Abstract
Purpose
Prostate cancer patients with locally advanced disease after radical prostatectomy (RP) are candidates for secondary therapy. However, this higher risk population is heterogeneous and many will not metastasize even when conservatively managed. Given the limited specificity of pathologic features to predict metastasis, newer risk-prediction models are needed. This represents a validation study of a genomic classifier (GC) that predicts post-RP metastasis in a high-risk population.
Materials and Methods
A case-cohort design was used to sample 1,010 post-RP patients at high risk of recurrence treated between 2000-2006. Patients had preoperative PSA >20 ng/mL, Gleason ≥8, pT3b or GPSM score ≥10. Patients with metastasis at diagnosis or any prior treatment for prostate cancer were excluded. 20% random sampling created a subcohort that included all cases with metastasis. 22-marker GC scores were generated for 219 patients with available genomic data. Receiver operating characteristic and decision curves, competing risk, and weighted regression models assessed GC performance.
Results
GC had area under the curve of 0.79 for predicting 5-year metastasis post-RP. Decision curves showed that net benefit of GC exceeded clinical-only models. GC was the predominant predictor of metastasis in multivariable analysis. Cumulative incidence of metastasis at 5 years post-RP was 2.4%, 6.0% and 22.5% for patients with low (60% of patients), intermediate (21% of patients), and high (19% of patients) GC scores, respectively (p<0.001).
Conclusions
These results indicate that genomic information from the primary tumor can identify patients with adverse pathology who are most at risk for metastasis and potentially lethal prostate cancer.
Keywords: prostate cancer, prognosis, metastasis, transcriptome analysis
INTRODUCTION
Prostate cancer is the most common cancer in men and the global burden is expected to increase to 1.7 million new cases and 499,000 deaths annually by 2030.1 In the US, among the ~2.5 million men with prostate cancer, 5-year relative survival for loco-regional disease (~96% of diagnoses) is nearly 100%.2 However, this overall favorable statistic hides the fact that 29,000 men died from prostate cancer in 2012.3 Most men who die of prostate cancer have localized disease on initial biopsy, but after radical prostatectomy (RP) are found to have tumors with one or more adverse pathological features.4 These ‘high-risk’ men often developed rising prostate-specific antigen (PSA) or biochemical recurrence (BCR). Following RP, some high-risk men developed bone metastasis and/or died from disease (typically within 10 years of diagnosis).5, 6 Unfortunately, in men with high-risk prostate cancer the mortality rate has not effectively improved over the last 20 years.2
These findings highlight the need for identification at diagnosis of truly aggressive tumors with an inherently greater potential for early metastasis.7 These are the men who have the most to gain from early secondary therapies and clinical trials.8-11 Currently, adverse pathological features such as Gleason pattern ≥4, positive surgical margins (SM+), seminal vesicle invasion (SVI), extra-capsular extension (ECE) and preoperative PSA ≥20 ng/ml are used to identify high-risk men. These are candidates for secondary therapy such as postoperative radiation;12 however, few will develop metastasis and die of prostate cancer – even when managed expectantly.6, 13 Thus clinicians are reluctant to recommend postoperative radiation, despite evidence8-10 that demonstrates its efficacy in high-risk men.14 Metastatic progression can be delayed if not prevented by early secondary intervention.4 However, clinicians perceive that the sum of costs and morbidity of secondary therapy will exceed its benefits if applied to all patients with adverse pathology.13
We previously reported the development and validation of a post-RP genomic classifier (GC, Decipher™) that predicts early metastasis through an oligonucleotide microarray to profile RNA from formalin-fixed, paraffin-embedded RP specimens. Using a retrospective, nested, case-control design with a median follow-up of 16.9 years, we found unique patterns of differential expression for 192 early metastasis cases (i.e., within 5 years of rising PSA) in comparison to 271 controls irrespective of BCR.15 We used these expression patterns to develop and validate a 22-marker GC to predict early clinical metastasis.16 The GC was a more specific predictor of aggressive disease than clinical variables or previously reported gene signatures. Here, we report a blinded study of a prospectively-designed cohort to evaluate GC for predicting clinical metastasis in a contemporary, high-risk population of patients treated with RP.
MATERIALS AND METHODS
Study Design
Patients treated with RP between 2000 and 2006 were identified from the Mayo Clinic tumor registry for a case-cohort study design. This involved identification of all patients with metastasis and a representative of the full cohort (see Appendix). Thus, men at high risk of recurrence post-RP (open/robotic) with no prior neoadjuvant/prostate cancer treatment were selected based on any of preoperative PSA >20 ng/mL, pathological Gleason score (GS) ≥8, SVI, or GPSM (GS; preoperative PSA; SVI; margins) score ≥10.17 The cohort of 1,010 men included 73 cases with metastasis as evidenced by CT or bone scan. A 20% random sample (n=202) was drawn from the cohort. This included 19 of 73 metastatic cases. To increase sampling of metastasis, the remaining 54 metastatic cases were added (Table S1), resulting in a final study set of 256 patients (Figure S1). Patients not experiencing metastasis regardless of BCR (defined as follow-up PSA ≥0.4 ng/mL >30 days post-RP) were censored at last follow-up. The study was approved by Mayo Clinic Institutional Review Board.
Tissue Processing and Application of Prognostic Classifiers
Following histopathological re-review, the dominant Gleason lesion (highest grade) was macrodissected for 238 available patient samples for microarray analysis. GC scores were computed for 219 samples that passed quality control based on the predefined 22-marker classifier.16 Study participants except Mayo Clinic statisticians who selected the study population were blinded to outcomes and clinical data. Previously described clinical-only (CC), combined genomic-clinical classifiers16 and scores for two validated prediction models, GPSM17 and Stephenson nomogram18 were evaluated. GC was also assessed on 384 additional post-RP patients from three independent datasets (see Appendix).
Statistical Analysis
Discrimination was measured by area under the receiver-operating characteristic curve (AUC) for censored survival data (survival ROC). Net clinical benefit was estimated using an extension of decision curve analysis for survival data. Univariable (UVA) and multivariable (MVA) Cox proportional hazards models were used for risk ratio estimation.19 Cumulative incidence curves were constructed using Fine-Gray competing risks analysis.20 Survival analyses were weighted to estimate parameters in the cohort (For further details see Appendix).
RESULTS
This study profiled RNA from formalin-fixed, paraffin-embedded primary prostate cancer specimens from patients treated with RP. After exclusion for tissue unavailability and quality control, the study consisted of 219 patients including 69 cases with metastasis, with a median follow-up of 6.7 years (Figure S1). BCR rates at 3 years (35%) and metastasis at 5 years (6%) post-RP were similar to the original cohort, indicating representative sampling (Table S1). Median age was 63 years (46-78 years) and 93% of tumors were GS ≥7, 47% pT3, and 56% SM+ (Table 1). Interestingly, most patients with adverse pathology (55%) met criteria for low-intermediate D'Amico risk groups prior to RP, suggesting many were significantly up-graded and up-staged post-RP. Median times to BCR and metastasis post-RP were 1.2 and 3.1 years, respectively.
Table 1.
Clinical characteristics of patients with GC scores in the study (N=219).
| Total n (%) | With Metastasis n (row %) | Without Metastasis n (row %) | |
|---|---|---|---|
| 219 | 69 (32) | 150 (68) | |
| Preoperative Prostate-specific Antigen | |||
| <10 ng/mL | 119 (54) | 33 (28) | 86 (72) |
| 10-20 ng/mL | 59 (27) | 20 (34) | 39 (66) |
| >20 ng/mL | 41 (19) | 16 (39) | 25 (61) |
| Preoperative D'Amico Risk Group | |||
| Low | 38 (17) | 6 (16) | 32 (84) |
| Intermediate | 83 (38) | 23 (28) | 60 (72) |
| High | 98 (45) | 40 (41) | 58 (59) |
| Pathological Gleason Score | |||
| ≤6 | 15 (7) | 0 (0) | 15 (100) |
| 7 | 111 (51) | 29 (26) | 82 (74) |
| ≥8 | 93 (42) | 40 (43) | 53 (57) |
| Pathological Stage | |||
| pT2N0M0 | 85 (39) | 14 (17) | 71 (84) |
| pT3/4N0M0 | 102 (47) | 40 (39) | 62 (61) |
| pTanyN+M0 | 32 (15) | 15 (47) | 17 (53) |
| Positive Surgical Margins | 123 (56) | 39 (32) | 84 (68) |
| Extra-capsular Extension | 95 (43) | 43 (45) | 52 (55) |
| Seminal Vesicle Invasion | 81 (37) | 36 (44) | 45 (56) |
| Postoperative Treatment | |||
| Adjuvant Radiation1 | 24 (11) | 9 (38) | 15 (63) |
| Adjuvant Hormone1 | 74 (34) | 32 (46) | 42 (57) |
| Salvage Radiation2 | 68 (31) | 34 (50) | 34 (50) |
| Salvage Hormone2 | 86 (39) | 62 (72) | 24 (28) |
| Biochemical Recurrence | |||
| Event | 110 (50) | 69 (63) | 41 (37) |
| Prostate Cancer-specific Mortality | |||
| Event | 28 (13) | 28 (100) | 0 (0) |
Adjuvant therapy administrated within 90 days post-RP
Salvage therapy administrated anytime after 90 days post-RP
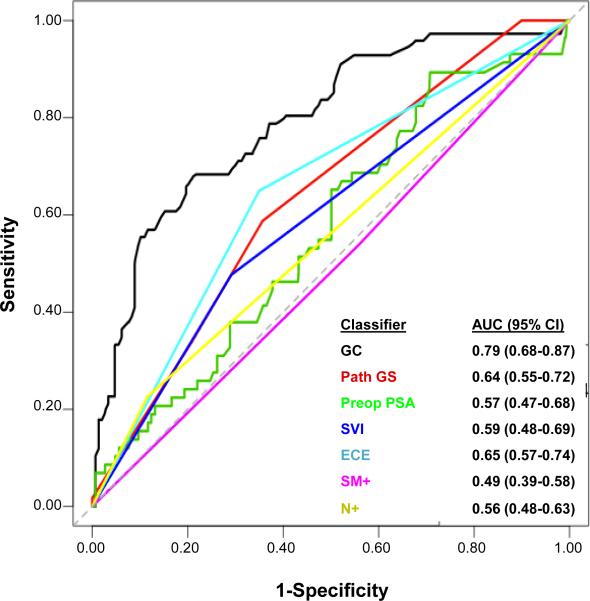
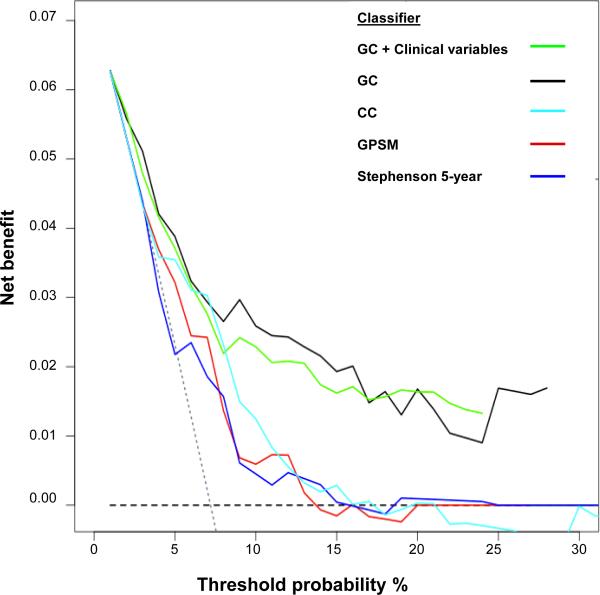
Survival ROC curves were used to assess classifier discrimination. The AUC for GC was 0.79 (95% CI: 0.68-0.87), outperforming all clinical variables (AUCs: 0.49-0.65; Figure 1). Incorporating all clinical variables into GC marginally increased the AUC to 0.82 (95% CI: 0.72-0.88, data not shown). Median GC scores were consistently higher in patients who metastasized compared to those who did not experience metastasis at last follow-up in this study and in three independent datasets (Figure S2). Decision curves compared the net clinical benefit (i.e., clinical implication of false positives) of genomic-based classifiers with clinical-only models. Overall, the higher net benefit of genomic-based classifiers compared to clinical-only risk models suggests that GC models have increased specificity (i.e., lower false positives), without sacrificing sensitivity (Figure 2).
Figure 1. Cumulative survival ROC curves comparing the GC score and individual clinicopathologic factors for predicting clinical metastasis at 5 years post-RP.
GC demonstrates noticeably higher discrimination than individual clinicopathologic factors. GC, genomic classifier; Path GS, pathological Gleason score; Preop PSA, preoperative prostate specific antigen; SVI, seminal vesicle invasion; ECE, extracapsular extension; SM+; positive surgical margins; N+, lymph node involvement.
Figure 2. Survival decision curve analysis comparing the net benefit of genomic-based classifiers GC and GC combined with clinical variables, with clinical-only models (CC,GPSM, Stephenson nomogram).
Performance of models is compared to extremes of classifying all patients as at risk for clinical metastasis (thus warranting treatment of all patients; sloping gray dotted line), versus classifying no patients at risk (thus treating none; horizontal black dashed line). The “decision-to-treat” threshold, the probability of metastasis used to trigger the decision to treat is varied from 0 to 1, with sensitivity and specificity of each prediction model calculated at each threshold to determine net benefit. An optimal classifier has high net benefit above the gray dotted “treat all” line. At a wide range of “decision-to-treat” thresholds the net benefit of the GC-based models are superior. GC, genomic classifier; GC+clinical variables, genomic classifier combined with clinical variables; CC, clinical-only classifier; GPSM, (Gleason score, preoperative PSA, SVI and margins algorithm from Mayo Clinic); Stephenson 5 year, (Stephenson nomogram derived 5-year probability of survival).
By UVA, GC had the highest significant hazard ratio (HR) among classifiers (1.58 for each 10% score increase). Among clinical variables, ECE, GS and SVI were significant prognostic factors (Table S2). Node positivity, preoperative PSA and SM+ were not significant. Patients receiving adjuvant hormone therapy had increased HR (1.97, p=0.02) for metastasis, likely because they were patients who were perceived most at risk. By MVA, only GC retained a significant HR when adjusted for clinical variables and postoperative adjuvant therapy (Table 2; p<0.001). GS was alternatively parameterized (e.g., 3+4, 4+3, 8, 9-10), but this did not change the significance of GC (Table S3). Three additional MVAs were performed to model GC with clinical-only nomograms. Only the Stephenson nomogram retained a significant HR (p=0.04) with GC as the dominant variable (Table S4).
Table 2.
Multivariable Cox proportional hazards modeling of GC scores after adjusting for clinicopathologic factors and adjuvant treatments.
| Hazard Ratio (95% CI) | P | |
|---|---|---|
| GC 1 | 1.51 (1.29-1.76) | < 0.001 |
| Pathological Gleason Score ≥8* | 1.59 (0.77-3.30) | 0.21 |
| Preoperative Prostate-specific Antigen 2 | 1.28 (0.85-1.93) | 0.24 |
| Seminal vesicle invasion | 2.16 (0.90-5.16) | 0.08 |
| Positive Surgical Margins | 1.26 (0.58-2.75) | 0.56 |
| Extra-capsular Extension | 1.35 (0.60-3.00) | 0.47 |
| Lymph Node Involvement | 0.73 (0.20-2.62) | 0.63 |
| Adjuvant Radiation | 0.65 (0.17, 2.47) | 0.52 |
| Adjuvant Hormone | 0.80 (0.31, 2.03) | 0.63 |
Hazard ratio reported for 10% increase of GC score.
Hazard ratio reported for 10% increase of log-transformed level.
Pathological Gleason Score was dichotomized to compare Gleason Score ≥ 8 to Gleason Score < 8
Abbreviations - CI: confidence interval; GC: genomic classifier.
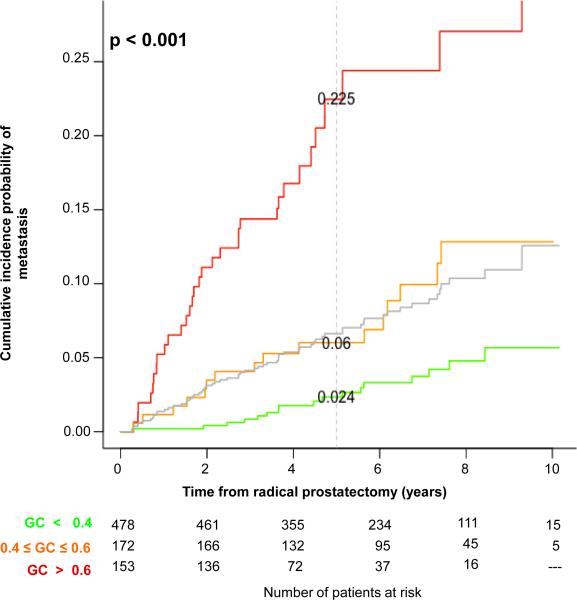
To investigate the magnitude of HR for increments in GC score, we evaluated the effect size of each 10% increase for predicting metastasis after adjusting for postoperative treatment (Table S5, Appendix).21 We observed a general trend of increasing HR and decreasing probability of metastasis-free survival with increasing deciles (Cochran-Armitage trend independence test, p<0.035), although differences between deciles were not statistically significant, likely due to fewer patients in higher GC score deciles. Score deciles were then incrementally collapsed to create three GC risk groups (GC<0.4, 0.4-0.6, >0.6), which showed significant differences in HR (and metastasis-free survival) in comparison to the reference group and to the prior level (Table 3). Progression-free probability estimates and cumulative incidence plots (Figure 3) showed that 60% of patients had GC<0.4 (i.e., number at risk at t=0), and they have a 5-year cumulative incidence of metastasis of just 2.4%. In contrast, the 20% of patients with GC>0.6 had 22.5% 5-year cumulative incidence (p<0.001). Similar results were obtained when patients receiving adjuvant treatment were excluded (Figure S3).
Table 3.
Survival analysis of GC score risk groups.
| reference = GC<0.4 | reference= prior GC group | Clinical Metastasis-free Probability | ||||||
|---|---|---|---|---|---|---|---|---|
| GC risk categories | % of Patients | Hazard Ratio (95% CI) | P | Hazard Ratio (95% CI) | P | 3- year | 5- year | 8- year |
| < 0.4 | 60% | 99% | 98% | 95% | ||||
| 0.4-0.6 | 21% | 2.39 (1.10-5.17) | 0.03 | 2.39 (1.10-5.17) | 0.03 | 96% | 94% | 87% |
| > 0.6 | 19% | 7.30 (3.51-15.14) | < 0.001 | 3.06 (1.40-6.72) | 0.005 | 86% | 78% | 73% |
* Model adjusted for adjuvant treatments
Figure 3. Cumulative incidence of clinical metastasis based on GC score risk groups.
The grey curve indicates the underlying cumulative incidence rate of metastasis in the full cohort obtained by resampling controls. Sixty percent of patients had low GC scores (<0.4, green) with incidence of metastasis lower (2.4% at 5 years) than the average risk of the cohort at any time point. Patients with high GC scores (>0.6, red) had a much higher cumulative incidence reaching 22.5% at 5 years. Total number of patients at risk was 803 at t=0 from weighting controls sampled from the original population after excluding those with unavailable tissue. The dashed grey line indicates the 5-year time point following radical prostatectomy.
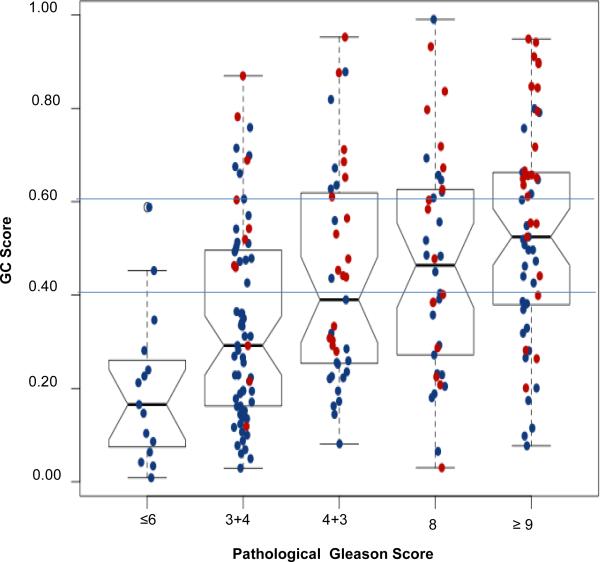
Since GS was the strongest clinical predictor of metastasis, we compared the distribution of GC scores across GS groups; as expected median GC increased with higher GS groups (Figure 4, Table 4). Among patients with GS ≤6, none had GC>0.6 or had clinical metastasis on study follow-up. Among patients with GS 7 tumors, 41% had GC≥0.4 and 44% of these men had clinical metastasis. Conversely, for GS 7 tumors with low GC (<0.4), 86% of them did not metastasize and only 3% died of their disease. As expected, 40% of patients with GS ≥8 had high GC scores (>0.6), of whom 62% experienced metastasis and 41% died of their disease. However, more than a third of patients with GS ≥8 (36%) had low GC scores, and the majority of these men did not have metastasis (77%) or die of prostate cancer (85%). This reclassification was significant (McNemar's chi square p=2.2×10−16). The data suggests that GC may identify a subset of men with ‘high-risk’ GS ≥8 tumors that may never develop clinical metastasis and, conversely, among patients with ‘intermediate risk’ GS 7 tumors, a subset enriched for clinical metastasis events, although such interpretations may need to be made in the context of additional clinical data.
Figure 4. Distribution of GC scores across Gleason score groups.
GC scores (y-axis) are plotted for each Gleason score group (x-axis). Clinical metastasis patients (red) and those without metastasis patients (blue) on study follow-up are shown for each group. The horizontal lines indicate the GC risk groups depicted in Figure 3. The median value of GC scores increases with Gleason score, but GC discriminates clinical metastasis cases in all Gleason score groups.
DISCUSSION
This blinded, prospectively designed study independently validates a novel GC for prediction of prostate cancer metastasis following RP. The results show that GC has improved performance over any individual clinicopathologic variable or multivariable prediction model. In this contemporary (2000-2006) population of 1,010 men at risk of recurrence following RP, the cumulative incidence of metastasis was only 6% at 5 years (7.2% at 10 years). The GC score re-stratified this population such that low-score group patients had nearly three times lower cumulative incidence of metastasis than average and high-score group patients had nearly four times higher cumulative incidence of metastasis. Even after adjusting for postoperative therapy and clinical variables, GC provided independent prediction of metastasis. When assessed in 384 patients from independent datasets, GC retained its prognostic potential with higher scores in patients who metastasized (see Appendix). Further, clinical utility studies indicate that GC can potentially modify patient management,22 and this is also evident at the individual patient level.
Stephenson et al. previously developed and validated a nomogram to predict the probability of prostate cancer-specific mortality using standard clinical parameters.23 Few of the patients in Stephenson et al. had a predicted 15-year metastasis incidence greater than 5%. The authors concluded that there is a “difficulty in identifying patients at substantially increased risk on the basis of clinical parameters alone and the need for novel markers specifically associated with the biology of lethal prostate cancer is evident”.23 Drawing from advances in expression profiling and genomics, many signatures of aggressive disease have been proposed,24 but to our knowledge none have demonstrated significant improvements in prediction of aggressive prostate cancer over risk prediction models such as the Stephenson nomogram. This is probably because most studies involving RP patients used BCR as an endpoint, a non-specific surrogate for metastatic and lethal disease.25 In fact, our investigations using transcriptome-wide expression profiling to compare patients who develop BCR (but not metastasis with long term follow-up) and those with no evidence of disease in the absence of secondary treatment have revealed very few differences in RNA expression between these groups.15 This contrasts with patients who develop metastasis. Their primary tumors show many thousands of differentially expressed RNAs when compared to patients with no evidence of disease or BCR-only. Furthermore, most previous studies represented a broader prostate cancer population, which, in the era of PSA screening, consisted of RP cases that are not necessarily representative of current cancer presentations.25
In our previous study, we used high-density (~1.4 million features) expression arrays to discover and validate primary tumor differential expression patterns associated with prostate cancer death and/or metastasis as endpoints.16 The transcriptome-wide approach allowed interrogation of a richer genomic dataset, including thousands of non-coding RNAs (ncRNA), compared to previous efforts that were primarily protein-coding “gene-centric”.26 Indeed, recent investigations of ncRNA have demonstrated key regulatory functions on genes involved in metastatic disease progression.27 Perhaps ncRNAs in GC encode additional prognostic information missing from previous protein-coding gene expression signatures and can therefore provide improved prediction of aggressive disease over clinicopathologic variables and nomograms in the clinically high-risk population.26
Although majority of patients with aggressive prostate cancer have adverse pathology post-RP, patients with intermediate risk tumors (e.g., GS 7 and SM-) may also progress to advanced disease.4 Indeed, based on preoperative clinical criteria, most patients in this study had low-intermediate D'Amico risk disease. Therefore additional studies are warranted to determine whether GC scores obtained from diagnostic biopsy specimens can predict metastasis as well as in postoperative specimens. In this cohort, nearly 15% of patients were N+ and 45% received adjuvant therapy; the overall validation of GC on an adjuvant therapy-rich cohort may represent a limitation. While adjusting for secondary therapy in multivariable analysis showed that GC remained an independent and significant predictor of metastasis, we could not determine whether GC predicts benefit from local (i.e, radiation) or systemic (e.g., hormone) therapies as patients were not randomized to these treatments. Evaluation of GC in randomized clinical trial datasets will help to better understand the relationship between GC and benefit from specific therapies, and in turn can also lead to better selection of truly high-risk patients for future trials.
Experience over the last decade suggests that RP is used more frequently to manage more aggressive disease.28 Recent PSA screening guidelines may also likely result in greater proportions of men presenting with more aggressive disease features.29 As a result, many contemporary patients with adverse pathology may require additional post-RP intervention as long-term cancer control may not be achieved by surgery alone.4 Three large randomized trials have shown improved outcomes for patients with adverse pathology when treated with adjuvant radiotherapy.8-10 Initial reports from the RTOG 96-01 trial indicated that intensification with multimodal therapy post-RP reduced the incidence of metastases.30 Despite this evidence, application of postoperative secondary therapy for individual patients remains challenging. Concerns for over-treatment causing toxicity and morbidity must be weighed with the potential harm of disease progression. The alternative is often observation with intervention only when the PSA rises, which may prevent over-treatment but may delay treatment until the disease has already disseminated. A better predictor of metastasis from analysis of the primary tumor, long before metastasis manifests clinically, would enable more tailored application of multimodal therapy and enhanced clinical trial design for high-risk prostate cancer.
CONCLUSIONS
We show that genomic information significantly improves prediction of metastatic disease compared to established clinicopathologic risk factors in a contemporary cohort of men at high-risk of recurrence post-RP. Furthermore, we document that most of the prognostic information for predicting metastatic disease is captured by genomic variables measured in the primary tumor. These data suggest that genomic alterations in aggressive prostate cancer manifest early, many years before the metastatic disease is detected clinically. Use of genomic information to characterize the true biological potential of a tumor to metastasize may ultimately lead to improved treatment of prostate cancer patients.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Stephanie Fink for her assistance with these experiments.
Funding support: NIH P50 CA91956 Mayo Prostate Cancer SPORE grant; Richard M. Schulze Family Foundation; National Research Council of Canada Industrial Research Assistance Program; GenomeDx Biosciences Inc.
Footnotes
DISCLOSURE / CONFLICTS OF INTEREST
This study was partly funded by GenomeDx Biosciences Inc. Elai Davicioni, Mercedeh Ghadessi, Christine Buerki, Anamaria Crisan, Nicholas Erho, Ismael A. Vergara, Lucia L. Lam, Zaid Haddad, Benedikt Zimmermann, Thomas Sierocinski, and Timothy J. Triche are employees of GenomeDx Biosciences Inc. Darby J. S. Thompson and Peter C. Black are consultants for GenomeDx Biosciences Inc.
REFERENCES
- 1.Center MM, Jemal A, Lortet-Tieulent J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 2.Brawley OW. Prostate cancer epidemiology in the United States. World J Urol. 2012;30:195. doi: 10.1007/s00345-012-0824-2. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 4.Swanson GP, Basler JW. Prognostic factors for failure after prostatectomy. J Cancer. 2011;2:1. [PMC free article] [PubMed] [Google Scholar]
- 5.Uchio EM, Aslan M, Wells CK, et al. Impact of biochemical recurrence in prostate cancer among US veterans. Arch Intern Med. 2010;170:1390. doi: 10.1001/archinternmed.2010.262. [DOI] [PubMed] [Google Scholar]
- 6.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 7.Bastian PJ, Boorjian SA, Bossi A, et al. High-risk prostate cancer: from definition to contemporary management. Eur Urol. 2012;61:1096. doi: 10.1016/j.eururo.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 8.Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;181:956. doi: 10.1016/j.juro.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolla M, van Poppel H, Tombal B, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). The Lancet. 2012;380:2018. doi: 10.1016/S0140-6736(12)61253-7. [DOI] [PubMed] [Google Scholar]
- 10.Wiegel T, Bottke D, Steiner U, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009;27:2924. doi: 10.1200/JCO.2008.18.9563. [DOI] [PubMed] [Google Scholar]
- 11.Schubert M, Joniau S, Gontero P, et al. The role of adjuvant hormonal treatment after surgery for localized high-risk prostate cancer: results of a matched multiinstitutional analysis. Adv Urol. 2012;2012:612707. doi: 10.1155/2012/612707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roehl KA, Han M, Ramos CG, et al. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172:910. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 13.Boorjian SA, Thompson RH, Tollefson MK, et al. Long-term risk of clinical progression after biochemical recurrence following radical prostatectomy: the impact of time from surgery to recurrence. Eur Urol. 2011;59:893. doi: 10.1016/j.eururo.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman KE, Nguyen PL, Chen MH, et al. Recommendations for post-prostatectomy radiation therapy in the United States before and after the presentation of randomized trials. J Urol. 2011;185:116. doi: 10.1016/j.juro.2010.08.086. [DOI] [PubMed] [Google Scholar]
- 15.Crisan A, Vergara I, Ghadessi M, et al. Clinical and genomic analysis of metastatic disease progression in a background of biochemical recurrence. J Urol. 2013;189:e918. doi: 10.1111/bju.13013. [DOI] [PubMed] [Google Scholar]
- 16.Erho N, Crisan A, Vergara IA, et al. Discovery and Validation of a Prostate Cancer Genomic Classifier that Predicts Early Metastasis Following Radical Prostatectomy. PLoS One. 2013 doi: 10.1371/journal.pone.0066855. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson RH, Blute ML, Slezak JM, et al. Is the GPSM scoring algorithm for patients with prostate cancer valid in the contemporary era? J Urol. 2007;178:459. doi: 10.1016/j.juro.2007.03.124. [DOI] [PubMed] [Google Scholar]
- 18.Stephenson AJ, Scardino PT, Eastham JA, et al. Postoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Clin Oncol. 2005;23:7005. doi: 10.1200/JCO.2005.01.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin DY, Ying Z. Cox Regressio with Incomplete Covariate Measurements. Journal of American Statistical Association. 1993;88:1341. [Google Scholar]
- 20.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J AM Stat Assoc. 1999;94:496. [Google Scholar]
- 21.Cooperberg MR, Hilton JF, Carroll PR. The CAPRA-S score: A straightforward tool for improved prediction of outcomes after radical prostatectomy. Cancer. 2011;117:5039. doi: 10.1002/cncr.26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Badani K, Thompson DJ, Buerki C, et al. Impact of a genomic classifier of metastatic risk on postoperative treatment recommendations for prostate cancer patients: a report from the DECIDE study group. Oncotarget. 2013;4:600. doi: 10.18632/oncotarget.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stephenson AJ, Kattan MW, Eastham JA, et al. Prostate cancer-specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. J Clin Oncol. 2009;27:4300. doi: 10.1200/JCO.2008.18.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorensen KD, Orntoft TF. Discovery of prostate cancer biomarkers by microarray gene expression profiling. Expert Rev Mol Diagn. 2010;10:49. doi: 10.1586/erm.09.74. [DOI] [PubMed] [Google Scholar]
- 25.Cuzick J, Swanson GP, Fisher G, et al. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: a retrospective study. Lancet Oncol. 2011;12:245. doi: 10.1016/S1470-2045(10)70295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vergara IA, Erho N, Triche TJ, et al. Genomic “Dark Matter” in Prostate Cancer: Exploring the Clinical Utility of ncRNA as Biomarkers. Front Genet. 2012;3:23. doi: 10.3389/fgene.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prensner JR, Iyer MK, Balbin OA, et al. Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nat Biotechnol. 2011;29:742. doi: 10.1038/nbt.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silberstein JL, Vickers AJ, Power NE, et al. Reverse stage shift at a tertiary care center: escalating risk in men undergoing radical prostatectomy. Cancer. 2011;117:4855. doi: 10.1002/cncr.26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moyer VA. Screening for Prostate Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2012;157:120. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 30.Shipley WU, Hunt D, Lukka H, et al. Initial Report of RTOG 9601: A Phase III Trial in Prostate Cancer: Anti-androgen Therapy (AAT) with Bicalutamide during and after Radiation Therapy (RT) Improves Freedom from Progression and Reduces the Incidence of Metastatic Disease in Patients following Radical Prostatectomy (RP) with pT2-3, N0 Disease, and Elevated PSA Levels. International Journal of Radiation Oncology Biology Physics. 2010;78:S27. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.