143 patients mentioned above and stored at room temperature. HE slides from these patients were viewed under a light microscope by pathologists and 4-μm-thick tissue sections were cut from corresponding blocks containing representative tumor regions.
Immunohistochemical stainning
Sections were incubated with antibody of MICA (1:100, Abcam). Visualization signal was developed with Invitrogen Histostain Plus kit. The intensity and extent of MICA immunostaining were evaluated for all samples under double-blinded conditions. In brief, the percentage of positive staining was scored as 0 (0-9%), 1 (10%-25%), 2 (26%-50%), 3 (51%-75%) or 4 (76%-100%), and the intensity as 0 (no staining), 1 (weak staining), 2 (moderate staining) or 3 (dark staining). The total score was calculated as the product of intensity and extent, ranging from 0 to 12.
Statistical analysis
All statistical analyses were performed using the SPSS Statistics 19.0 software (IBM). χ2 test was used to analyze the relationships between MICA expression and various clinicopathological parameters. Kaplan-Meier survival function was calculated and compared with log-rank test. Cox proportional hazard regression model was used for univariate and multivariate analyses to explore the effects of the clinicopathological variables and MICA expression on survival. All statistical tests were two-tailed and P < 0.05 was considered to be significant.
Results
Characteristics of patients
The characteristics of 143 HCC patients involved in this study are shown in Table 1. 118 patients (82.5%) were female and 25 (17.5%) were male. The medium age was 52 years, with a range of 25 to78. The tumor size of 57 HCC patients (39.9%) was smaller than 5 cm and that of 86 patients (60.1%) was larger than 5 cm (including 5 cm). Poorly differentiated tumor was the most common (65.7%), followed by moderately (30.8%) and well-differentiated (3.5%) tumors. According to the International TNM (Tumor Node Metastasis) Classification, 86 (60.1%), 20 (14%), 32 (22.4%), and 5 (3.5%) of 143 HCC patients were classified as TNM stages I, II, III, and IV, respectively. During this study, 57 (39.8%) patients died and 94 (65.7%) patients developed recurrence.
Table 1.
Associations of MICA expression with characteristics of HCC patients
| Variables | No. of cases | MICA_HCC expression | P (χ2 test) | |
|---|---|---|---|---|
|
| ||||
| Low | High | |||
| Total | 143 | 79 | 64 | |
| Gender | 0.158 | |||
| Male | 25 | 17 | 8 | |
| Female | 118 | 62 | 56 | |
| age | 0.243 | |||
| Age ≤ 54 | 77 | 46 | 31 | |
| Age > 54 | 66 | 33 | 33 | |
| HBsAg | 0.828 | |||
| HBsAg negative | 5 | 3 | 2 | |
| HBsAg positive | 138 | 76 | 62 | |
| AFP (μg/L) | 0.000 | |||
| AFP < 200 | 81 | 34 | 47 | |
| AFP ≥ 200 | 62 | 45 | 17 | |
| Maximal diameter (cm) | 0.609 | |||
| < 5 | 57 | 30 | 27 | |
| ≥ 5 | 86 | 49 | 37 | |
| Tumor number | 0.080 | |||
| Single | 106 | 54 | 52 | |
| Mutiple | 37 | 25 | 12 | |
| Differentiation | 0.505 | |||
| Well | 5 | 2 | 3 | |
| Moderately | 44 | 22 | 22 | |
| Poorly | 94 | 55 | 39 | |
| TNM | 0.003 | |||
| 1 | 86 | 38 | 48 | |
| 2 | 20 | 16 | 4 | |
| 3 | 32 | 20 | 12 | |
| 4 | 5 | 5 | 0 | |
| PVTT | 0.152 | |||
| No PVTT | 123 | 65 | 58 | |
| PVTT | 20 | 14 | 6 | |
| therapy | 0.140 | |||
| hepatectomy | 113 | 66 | 47 | |
| hepatectomy + TACE | 30 | 13 | 17 | |
CEA, carcinoembryonic antigen; MICA, MHC class I polypeptide-related sequence A.
MICA expression was significantly decreased in HCC cells
We performed immunohistochemical analyses of 143 HCC tissue blocks, and MICA was detectable in all analyzed clinical specimens. As shown in Figure 1, MICA expression was lower in cancer tissues than in adjacent noncancerous tissues, and this difference was statistically significant (P < 0.001). By immunohistochemistry, MICA has a membrane-staining and cytoplasmic pattern, which is in accord with the membrane-bound and soluble forms of MICA.
Figure 1.
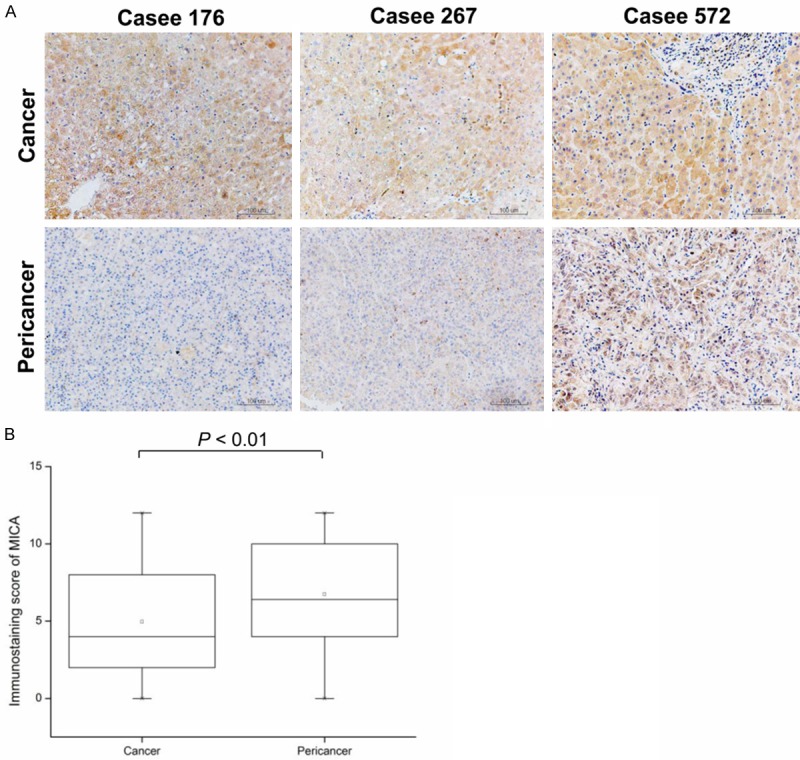
MICA expression in HCC tissues examined by immunohistochemistry. A: Representative intratumoral and peritumoral immunohistochemical staining of MICA is shown. B: Mann-Whitney test showed elevated MICA expression in HCC tissues compared to pericancer tissues.
Association between expression of MICA and clinicopathologic characteristics of HCCs
Patients were divided into two groups based on the overall expression level of MICA: a high MICA expression group (n = 64) and a low MICA expression group (n = 79). The association between the MICA expression level and different clinicopathologic variables are shown in Table 1. No statistically significant associations were observed between MICA expression and gender, age at diagnosis, HBsAg, tumor size, tumor number, differentiation status, and PVTT status. Instead, the low level of MICA expression were found to be significantly associated with high AFP class (P < 0.001) and advanced TNM staging (P = 0.003) (Figure 2).
Figure 2.
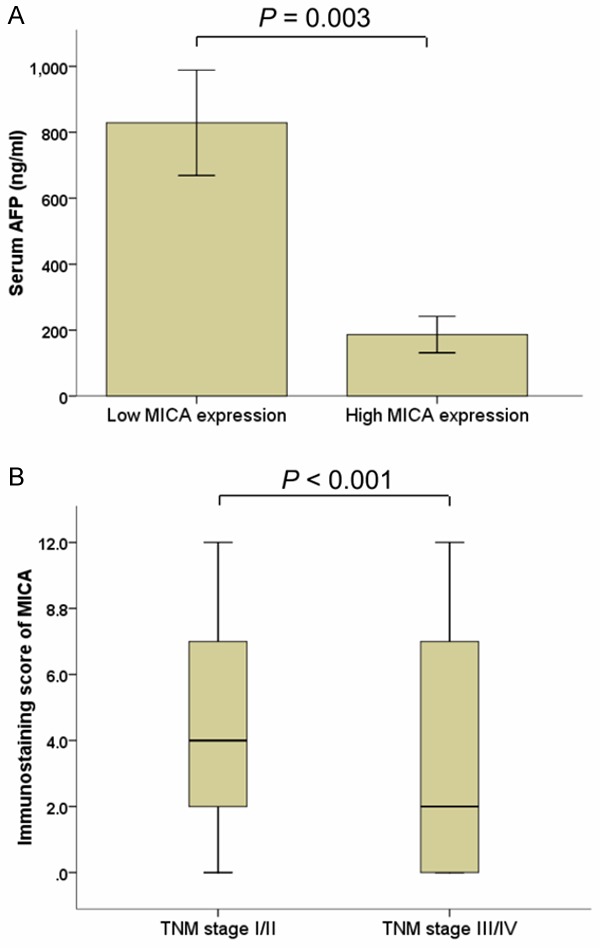
Associations of MICA expression with characteristics of HCC patients. MICA expression is negatively related to AFP class (A) and TNM staging (B).
Association between the MICA expression and disease-free survival in HCC patients
The postoperative median follow-up duration was 24 months, and the Kaplan–Meier analysis was used to evaluate the disease-free survival of HCC patients with high or low MICA expression. Our data showed that HCC patients with high MICA expression had better disease-free survival than those with low MICA expression (Figure 3A, log-rank test: P = 0.05). The postoperative mean disease-free survival time of all eligible patients with HCC was 19.26 months (95% CI: 16.39-22.13). The postoperative mean disease-free survival time of patients with high expression of MICA was 23.01 months (95% CI: 18.03-27.97) whereas that of patients with low MICA expression was 17.26 months (95% CI: 13.83-20.68). AFP, tumor size, tumor number, differentiation status, TNM stage, and PVTT status were proved to be associated with disease-free survival of patients with HCC. Patients with AFP of greater than 200 μg/L (including 200 μg/L) and HCC patients with greater tumor size or number, poor differentiation, advanced TNM stage or PVTT positive had shorter disease-free survival and higher risk to relapse than those without. However, gender, age, HBsAg class, or therapy status had no prognostic value on disease-free survival of patients with HCC. Unadjusted hazard ratio (HR) is shown in Table 2.
Figure 3.
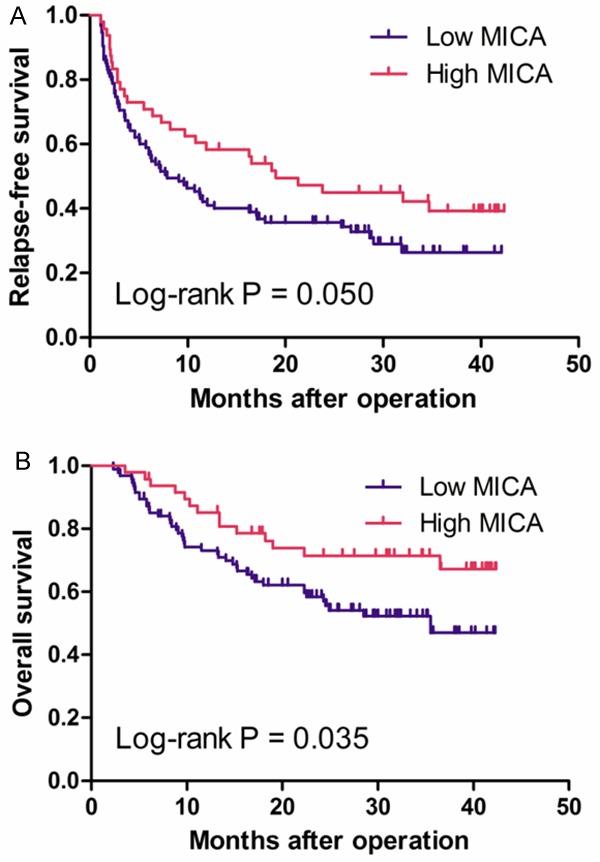
Kaplan-Meier survival curves of patients with different MICA expression levels. There is significant difference in the RFS (A) and OS (B) of HCC patients with different MICA levels.
Table 2.
Cox regression analysis of prognostic factors in hepatocellular carcinoma
| Variables | OS | RFS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Unadjusted analysisa | Adjusted analysisb | Unadjusted analysisa | Adjusted analysisb | |||||||
|
|
|
|
|
|||||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |||
| MICA expression | 0.695 (0.408-1.184) | 0.181 | 1.094 (0.592-2.022) | 0.774 | 0.928 (0.617-1.395) | 0.720 | 1.425 (0.896-2.267) | 0.135 | ||
| Gender | 1.021 (0.531-1.963) | 0.951 | 1.284 (0.629-2.620) | 0.492 | 1.046 (0.612-1.789) | 0.869 | 1.323 (0.743-2.355) | 0.342 | ||
| Age_class | 1.009 (0.607-1.678) | 0.972 | 1.106 (0.633-1.931) | 0.723 | 1.095 (0.735-1.633) | 0.655 | 1.120 (0.727-1.725) | 0.608 | ||
| HBsAg | 0.877 (0.214-3.598) | 0.855 | 0.893 (0.282-2.822) | 0.847 | ||||||
| AFP_class | 2.040 (1.224-3.399) | 0.006 | 1.875 (1.031-3.410) | 0.039 | 1.712 (1.148-2.554) | 0.008 | 1.749 (1.100-2.780) | 0.018 | ||
| HCC_size | 2.489 (1.401-4.421) | 0.002 | 1.769 (1.163-2.691) | 0.008 | ||||||
| Tumor_No | 2.273 (1.334-3.871) | 0.003 | 2.129 (1.385-3.272) | 0.001 | ||||||
| Differentiation | 1.717 (1.026-2.876) | 0.040 | 1.396 (0.801-2.433) | 0.240 | 1.647 (1.109-2.447) | 0.013 | 1.463 (0.963-2.223) | 0.074 | ||
| TNM | 1.953 (1.524-2.503) | 0.000 | 1.943 (1.489-2.536) | 0.000 | 1.661 (1.364-2.022) | 0.000 | 1.688 (1.368-2.083) | 0.000 | ||
| PVTT | 4.564 (2.527-8.244) | 0.000 | 3.869 (2.320-6.452) | 0.000 | ||||||
| therapy | 0.722 (0.366-1.424) | 0.347 | 0.632 (0.304-1.313) | 0.219 | 0.722 (0.432-1.205) | 0.212 | 0.597 (0.344-1.038) | 0.067 | ||
Hazard ratios in univariate models.
Hazard ratios in multivariable models.
Abbreviations: CI, confidence interval; MICA, MHC class I polypeptide-related sequence A; HR, hazard ratio; OS, overall survival; RFS, relapse-free survival; PVTT, Portal Vein Tumor Thrombus.
To verify the independent prognostic value of MICA expression, the Cox proportional hazards model adjusted for gender, age, HBsAg, AFP class, tumor size, tumor number, differentiation status, TNM stage, PVTT, and therapy status was utilized to control for other prognostic factors. As a result, MICA protein level seems to be not an independent prognostic factor after controlling for all other life style and clinicopathologic factors. Whereas, AFP class and TNM stage were proved to be independent prognostic factors for disease-free survival of patients with HCC.
Association between MICA expression and overall survival of HCC patients
A statistically significant association between poor overall survival and reduced MICA expression level was found in patients with HCC. The Kaplan–Meier analysis for postoperative overall survival showed that HCC patients with high MICA expression had better overall survival than patients with low expression of MICA (Figure 3B, log-rank test: P = 0.035). The postoperative mean overall survival time of all eligible patients with HCC was 29.76 months (95% CI: 27.16-32.35). The postoperative mean overall survival time of patients with high expression of MICA was 33.57 months (95% CI: 29.57-37.58), whereas that of patients with low expression of MICA was 27.62 months (95% CI: 24.34-30.89). Similar to results of disease-free survival, AFP, tumor size, tumor number, differentiation status, TNM stage, and PVTT status proved to be prognostic factors for overall survival of patients with HCC. Patients with greater tumor size or number, poor differentiation, advanced TNM stage or PVTT positive had shorter overall survival. However, MICA expression, gender, age, HBsAg class, or therapy status had no prognostic value on overall survival of patients with HCC. Unadjusted HR values are shown in Table 2.
Multivariate analysis showed that AFP class and TNM stage were independent prognostic factors for overall survival of patients with HCC. Whereas, MICA expression level was proved to be not a prognostic factor for overall survival of HCC patients. In addition, no statistically significant correlation between age, gender, HBsAg, vascular invasion, differentiation or PVTT status and overall survival was found among patients with HCC (Table 2).
Discussion
In this study, we examined the expression of MICA and its association with clinical characteristics in HCC patients. We found that MICA expression was decreased in HCC tissues when compared with adjacent noncancerous tissues. Additionally, the expression levels of MICA were significantly associated with advanced TNM stage and AFP production, suggesting that decreased MICA expression might be of clinical relevance in the aggressiveness of hepatocellular carcinoma. We also explored the prognostic value of MICA in HCC patients. To our best knowledge, this is the first study to investigate the expression and clinical significance of MICA in HCC.
Immune responses against cancer cells is crucial for the prevention of tumor recurrence [28]. One important mechanism that prevents cancer metastasis is immune surveillance against cancer cells, in which NK cells play a crucial role [29]. The lysis of cancer cells by NK cells needs the symphony of KARs, such as NKG2D, DNAM-1, NKp46, and NKp30. It has been well documented that the interaction between MICA and NKG2D especially contributes to the effector responses of NK cells [14]. MICA expressed in many carcinoma cells and may serve as an important ‘on’ signal for NK cell-mediated innate immune surveillance against tumor cells [15,16,30]. Wu et al have demonstrated that MICA is induced at the early stage of prostate luminal epithelial cell transformation, and loss of predominant surface localization of MICA is associated with progression to invasive tumor or to progressively higher grades [16]. In addition, Maccalli et al have shown that the MICA/NKG2D interaction promotes the lysis of tumor cells by T cells [18]. In consistence with these findings, we found that decreased MICA expression in HCC cells was associated with high-grade of HCC and worth OS of patients, suggesting the critical role of MICA/NKG2D interaction in the prevention of malignization of HCC by NK cells. However, the underlying mechanisms need to be further investigated.
Limited information is available about how MICA expression is regulated, particularly in specific tumors. The MICA gene transcriptional regulatory sequences contain heat shock elements similar to those in the hsp70 promoter, and previous studies have shown that MICA expression can be upregulated by heat shock treatment [31]. It had already shown that oxidative stress, viral and bacterial infections induced MICA expression, and activation of the MAPK intracellular signaling pathway upregulates the MICA expression on activated T lymphocytes [32-35]. However, the specific mechanisms of the induction of MICA expression remain unclear.
According to current available data, MICA is induced on a broad range of epithelial tumor cells, while is absent from normal tissues [15,27,30]. However, our current study clearly demonstrates that MICA is predominantly expressed in the adjacent nontumorous tissues rather than the HCC tumor cells. Human HCC has the unique characteristic of a development from chronic inflammatory liver diseases, and the adjacent “nontumorous tissues” is actually undergoing chronic inflammation. As cells expressing MICA on their surface are susceptible to NK and antigen-specific T cell immunity, the increased surface expression of MICA on adjacent nontumorous cells is proposed to mark nascent transformed cells for immune surveillance [36]. On the other hand, it has been demonstrated that NK cell activity decrease in patients with chronic liver diseases as well as HCC [37,38], and long-term immunosuppression increases the incidence of many forms of malignancies [16]. Decreased MICA expression may represent one of the factors involved in HCC tumor evasion of host immunity, which is in accordance with our findings that decreased MICA expression is of clinical relevance in the aggressiveness of HCC. However, definitive evidence is still lacking and the role of MICA in the development of HCC needs to be further investigated in future studies.
In summary, our study for the first time demonstrated the reduction of MICA expression levels in HCC tissues compared with adjacent nontumorous tissues. In addition, significant associations between the decreased expression levels of MICA were found with high AFP class and advanced TNM staging. However, we did not find any association of MICA expression with the prognosis of HCC patients. Our findings contribute to the current understanding on the tumor cell adaption to antagonize the immunologic defense, which warrant further investigation in the role of MICA in the procedure of HCC development in future studies.
Acknowledgements
This work was supported by Program for New Century Excellent Talents in University, National Natural Science Foundation (81171966) and National Key Technologies R&D Program (2011ZX09307-001-04) of China.
Disclosure of conflict of interest
None.
References
- 1.Avila MA, Berasain C, Sangro B, Prieto J. New therapies for hepatocellular carcinoma. Oncogene. 2006;25:3866–3884. doi: 10.1038/sj.onc.1209550. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273. e1261. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Mann CD, Neal CP, Garcea G, Manson MM, Dennison AR, Berry DP. Prognostic molecular markers in hepatocellular carcinoma: a systematic review. Eur J Cancer. 2007;43:979–992. doi: 10.1016/j.ejca.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Feng JT, Shang S, Beretta L. Proteomics for the early detection and treatment of hepatocellular carcinoma. Oncogene. 2006;25:3810–3817. doi: 10.1038/sj.onc.1209551. [DOI] [PubMed] [Google Scholar]
- 6.Sherman M. Hepatocellular carcinoma: New and emerging risks. Dig Liver Dis. 2010;42(Suppl 3):S215–222. doi: 10.1016/S1590-8658(10)60508-7. [DOI] [PubMed] [Google Scholar]
- 7.Arababadi MK, Nasiri Ahmadabadi B, Kennedy D. Current information on the immunologic status of occult hepatitis B infection. Transfusion. 2012;52:1819–1826. doi: 10.1111/j.1537-2995.2012.03575.x. [DOI] [PubMed] [Google Scholar]
- 8.Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2:850–861. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- 9.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 10.Moretta A. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat Rev Immunol. 2002;2:957–964. doi: 10.1038/nri956. [DOI] [PubMed] [Google Scholar]
- 11.Degli-Esposti MA, Smyth MJ. Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nat Rev Immunol. 2005;5:112–124. doi: 10.1038/nri1549. [DOI] [PubMed] [Google Scholar]
- 12.Cerwenka A, Lanier LL. Natural killer cells, viruses and cancer. Nat Rev Immunol. 2001;1:41–49. doi: 10.1038/35095564. [DOI] [PubMed] [Google Scholar]
- 13.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 14.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 15.Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc Natl Acad Sci U S A. 1999;96:6879–6884. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu JD, Higgins LM, Steinle A, Cosman D, Haugk K, Plymate SR. Prevalent expression of the immunostimulatory MHC class I chain-related molecule is counteracted by shedding in prostate cancer. J Clin Invest. 2004;114:560–568. doi: 10.1172/JCI22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauer S, Willie ST, Spies T, Strong RK. Expression, purification, crystallization and crystallographic characterization of the human MHC class I related protein MICA. Acta Crystallogr D Biol Crystallogr. 1998;54:451–453. doi: 10.1107/s0907444997015229. [DOI] [PubMed] [Google Scholar]
- 18.Maccalli C, Scaramuzza S, Parmiani G. TNK cells (NKG2D+ CD8+ or CD4+ T lymphocytes) in the control of human tumors. Cancer Immunol Immunother. 2009;58:801–808. doi: 10.1007/s00262-008-0635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jinushi M, Takehara T, Tatsumi T, Hiramatsu N, Sakamori R, Yamaguchi S, Hayashi N. Impairment of natural killer cell and dendritic cell functions by the soluble form of MHC class I-related chain A in advanced human hepatocellular carcinomas. J Hepatol. 2005;43:1013–1020. doi: 10.1016/j.jhep.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 20.Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165–171. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayakawa Y. Targeting NKG2D in tumor surveillance. Expert Opin Ther Targets. 2012;16:587–599. doi: 10.1517/14728222.2012.681378. [DOI] [PubMed] [Google Scholar]
- 22.Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, Knoblaugh S, Cado D, Greenberg NM, Raulet DH. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–580. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salih HR, Rammensee HG, Steinle A. Cutting edge: down-regulation of MICA on human tumors by proteolytic shedding. J Immunol. 2002;169:4098–4102. doi: 10.4049/jimmunol.169.8.4098. [DOI] [PubMed] [Google Scholar]
- 24.Waldhauer I, Goehlsdorf D, Gieseke F, Weinschenk T, Wittenbrink M, Ludwig A, Stevanovic S, Rammensee HG, Steinle A. Tumor-associated MICA is shed by ADAM proteases. Cancer Res. 2008;68:6368–6376. doi: 10.1158/0008-5472.CAN-07-6768. [DOI] [PubMed] [Google Scholar]
- 25.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 26.Doubrovina ES, Doubrovin MM, Vider E, Sisson RB, O’Reilly RJ, Dupont B, Vyas YM. Evasion from NK cell immunity by MHC class I chain-related molecules expressing colon adenocarcinoma. J Immunol. 2003;171:6891–6899. doi: 10.4049/jimmunol.171.12.6891. [DOI] [PubMed] [Google Scholar]
- 27.Vetter CS, Groh V, thor Straten P, Spies T, Brocker EB, Becker JC. Expression of stress-induced MHC class I related chain molecules on human melanoma. J Invest Dermatol. 2002;118:600–605. doi: 10.1046/j.1523-1747.2002.01700.x. [DOI] [PubMed] [Google Scholar]
- 28.Budhu A, Forgues M, Ye QH, Jia HL, He P, Zanetti KA, Kammula US, Chen Y, Qin LX, Tang ZY, Wang XW. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10:99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 29.Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27:5932–5943. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]
- 30.Jinushi M, Takehara T, Tatsumi T, Kanto T, Groh V, Spies T, Kimura R, Miyagi T, Mochizuki K, Sasaki Y, Hayashi N. Expression and role of MICA and MICB in human hepatocellular carcinomas and their regulation by retinoic acid. Int J Cancer. 2003;104:354–361. doi: 10.1002/ijc.10966. [DOI] [PubMed] [Google Scholar]
- 31.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science. 1998;279:1737–1740. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto K, Fujiyama Y, Andoh A, Bamba T, Okabe H. Oxidative stress increases MICA and MICB gene expression in the human colon carcinoma cell line (CaCo-2) Biochim Biophys Acta. 2001;1526:10–12. doi: 10.1016/s0304-4165(01)00099-x. [DOI] [PubMed] [Google Scholar]
- 33.Groh V, Rhinehart R, Randolph-Habecker J, Topp MS, Riddell SR, Spies T. Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat Immunol. 2001;2:255–260. doi: 10.1038/85321. [DOI] [PubMed] [Google Scholar]
- 34.Das H, Groh V, Kuijl C, Sugita M, Morita CT, Spies T, Bukowski JF. MICA engagement by human Vgamma2Vdelta2 T cells enhances their antigen-dependent effector function. Immunity. 2001;15:83–93. doi: 10.1016/s1074-7613(01)00168-6. [DOI] [PubMed] [Google Scholar]
- 35.Molinero LL, Fuertes MB, Fainboim L, Rabinovich GA, Zwirner NW. Up-regulated expression of MICA on activated T lymphocytes involves Lck and Fyn kinases and signaling through MEK1/ERK, p38 MAP kinase, and calcineurin. J Leukoc Biol. 2003;73:815–822. doi: 10.1189/jlb.0602329. [DOI] [PubMed] [Google Scholar]
- 36.Kim S, Iizuka K, Aguila HL, Weissman IL, Yokoyama WM. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc Natl Acad Sci U S A. 2000;97:2731–2736. doi: 10.1073/pnas.050588297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taketomi A, Shimada M, Shirabe K, Kajiyama K, Gion T, Sugimachi K. Natural killer cell activity in patients with hepatocellular carcinoma: a new prognostic indicator after hepatectomy. Cancer. 1998;83:58–63. doi: 10.1002/(sici)1097-0142(19980701)83:1<58::aid-cncr8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 38.Kawarabayashi N, Seki S, Hatsuse K, Ohkawa T, Koike Y, Aihara T, Habu Y, Nakagawa R, Ami K, Hiraide H, Mochizuki H. Decrease of CD56(+)T cells and natural killer cells in cirrhotic livers with hepatitis C may be involved in their susceptibility to hepatocellular carcinoma. Hepatology. 2000;32:962–969. doi: 10.1053/jhep.2000.19362. [DOI] [PMC free article] [PubMed] [Google Scholar]


