Abstract
Mucolipidosis type IV (MLIV) is an autosomal recessive disorder resulting from mutations in the MCOLN1 gene. This gene encodes the endosomal/lysosomal transient receptor potential channel protein mucolipin-1 (TRPML1). Affected patients suffer from neurodevelopmental abnormalities and progressive retinal dystrophy. In a prospective natural history study we hypothesized the presence of an additional slow cerebral neurodegenerative process. We have recruited 5 patients, tested their neurodevelopmental status, and measured cerebral regional volumes and white matter integrity using MRI yearly. Over a period of up to 3 years, MLIV patients remained neurologically stable. There was a trend for increased cortical and subcortical gray matter volumes, and increased ventricular size, while white matter and cerebellar volumes decreased. Mean diffusivity (MD) was increased and fractional anisotropy (FA) values were below normal in all analyzed brain regions. There was a positive correlation between motor scores of the Vineland scale and the FA values in the corticospinal tract (corr coef 0.39) and a negative correlation with the MD values (corr coef. −0.50) in the same brain region. We conclude from these initial findings that deficiency in mucolipin-1 affects the entire brain but that there might be a selective regional cerebral neurodegenerative process in MLIV. In addition, these data suggest that diffusion-weighted imaging might be a good biomarker for following patients with MLIV. Therefore, our findings may be helpful for designing of future clinical trials.
Introduction
Mucolipidosis type IV (MLIV) is an autosomal recessive inborn error of intracellular membrane trafficking that is associated with lysosomal inclusions in a variety of cell types[1]. An alteration in mucolipin-1 (also known as TRPML1), a transmembrane protein of the transient receptor potential channel family that is coded by the MCOLN1 gene causes MLIV [2]. All MLIV patients have a constitutive achlorhydria associated with secondary blood gastrin elevation and frequent malabsorption of iron from food [3]. Thus far, MLIV has been known to associate developmental brain abnormalities with a degenerative retinopathy [4]. The disease has currently no specific therapy and its natural history has not been determined or quantified [5, 6]. In order to design adequate future clinical trials, quantitative description of the natural history of the various aspects of MLIV needs to be obtained. In the current study we hypothesized that over the period of observation there would be an additional slow decline in neurological function accompanied by brain changes as measured by magnetic resonance (MRI) volumetry and diffusion-weighted imaging (DWI). Based on the reported findings of white matter pathology and hypomyelination [7] we expected lower FA and higher MD values in patients with MLIV.
Materials and Methods
Patients
Patients were recruited to participate in a prospective study termed ‘The Natural History of Mucolipidosis type IV’ RDCRN Protocol #6704 (ClinicalTrials.gov Identifier: NCT00015782). All patients were studied at the Baylor Research Institute under IRB Project Number 008-295. All patients’ parents gave written informed consent. Patients were seen at baseline and then once a year for a comprehensive evaluation that focused mostly on the neurological aspect of the disease.
Neuropsychological testing
Two main tests were administered to these markedly neurologically handicapped patients:
The Developmental Assessment of Young Children (DAYC) was designed for use with children from birth through 5 years, 11 months to measure the five areas of assessment: cognitive, communication, social-emotional development, physical development, and adaptive behavior. The assessment may be completed through observation of the child or interviews with the caregivers. Cognitive skills include abilities such as attention, memory, purposive planning, decision-making, and discrimination. Communication includes expressive and receptive language abilities and verbal and nonverbal expressions. The Social-Emotional subtest measures the child’s social awareness, social relationships, and social competence. Fine and gross motor skills were assessed though the Physical Development subtest. The Adaptive Behavior subtest examines self-help skills such as toileting, feeding, dressing, and personal responsibility. Items were scored as “passed” or “not passed.” Passed items earn 1 point, and items not passed are scored 0. The raw score (number correct) was converted to Standard Score with a mean of 100 and a Standard Deviation of 15. Because the children in the MLIV study are not within the normative sample, the age equivalent scores were utilized (Supplementary material).
The Vineland Adaptive Behavior Scales – Second Edition (Vineland-II) is additionally used to further assess adaptive behavior in the four broad domains of Communication, Daily Living Skills, Socialization, and Motor Skills. We have used it previously in studying patients with MLIV [1]. The parents complete this assessment with a rating form. Response options include usually (score of 2), sometimes or partially (score of 1), never (score of 0), or don’t know. Norms are available from birth through 90. The raw scores are converted to a V-Scale score (mean of 15 and standard deviation of 3) and Standard Scores (mean of 100 and standard deviation of 15). Age-equivalents are also available. Since age appropriate norms are available, they have been used in this study. Other cognitive tests were performed in some patients (Supplemental material).
MRI Acquisition Procedures
Subjects underwent the MRI imaging under general anesthesia. Subjects were scanned on a 1.5T MRI scanner (Signa HDxt; GE Healthcare, Milwaukee, WI) with an 8-channel head array coil. For structural T1-weighted image, three-dimensional axial brain volume imaging (3D BRAVO) sequence (TR/TE/TI 9.5–10.3/3.7–3.9/600 ms, flip angle 15°, matrix 256×256, slice thickness 1mm) was obtained. Two concatenated diffusion tensor scans per subject were performed with an axial single-shot echo-planar imaging sequence. The acquisition parameters were TR/TE 16000/85–96 m, flip angle 90°, number of axial sections=62, slice thickness of 2 mm with no gap, matrix of 128 × 128. Three volumes with diffusion weighting b-factor of 0 s/mm2 and 25 volumes with b-factor of 1000 s/mm2 were acquired. The de-identified data in DICOM format were transferred to the University of Minnesota for the analysis.
FreeSurfer processing
Volumetric brain segmentation of the T1-weighted scan was carried out with FreeSurfer version 5.3.0 (www.surfer.nmr.mgh.harvard.edu) installed on the Itasca supercomputer at the Minnesota Supercomputing Institute. Automated processing has been described previously [8]. A trained operator (IN) visually inspected each subject’s data to ensure accuracy of the segmentation. Hand editing, adding control points, and rerunning FreeSurfer analysis was employed in 10 MRI scans due to the improper segmentation of temporal lobes.
DWI processing
Diffusion-weighted images were analyzed with FMRIB’s Diffusion Toolbox (FDT) [9]. Eddy current distortions were corrected by affine registration of DWIs to the b0 image. After removal of non-brain tissue, the diffusion tensor was computed with FDT. MD and FA maps were generated. MD corresponds to a scalar measure of the total diffusion within a voxel and is a mean of the three eigenvalues. Higher MD values denote increased rate of diffusion within a voxel. FA is the degree of anisotropy (directional dependence) of a diffusion process in a given voxel. FA value of zero means that diffusion is isotropic and so independent in all directions. A value of one is when diffusion occurs only along one axis and so is fully restricted (dependent) [10]. To obtain MD and FA values of the major white matter tracts we used the tract based spatial statistics (TBSS) pipeline, which is a part of the FMRIB Software Library (FSL). All MD and FA images were non-linearly registered into a standard space (FMRIB58_FA template) and merged into a single 4D image file. The means of FA and MD images respectively were created and thinned to make mean skeleton representing the centres of all tracts common to the group. Each subject’s aligned mean FA and MD data were projected onto the mean skeleton. Resulting skeletonized mean FA and MD data were binarized to reduce the partial volumes between tissues borders with the threshold factor of FA > 0.2 [11]. The processed data were visually inspected for accuracy at each analysis step. The John Hopkins University white matter tractography atlas (JHU-WhiteMatter-labels-1mm-ROI, http://cmrm.med.jhmi.edu/) [12] coregistered to each subject’s TBSS processed brain was used to extract FA and MD values from the main white matter tracts.
Results
Five patients were recruited (Table 1). Patient 2,3 and 5 were Ashkenazi Jews and patients 1 and 4 were unrelated non-Jews but interestingly shared the same MCOLN1 genotype [13]. The Ashkenazi Jewish patients had a more severe disease than the other two patients. All patients were microcephalic (< 5th percentile for age and sex) except patient 4 whose head circumference was at the 50th percentile. The Vineland Adaptive Behavior Scores have remained fairly stable or slightly declined across multiple evaluations. It should be noted that skills are significantly impaired and functioning level was generally in the infant age range (Table 1). In general, the adaptive behavior and social emotional skills were the MLIV patient’s strengths, which is consistent with their presentation during the interview. Cognition and communication were primary areas of weakness. The scores in all areas assessed with the DAYC have remained stable across administrations; no significant improvements or declines have been observed (Supplementary Material).
Table 1.
Characteristics of patients and their neuropsychological and brain volumetric changes
| Patient | Age /Sex |
ML1 mutations |
Visit | Neurodevelopmental Testing - Vineland Adaptive Behavior Scales- II Age Equivalent Best Score (year:month) |
Brain Volumes corrected for Total Brain Volume (Fraction of total brain volume) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Communication | Daily Living |
Socialization | Motor | Cortex | White Matter |
Subcortical Gray Matter |
Cerebellum | Ventricles | ||||
| 1 | 12/F | c.1207C>TR403C and c.236_237ins93 | 1 | 2.5 | 2.9 | 1.1 | 1.3 | 0.5956 | 0.2568 | 0.048 | 0.098 | 0.0128 |
| 2 | 2:11 | 2:10 | 1:6 | 1:3 | 0.6107 | 0.2397 | 0.0515 | 0.0971 | 0.014 | |||
| 3 | 1:1 | 2:3 | 1:11 | 1:8 | 0.5968 | 0.2593 | 0.0502 | 0.0925 | 0.0142 | |||
| 2 | 10/F | g.5534A>G homozygote | 1 | 1:4 | 0:7 | 1:1 | 1:3 | NA | NA | NA | NA | NA |
| 2 | 1:4 | 0:7 | 0:6 | 0:6 | 0.5756 | 0.2834 | 0.0538 | 0.0847 | 0.027 | |||
| 3 | 19/F | g.5534A>G homozygote | 1 | 3:1 | 3:11 | 3:0 | 3:2 | 0.5906 | 0.2424 | 0.0518 | 0.1122 | 0.0185 |
| 2 | 2:6 | 1:9 | 1:1 | 1:8 | 0.5927 | 0.241 | 0.0548 | 0.1079 | 0.0199 | |||
| 4 | 7/F | c.1207C>TR403C and c.236_237ins93 | 1 | 2:9 | 3:2 | 2:1 | 2:7 | 0.6021 | 0.2325 | 0.0507 | 0.113 | 0.0105 |
| 2 | 3:5 | 3:6 | 2:7 | 3:0 | 0.6236 | 0.2241 | 0.04731 | 0.1037 | 0.0101 | |||
| 5 | 18/M | g.5534A>G homozygote | 1 | 1:1 | 0:7 | 0:9 | 0:9 | 0.5095 | 0.3602 | 0.0497 | 0.0758 | 0.0498 |
| 2 | 1:1 | 0:7 | 0:7 | 0:9 | 0.5721 | 0.2624 | 0.0538 | 0.0773 | 0.0562 | |||
NA – not available
MRI volumetric studies
Typical MRI abnormalities seen in MLIV patients are depicted in Figure 1. Baseline and follow-up brain volume data were available in 4 patients (Table 1). All brain volumes of interest were corrected for total brain volume. Cerebral cortex volume was stable or mildly increasing. Subcortical gray matter volumes increased in three subjects. White matter and cerebellar volume decreased in three patients while ventricular volume comprising both lateral, the third and the fourth ventricles increased in three of the four patients (Table 1).
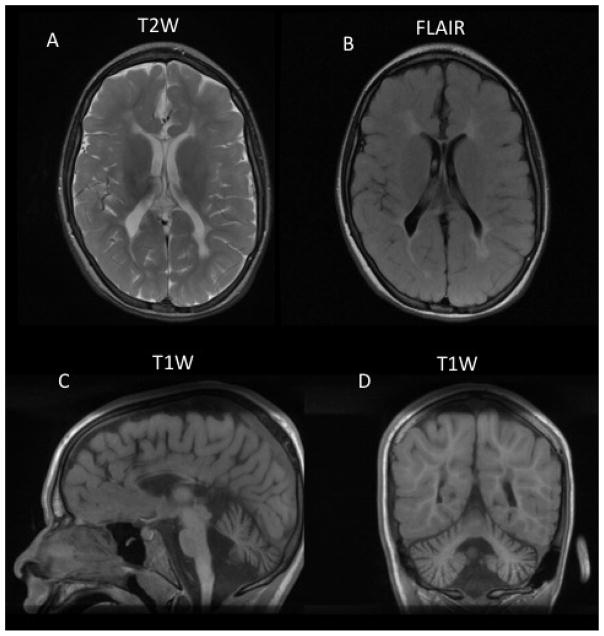
Figure 1.
Brain MRI of patient 2. A. T2-weighted (T2W) and B. Fluid Attenuated Inversion Recovery (FLAIR) images show increased signal in white matter suggesting dysmyelination (hypomyelination). C. Sagittal T1-weighted image (T1W) showing marked thinning of the corpus callosum and midline cerebellar hypoplasia or atrophy. D. Coronal T1-weighted image (T1W) showing normal white matter signal as well as hypoplastic or atrophic cerebellar hemispheres.
Diffusion weighted imaging
Mean diffusivity (MD) was increased and fractional anisotropy (FA) values were below normal in all 48 brain regions (e.g. Table 2).[14, 15] There was a positive correlation between motor scores in the Vineland and the FA values in the corticospinal tract (corr coef 0.39; p=0.1 one-tailed t test) and a negative correlation with the MD values (corr coef. −0.50: p=0.05 one-tailed t test) in the same brain regions. The extent of FA map and structural abnormalities on brain MRI paralleled differences in cognitive testing (Figure 2).
Table 2.
Characteristics of patients and their neuropsychological and Diffusion Weighted Imaging changes
| Patient | Age/Sex | ML1 mutations | Visit | Neurodevelopmental Testing - Vineland Adaptive Behavior Scales- II Age Equivalent Best Score (year:month) | Fractional Anisotropy (normal 0.52±0.04[25]) | Mean Diffusivity (103 × mm2/s) (normal 0.72±0.02[14]) | |||
|---|---|---|---|---|---|---|---|---|---|
| Communication | Daily Living | Socialization | Motor | Corticospinal tracts | |||||
| 1 | 12/F | c.1207C>TR403C and c.236_237ins93 | 1 | 2.5 | 2.9 | 1.1 | 1.3 | 0.288 | 0.82 |
| 2 | 2:11 | 2:10 | 1:6 | 1:3 | 0.312 | 0.793 | |||
| 3 | 1:1 | 2:3 | 1:11 | 1:8 | 0.303 | 0.824 | |||
| 2 | 10/F | g.5534A>G homozygote | 1 | 1:4 | 0:7 | 1:1 | 1:3 | 0.162 | 1.063 |
| 2 | 1:4 | 0:7 | 0:6 | 0:6 | 0.179 | 1.029 | |||
| 3 | 19/F | g.5534A>G homozygote | 1 | 3:1 | 3:11 | 3:0 | 3:2 | 0.216 | 0.941 |
| 2 | 2:6 | 1:9 | 1:1 | 1:8 | 0.188 | 1.019 | |||
| 4 | 7/F | c.1207C>TR403C and c.236_237ins93 | 1 | 2:9 | 3:2 | 2:1 | 2:7 | 0.263 | 0.829 |
| 2 | 3:5 | 3:6 | 2:7 | 3:0 | 0.286 | 0.825 | |||
| 5 | 18/M | g.5534A>G homozygote | 1 | 1:1 | 0:7 | 0:9 | 0:9 | 0.180 | 1.093 |
| 2 | 1:1 | 0:7 | 0:7 | 0:9 | 0.165 | 1.274 | |||
NA – not available
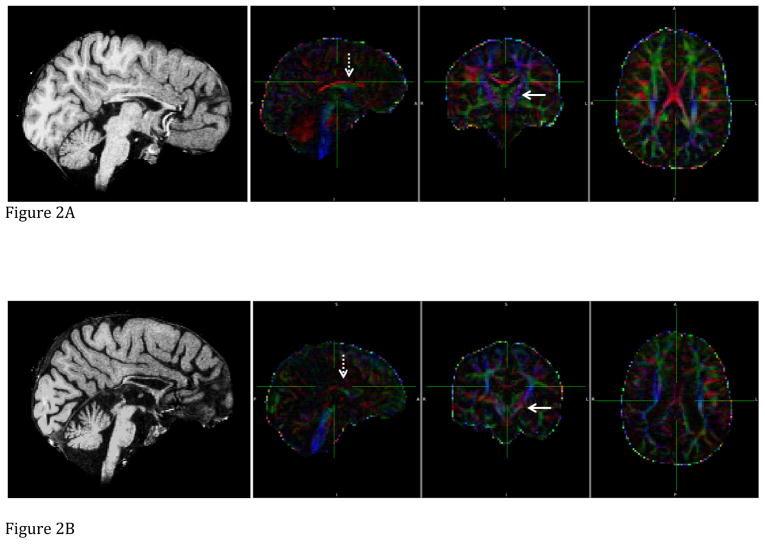
Figure 2.
The sagittal T1-weighted image, and sagittal, coronal, and axial FA maps (from left to right) of two MLIV patients. Line arrow points towards left corticospinal tract, dashed arrow towards corpus callosum. Color codes to give directions: Red is left (L) to right (R). Green is anterior (A) to posterior (P). Blue is superior (S) to inferior (I). FA value is coded by brightness, i.e. higher FA values are portrayed with brighter colors. a: Patient 1 (visit 3), b: Patient 5 (visit 1). Note lower FA values on FA map images, and thinner corpus callosum, presence of cerebellar atrophy, enlarged third ventricle on T1W images in patient 5 compared to patient 1
Discussion
We present here the initial natural history data that focus on the neurodevelopment and volumetric and diffusion MRI findings in 5 patients with MLIV. Based on our prior experience with MLIV patients, we hypothesized that the brain in MLIV has largely developmental abnormalities with only little evidence of cerebral neurodegeneration [1]. This is contrary to a clearly progressive degenerative course of retinal cells in the same patients leading to retinal dystrophy and blindness by the middle of their second decade of life [16]. The overall brain volumes were small in 4 of the 5 patients in accordance to their head circumference. However, normalized cerebellar and white matter were similar to the values obtained in other studies of healthy subjects [17, 18] and ventricular values were elevated in our MLIV patients [19]. We observed in this small group of patients clear volumetric trends despite the short follow-up of 2–3 years and a relatively stable neurodevelopmental status. Cerebral cortical volume increased in all 4 patients with available baseline data, while we observed in all but one patient a decrease in cerebellar and white matter volume in combination with increased ventricular volume. In MRI studies looking at normal brain development, both cortical volumes and total cerebellar volumes have been shown to follow an inverted U-shaped course. Cortical volumes peak during childhood whereas total cerebellar volumes do so during adolescence and then are very slowly decreasing [20–22]. In contrast, white matter and ventricular volumes have been shown to increase throughout late childhood and adolescence [21–23]. Our data in MLIV suggest continued cortical development in the face of neurodegeneration of the cerebral white matter and the cerebellum. Should these trends be confirmed in a longer follow-up of a larger group of patients, these findings will be the first confirmation of a selective cerebral neurodegenerative process in MLIV. Diffusion weighted imaging showed abnormal values in all patients and in all brain regions examined comprising the main white matter tracts in JHU-WhiteMatter atlas [12, 24]. FA was decreased and MD was increased throughout the brain of patients with MLIV. Although we did not include healthy controls in our study, normal FA range is 0.4–0.8 while in our MLIV patients it was 0.16–0.31 in the corticospinal tract and 0.3–0.43 in the cerebral peduncle [14, 15, 21, 25]. MD and FA of the cortical spinal tracts may be good imaging correlate of motor function in MLIV patients. These data, if confirmed on a larger number of patients and over a longer period of time, would suggest that diffusion weighted imaging is a good biomarker for following patients with MLIV.
Our findings are consistent with our cross-sectional studies in patients with MLIV as well as with pathological findings in one autopsied patient and those in the mouse model [1, 7, 26]. Standard MRI images showed a developmental abnormality of the white matter and the corpus callosum combined with frequent atrophy or hypoplasia of the cerebellum (Figure 1) [7]. MR spectroscopic imaging showed greater signs of neuronal alterations in the cerebellum and white matter compared to the basal ganglia and parietal cortex [26]. Although neuronal numbers have not thus far been examined in the MLIV mouse, marked cerebral cortical astrogliosis was seen [27]. Axonal spheroids were observed only in the cerebellum of the mouse model [27]. These abnormalities are similar to those found in an autopsy of a 23 year old MLIV patient [28]. In that study neuronal loss and astrogliosis were present in the thalamus, brain stem, cerebellar Purkinje cell layer, and other subcortical gray matter areas. Our diffusion weighted findings in the present study show that all areas of the brain are affected. These findings may reflect the combination of hypomyelination, neuronal vacuolization and gliosis described above. These findings are further strengthened by the correlation between the corticospinal tract diffusion weighed abnormality and the motor clinical scores.
In conclusion, the initial findings of the first longitudinal study of the brain of MLIV patients suggest a selective neurodegenerative process that is limited to the cerebellum and possibly the cerebral white matter. Further observations over a longer period of time in a larger patient cohort should clarify whether MLIV is a neurodegenerative process in addition to being a developmental brain disease.
Supplementary Material
Acknowledgments
This work was carried out in part using computing resources at the University of Minnesota Supercomputing Institute, and was funded in part by the ML4 Foundation and by NIH grant U54 NS065768. The Lysosomal Disease Network (U54NS065768) is a part of the National Institutes of Health (NIH) Rare Diseases Clinical Research Network (RDCRN), supported through collaboration between the NIH Office of Rare Diseases Research (ORDR) at the National Center for Advancing Translational Science (NCATS), the National Institute of Neurological Disorders and Stroke (NINDS) and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Altarescu G, Sun M, Moore DF, Smith JA, Wiggs EA, Solomon BI, Patronas NJ, Frei KP, Gupta S, Kaneski CR, Quarrell OW, Slaugenhaupt SA, Goldin E, Schiffmann R. The neurogenetics of mucolipidosis type IV. Neurology. 2002;59:306–313. doi: 10.1212/wnl.59.3.306. [DOI] [PubMed] [Google Scholar]
- 2.Sun M, Goldin E, Stahl S, Falardeau JL, Kennedy JC, Acierno JS, Jr, Bove C, Kaneski CR, Nagle J, Bromley MC, Colman M, Schiffmann R, Slaugenhaupt SA. Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Hum Mol Genet. 2000;9:2471–2478. doi: 10.1093/hmg/9.17.2471. [DOI] [PubMed] [Google Scholar]
- 3.Schiffmann R, Dwyer NK, Lubensky IA, Tsokos M, Sutliff VE, Latimer JS, Frei KP, Brady RO, Barton NW, Blanchette-Mackie EJ, Goldin E. Constitutive achlorhydria in mucolipidosis type IV. Proc Natl Acad Sci U S A. 1998;95:1207–1212. doi: 10.1073/pnas.95.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldin E, Slaugenhaupt SA, Smith JA, Schiffmann R. Mucolipidosis type IV. In: Scriver CR, Beaudet AL, Valle D, Sly WS, Vogelstein B, Childs B, Kinzler KW, editors. Metabolic and Molecular Bases of Inherited Diseases. McGraw-Hill; 2005. [Google Scholar]
- 5.Amir N, Zlotogora J, Bach G. Mucolipidosis type IV: clinical spectrum and natural history. Pediatrics. 1987;79:953–959. [PubMed] [Google Scholar]
- 6.Chitayat D, Meunier CM, Hodgkinson KA, Silver K, Flanders M, Anderson IJ, Little JM, Whiteman DA, Carpenter S. Mucolipidosis type IV: clinical manifestations and natural history. Am J Med Genet. 1991;41:313–318. doi: 10.1002/ajmg.1320410310. [DOI] [PubMed] [Google Scholar]
- 7.Frei KP, Patronas NJ, Crutchfield KE, Altarescu G, Schiffmann R. Mucolipidosis type IV: characteristic MRI findings. Neurology. 1998;51:565–569. doi: 10.1212/wnl.51.2.565. [DOI] [PubMed] [Google Scholar]
- 8.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 9.Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- 10.Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 11.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W, Olivi A, Hertig SJ, van Zijl P, Mori S. Automated fiber tracking of human brain white matter using diffusion tensor imaging. Neuroimage. 2008;42:771–777. doi: 10.1016/j.neuroimage.2008.04.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldin E, Stahl S, Cooney AM, Kaneski CR, Gupta S, Brady RO, Ellis JR, Schiffmann R. Transfer of a mitochondrial DNA fragment to MCOLN1 causes an inherited case of mucolipidosis IV. Hum Mutat. 2004;24:460–465. doi: 10.1002/humu.20094. [DOI] [PubMed] [Google Scholar]
- 14.Snook L, Paulson LA, Roy D, Phillips L, Beaulieu C. Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage. 2005;26:1164–1173. doi: 10.1016/j.neuroimage.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Bonekamp D, Nagae LM, Degaonkar M, Matson M, Abdalla WM, Barker PB, Mori S, Horska A. Diffusion tensor imaging in children and adolescents: reproducibility, hemispheric, and age-related differences. Neuroimage. 2007;34:733–742. doi: 10.1016/j.neuroimage.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith JA, Chan CC, Goldin E, Schiffmann R. Noninvasive diagnosis and ophthalmic features of mucolipidosis type IV. Ophthalmology. 2002;109:588–594. doi: 10.1016/s0161-6420(01)00968-x. [DOI] [PubMed] [Google Scholar]
- 17.Kerbrat A, Aubert-Broche B, Fonov V, Narayanan S, Sled JG, Arnold DA, Banwell B, Collins DL. Reduced head and brain size for age and disproportionately smaller thalami in child-onset MS. Neurology. 2012;78:194–201. doi: 10.1212/WNL.0b013e318240799a. [DOI] [PubMed] [Google Scholar]
- 18.Koudijs SM, van der Grond J, Hoogendoorn ML, Hulshoff Pol HE, Schnack HG, Witkamp TD, Gooskens RH, van Nieuwenhuizen O, Braun KP. MRI, volumetry, 1H spectroscopy, and cerebropetal blood flowmetry in childhood idiopathic anatomic megalencephaly. J Magn Reson Imaging. 2006;24:282–287. doi: 10.1002/jmri.20628. [DOI] [PubMed] [Google Scholar]
- 19.Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Archives of neurology. 2003;60:989–994. doi: 10.1001/archneur.60.7.989. [DOI] [PubMed] [Google Scholar]
- 20.Tiemeier H, Lenroot RK, Greenstein DK, Tran L, Pierson R, Giedd JN. Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. Neuroimage. 2010;49:63–70. doi: 10.1016/j.neuroimage.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamnes CK, Ostby Y, Fjell AM, Westlye LT, Due-Tonnessen P, Walhovd KB. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex. 2010;20:534–548. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- 22.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 23.Xenos C, Sgouros S, Natarajan K. Ventricular volume change in childhood. J Neurosurg. 2002;97:584–590. doi: 10.3171/jns.2002.97.3.0584. [DOI] [PubMed] [Google Scholar]
- 24.Mella N, de Ribaupierre S, Eagleson R, de Ribaupierre A. Cognitive intraindividual variability and white matter integrity in aging. Scientific World Journal. 2013;2013:350623. doi: 10.1155/2013/350623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rha DW, Chang WH, Kim J, Sim EG, Park ES. Comparing quantitative tractography metrics of motor and sensory pathways in children with periventricular leukomalacia and different levels of gross motor function. Neuroradiology. 2012;54:615–621. doi: 10.1007/s00234-011-0996-2. [DOI] [PubMed] [Google Scholar]
- 26.Bonavita S, Virta A, Jeffries N, Goldin E, Tedeschi G, Schiffmann R. Diffuse neuroaxonal involvement in mucolipidosis IV as assessed by proton magnetic resonance spectroscopic imaging. J Child Neurol. 2003;18:443–449. doi: 10.1177/08830738030180070701. [DOI] [PubMed] [Google Scholar]
- 27.Micsenyi MC, Dobrenis K, Stephney G, Pickel J, Vanier MT, Slaugenhaupt SA, Walkley SU. Neuropathology of the Mcoln1(−/−) knockout mouse model of mucolipidosis type IV. J Neuropathol Exp Neurol. 2009;68:125–135. doi: 10.1097/NEN.0b013e3181942cf0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Folkerth RD, Alroy J, Lomakina I, Skutelsky E, Raghavan SS, Kolodny EH. Mucolipidosis IV: morphology and histochemistry of an autopsy case. J Neuropathol Exp Neurol. 1995;54:154–164. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.