Abstract
The clear cell renal cell carcinoma (ccRCC) is the most common subtype in renal cell carcinomas. Rapidly accumulating studies show that the long non-coding RNAs (lncRNAs) may play essential roles in cancers. In this study, we investigated the expression pattern of a novel lncRNA CADM1-AS1 in ccRCC by quantitative real time PCR. The results showed that CADM1-AS1 expression was down-regulated in tumor tissues in 64 patients with ccRCC compared with adjacent non-tumor tissues. Furthermore, the expression of CADM1-AS1 was positively correlated with the expression of mRNA CADM1 in ccRCC specimens (R = 0.611, P <0.0001). Decreased CADM1-AS1 expression was correlated with the progression of AJCC stage (P = 0.039) and worse survival of ccRCC patients (P <0.05). Also, multivariate analysis identified low CADM1-AS1 expression as an independent prognostic factor for ccRCC (P <0.001, HR = 0.211, 95% CI = 0.088-0.504). In addition, we used small interfering RNA (siRNA) to evaluate the biological function of CADM1-AS1 in vitro. The results showed that CADM1-AS1 expression was positively associated with CADM1 mRNA expression in 786-O cells and ACHN cells. Functional experiments demonstrated markedly enhanced ability of growth and migration, and reduced apoptotic rate in CADM1-AS1 knocking down in 786-O cells. Conversely, overexpression of CADM1-AS1 showed a significant decrease in growth and migration, along with an increase in apoptotic rate in ACHN cells. In conclusion, our data demonstrated CADM1-AS1 is a new tumor suppressor in ccRCC which regulates cell proliferation, apoptosis and migration via the expression pattern of “CADM1-AS1/CADM1 mRNA gene pairs”. CADM1-AS1 may be a potential biomarker and therapeutic target in patients with ccRCC.
Keywords: lnc RNAs, CADM1-AS1, clear cell renal cell carcinoma
Introduction
Renal cell cancer (RCC) represents 2% of all cancers and is the most lethal of all urological malignancies [1,2]. The clear cell renal cell carcinomas (ccRCC) is the most common subtype and represents about 70% of all renal tumors [3]. Metastasis is a common event for ccRCC and about one third of ccRCC patients have metastasis at the time of diagnosis [4,5]. To identify predictive biomarkers is therefore crucial for early detection and prevention of ccRCC.
An increasing number of studies have shown that the long non-coding RNAs (lncRNAs), the largest transcript class in human genome, may play a fundamental role in a variety of biological cellular processes and multiple diseases, including cancer [6,7]. There are a few prior data on the relationship between RCC and lncRNAs [8], which prompted us to conduct the current study.
It is known that many of these lncRNA transcripts originate from transcription at promoters or other nearby location of protein-coding genes [9,10]. Using bioinformatics analysis, we found a new lncRNA (RNA176206|ENST00000546273) located at the antisense direction of a coding exon of the cell adhesion molecule1 (CADM1). We gave this lncRNA a new name “CADM1-AS1”. CADM1 encodes a cellular adhesion molecule and regulates apoptosis, differentiation and cell cycle as a tumor suppressor [11]. And it is down-regulated in many solid tumors, including lung, liver, pancreatic, breast, brain and prostate cancers [12-15].
The main objective of our study was to investigate the expression of the “CADM1-AS1/ CADM1 mRNA gene pair” in ccRCC tumor tissues and adjacent non-tumor tissues, and to explore the correlation between CADM1-AS1 expression and clinicopathological characteristics and patient survival, in order to evaluate CADM1-AS1 as a potential biomarker for ccRCC prognosis. This study may provide a new biomarker and therapeutic target that facilitates early diagnosis and treatment for ccRCC.
Materials and methods
Patient samples
A total of 64 patients with ccRCC were recruited during 2006-2012 at Chinese PLA General Hospital. All patients had underwent surgery and were histologically confirmed. Tumor and paired adjacent non-tumorous specimens (≤1.5 cm away from the tumor) were presented in our study after nephrectomy operation. The study was carried out with pre-approval from the hospital’s Ethical Research Committee and all enrolled patients provided written informed consent for tissue donation and analysis, and for publication of findings. No patient had received therapy before resection. All patients were followed up from the date of surgery until either the date of death or December 2012. For analysis of patient survival, the patients who were lost to follow-up or those who died from causes other than RCC were regarded as censored data.
Cancer cell lines
The renal carcinomas cell 786-O and ACHN were purchased from American Type Culture Collection. 786-O and ACHN cell lines were cultured in DMEM and MEM medium, respectively, at 37°C with 5% CO2, with each medium containing 10% fetal bovine serum (Gibco, USA).
RNA extraction and real-time quantitative PCR
Total RNA was extracted from frozen cancer tissues, matched adjacent normal tissue using the Trizol Total RNA Reagent (Invitrogen, USA). The concentration and A260/280 ratio were measured by NanoveuPlus (GE Healthcare, UK). The RNA integrity and absence of genomic DNA contamination were evaluated by formaldehyde-agarosegel electrophoresis.
Complementary DNA was synthesized from 1 ug total RNA using a Revert Aid First Strand cDNA Synthesis Kit (Thermo Scientific, USA), according to the manufacturer’s instructions.
Quantitative PCR was performed using the SYBR Prime Script RT-PCR kit (Takara, Japan) in an Applied Biosystems 7500 Fluorescent Quantitative PCR System (Applied Biosystems, USA). The reaction mixtures were incubated at 95°C for 30 s, followed by 40 amplification cycles of 95°C for 5 s and 60°C for 34 s. The comparative Ct method was used to quantify relative expression of mRNA and lncRNA. Expression level of housekeeping gene GADPH was used to normalize gene-of-interest expression. The primers sequences in ourstudy were as follows: CADM1-AS1 Forward 5’-TGACAAAGGCAGGAGGTA-3’, Reverse 5’-GCACTATGGCTGAGGAAA-3’; CADM1 Forward 5’-ATGGCGAGTGTAGTGCTGC-3’; Reverse 5’-GATCACTGTCACGTCTTTCGT-3’.
Transfection of siRNA
We designed small interfering RNA (siRNA1, siRNA2) for knock down of CADM1-AS1 in 786-O cells. And non-targeting siRNA (NC SiRNA) was negative control and obtained from GenePharma (Shanghai, China). The sequences were as follows: siRNA1 Sense strand 5’-rGrUrArCrCrUrCrCrUrGrCrCrUrUrUrGrUrCrArArGrCrCAA-3’; Antisense strand 5’-rUrUrGrGrCrUrUrGrArCrArArArGrGrCrArGrGrArGrGrUrArCrArA-3’; siRNA2Sense strand 5’-rGrArCrCrUrArUrCrGrArGrArArCrUrGrArGrArGrCrGrACA-3’; Antisense strand 5’-rUrGrUrCrGrCrUrCrUrCrArGrUrUrCrUrCrGArUrArGrGrUrCrArG-3’.
Overexpression of CADM1-AS1 in ACHN cells
Plasmid pcDNA-CADM1-AS1 was constructed by introducing a BamHI-EcoRI fragment containing CADM1-AS1cDNA into the same sites in pcDNA3.1. The ACNH cells were transfected with pcDNA-CADM1-AS1 using Lipofectamine 2000 (Invitrogen, USA). And pcDNA-control was used as a negative control.
Cell growth curve
786-O cells (5×104) and ACHN cells (5×104) in exponential growth were seeded into four 12-well plates. The plates were incubated at 37°C in a humidified 5% CO2 atmosphere. The 786-O cells and ACHN cells were counted by hemocytometer after transfection at 24 h, 48 h, and 72 h.
Scratch wound healing assay
For the scratch wound healing assay, 786-O cells and ACHN cells were grown in complete growth medium until 90% confluency after transfection. A 3 mm wound was introduced across the diameter of each plate. Cell migration was observed by microscopy at 24 and 48 h later and analyzed objectively using Image J.
Apoptosis detection by flow cytometry
786-O cells and ACHN cells were cultured in Serum-Free Medium after transfection. Apoptosis analysis was performed by the PE Annexin V Apoptosis Detection Kit I (BD, USA) after culturing in serum-free medium about 48 h. Samples were analyzed using the FACS Caliber flow cytometer (BD, USA). Data were analyzed by Cell Quest software (BD, USA).
Statistical analysis
Differences between groups were analyzed using Student’s t test. Correlation between gene expressions was studied by using Pearson’s correlation. Chi-square test was performed to explore the associations between CDAM1-AS1 expression and clinical characteristics. Survival curves were estimated by the Kaplan-Meier method. The log-rank test was used to estimate the statistical difference between survival curves.
Cox proportional hazards analysis was performed to calculate the hazard ratio (HR) and the 95% confidence interval (CI) to evaluate the association between CDAM1-AS1 expression and overall survival time. In addition, a multivariate Cox regression was performed to adjust for other covariates. Statistical analyses were performed by using SPSS version 18.0. For all statistical analyses, P <0.05 was considered statistically significant.
Results
CADM1-AS1 expression was down-regulated and correlated with the mRNA expression of CADM1 in ccRCC tumor tissues
CADM1-AS1 expression and the mRNA expression of CADM1 were assessed in 64 patients with ccRCC by Q-PCR. For each ccRCC patient, CADM1-AS1 expression and the mRNA expression of CADM1 were isolated from cancerous tissues and adjacent non-tumorous tissues. Adjacent non-tumorous tissues were higher than cancerous tissues in CADM1-AS1 expression and the mRNA expression of CADM1. The results showed that the expression of CADM1-AS1 was significantly correlated with the mRNA expression of CADM1 in ccRCC tissue samples (R = 0.611, P <0.0001, Figure 1).
Figure 1.
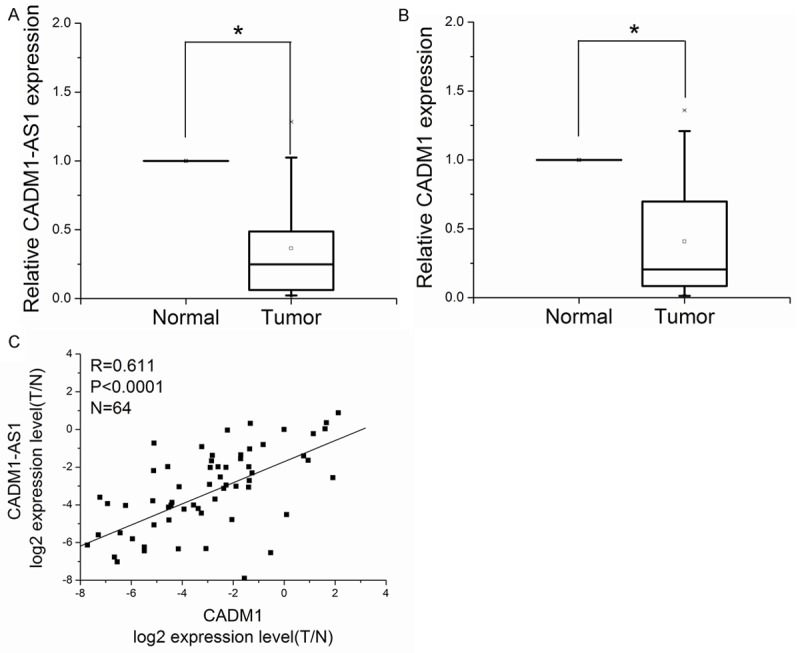
The relationship between CADM1-AS1 and CADM1 in ccRCC. A. CADM1-AS1 expression was detected in 64 pairs of ccRCC tissues by Q-PCR. B. The mRNA expression of CADM1 was detected in 64 pairs of ccRCC tissues by Q-PCR. C. The correlation between CADM1-AS1 expression and the mRNA expression of CADM1 in ccRCC.
Relationship between CADM1-AS1 expression and clinicopathologic factors in ccRCC
We examined the correlation of CADM1-AS1 expression level with the clinicopathologic factors in 64 patients with ccRCC. Decreased CADM1-AS1 expression significantly correlated with the progression of ccRCC from AJCC stages I & II to AJCC stages III & IV. Decreased CADM1-AS1 expression was associated with more advanced AJCC stage (P = 0.039, Figure 2). No relationship between CADM1-AS1 expression and other factors, e.g., age (≤60, >60), gender (male, female), tumor diameter (<4, ≥4) or differentiation (poor differentiation: III/IV; well differentiation: I/II), was found in our study (Table 1).
Figure 2.
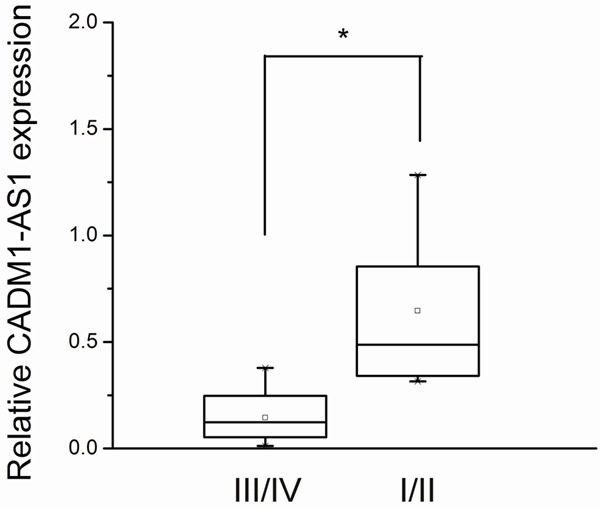
CADM1-AS1 expression was significantly lower in tumor with AJCC stage III/IV than AJCC stage I/II.
Table 1.
Relationship between expression of CADM1-AS1 and clinicopathologic factors in 64 patients with ccRCC
| Characteristics | Cases | Expression of CADM1-AS1 | P value | |
|---|---|---|---|---|
|
| ||||
| Low | High | |||
| Age, year | ||||
| ≤60 | 24 | 13 | 11 | 0.606 |
| >60 | 40 | 19 | 21 | |
| Gender | ||||
| Male | 38 | 20 | 18 | 0.611 |
| Female | 26 | 12 | 14 | |
| Tumor diameter (cm) | ||||
| <4 | 18 | 8 | 10 | 0.578 |
| ≥4 | 46 | 24 | 22 | |
| Differentiation | ||||
| I/II | 30 | 12 | 18 | 0.133 |
| III/IV | 34 | 20 | 14 | |
| AJCC stage | ||||
| I/II | 54 | 24 | 30 | 0.039※ |
| III/IV | 10 | 8 | 2 | |
P <0.05.
CADM1-AS1 expression is an independent predictor for overall survival
Kaplan-Meier analysis of the survival of patients was performed to evaluate CADM1-AS1 expression in ccRCC. We divided the samples into high (above the mean, n = 25) and low (below the mean, n = 25) CADM1-AS1 expression groups according to the mean value of CADM1-AS1 level. As shown in Figure 3, patients with low expression of CADM1-AS1 had significantly worse overall compared with patients with higher expression of CADM1-AS1 expression (P <0.05).
Figure 3.
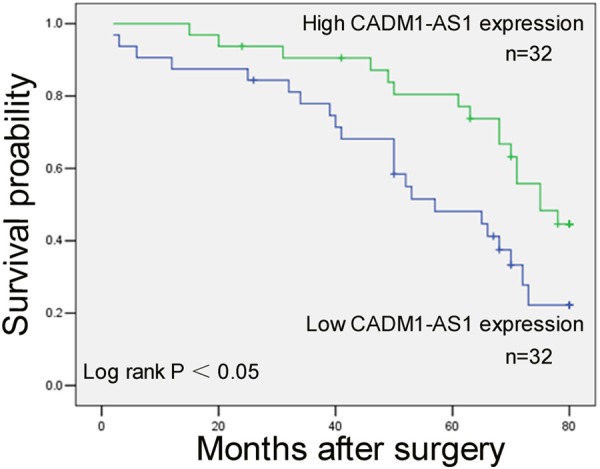
Patients with lower expression of CADM1-AS1 expression showed decreased overall survival compared with patients with higher expression of CADM1-AS1 expression.
Univariate analysis identified five prognostic factors: tumor diameter (<4 cm, ≥4 cm), differentiation (I/II, III/IV), AJCC stage (I/II, III/IV), gender (male, female) and CADM1-AS1 expression (P <0.05, Table 2). Age was not statistically significant prognosis factors. More importantly, multivariate analysis of the prognostic factors confirmed that low CADM1-AS1 expression was a significant independent predictor of poor survival of ccRCC (P <0.001, HR 0.211, 95% CI 0.088-0.504), in addition to tumor diameter, differentiation and AJCC stage (Table 2).
Table 2.
Univariate and multivariate analysis of clinicopathologic factors for overall survival in 64 patients with ccRCC
| Risk factors | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR※ | P value | 95% CI | HR※ | P value | 95% CI | |
| CADM1-AS1 expression (low/high) | 0.461 | 0.021※※ | 0.239-0.889 | 0.211 | 0.001※※ | 0.088-0.504 |
| Tumor diameter (<4 cm, ≥4 cm) | 4.655 | 0.001※※ | 2.379-9.109 | 4.054 | 0.001※※ | 1.986-8.277 |
| Differentiation (I/II, III/IV) | 0.242 | 0.001※※ | 0.123-0.473 | 0.130 | 0.001※※ | 0.050-0.337 |
| AJCC stage (I/II, III/IV) | 10.661 | 0.001※※ | 4.127-27.540 | 5.256 | 0.005※※ | 1.661-16.626 |
| Age (≤60, >60) | 1.283 | 0.473 | 0.650-2.530 | |||
| Gender (male, female) | 0.428 | 0.015※※ | 0.216-0.848 | |||
HR hazard ratio.
P <0.05.
Knockdown of CADM1-AS1 by siRNA in 786-O cells
Transfection of siRNA1 and siRNA2 were performed to knock down of CADM1-AS1 in 786-O cells. Quantification analysis showed that CADM1-AS1 expression levels in siRNA1 and siRNA2 were significantly knocked down. The expression level was obviously decreased in siRNA2. In order to exclude “off target effect”, we measured CADM1-AS1 expression and the mRNA expression of CADM1 after transfection by sense strand and antisense strand of siRNA in 786-O cells. The results showed that CADM1-AS1 expression and the mRNA expression of CADM1 were significantly knocked down after transfection by sense strand of siRNA in786-O cells (Figure 4).
Figure 4.
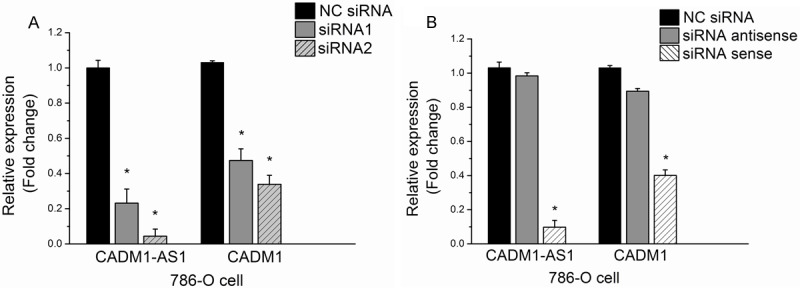
Knockdown of CADM1-AS1 by sense strand and antisense strand of siRNA in 786-O cells. A. Knockdown of CADM1-AS1 by siRNA1 and siRNA2 in 786-O cells. B. Knockdown of CADM1-AS1 by sense strand and antisense strand of siRNA in 786-O cells.
Overexpression of CADM1-AS1 by pcDNA-CADM1-AS1 in ACHN cells
The results showed that CADM1-AS1 expression and the mRNA expression of CADM1 were increased after transfection by pcDNA-CADM1-AS1 in compare with pcDNA-control at 24 h in ACHN cells (Figure 5).
Figure 5.
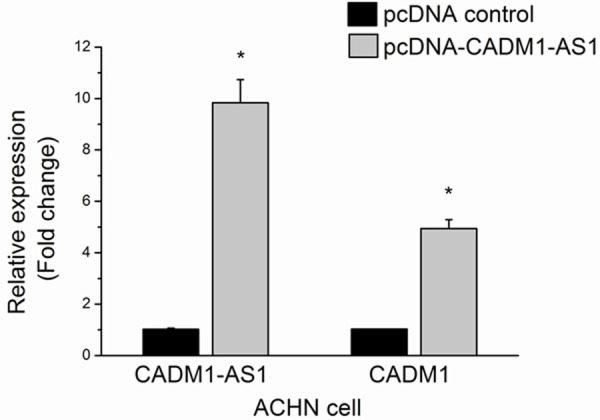
ACHN cells were transfected by pcDNA-CADM1-AS1.
Cell growth curve
By directly cell counting, the results showed that the group of siRNA evidently accelerated the growth compared with the group of NC SiRNA in 786-O cells. And the pcDNA-CADM1-AS1 transfection remarkably decreased the growth compared with the pcDNA-control transfection in ACHN cells (Figure 6).
Figure 6.
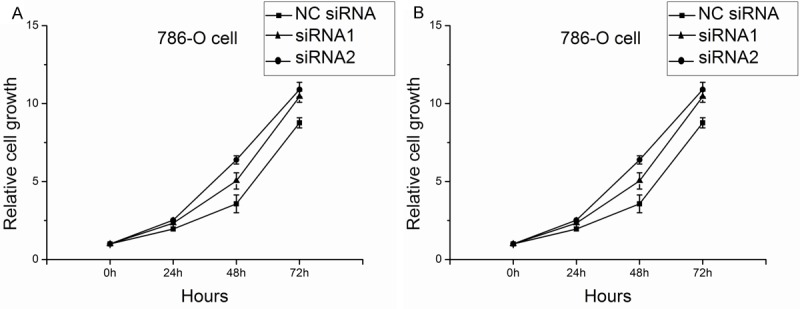
Cell growth cure of 786-O cells and ACHN cells. A. Cell growth cure of 786-O cells after knockdown CADM1-AS1 by siRNA. B. Cell growth cure of ACHN cells after overexpression of CADM1-AS1 by pcDNA-CADM1-AS1.
Scratch wound healing assay
The migration of 786-O cells was increased after transfection of siRNA at 48 h. And the migration of ACHN cells was decreased after transfection of pcDNA-CADM1-AS1 at 48 h (Figure 7). Quantitative analysis of Image J2x indicated a significant increase of 786-O cells migration after siRNA transfection (P <0.05). And the results showed a significant decrease of ACHN cells migration after transfection of pcDNA-CADM1-AS1 (P <0.05).
Figure 7.
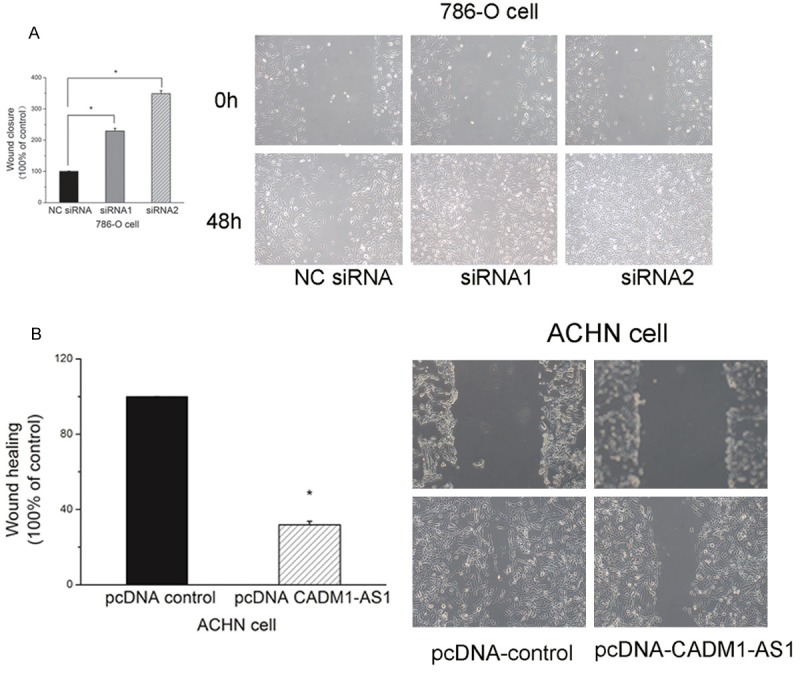
Scratch wound healing assay of 786-O cells and ACHN cells. A. Scratch wound healing assay of 786-O cells after knockdown CADM1-AS1 by siRNA. B. Scratch wound healing assay of ACHN cells after overexpression of CADM1-AS1 by pcDNA-CADM1-AS1.
Apoptosis detection by flow cytometry
The results showed that the percentage of apoptotic cells in the group of NC SiRNA was higher than the group of siRNA. The percentage of apoptotic cells in the group of pcDNA-CADM1-AS1 was higher than the group of pcDNA-control. (P <0.05, Figure 8).
Figure 8.
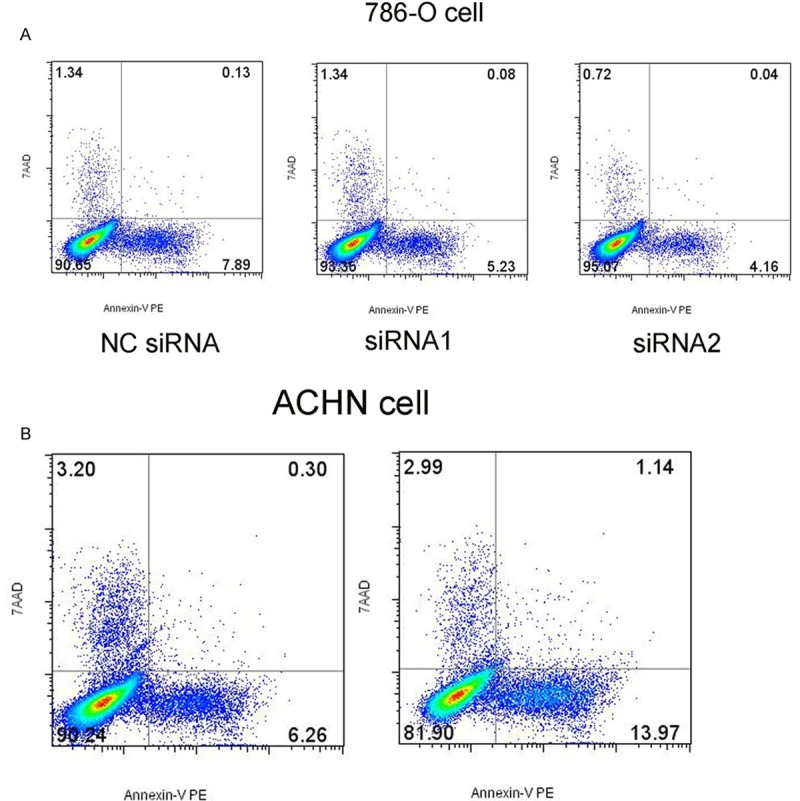
Apoptosis detection by flow cytometry of 786-O cells and ACHN cells. A. Apoptosis detection of 786-O cells after knockdown CADM1-AS1 by siRNA. B. Apoptosis detection of ACHN cells after overexpression of CADM1-AS1 by pcDNA-CADM1-AS1.
Discussion
LncRNAs constitute the biggest class of non-protein-coding RNAs (ncRNAs) [16]. LncRNAs are longer than 200 nucleotides and distinguished from the small ncRNAs [17]. LncRNAs may regulate protein-coding genes expression at both the post-transcriptional and transcriptional level [18]. In post-transcriptional regulation, lncRNAs act as competing endogenous RNAs to regulate microRNA and modulate mRNA stability. In transcriptional regulation, lncRNAs transcription leads to gene silencing or activation. A growing body of literature has suggested a major role of lncRNAs in regulating the development of cancer [19,20]. In these cancer related lncRNAs, a common type is the antisense partner of a protein coding gene. Examples include ANRIL (CDKN2B-AS1) [21,22], CTBP1-AS [23], HNF1A-AS1 [24], and GAS6-AS1 [25].
Through bioinformatics analysis, we found a new lncRNA CADM1-AS1, which is located in the antisense direction of a coding exon of tumor suppressor gene CADM1 [11]. A large number of these lncRNA transcripts originate from transcription at the promoters or other nearby locations of protein-coding genes. These “lncRNA/mRNA gene pairs” interact with each other in expression and in their function [26]. This leads us to presume that CADM1-AS1 may modulate CADM1 expression via the formation of “CADM1-AS1/CADM1mRNA gene pair”. This hypothesis requires further testing.
The ccRCC-related lncRNAs has rarely been reported yet. In this study, we investigated the expression pattern of the “CADM1-AS1/CADM1mRNA gene pairs” in 64ccRCC specimens. Through analysis we discovered that CADM1-AS1 expression and the mRNA expression of CADM1 were down-regulated in tumor tissues in 64 patients with ccRCC compared with adjacent non-tumor tissues. The analysis revealed that CADM1-AS1 expression was positively correlated with mRNA expression of CADM1 in ccRCC. This is the first time to report that CADM1-AS1 expression was down-regulated in tumor tissues than adjacent non-tumor tissues in ccRCC.
Next, we explored the correlation between CADM1-AS1 expression level and clinicopathological characteristics in patients with ccRCC. We showed that decreased CADM1-AS1 expression was negatively correlated with AJCC stage. Kaplan-Meier survival analysis showed a significant correlation between low CADM1 expression and poor clinical outcome in ccRCC patients after operation.
It is more important that low CADM1-AS1 expression was a significant independent predictor of poor survival of ccRCC, in addition to tumor diameter, differentiation and AJCC stage. These results suggested that lncRNA CADM1-AS1 might be involved in the development and progression of ccRCC.
In an effort to investigate the relationship between the CADM1-AS1 and CADM1 in vitro, we designed siRNA to knock down the expression of CADM1-AS1 in 786-O cells. When the expression of CADM1-AS1 was significantly knocked down by siRNA, the expression of CADM1 was also suppressed, which suggests that CADM1-AS1 may modulate CADM1 expression. Then we investigated the biological functions of CADM1-AS1. Inhibition of CADM1-AS1 increased the growth and migration. The percentage of apoptotic was remarkably changed concomitantly with the reduction in the expression of CADM1-AS1. Following overexpression of CADM1-AS1, the results were reversed in ACHN cells.
In conclusion, CADM1-AS1 is a tumor suppressor gene and its expression is positively correlated with the mRNA expression of CADM1 in ccRCC. CADM1-AS1 regulates CADM1 expression on proliferation, apoptosis and migration via the expression pattern of “CADM1-AS1/CADM1 mRNA gene pairs” in vitro. For the first time, we reported that CADM1-AS1 was an independent prognostic factor in patients with ccRCC. Our findings suggested that CADM1-AS1 may be useful in clinic as a potential diagnostic target and therapeutic target for ccRCC.
Disclosure of conflict of interest
None.
References
- 1.Hofmann HS, Neef H, Krohe K, Andreev P, Silber RE. Prognostic factors and survival after pulmonary resection of metastatic renal cell carcinoma. Eur Urol. 2005;48:77–82. doi: 10.1016/j.eururo.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Martel CL, Lara PN. Renal cell carcinoma: current status and future directions. Crit Rev Oncol Hematol. 2003;45:177–190. doi: 10.1016/s1040-8428(02)00076-8. [DOI] [PubMed] [Google Scholar]
- 4.Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373:1119–1132. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Bander NH, Nanus DM. Renal cell carcinoma. N Engl J Med. 1996;335:865–875. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- 6.Wilusz JE, Sunwoo H, Spector DL. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 2009;23:1494–1504. doi: 10.1101/gad.1800909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Xuan Z, Liu C. Long non-coding RNAs and complex human diseases. Int J Mol Sci. 2013;14:18790–18808. doi: 10.3390/ijms140918790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu G, Yao W, Wang J, Ma X, Xiao W, Li H, Xia D, Yang Y, Deng K, Xiao H, Wang B, Guo X, Guan W, Hu Z, Bai Y, Xu H, Liu J, Zhang X, Ye Z. LncRNAs expression signatures of renal clear cell carcinoma revealed by microarray. PLoS One. 2012;7:e42377. doi: 10.1371/journal.pone.0042377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiu MT, Hu JW, Yin R, Xu L. Long noncoding RNA: an emerging paradigm of cancer research. Tumour Biol. 2013;34:613–620. doi: 10.1007/s13277-013-0658-6. [DOI] [PubMed] [Google Scholar]
- 10.Clark MB, Mattick JS. Long noncoding RNAs in cell biology. Semin Cell Dev Biol. 2011;22:366–376. doi: 10.1016/j.semcdb.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Murakami Y. Involvement of a cell adhesion molecule, TSLC1/IGSF4, in human oncogenesis. Cancer Sci. 2005;96:543–552. doi: 10.1111/j.1349-7006.2005.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuramochi M, Fukuhara H, Nobukuni T, Kanbe T, Maruyama T, Ghosh HP, Pletcher M, Isomura M, Onizuka M, Kitamura T, Sekiya T, Reeves RH, Murakami Y. TSLC1 is a tumor-suppressor gene in human non-small-cell lung cancer. Nat Genet. 2001;27:427–430. doi: 10.1038/86934. [DOI] [PubMed] [Google Scholar]
- 13.Fukuhara H, Kuramochi M, Fukami T, Kasahara K, Furuhata M, Nobukuni T, Maruyama T, Isogai K, Sekiya T, Shuin T, Kitamura T, Reeves RH, Murakami Y. Promoter methylation of TSLC1 and tumor suppression by its gene product in human prostate cancer. Jpn J Cancer Res. 2002;93:605–609. doi: 10.1111/j.1349-7006.2002.tb01297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jansen M, Fukushima N, Rosty C, Walter K, Altink R, Heek TV, Hruban R, Offerhaus JG, Goggins M. Aberrant methylation ofthe 5’CpG island of TSLC1 is common in pancreatic ductal adenocarcinoma and is first manifest in high-grade PanlNs. Cancer Biol Ther. 2002;1:293–296. doi: 10.4161/cbt.84. [DOI] [PubMed] [Google Scholar]
- 15.Heller G, Geradts J, Ziegler B, Newsham I, Filipits M, Markis-Ritzinger EM, Kandioler D, Berger W, Stiglbauer W, Depisch D, Pirker R, Zielinski CC, Zöchbauer-Müller S. Downregulation of TSLC1 and DAL-1 expression occurs frequently in breast cancer. Breast Cancer Res Treat. 2007;103:283–291. doi: 10.1007/s10549-006-9377-7. [DOI] [PubMed] [Google Scholar]
- 16.Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG, Lagarde J, Veeravalli L, Ruan X, Ruan Y, Lassmann T, Carninci P, Brown JB, Lipovich L, Gonzalez JM, Thomas M, Davis CA, Shiekhattar R, Gingeras TR, Hubbard TJ, Notredame C, Harrow J, Guigó R. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22:1775–1789. doi: 10.1101/gr.132159.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilusz JE, Sunwoo H, Spector DL. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 2009;23:1494–1504. doi: 10.1101/gad.1800909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Ernst C, Morton CC. Identification and function oflong non-coding RNA. Front Cell Neurosci. 2013;7:168. doi: 10.3389/fncel.2013.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maruyama R, Suzuki H. Long noncoding RNA involvement in cancer. BMB Rep. 2012;45:604–611. doi: 10.5483/BMBRep.2012.45.11.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasmant E, Laurendeau I, Heron D, Vidaud M, Vidaud D, Bieche I. Characterization of a germ-line deletion, including the entire INK4/ARF locus, in a melanoma-neural system tumor family: identification of ANRIL, an antisense noncoding RNA whose expression coclusters with ARF. Cancer Res. 2007;67:3963–69. doi: 10.1158/0008-5472.CAN-06-2004. [DOI] [PubMed] [Google Scholar]
- 22.Yu W, Gius D, Onyango P, Muldoon-Jacobs K, Karp J, Feinberg AP, Cui H. Epigenetic silencing of tumour suppressor gene p15 by its antisense RNA. Nature. 2008;451:202–206. doi: 10.1038/nature06468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takayama K, Horie-Inoue K, Katayama S, Suzuki T, Tsutsumi S, Ikeda K, Urano T, Fujimura T, Takagi K, Takahashi S, Homma Y, Ouchi Y, Aburatani H, Hayashizaki Y, Inoue S. Androgen-responsive long noncoding RNA CTBP1-AS promotes prostate cancer. EMBO J. 2013;32:1665–1680. doi: 10.1038/emboj.2013.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X, Song JH, Cheng Y, Wu W, Bhagat T, Yu Y, Abraham JM, Ibrahim S, Ravich W, Roland BC, Khashab M, Singh VK, Shin EJ, Yang X, Verma AK, Meltzer SJ, Mori Y. Long non-coding RNA HNF1A-AS1 regulates proliferation and migration in oesophageal adenocarcinoma cells. Gut. 2014;63:881–90. doi: 10.1136/gutjnl-2013-305266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han L, Kong R, Yin DD, Zhang EB, Xu TP, De W, Shu YQ. Low expression of long noncoding RNA GAS6-AS1 predicts a poor prognosis in patients with NSCLC. Med Oncol. 2013;30:694. doi: 10.1007/s12032-013-0694-5. [DOI] [PubMed] [Google Scholar]
- 26.Sigova AA, Mullen AC, Molinie B, Gupta S, Orlando DA, Guenther MG, Almada AE, Lin C, Sharp PA, Giallourakis CC, Young RA. Divergent transcription of long noncoding RNA/mRNA gene pairs in embryonic stem cells. Proc Natl Acad Sci U S A. 2013;110:2876–2881. doi: 10.1073/pnas.1221904110. [DOI] [PMC free article] [PubMed] [Google Scholar]


