Abstract
An array of studies implicate different classes of protease and their endogenous inhibitors in multiple sclerosis (MS) pathogenesis based on expression patterns in MS lesions, sera, and/or cerebrospinal fluid (CSF). Growing evidence exists regarding their mechanistic roles in inflammatory and neurodegenerative aspects of this disease. Proteolytic events participate in demyelination, axon injury, apoptosis, and development of the inflammatory response including immune cell activation and extravasation, cytokine and chemokine activation/inactivation, complement activation, and epitope spreading. The potential significance of proteolytic activity to MS therefore relates not only to their potential use as important biomarkers of disease activity, but additionally as prospective therapeutic targets. Experimental data indicate that understanding the net physiological consequence of altered protease levels in MS development and progression necessitates understanding protease activity in the context of substrates, endogenous inhibitors, and proteolytic cascade interactions, which together make up the MS degradome. This review will focus on evidence regarding the potential physiologic role of those protease families already identified as markers of disease activity in MS; that is, the metallo-, serine, and cysteine proteases.
1 Introduction
Multiple Sclerosis (MS) is considered a complex and heterogeneous multifocal demyelinating disease of the central nervous system (CNS) which is of undetermined origin. Several theories regarding the initiating event have emerged, and these include a viral, or autoimmune cause [61], or metabolically dependent neurodegenerative changes that promote inflammation, all on a background of genetic susceptibility [13, 28]. While the initiating event in MS is not known and likely a combination of factors participate, the prevailing view of pathogenesis is that it involves a pro-inflammatory assault on CNS white matter driven by autoreactive T cells and activated macrophages, among other immune cells, which results in myelin destruction, axon and neuron degeneration, and irreversible neurological deficits [24, 266]. Regardless of whether the triggering event is viral, autoimmune, and/or metabolic, the effector processes promoting demyelination and axon injury are multifactorial and include cytokines, chemokines, and the focus of this review, proteases. The range of potential involvement of proteolytic activity in MS pathogenesis extends from parenchymal degenerative events, including myelin destruction and axon injury, to release of antigenic self epitopes, immune cell activation, and permeabilization of the blood–brain barrier (BBB).
Since the overall function of proteases involves hydrolytic breakdown of proteins and polypeptides, they have found functional roles in an array of physiologic activities including digestion, fertilization, cellular proliferation and differentiation, cell signaling and migration, wound healing, apoptosis, angiogenesis, and inflammatory responses. While proteases were once viewed as nonspecific degradative enzymes associated with protein catabolism, it is now clear that many proteases hydrolyze highly specific peptide bonds, resulting in limited substrate modification. Limited proteolysis is dictated by protease specificity and activation, accessibility of substrate peptide bonds, and availability of protease inhibitors, or a combination of these, and represents an essential mechanism mediating precise control of cellular processes. By processing bioactive molecules, proteases regulate the availability and function of a wide range of proteins responsible for initiation, modulation, and termination of important cellular activities. Physiologic roles in maintaining a healthy state and in driving inflammatory, respiratory, cardiovascular, neurodegenerative, and immunological diseases, as well as certain types of cancer and viral infections, have been described. Widespread involvement in health and disease makes proteases very attractive targets for development of new drugs for treatment of a variety of conditions including MS.
2 Multiple Classes of Protease Implicated in MS
Proteases (proteinases, peptidases, proteolytic enzymes) catalyze the hydrolysis of covalent peptide bonds in proteins. As of 2007, more than 565 genes encoding proteases or protease-like proteins have been identified in human, and proteases are estimated to comprise 1.7% of the human genome [154, 198, 213]. Despite evidence for roles in a variety of pathological conditions, the biological activities of many proteases remain undefined, including their relevant substrates, activators, and endogenous inhibitors. Understanding in vivo roles is complicated by the fact that proteases generally operate in cascades with other proteases, substrates, binding proteins, and inhibitors in the cellular microenvironment.
Five distinct protease catalytic classes have been defined: metallo-, serine, cysteine, threonine, and aspartic. These five classes are further divided into families according to MEROPS database criteria, which include amino acid sequence and three-dimensional folding. Metallo- and serine protease families are the largest, known to consist of 187 and 176 members, respectively, followed by cysteine (143 members), threonine (27 members), and aspartic (21 members). Protease activity is essential to life but must be tightly controlled to prevent damage to the producing cells and surrounding tissues.
Proteases are produced as inactive zymogen precursors, and activation most commonly occurs by cleavage of a C-terminal pro-peptide with the arrival of a specific protease to intracellular compartments such as lysosomes, or in the extracellular environment in the case of secreted proteases. There is an additional level of control in most cases involving a series of endogenous inhibitors that bind and block catalytic activity. Approximately 18 protease inhibitor families have been identified. A delicate balance exists between proteases, their activating enzymes, and endogenous inhibitors, which control activation and catabolism of intracellular and extracellular proteins. To understand the contribution of proteases to development of pathology, individual proteases must therefore be viewed as part of a system which includes their specific activities, level of redundancy, temporal and spatial distribution and expression level, activation state, turnover, and inhibition properties [154, 170]. We propose the term “MS degradome” to encompass the set of proteases, substrates, and protease inhibitors involved in development and progression of MS.
2.1 Matrix Metalloproteases
Matrix metalloproteases (MMPs) have received most attention regarding their role in MS pathogenesis [298], and in experimental models of MS including experimental autoimmune encephalomyelitis (EAE) [48, 265, 288] and Theiler’s murine encephalomyelitis virus (TMEV) [273] (Table 1 ). MMPs make up an expanding family of enzymes with a zinc-binding motif that degrade and remodel structural proteins in the extracellular matrix (ECM), such as collagens, aggrecan, fibronectin, and laminin. The 23 identified MMPs compose four major groups differing in structure and substrate specificity, the collagenases (MMP-1, −8, −13), gelatinases (MMP-2, −9), stromelysins (MMP-3, −7, −10, −11), and membrane-type MMPs (MMP-14, −15, −16, −24). In mature CNS, MMPs are low or nondetectable, but several become upregulated in cases of neurological disease including MS, malignant glioma, and stroke. In brain, the major MMPs identified include MMP-2 (gelatinase A), MMP-3 (stromelysin 1), MMP-7 (matrilysin), MMP-9 (gelatinase B), and membrane-type metalloproteases [298]. The proteolytic activity of MMPs is tightly controlled by transcription, activation, the tissue inhibitors of metalloproteases (TIMPs), and α2-macroglobulins. While traditionally viewed as effectors of ECM catabolism participating in tissue morphogenesis and wound healing, MMP activities are also linked to modification of matrix–integrin contacts, resulting in signaling events that alter cell survival [42, 169, 252]. Significantly, MMP-knockout mice are associated with relatively minor alterations in matrix turnover, suggesting MMPs are not essential to normal matrix remodeling. Emerging studies indicate MMPs are capable of cleaving a wide range of bioactive substrates including cytokines, growth factors, Fas ligand, and chemokines, and therefore MMPs may exert regulatory effects on cell responses involved in host defense [152, 170]. Abnormal expression of several MMP members has been linked not only to MS but also to arthritis, cancer, atherosclerosis, lung disease, and cardiac dysfunction [181, 252].
Table 1.
Summary of matrix metalloproteases implicated in MS pathogenesis or in the pathogenesis of animal models
| Pathogenesis | Protease | Disease association |
|---|---|---|
| MS Sera | MMP-9 | Elevated prior to Gd-enhancing lesions [267, 286] |
| TACE | Reduced by IFN-β [299] and methylprednisolone [220] | |
| ADAM-17 | Correlates with TNF-α during relapse [50] Elevated fracktalkine levels [127] |
|
| MS CSF | MMP-9 | Elevated with Gd-enhancing lesions [164] |
| MMP-9/TIMP-1 | Elevations predictive of new Gd-enhancing lesions [126, 287] | |
| MMP-2 | Constitutive | |
| ADAM-17 | Elevated fracktalkine levels [127] | |
| MS PBMC | MMP-2 | Elevated in monocytes [14] |
| MMP-2, −14, TIMP-1 | mRNA elevated [14] | |
| TACE | Levels correlate with new Gd-enhancing lesions [239] | |
| MS lesions | MMP-3, −9 | Capillary endothelial cells [162] |
| MMP-2, −3, −9 | Activated astrocytes and microglia [51, 103] | |
| MMP-2, −7, −9, 12 | Elevated in macrophages [6, 51, 281] | |
| MMP-2, −7, −9 | Elevated in plaque and NAWM [62, 151] | |
| MMP-3 | Elevated in acute lesions prior to demyelination [151] | |
| TACE | T lymphocytes in chronic active plaques | |
| Animal models | MMP-9 | Elevated in EAE CNS [48, 201] |
| MMP-9−/− resistant to EAE [68] | ||
| Synthetic inhibitors block BBB damage and EAE [88, 107, 192] | ||
| MMP-8 | Elevated in EAE CNS [194, 265] | |
| MMP-3 | Elevated 10-fold prior to demyelination in DM-20-overexpressing mice [67] | |
| MMP-3, TIMP-1 | Elevated early TMEV CNS [273] | |
| MMP-12 | Elevated late TMEV CNS, microglia, macrophages [273] | |
| MMP-2 | Elevated EAE CNS at peak disease [288] MMP-12−/− EAE disease exacerbation [288] MMP-2−/− EAE disease exacerbation [73] |
There is ample evidence that MMP-9 levels are elevated in sera and CSF of MS patients [87, 200, 220]. In sera, MMP-9 levels increase prior to an acute attack and the appearance of gadolinium-enhancing lesions [267, 286]. In primary progressive MS, interferon beta (IFN-β) treatment reduces sera MMP-9 levels [299]. High-dose intravenous methylprednisolone treatment, the first-line treatment for acute relapses, also returns MMP-9 to normal levels [220]. In CSF, MMP-9 levels are increased in MS patients with gadolinium-enhancing lesions, but not in CSF from Devic’s neuromyelitis optica patients [164]. The availability of TIMPs plays an important role in determining MMP-9’s effects, since a higher MMP-9/TIMP-1 ratio predicts new enhancing MRI lesions [126, 287].
The likely integral role of other MMPs in the MS degradome is underscored by the fact that MMP-9 can be activated by both MMP-2 [79] and MMP-3 [195], and each of these, among other MMPs, have been associated with MS pathogenesis. MMP-2 is constitutively found in CSF and elevated in monocytes from MS patients [14]. Both monocytes and neutrophils contribute to altered MMP activity [108], but endogenous CNS sources are also likely. For example, capillary endothelial cells of MS brain are both MMP-3- and MMP-9-positive [162], and isolated cerebral capillaries produce MMP-9 in response to inflammatory stimuli [51, 103]. Activated astrocytes and microglia also produce MMP-2, −3 and −9 [49, 90]. Real-time PCR analysis of 23 MMPs in subsets of human leukocytes indicates MMP-11, −26, and −27 are enriched in B cells, MMP-15, −16, −24, and −28 are prominent in T lymphocytes, and the majority of MMPs are detected in monocytes. Notably, these studies showed only MMP-2, MMP-14, and TIMP-1 to be elevated in peripheral blood monocytes isolated from MS patients [14]. In MS lesions, MMP-2, −7, −9, and −12 are each detected at elevated levels in macrophages [6, 51, 281]. Of interest, MMP-2, −7, and −9 proteins are expressed not only in plaques but also in adjacent normal-appearing white matter (NAWM) [62, 151]. MMP-3 mRNA has also been observed within early MS lesions, prior to demyelination [151].
Supporting findings in MS patients, the induction or enhanced expression of several MMP family members has been linked to promotion of disease in animal models of MS [48, 130]. For example, MMP-9 is elevated in brain of EAE mice [48, 201], and MMP-9-deficient young mice are resistant to EAE development [68]. Further, synthetic inhibitors of MMP-9 activity partially block BBB damage and manifestation of EAE [88, 107, 192]. MMP-8 (neutrophil collagenase) likewise increases in CNS in response to EAE and correlates with symptom severity [194, 265]. A comprehensive examination of 11 MMPs, and all four TIMPs, in TMEV-infected SJL/J mouse spinal cord points to a prominent role for MMP-3 and TIMP-1 in early stages of disease, while MMP-12 is prominent in microglia/macrophages at more chronic stages, suggestive of a role in ongoing demyelination [273]. MMP-12, also known as macrophage metalloelastase, is known to promote ECM proteolysis and tissue invasion [243]. Of interest with regard to MMP-3, DM-20-overexpressing mice, a transgenic model of demyelination, upregulate MMP-3 in CNS by 10-fold prior to onset of demyelination [67]. MMP-3 can also be induced in CNS resident cells by lipopolysaccharide (LPS) [97]. As seen in TMEV, MMP-12 is dramatically upregulated in EAE at the peak of disease [201]. However, MMP-12-null mice have a significantly worse disease course, suggesting MMP-12 plays a protective role in EAE pathogenesis [288]. Notably, EAE is also exacerbated in MMP-2-null mice, and in this case, disease exacerbation is associated with elevated MMP-9 [73]. Since proteases are generally members of activation/inactivation cascades, it is difficult to determine whether phenotype in knockout mice is due to alteration of a target protease or secondary to alteration of a downstream effector protease or substrate.
2.1.1 A Disintegrin and Metalloproteases
The cell surface A disintegrin and metalloproteases (ADAMs), or secreted ADAMTS, are a subfamily of metalloproteases (Adamalysins). Over 40 ADAM family members are known, with characterized roles in modulation of cell adhesion molecule function [114]. The disintegrin domain is capable of interacting with integrins to modify adhesion. The metalloprotease domain cleaves membrane-bound adhesion molecules, thereby mediating anti-adhesive function. ADAMs also regulate shedding of cell surface-anchored cytokines, such as tumor necrosis factor (TNF-α), cytokine receptors for interleukin (IL) −1, IL-6, and chemokines. Metalloprotease-mediated shedding can result in release of soluble ectodomains, which influence intracellular signaling pathways.
TNF-α converting enzyme (TACE), also known as ADAM-17, is responsible for release of the Th1 cytokine TNF-α from its cell-bound inactive precursor. In MS, TACE mRNA levels in peripheral blood mononuclear cells (PBMCs) are correlated with the number of new gadolinium-enhancing lesions [239]. TACE is localized to invading T lymphocytes in active and chronic active MS plaques [131]. Moreover, TACE levels in serum are correlated with TNF-α levels in relapsing-remitting MS patients during relapse [50]. ADAM-17 is also responsible for shedding of CX3CL1 (fractalkine), which is found in CSF and/or serum of patients with CNS inflammatory disease, as well as other inflammatory conditions, and may therefore serve as an inflammatory marker. CSF fractalkine levels are increased in Guillain-Barre Syndrome, MS, and viral and bacterial meningitis, relative to controls; however, in serum, fractalkine levels are elevated only in MS [127]. Soluble fractalkine has several proposed functions, including activation of circulating leukocytes [160] in addition to anti-inflammatory actions in blocking adhesion of leukocytes to membrane-bound fractalkine [127].
2.2 Serine Proteases
There are a growing number of links between inflammation and the coagulation/fibrinolytic systems governed by serine proteases. Serine protease activity depends on a catalytic triad comprising the active site, which includes serine, histidine, and aspartate residues. The major clans of serine protease include chymotrypsin-like, subtilisn-like (prokaryotes), alpha/beta hydrolases, and signal peptidases. Chymotrypsin-like proteases (S1 peptidases) include pancreatic digestive enzymes, chymotrypsin, trypsin and elastase, the clotting factors, factor Xa, factor XI, thrombin, plasmin, plasminogen activators, kallikreins, granzymes, cathepsin G, and complement factors including C1r, C1s, and the C3 convertases. In each case, serine proteases are produced as inactive zymogen precursors, requiring proteolytic removal of a pro-peptide for bioactivity. Serine protease activation/inactivation cascades are integrally involved in a diverse array of physiological functions ranging from digestive and degradative processes to blood clotting, fibrinolysis, cellular and humoral immunity, fertilization, embryogenesis, and tissue remodeling. Serine protease activity is further tightly regulated by serine protease inhibitors, or serpins, that mimic the 3D structure of the protease substrate and block substrate binding or result in protease destruction. Endogenous serpins include antithrombin, α1-antitrypsin, complement 1-inhibitor, α1-antichymotrypsin, plasminogen activator inhibitor 1 (PAI-1), and neuroserpin. Reflecting their importance, approximately 20% of proteins found in blood plasma are serpins. Underscoring the critical role of serpin inhibition is the fact that a small activating event, through cascade interactions, results in rapid amplification of serine protease activity.
As with MMPs, substantial evidence exists for alterations in serine protease levels in MS sera, CSF, and CNS lesions (Table 2). Indeed, alterations in serine protease activity are among the first detectable signs of inflammatory demyelination [55]. Importantly, with BBB breakdown, serum proteins such as thrombin and the plasminogen activators, tissue plasminogen activator (tPA), and urokinase plasminogen activator (uPA) are able to enter the CNS prior to signs of clinical disease and demyelination [129, 133, 282]. Considerable evidence suggests that the activities of these enzymes are altered in response to various injuries including ischemia, inflammation, and excitotoxicity. Cumulatively, data point to a functional loss in fibrinolytic potential in NAWM and in demyelinating MS lesions prior to CNS inflammation and clinical manifestation [129, 139, 282].
Table 2.
Summary of serine proteases implicated in MS pathogenesis or in the pathogenesis of animal models
| Pathogenesis | Protease Subfamily | Disease Association |
|---|---|---|
| MS Sera | Elastase | Elevated thrombomodulin [82] |
| MS CSF | Plasminogen activator | |
| tPA | Elevated [4] | |
| PAI-1 | Elevated [4] | |
| uPA, α 2-antiplasmin | Detected [4, 55] | |
| Elastase | Elevated [222] | |
| MS PBMC | NA | NA |
| MS lesions | Plasminogen activator | |
| tPA | Elevated [55], localized to nonphosphorylated neurofilament positive axons [99, 100] | |
| PAI-1 | Elevated with fibrin deposition [47, 99] | |
| uPA, α 2-antiplasmin | Localized to neurons in areas of demyelination | |
| Elastase | Mast cells chronic active plaques [115, 136] | |
| Tissue kallikreins | ||
| K6 | Elevated [231] | |
| Animal models | Plasminogen activator | |
| tPA, PAI-1 | Elevated activated astrocytes EAE [261] | |
| uPA, uPAR | Inflammatory cells, EAE [261] Fibrin deposition precedes clinical disease [118] Fibrin removal suppresses EAE [3, 118, 204] |
|
| tPA | tPA−/− more severe acute EAE, axon injury [70] | |
| uPAR | uPAR−/− delayed acute disease, exacerbated chronic disease [70] | |
| Thrombin | Fibrin deposition correlates with TMEV susceptibility [119] | |
| PN1, ATIII | Batroxobin, reduced TMEV clinical disease [119] Elevated in EAE [18, 117] |
|
| Elastase | Mast cell-deficient mice reduced EAE [235] | |
| Tissue kallikreins | Elevated in astrocytes, T cells, macrophages EAE and TMEV [26, 27, 45, 229] | |
| K6 | Elevated in oligodendroglia in EAE [262] | |
| K8 | K8−/− delayed demyelination in EAE [262] |
2.2.1 Plasminogen Activators
Tissue plasminogen activator is constitutively expressed by CNS neurons and microglia, and although experimental evidence supports a role in regulation of synaptic plasticity, deregulation is believed to contribute to neuronal degeneration [37, 236, 270]. Contributing to altered fibrinolytic activity in MS lesions are broad-spectrum protease inhibitors, such as α2-macroglobulin and α1-antitrypsin, which enter the CNS through the damaged BBB. Further, PAI-1, which inhibits tPA, while present in very low levels in plasma, is rapidly induced by pro-inflammatory cytokines IL-1β and TNF-α [159]. As a result, while tPA has been reported to be elevated in CSF of MS patients by 10-fold relative to controls [4], and in MS lesions [55], the concomitant increase in PAI-1, along with formation of tPA:PAI-1 complexes in MS tissues, serves to decrease active tPA, thereby decreasing fibrinolytic activity and contributing to fibrin deposition [47, 99]. Suggestive of a role in axon injury, tPA is co-localized in MS lesions with demyelinated axons that stain positively for nonphosphorylated neurofilament and fibrin [99, 100].
The other major fibrinolytic protease, uPA, along with its endogenous inhibitor α2-antiplasmin, is seen in MS CSF and is localized to neurons in areas of demyelination [4, 55]. High levels of uPA, uPA receptor (uPAR), and PAI-1 occur in acute MS lesions in association with mononuclear cells and foamy macrophages suggestive of a role in facilitation of CNS cellular infiltration [7, 55, 99]. uPAR is constitutively expressed by leukocytes, including monocytes and activated T cells, and plays a prominent role in adhesion and migration to sites of inflammation by interactions with vitronectin and integrins [259, 283].
Studies regarding the plasminogen activator system in animal models of MS support the idea that the level of fibrinolysis modulates both inflammatory and degenerative events in CNS. As in MS, fibrin deposition in EAE precedes clinical manifestation [118]. Removal of fibrin in EAE suppresses disease development and neurological deficits [3, 118, 204]. Supporting the important role of fibrinolysis in CNS inflammatory disease, tPA−/− mice show early and more severe acute disease and incomplete recovery from EAE with significantly higher CNS levels of PAI-1 and fibrin accumulation in association with nonphosphorylated neurofilament axons [70, 155]. By contrast, uPAR−/− mice show delayed, less acute disease with delayed infiltration of inflammatory cells. However, uPAR−/− mice develop chronic disease as a result of steadily increasing inflammation, increased levels of uPA, and a greater degree of demyelination [70]. Combined results point to complex roles for tPA and uPA activity in development of CNS inflammatory disease with critical activities in regulating CNS levels of blood proteins, which may enter through a leaky BBB [155].
2.2.2 Thrombin
Thrombin is another serum protein that may enter the CNS through a damaged BBB, although both pro-thrombin and thrombin-receptor (PAR-1) are expressed by CNS resident cells [64]. Thrombin is a multifunctional serine protease best characterized for its role in cleaving fibrinogen to yield fibrin, but additional roles include hormone-like effects in numerous cell types mediated by activation of protease-activated receptors (PAR-1, −3, and −4), such as proliferation of astrocytes and neurite retraction [66, 98]. Thrombin also induces mast cell degranulation, monocyte and neutrophil chemotaxis, induction of cytokine expression, increased vascular permeability, and promotion of transendothelial migration of neutrophils [46]. Little information exists regarding the activity of thrombin in MS patients, but studies in animal models point to roles in pathogenesis, which like plasminogen activators, appears to be related in large part to altered fibrin levels. In the case of TMEV-induced disease, fibrin deposition is greater in susceptible SJL/J mice compared to the resistant C57BL/6 background. Moreover, batroxobin, a thrombin-like defibrinogenating enzyme, reduces clinical signs of disease but not perivascular monocular cell infiltration [119]. The important relationship between thrombin and serpins in disease pathogenesis is suggested by studies showing elevated endogenous inhibitors of thrombin, PN1 and ATIII in CNS of mice with EAE, with PN1 being most abundant prior to clinical disease and ATIII levels paralleling the peak of clinical disease [18, 117].
2.2.3 Elastase
Several other serine proteases associated with inflammatory cell subsets, including mast cell tryptase and neutrophil elastase, are also implicated in MS. Tryptase is the major secretory product of mast cells released in response to mast cell activation along with histamine, heparin, and other proteases. Tryptase is implicated in a number of different inflammatory and allergic conditions such as conjunctivitis, rhinitis, and asthma, and is elevated in CSF of MS patients [222]. While T cells and macrophages have long been established as the main effector cells in MS, a role for mast cells, traditionally associated with allergic reactions, is suggested, since mast cells are associated with chronic active plaques [115, 136] and mast cell-deficient mice exhibit markedly reduced EAE disease progression and severity [235]. Neutrophil elastase is released in response to inflammatory stimuli and rapidly degrades connective tissue proteins, thereby contributing to tissue destruction. Thrombomodulin is an endothelial cell transmembrane glycoprotein that is cleaved to its soluble form by neutrophil elastase. Soluble thrombomodulin can be used as a measure of damage to the BBB and has a role in binding thrombin and activation of the natural anticoagulant protein C [33]. Serum levels of thrombomodulin increase in acute relapsing MS and in chronic progressive MS, relative to controls [82]. Neutrophils are also rich in other serine proteases including cathepsin G, proteinase 3, and serprocidins, which have combined proteolytic and bactericidal activities.
2.2.4 Tissue Kallikreins
Tissue kallikreins are a serine protease subfamily with trypsin- or chymotrypsin-like enzymatic activity. Fifteen tissue kallikreins have been identified located in tandem on human chromosome 19q. The classical kallikreins were the first to be identified, K1 (tissue/renal/pancreatic kallikrein), K2 (prostate specific glandular kallikrein), and K3 (prostate-specific antigen). Evidence suggests that K1 participates in blood flow regulation, sodium equilibrium, tumor cell invasiveness, and inflammation [36], while K2 and K3 are widely used biomarkers for prostate cancer prognosis [183]. Far less is currently known regarding the newly identified neo-kallikreins, but value as disease biomarkers in steroid hormone-related cancers has been described [30]. Emerging studies link altered tissue, sera, or CSF kallikrein levels to neurological disorders with levels of K6 downregulated in brain lesions [302] and sera [174] of Alzheimer’s patients but upregulated in active MS lesions [229] and stroke [272]. Other tissue kallikreins have also been implicated in CNS disease; for example, K8 has been linked to epileptogenesis in mice [57]. Kallikreins, like other serine proteases, may be involved in discrete proteolytic processing such as growth factor or cell surface receptor activation, and/or more nonselective degradative activities.
Investigators are only beginning to study the potential activity of kallikreins in the pathogenesis of MS because most members of this family have only been recently discovered [30]. A number of kallikreins are clearly in a position to participate in MS, since their expression bridges both the CNS and immune system, and several are transcriptionally regulated by immune cell activation [230]. Additionally, while not fully elucidated, kallikreins likely participate in activation/inactivation cascades with other serine proteases [25] and, indeed, may be regulated by overlapping endogenous inhibitors such as α1-antichymotrypsin, α1-antitrypsin, and α2-macroglobulin [157]. Already, kallikrein 6 (K6), the most abundant kallikrein in CNS with preferential expression by neurons and oligodendroglia, has been shown to be upregulated at sites of active demyelination in MS lesions, in TMEV, and in EAE models [26, 27, 45, 229]. Importantly, inhibiting K6 enzymatic activity delays the onset of disease and reduces clinical and histological signs in EAE. Notably, disease attenuation parallels diminished Th1 responses, which points to a prominent role for K6 not only in lesion development but also in modulation of the inflammatory response [27]. Furthermore, it was recently demonstrated that kallikreins are not only capable of modifying the extracellular environment but, like other serine proteases, may serve in a hormone-like capacity by virtue of their ability to activate select PAR. Several lines of evidence indicate that K6 specifically activates PAR-2 [5], mediating intracellular Ca2+ flux [196]. Of great interest in this regard, PAR-2 expression has been demonstrated in association with macrophages in MS lesions, and reduced clinical disease in association with EAE is observed in PAR-2 knockout mice [191]. Neuropsin, also known as K8, has a demonstrated role in neuronal plasticity and is elevated in oligodendroglia in EAE. Additionally, K8−/− mice show delayed onset and progression of clinical disease with reduced demyelination and oligodendroglial apoptosis [262]. Supporting the likely role of trypsin and chymotrypsin-like kallikreins in pathogenesis of CNS inflammatory disease, oral administration of Bowman-Birk Inhibitor, a soy-derived inhibitor of serine proteases with trypsin and chymotrypsin activities, suppresses clinical and histological signs of EAE including T cell autoreactivity [92].
2.3 Cysteine Proteases
Cysteine proteases have a nucleophilic cysteine thiol in the catalytic triad. Cysteine proteases include lysosomal cathepsins, cytosolic Ca2+-activated calpains, and caspases. Like other proteases, cysteine proteases are produced as inactive precursor proteins requiring proteolytic processing for activation. In the case of caspases, this activation depends critically on recruitment platforms such as the apoptosome for caspase-9 and the inflammasome for caspase-1 [167].
2.3.1 Caspases
As a fundamental player in apoptosis, the caspase family is the subject of multiple studies regarding its role in both MS lesion pathogenesis and regulation of inflammatory cell turnover (Table 3). Additionally, a growing body of literature indicates that certain caspases have an important role in regulation of inflammatory responses beyond their actions in cell execution. The caspase family includes 13 members in humans. Both inflammatory caspases (−1, −4, −5 in humans, and −1, −11, and −12 in mouse), and apoptotic caspases (−7, −3, −6, −8, −10, −2, −9), are synthesized as inactive zymogen precursors. Cell death caspases are either initiators (caspases-2, −8, −9, and −10) or executioners (caspases-3, −6, and −7) of apoptosis. Caspase-mediated cell death is a final common pathway of many neurological and non-neurological disorders. Some caspases, such as caspase-8, have additional roles unrelated to cell death, including T cell homeostasis, proliferation and activation [227], and cell motility [105]. Not surprisingly, caspases have been shown to activate several kinases, including protein kinase C isoforms, mitogen-activated protein kinase/extracellular-signal regulated kinase (MAPK/ERK), and kinase kinase (MEKK-1), and to inactivate p53, focal adhesion kinase (FAK), transcription factors nuclear factor-kB (NF-kB), and signal transducer and activator of transcription-1 (STAT-1) [69].
Table 3.
Summary of cysteine, threonine, and aspartic proteases implicated in MS pathogenesis or in the pathogenesis of animal models
| Pathogenesis | Protease Subfamily | Disease Association |
|---|---|---|
| MS Sera | ||
| MS CSF | Caspase | |
| Caspase-1 | Elevated acute MS [78] | |
| Cathepsins | ||
| Cathepsin B | Elevated [179] | |
| MS PBMC | Caspase | |
| Caspase-1 | Elevated [83, 112] | |
| Cathepsins | ||
| Cathepsin B | Elevated [22] | |
| MS lesions | Caspase | |
| Caspase-1 | Elevated microglia, inflammatory cells, and oligodendrocytes [173] | |
| Calpain-1 | Elevated [62, 242] | |
| Cathepsins | ||
| Cathepsin B | Elevated [23] | |
| Cathepsin C | Elevated [39] | |
| Aspartic | ||
| Cathepsin D | Elevated [39] | |
| Animal models | Caspase | |
| Caspase-1 | Caspase-1−/− reduced incidence and severity of EAE [84] | |
| Calapain-1 | Elevated EAE CNS, astrocytes, oligodendrocytes, inflammatory cells [241] Calpain inhibitor reduces inflammation, demyelination, and clinical EAE [104] |
|
| Cathepsins | ||
| Cathepsin B, L, H | Elevated PLP transgenic mice [158] | |
| Cathepsin A and C | Elevated with clinical symptoms [166] | |
| Threonine | PS-519 proteosome inhibitor reduces EAE clinical disease [278] | |
| Aspartic | ||
| Cathepsin D | Pepstain suppresses EAE [29] |
Critical roles for inflammatory caspases have been demonstrated in maturation of pro-inflammatory cytokines, IL-1β, and IL-18 [188]. Caspase-1 (IL-1B converting enzyme (ICE)) is responsible for the proteolytic activation of the inflammatory mediator IL-1β, and blocking caspase-1 decreases neurological injury in several disease models [304]. Elevations in caspase-1 appear to play a prominent role in MS pathogenesis. Caspase-1 levels correlate with ongoing inflammatory responses as caspase-1 protein is found in CSF of patients with acute, but not stable, MS [78]. Moreover, both caspase-1 and IL-18 mRNA are significantly elevated in PBMCs derived from MS patients compared to controls [83, 112]. Caspase-1 is also upregulated in acute and chronic MS plaques, with expression associated with microglia, infiltrating perivascular mononuclear cells, and oligodendrocytes [173]. In EAE, caspase-1 mRNA is elevated in CNS, and caspase-1-deficient mice show reduced incidence and severity [84]. Caspase-1 is likely to participate both in inflammatory and cell death processes in the evolution of MS lesions, since a caspase-1 inhibitor, but not an inhibitor of caspase-3, reduced oligodendroglial death in response to cytokine challenge in vitro [173].
2.3.2 Calpain
Calpains are nonlysosomal Ca2+-dependent intracellular proteases that promote limited cleavage of intracellular proteins involved in cell motility and adhesion. There are 14 human calpains that proteolyze many signaling-related substrates, including protein kinase C, a subunit of G-proteins, and protein tyrosine phosphatases. Calpains are upstream of the Rho GTPase family, Rac1 and RhoA, which promote lamellipodia formation. Calapin-1 is abundant in most tissues and well characterized in MS (Table 3). Under normal circumstances, Calpain-1 is associated with calpastatin, its specific endogenous inhibitor. Cytosolic calpain-1 is associated with cytoskeletal rearrangement, but calpain-1 may also be secreted from activated macrophages and T cells [60, 248] and degrades all major myelin proteins [10]. Calpain-1 expression occurs in MS plaques [62, 242] and in association with activated astrocytes, microglia, and infiltrating inflammatory cells in EAE [241]. Notably, the onset of calpain-1 expression and activity in CNS correlates with onset of clinical signs of EAE [231]. Cysteic-leucyl-argininal, a novel calpain-1 inhibitor that readily crosses the BBB, reduces CNS demyelination, inflammatory cell infiltration, and clinical signs of EAE [104].
2.3.3 Cathepsins
Eleven endosomal/lysosomal cathepsins are known in human: cathepsin B, C, F, H, K, L, O, S, V, X and W. Cathepsins are related to degranulation of cytotoxic lymphocytes [8], processing of MHC II antigens and maturation of MHC class II molecules [93], and activation of granzymes, neutrophil elastase, and trypsinogen [101, 147]. Cathepsin B levels are elevated in CSF [179], CNS [23], and peripheral blood monocytes of MS patients [22]. Cathepsin C is also upregulated in MS plaques [39]. Both cathepsin A and C increase in the spinal cord with the appearance of clinical symptoms in EAE [166]. cDNA microarray analysis of PLP transgenic mice, a model of remyelination failure in MS, showed up regulation of cathepsin B, L, and H expression [158] (Table 3).
2.4 Threonine and Aspartic Proteases
Proteosomes belong to the threonine class of proteases. The active site threonine residues are associated with three distinct cleavage preferences; that is, chymotryptic, tryptic and peptidylglutamyl. The proteosome is a proteolytic complex in which proteins are ubiquitin-tagged for selective destruction, although other proteins can be degraded within the proteosome without ubiquitination. Proteosome hydrolases are regulated by TMC-95s, endogenous proteasome inhibitors that bind to active site threonines. The ubiquitin proteosome process regulates levels and activity of numerous intracellular proteins including those involved in inflammation, regulation of the cell cycle, and gene expression. Proteosome inhibitors successfully alleviate symptoms in several inflammatory models [72, 202, 207]. The PS-519 proteasome inhibitor reduces clinical disease and results in fewer relapses in EAE [278], with inhibition of NF-kB activity thought to play a major role in disease attenuation. Ritonavir, which modulates proteasome function by inhibiting chymotrypsin-like and enhancing trypsin-like activity, also confers partial protection from EAE [110] (Table 3).
Far less is known regarding the role of aspartic proteases, cathepsins D and E, in MS. Cathepsin D degrades myelin basic protein (MBP) [15, 291], and at least one study has shown that pepstain, an inhibitor of cathepsin D, suppresses clinical and histological signs of EAE [29]. Of interest, cathepsin D was among the genes upregulated by more than 2.5-fold in a large-scale cDNA sequencing study of MS plaques [39].
3 Functional Roles of Proteases in MS
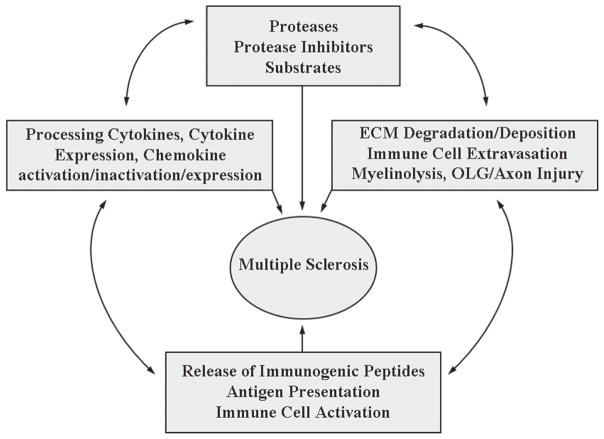
Regardless of the initiating event in MS, albeit tissue injury due to metabolic abnormality, infection, or autoimmune reaction, the host response designed to restore homeostasis is integrally dependent on the action of numerous extracellular and intracellular proteases. Partially overlapping proteolytic mechanisms govern blood clotting, fibrinolysis, complement activation, inflammation, and immunity, and these processes have co-evolved from common ancestral activation pathways [135]. Underscoring the importance of proteases in host defense, extracellular proteases often have multiple targets and are poised for rapid action and amplification. [300]. By virtue of their ability to release, activate, or inactivate bioactive molecules, proteolytic events are important post-translational control check points regulating hemostasis and immune function, and evidence suggests they play key roles in both the immune and neurodegenerative arms of MS (Fig. 1).
Fig. 1.
The MS degradome is that set of proteases, their inhibitors, and substrates that contribute to the development and progression of MS. Proteolysis plays a direct role in ECM turnover, immune cell extravasation, myelinolysis, and injury to oligodendrocytes (OLG) and axons. Limited proteolytic events also play key roles in regulating the activity and expression of cytokines, chemokines, and their receptors. Combined, these proteolytic activities promote the release of immunogenic peptides, antigen presentation, and immune cell activation. In turn, these events collectively affect the availability of proteases, their inhibitors, and substrates and, therefore, the development and progression of CNS inflammatory demyelinating disease
3.1 Proteolytic Modulation and Mediation of Immune Cell Effector Function
3.1.1 Regulation of Cytokine Activity
Proteolysis is critically involved in the activation of latent immunologic cytokines. Like proteases, cytokines are produced as precursor proteins necessitating cleavage of a pro-peptide for bioactivity. For example, plasmin activates such factors as latent transforming growth factor-β [134], complement C3 [285], and the leukocyte chemoattractant CCL14 [274]. MMP-9 cleaves pro-IL-1β [234], and pro-TNF-α [86]. TACE and possibly MMP-12 also cleave, thereby releasing membrane-bound pro-TNF-α [41]. Neutrophil proteinase 3 can activate pro-TNF-α, pro-IL-1β, and pro-IL-18 [290]. Inflammatory caspases are also integrally involved in cytokine activation. Caspase-1 mediates maturation of pro-IL-1β and pro-IL-18 to their biologically active forms [38, 263]. IL-18 in turn stimulates production of interferon-γ and other pro-inflammatory cytokines, participates in T cell polarization, stimulates nitric oxide (NO) synthesis, activates natural killer cells, and upregulates adhesion molecules [65, 178]. IL-1β initiates and amplifies a wide range of activities associated with innate immunity and host response to infection and tissue injury, including promotion of collagenase and metalloprotease synthesis [65]. In cuprizone-induced demyelination, IL-1β plays a critical role in remyelination [168]. Notably, underscoring overlap in hemostatic and immune pathways, IL-1β affects vessel wall elements promoting coagulation and thrombosis [260].
Mice deficient in caspase-1 have defective maturation of IL-1β and IL-18 and are resistant to endotoxicity [138, 148]. More recently, IL-33 has also been described as a possible substrate for caspase-1 [233]. Caspase-1 is activated in the inflammasome, an NALP (cryopyrin)-driven platform for caspase oligomerization. Mutation of the NALP3 gene results in defective control of inflammasome proteins, resulting in constitutively elevated IL-1β maturation and secretion, and this contributes to a clinical spectrum of autoinflammatory diseases such as Muckle Wells syndrome and familial cold urticaria, characterized by spontaneous systemic inflammation and episodes of fever [167]. Caspase-12 may inhibit caspase-1 activity, since caspase-12−/− mice secrete higher levels of IL-1β and IL-18 [226]. Caspase 12 polymorphisms in humans result in hyporesponsiveness to LPS-induced cytokine production [226]. Much remains to be learned regarding the substrates of inflammatory caspases and their role in MS.
Proteases not only activate pro-cytokines but, in turn, are regulated by cytokines. For example, MMP-9 is upregulated by Th1 cytokines, while Th2 cytokines, such as IL-4 and IL-10, have the opposite effect [1, 140]. MMP-12, which has a protective effect in EAE [140, 288], is elevated in macrophages after stimulation with cytokines IL-1β, TNF-α, macrophage colony stimulating factor, and growth factors, such as platelet derived growth factor and vascular endothelial growth factor. Transforming growth factor-β1 inhibits cytokine-mediated induction of MMP-12 [75]. Interestingly, vasoactive intestinal peptide promotes Th2 responses by suppressing upregulation of granzyme B in Th2 but not Th1 effector cells [240]. In Jurkat T cells, K2, K6, K10, and K11 are upregulated by IL-2, while K7 and K13 are simultaneously downregulated [230]. Notably, inhibition of K6 in EAE results in reduced sera levels of IFN-γ [27].
3.1.2 Regulation of Chemokine Activity
Ligands for many chemokine receptors undergo some level of proteolytic processing to enhance or inhibit local chemoattractant activity [300]. Carboxypeptidase modification is a common proteolytic regulatory mechanism for effector proteins of the innate immune response including the anaphylatoxins C5a, C3a, and C4a, for which cleavage results in partial inactivation. Chemerin is proteolytically activated by plasmin, mast cell tryptase, cathepsin G, and neutrophil elastase by carboxyl-terminal cleavage. CXCL12 (stromal-derived factor-1), a potent chemoattractant for leukocytes, is also proteolytically modified at the carboxy terminus, and this results in reduced activity [58]. CXCL10 (IP-10) is cleaved at the carboxy terminus by MMP-8, and CXCL9 (MIG) by MMP-9 [275], although the biological outcome is not defined. Carboxy terminus modification of CXCL7 (NAP2) by an unknown protease enhances receptor binding and triggers neutrophil degranulation [71]. CXCL7 itself is generated from CTAP-III by proteolytic cleavage and is a potent neutrophil chemoattractant acting at CXCR1 and CXCR2 [255].
Amino-terminal proteolysis is even more common among chemoattractant proteins than carboxyterminus modifications, likely due to the importance of this region in chemokine-receptor binding. Cathepsin G, elastase, and mast cell chymase cleave the amino-terminal of CCL15 and CCL23, promoting recruitment of CCR1+ and CCR3+ leukocytes to sites of neutrophil degranulation [20, 216]. Aside from neutrophil granule proteases, CD26/dipeptidyl-peptidase IV (DPP IV), a serine protease, has the largest number of chemoattractant targets removing dipeptides from their amino-terminus. CD26/DPP IV inactivates CXCL11, limiting chemotactic responses, and CXCL12, a chemokine essential for hematopoiesis [211, 212]. Other cell surface proteases, such as ADAM-17 and ADAM-10, promote ectodomain shedding of CX3CL1 [85, 113], and since both are expressed by inflamed vessels, they may play a role in the recruitment of monocytes from vasculature into tissues. CXCL16 is expressed by antigen-presenting cells and binds oxidized low-density lipoprotein and bacteria; however, upon ectodomain shedding mediated by ADAM-10, it is converted to a soluble chemoattractant for activated CD4+ and CD8+ lymphocytes acting through CXCR6 [91].
Using an exosite scanning approach, the Overall lab demonstrated that chemokines are substrates for MMP-2, pointing to novel roles for MMPs in the regulation of inflammation [170, 171]. For example, MMP-2 was shown to cleave monocyte chemoattractant protein-3 (MCP-3/CCL-7), resulting in loss of MCP-3 bioactivity [171]. Further, the cleavage product MCP-3 (amino acids 5–76) forms a receptor antagonist for native MCPs and for other chemokines such as macrophage inflammatory protein-1-alpha in vitro and in vivo. In vivo, MMP-2-cleaved MCP-3 reduces mononuclear infiltration in models of inflammation and, therefore, may serve as a natural anti-inflammatory agent [171]. Notably, other MMPs located in neutrophil granules also target chemoattractant proteins to enhance or inhibit activity. MMP-9 inactivates CXCL5, limiting neutrophil accumulation at sites of inflammation, while MMP-8 and MMP-9 inactivate CXCL6 [276]. Elastase released from neutrophils cleaves and inactivates CXCL12, blocking transendothelial cell migration of CD3+ T cells [215]. Conversely, certain MMPs activate chemoattractants serving to attract neutrophils. MMP-3 and mast cell chymase activate CXCL7 [137, 232].
3.1.3 Complement Activation
Components of the complement system are activated by serine proteases, such as C1r, C1s, the C3 convertases, and mannan-associated serine protease-1 and −2 [246]. Activation culminates in formation of C5b-9, the membrane attack complex (MAC) that damages membranes, induces release of proinflammatory mediators, controls entry into the cell cycle, and has apoptotic or anti-apoptotic effects [184]. Numerous studies link complement activation with MS [156] and its animal models [172]. MAC deposition occurs at the edge of active plaques [253] and in association with myelin in newly forming lesions [13]. While C5b-9 may be pro-inflammatory during the acute phase of disease and may promote demyelination, other studies point to a possible neuroprotective role in rescuing oligodendrocytes from apoptosis at sublytic concentrations in vitro [249]. Moreover, mice deficient in C5 show enhanced oligodendrocyte apoptosis in EAE [190, 224] and are more susceptible to kainic acid (KA)-induced neurotoxicity [264].
3.1.4 Direct Modulation of Immune Effector Function
A large body of experimental data indicates proteases with classic activities in the blood coagulation system also directly affect immune responses by binding to and cleaving PAR. Activation of these G-protein-linked receptors promotes production of cytokines, growth factors, reactive oxygen species (ROS), and MMPs, permitting rapid response to environmental stimuli. Proteolysis of PARs results in the formation of a tethered ligand that can bind and activate the receptor, resulting in activation of a wide range of signaling cascades [52]. Thrombin is known to activate PAR-1, −3, and −4, while trypsin, mast cell tryptase, mast cell granzymes, and factor Xa activate PAR-2. PARs are widely expressed on endothelial cells, leukocytes, and in brain and therefore are poised to be mediators of pathogenesis of MS at multiple levels. PAR-1 activation promotes mast cell degranulation [46], mediates thrombin-induced macrophage production of IL-1, IL-6, and MMP-13, and inhibits IL-4 production [296] and thrombin-induced inflammation in crescentic glomerulonephritis [54]. Thrombin’s effects on microglia appear to occur by a nonproteolytic mechanism as the effects are not diminished by hirudin. PAR-2 activation induces leukocyte rolling and adhesion and can trigger edema [52, 280] and allergic dermatitis [128]. Trypsin induces lymphocyte adhesion, production of ROS [150], and induction of dendritic cell maturation [53]. Tryptase activates peripheral mononuclear cells, inducing the synthesis and release of TNF-α, IL-6, and IL-1β but not IL-12, INF-γ, IL-4, or IL-10. Of particular interest, tryptase-induced cytokine secretion was greater in PBMC derived from secondary progressive and relapsing-remitting MS patients relative to healthy controls [163]. K14 activates PAR-1, −2, and −4, and K5 and K6 activate PAR-2 [196]. Notably, PAR-2-deficient mice are protected from CFA-induced arthritis [32] and are more resistant to EAE [191].
Immune homeostasis and maintenance of tolerance also depends heavily on apoptotic mechanisms and clearance of dead cells mediated, in part, by cell death caspases [244]. Autoimmune disease has been linked to defective apoptosis of autoreactive T or B cells, resulting in tissue destruction or defective clearance [143]. Removal of certain immune cell subsets, such as neutrophils, which contain proteolytic enzymes, ROS, and other bacteriocidal factors, is likewise induced by caspase-mediated apoptosis, since simple disintegration with release of inflammatory mediators would prolong inflammation [74]. Activation of caspase-3 is linked to release of Ca2+-independent phospholipase A2, promoting attraction of macrophages and ensuring efficient removal of dying cells [143]. In addition to caspase-mediated apoptosis, granzyme B is involved in activation-induced apoptosis of T helper cells [240].
3.2 Proteases as Mediators of Immune Cell Extravasation
Inflammation in MS necessitates the recruitment of systemic leukocytes across the BBB into the brain parenchyma, a process mediated, in part, by secretion of proteases [106]. The BBB partially protects the CNS from abrupt changes in blood composition. The luminal endothelium of cerebral capillaries are joined by tight junctions and are separated from circumferential astrocyte end-feet by an intervening ECM, which cumulatively forms a unique barrier to the flow of blood proteins and cells. T cell transmigration involves both transient adhesion to vascular endothelium and focal degradation of endothelial basement membrane and surrounding ECM. BBB changes are deemed critical to pathogenesis of MS and imaging reveals dynamic and ongoing disruption.
MMPs may be one of the major players in the process of leukocyte transmigration into tissues. MMP-2 and MMP-9 degrade components of the capillary basal lamina, including collagen type IV, laminin, fibronectin, and gelatin, and therefore may play key roles in increasing BBB permeability and in T cell extravasation [89]. MMP-9 is produced by both endothelial cells and astrocytes, and upregulation may be a key event in breakdown of the BBB [219]. TNF-α induces expression of MMP-9 and, therefore likely contributes to opening of the BBB in inflammatory disease [219].
MMP-2 and MMP-3 also affect the integrity of the BBB. Like MMP-9, MMP-2 is constitutively expressed by astrocytes, including those astrocyte end-feet so critical to formation of the BBB. Pericytes are a type of macrophage embedded in the endothelial basal lamina and secrete both MMP-3 and MMP-9 in addition to other inflammatory mediators. MMPs arising from components of the BBB can therefore attack tight junction proteins. Intracerebral injection of MMP-2 opens the BBB [219]. MMP-3 knockout mice show reduced BBB damage in response to LPS [97]. TIMP-1 also inhibits transmigration of monocytes across an endothelial barrier in vitro [237]. Synthetic inhibitors of MMP activity also attenuate transmigration of T cells across basement membrane matrices in vitro [31, 293]. The most convincing evidence for the role of MMPs in leukocyte transmigration comes from studies demonstrating that IFN-β, used therapeutically for relapsing remitting MS, inhibits T cell MMP-9 production and restores BBB integrity [126, 146, 256].
Serine proteases such as tPA, thrombin, and plasmin, traditionally viewed as part of the blood homeostasis system, also contribute to BBB permeability. For thrombin stimulates release of prostaglandin PGI2 example, from endothelial cells, increases vascular permeability, and promotes transendothelial migration by neutrophils [254]. Thrombin is characterized by its ability to cause retraction of astrocytic processes [98], a clear mechanism by which it may contribute to disruption of the BBB. Additionally, PAR-1 is associated with capillaries and may take part in response to influx of thrombin. Specialized receptors capable of binding tPA, annexin II tetramer (AIIt), and low-density lipoprotein receptor-related protein (LRP) are localized to BBB endothelial cells and surrounding microglia and therefore provide a mechanism to enhance localized proteolytic and nonproteolytic effects of tPA. A significant increase in AIIt and plasminogen is seen in MS lesions compared to NAWM [100]. As new proteases are identified and characterized, additional players in BBB permeabilization will be identified. For example, K6 is also capable of degrading all major extracellular components of the endothelial basal lamina, is produced by activated immune cells and astroglia, and inhibition of K6 activity reduces SDF-1-α-mediated immune cell invasion in a modified Boyden chamber assay [27].
Adhesion receptors expressed by cells of the cerebral microvasculature and astrocyte end-feet, including integrin and dystroglycan family members, also play key roles in maintaining BBB integrity in part by evoking changes in protease expression [59]. Engagement of T cell VLA-4 and endothelial cell VCAM-1 induces MMP-2 expression and activation, consistent with manifestation of an invasive phenotype in T cells and an activated phenotype in endothelial cells, resulting in proteolysis of basement membrane and interstitial matrix components, facilitating T cell extravasation toward the site of inflammation [161].
Proteases that mediate shedding also participate in migration of leukocytes from blood to inflamed tissues [116]. Limited proteolysis mediated by ADAMs at the level of both leukocytes and endothelial cells controls in part the activity of selectins, integrins, cell adhesion molecules, and transmembrane cytokines, that promote leukocyte extravasation [180, 247]. ADAM-10 and −17 are both expressed by endothelial cells and leukocytes and participate in shedding of L-selectin, CD44, transmembrane chemokines, and VCAM.
3.3 Neurodegenerative Activities
Neurodegenerative changes in the MS lesion include both acute and chronic demyelination, oligodendrocyte loss, axon injury, and neuronal degeneration. These changes encompass both white and gray matter and ultimately result in significant brain atrophy. Ample evidence suggests that alterations in both extracellular and intracellular proteolytic activity occurring in association with areas of inflammation and demyelination, and in areas of NAWM and normal-appearing gray matter (NAGM), play important roles in neurodegenerative events.
3.3.1 Myelinolysis
Proteases of multiple classes degrade myelin proteins. These include MMP-9 [40, 88, 210], MMP-2, MMP-3, MMP-7, MMP-12, interstitial collagenase [41, 94], thrombin [144], plasminogen activators [34, 193], plasmin [120], K6 [21, 26], calpain-1 [10, 11], and mast cell proteases, such as tryptase [63]. An important consideration is that two proteases known to degrade myelin, K6 and MMP-12, also appear critical to myelin formation [9, 142]. There is great interest in further understanding the regulation of myelinolytic proteases and their suitability as therapeutic targets.
All proteases shown to degrade myelin proteins are able to generate encephalitogenic epitopes triggering activation of autoreactive T cells, epitope spreading, and exacerbation of demyelination [88, 210]. This concept has been formalized as the Remnant Epitopes Generate Autoimmunity (REGA) model [197]. In this model, an external factor stimulates inflammatory processes resulting in production of cytokines and chemokines that, in turn, promote protease release. As described, depending on substrate specificity, proteases operate in cascades not only as extracellular matrix and myelin-degrading factors, but also as key modulators of cytokine and chemokine activity. While the brain is classically considered immunologically privileged, antigens drain from brain to cervical lymph nodes, and leukocyte recruitment takes place in postcapillary venules [228]. The net effect of extracellular proteolysis therefore alters the antigen repertoire qualitatively and quantitatively. This thesis holds that proteolytic events drive autoimmunity, such that inhibition of certain proteases may be beneficial to the treatment of autoimmune disease [197].
Extracellular proteolytic processing appears necessary for generation of encephalitogenic peptides. Both MMP-9 and MMP-7 degrade myelin proteins into immunogenic peptides [40, 210]. Proteolytic involvement continues through an intracellular pathway; most antigens enter antigen-presenting cells via an endocytic pathway, where endoproteases process antigens into appropriately sized peptides for MHC class II presentation. While the list of proteases likely to be involved in antigen processing in the endosomal/lysosome pathway may be incomplete, it includes cysteine proteases, such as cathepsin B, H, L, S, F, Z, V, O, C, and K, the aspartic proteases cathepsins D and E, and asparaginyl endopeptidase. IFN-γ regulation of several cathepsins may permit fine tuning of the antigen-processing machinery [17]. Moreover, the cysteine protease cathepsin S is necessary for degradation of invariant chain (Ii) from MHC class II-Ii complexes, allowing peptide binding in professional APCs [217]. Cathepsin S-null mice are associated with impaired invariant chain degradation, antigen presentation, and diminished collagen-induced arthritis [182].
Recent studies have also implicated heat shock protein αβ-crystallin as an important immunodominant protein in MS. αβ-crystallin is the most upregulated mRNA in MS lesions [39] and, like myelin peptides, is a candidate autoantigen in MS [277]. Notably, cleavage of αβ-crystallin by gelatinase B produces immunodominant T cell activating peptides in human [44] and a variety of mouse strains [251]. The identification of proteases converting self-proteins, such as myelin and αβ-crystallin, into class II MHC-peptide complexes that trigger autoimmune responses may point to new therapeutic targets whose activities could be inhibited to blunt the severity of the autoimmune attack.
3.3.2 Neurotoxicity
Axon damage occurs in both chronic and acute MS lesions, although the mechanisms involved at each stage and whether demyelination is a necessary prerequisite are far from clear [76]. Axonal loss appears to be associated with every newly formed lesion, and cumulative axon loss may be a major contributor to progressive and irreversible neurological disability [186]. While undoubtedly a variety of factors contribute to axon injury, likely culprits include a loss of trophic support and events mediated directly by resident and infiltrating inflammatory cells and the toxic factors they secrete, such as NO, oxygen radicals, glutamate, complement, cytokines, and proteases.
As in most other aspects of MS pathogenesis, MMPs are implicated in direct neurotoxicity, particularly since they are capable of damaging both myelin and axons [96, 123, 187]. MMP-2 [62], −7, and −9 [151] are expressed in both active plaques and adjacent NAWM, suggesting a role in axon damage. Microinjection of MMP −2, −7, or −9 into rodent subcortical white matter results in rapid and marked axon injury even in the absence of inflammation [187, 206]. TIMP-1 is rapidly upregulated in astrocytes by kainate-induced excitotoxic injury and is neuroprotective, although it does not prevent apoptosis [218, 257].
Serine proteases, their substrates, and cleavage products are also implicated in neuron/axon injury. Neuroserpin, an endogenous inhibitor of tPA and uPA, decreases cell death after carotid artery occlusion [297]. Importantly, tPA-deficient mice are more resistant to hippocampal degeneration in response to either KA-induced excitotoxic injury or middle cerebral artery occlusion [268]. The ability of tPA to mediate direct neurotoxic effects has been linked to NO and peroxynitrite formation such that these compounds restore the toxic effects of KA in tPA−/− mice [203]. As a corollary, plasminogen-deficient mice are also resistant to hippocampal neurodegeneration mediated by excitotoxic events [269]. Paralleling this observation, exogenous application of α-2-antiplasmin, an endogenous inhibitor of plasmin, is neuroprotective in KA-induced hippocampal injury [268]. Also, direct injection of either plasminogen or plasmin into CNS promotes neuronal apoptosis and an increase in T cells, neutrophils, and macrophages/microglia [294]. Underscoring the concept of proteolytic cascades operating in parallel and serial pathways, tPA may increase tissue damage by activating MMP-9, while plasmin activates both MMP-2 and −9 [12, 149].
Highlighting the concept that the net effect of proteolytic activity depends heavily on its interactions with available substrates and inhibitors is growing evidence that reduced fibrinolytic activity contributes to axonal damage in MS. Opening of the BBB permits fibrin deposition in MS while at the same time fibrinolytic activity appears to be decreased due to increased PAI-1 inhibitor levels. Since fibrin localizes to demyelinated axons that stain for nonphosphorylated neurofilament, fibrin may contribute to axon damage [99]. Studies support the concept that fibrin accumulation in tPA-deficient mice exacerbates both axon damage and demyelination in response to sciatic nerve injury [2]. Notably, tPA-PAI-1 complexes increase in both NAWM and NAGM of MS lesions, indicating roles for reduced fibrinolysis in axon injury, even in apparently normal tissue. One suggested approach to axon damage reduction would be to remove inhibitors of the plasminogen activator system or otherwise stimulate local fibrinolytic activity.
Thrombin has both neuroprotective and pro-apoptotic effects in vitro [98]. At low concentrations, thrombin protects both hippocampal neurons and astrocytes from death [208]. However, at higher concentrations, thrombin inhibits neurite outgrowth [98]. Infusion of thrombin into CNS induces seizure [145], conduction block [43], tissue damage [294], inflammation, and gliosis [205]. Both thrombin and MMP-9 are toxic to cultured human fetal neurons, and these effects are enhanced in combination [295]. Glia-derived nexin-1 (PN-1), an endogenous inhibitor of thrombin, protects hippocampal neurons from injury [238]. PAR-1, −3, and −4, all activated by thrombin, are expressed in rodent CNS [121, 189, 289] and human brain [124] and likely mediate, in large part, thrombin’s neurotoxic effects. PAR-1 overexpression in motor neurons promotes degeneration [77]. Also, PAR-1-deficient mice exhibit enhanced neuronal survival in optic nerve injury [81]. Of particular interest, the neuroprotective effect of autoimmune T cells in models of axon injury appears to relate to their production and secretion of ATIII [80].
Granzymes are a subfamily of serine proteases that includes granzyme A, B, H, K, and M. Granzymes A and B play important roles in destruction of target cells by cytotoxic lymphocytes. Granzyme B is stored in CD8+, CD4+, and gamma delta T cell granules along with other cytotoxic factors, including perforin [279]. Granzyme B is classically thought to cross the plasma membrane to activate caspases in a perforin, or manose-6-phosphate receptor-dependent manner. Perforin has been implicated in exacerbation of TMEV-induced disease with enhanced levels of demyelination, viral load, Th1 responses, and axon injury [111, 176]. Recently, activated T cells were shown to release soluble granzyme B, which had neurotoxic properties independent of perforin or mannose-6-phosphate receptor, although perforin enhanced the toxic effects [284]. In this case, soluble granzyme B-mediated neurotoxicity was found to involve a G-protein receptor and to activate caspase-3.
Calpains have an established role in axon damage in several models of axon injury such as stretch, crush, and ischemia [122, 225]. Suggesting a role in mediating axon injury in MS, an increasing gradient of calpain-1 expression appears in areas of no axon damage to areas of maximal damage [62]. Expression of MMP-2 and calpain-1 in periplaque white matter of acute MS lesions, prior to axon injury, suggests upregulation represents an early event in plaque evolution. Moreover, peak calpain-1 and MMP-2 expression parallel peak expression of β-amyloid precursor protein, at the border of all MS lesion subtypes, further supporting activities in axon injury [62].
Oligodendrocyte apoptosis is also an important component of pathogenesis in the MS lesion and can occur prior to manifestation of an inflammatory response [13]. Experimental evidence for a role of calpains in oligodendrocyte death in response to kainate- and NO-induced injury has been described [19]. Oligodendrocyte injury by TNF-α has also been described in experimental models [125, 301]. Caspases, particularly caspase-3, appears to be involved in TNF-induced apoptosis of embryonic mouse oligodendrocytes [95, 109], although mitochondrial-derived apoptosis-inducing factor (AIF) may mediate TNF-induced apoptosis of mature oligodendrocytes [125, 141]. K8-deficient mice exhibit both attenuated demyelination and delayed oligodendroglial death in EAE [262]. Interestingly, PAR-2 activation of macrophages results in release of an oligotoxic factor [191]. Oligodendrocytes also express MHC class I and are therefore susceptible to CD8+ T cell-mediated cytotoxicity [303].
4 Novel Hypothesis Regarding Role of Proteases in MS
Despite the dominant view that MS is a T-cell-dependent, macrophage-mediated disease that results in an autoimmune attack on the myelin sheath, growing MRI and pathological evidence indicates that alterations occur in normal-appearing myelin prior to the phagocytic attack [13]. T2-weighted MRI shows that NAWM and NAGM are always involved in MS, extending well beyond the plaque edge. Also, magnetic resonance spectroscopy points to metabolic changes in NAWM surrounding acute plaques, suggesting metabolic abnormalities precede inflammatory demyelination [56]. Magnetization transfer imaging, which probes proton pools, indicates that NAWM without inflammatory infiltrate, in addition to MS lesions, shows significant changes in MS patients relative to controls [245]. Taken together, these data support the emerging concept that changes in NAWM precede myelin degradation and inflammation. Disruption of the myelin sheath may initiate macrophage activity, which subsequently leads to amplification of an inflammatory response.
MBP is extensively post-translationally modified by deimination, phosphorylation, deamidation, methylation, and N-terminal acylation. One novel hypothesis, which may underlie biochemical changes in NAWM, relates to the degree of MBP cationicity as a result of peptidylarginine deiminase 2 (PAD2 EC 3.5.3.15) activity [175]. PAD2 converts arginyl residues to citrulline. The compaction of myelin relies, in part, on the ability of MBP to bind the cytoplasmic faces of the oligodendrocyte membrane, and this occurs by virtue of its net positive charge and through protein–lipid interactions. Therefore, alterations in the net charge of MBP represent an important regulatory mechanism for myelin turnover and assembly. Deimination gives rise to MBP charge variants referred to as C1 and C8, with C1 being the least modified and most cationic isomer and the most abundant form in the adult human CNS. The lesser cationic component, C8, has extensive deimination of arginyl residues. Each deiminated arginine results in loss of one positive charge. Deiminated MBP is structurally less ordered and more susceptible to vesiculation and proteolysis [15, 35, 209]. The C8, deiminated form of MBP, is elevated both in MS lesions and infants [175]. MS severity strongly correlates with the degree of arginine loss caused by deimination. In Marburg’s syndrome, an aggressive fulminating type of MS, more than 80% of MBP is found as C8, compared to 45% in chronic MS, and 20% in normal adult brain [292]. MBP deimination may represent a primary defect preceding neurodegenerative events and autoimmune attack [177]. Deiminated C8 MBP is more susceptible to proteolytic digestion by MMP-3. C1 MBP isolated from MS patients is, interestingly, also more susceptible to MMP-3 cleavage relative to controls. Moreover, MMP-3 cleavage results in release of several peptides, including one containing an immunodominant epitope. Cathepsin D also digests the MBP C8 isomer 3-fold faster than C1 isomers [177]. Other post-translational modifications of MBP may also affect myelin stability. For example, phosphorylation of MBP C1 stabilizes β structure [214] and is associated with enhanced resistance to calpain-1 proteolysis [271].
5 Proteases as Targets for Rationale Drug Design
The MS lesion is a complex cellular and molecular environment that can differ in early active, chronic active, inactive, and remyelinating lesions. Infiltrating inflammatory and resident CNS cells alter levels of cytokines, chemokines, growth factors and proteases, their substrates, and endogenous inhibitors, and therefore responsiveness of local cells. This reality offers both the promise of identification of new therapeutic targets, but clearly indicates that targeting any one factor is likely an inefficient strategy to promote repair. Since certain proteases have clear roles in either the inflammatory or neurodegenerative aspects of MS and several are easily detectable in serum and/or CSF, there is continuing interest in determining their utility as potential surrogate markers to aid in patient classification, treatment selection, and disease monitoring. Additionally, there is great interest in determining whether proteases may serve as unique therapeutic targets that could be used selectively to target different aspects of pathogenesis alone, in combination with each other, or with other existing therapeutic regimes.
As outlined, layered on the complex environment of the MS lesion, are secreted proteases of various families that directly impact not only cellular viability, myelin turnover, and the integrity of the extracellular matrix, but may also modify the activation state of key cellular mediators of MS, either directly or indirectly by processing cytokine precursor proteins and influencing the activation state of chemokines and their receptors. A single secreted protease, therefore, may drive pathogenesis along multiple pathways. Targeting certain secreted proteases therapeutically may therefore attenuate pathogenic activity in the MS lesion on multiple levels. An increased understanding of protease systems will be critical to the design of potential inhibitors either directed toward a single protease or capable of more broad-spectrum inhibitory activity.
Several therapies currently in use for MS or in clinical trials owe their efficacy, in part, to their effects on production of MMP. For example, IFN-βs have several proposed mechanisms of action including interaction with T cell receptors to decrease antigen presentation and Th1 activation, decreased expression of T cell adhesion molecules, and relevant to the present discussion, reduced production of MMPs. Suppression of MMP activity may also be part of the mechanism of action of statins as immune modulators [185]. Minocycline, a tetracycline antibiotic now shown to be of considerable benefit in several animal models of neurological injury or disease including MS, Parkinson’s, Huntington’s, amyotrophic lateral sclerosis, stroke, and spinal cord injury, also attenuates MMP production, resulting in decreased T cell proliferation and migration, reduced microglial activation, and inhibition of inflammatory cytokines [221].
The inflammatory caspase, caspase-1, is a suggested drug target for several inflammatory diseases. Pralnacasan, an orally bioavailable nonpeptidic inhibitor of caspase-1, inhibits serum cytokine levels in a murine osteoarthritis model [223] and Th1 activation in a murine model of colitis [153]. VX-765, a more recently identified caspase-1 inhibitor, also blocks IL-1β secretion from LPS stimulated PBMCs [250]. The role of caspase-1 in MS needs further exploration.
Other studies suggest therapeutic uses for oral proteases in inflammatory disease. Phlogenzym, consisting of the hydrolytic enzymes bromelain and trypsin as well as the antioxidant rutosid, is an oral therapeutic used in clinical trials in Western Europe to treat T cell-mediated inflammatory conditions including MS, type 1 diabetes, and rheumatoid arthritis [132, 199]. Oral phlogenzym confers complete protection from EAE. In these studies, T cell responses were shifted toward Th2 cytokines and showed increased activation thresholds. Notably, accessory molecules necessary for T cell co-stimulation, CD4, CD44, and B7-1 were hydrolyzed by Phlogenzym, while CD3 and MHC class II molecules, and LFA-1, were not affected, suggesting the neuroprotective effect may be due to cleavage of molecules necessary for T cell interactions with antigen-presenting cells [258]. Oral bromelain also decreased the incidence and severity of spontaneous colitis in IL-10-deficient mice [102]. Oral Phlogenzym lowered CD4+ cell number and IFN-γ production in splenocytes of endotoxemic mice [165]. Phlogenzym may also be an effective nonsteroidal anti-inflammatory agent for treatment of osteoarthritis [132]. A recent randomized, double-blind, placebo-controlled study of orally administered hydrolytic enzymes in MS, however, found no treatment effect on clinical or MRI parameters [16].
Conclusion
Clearly, matrix metalloproteases are only one component of the MS degradome. Additional research regarding the activities of proteases of each major class and their likely activation/inactivation interactive cascades will be necessary to understand the full scope of action of proteases in MS pathogenesis and their potential therapeutic usefulness. It is also evident that proteases typically have multiple targets and that the consequence of their over- or underexpression in the MS lesion needs to be considered in terms of the full complement of other available proteases, substrates, and inhibitors. Given the identified roles of members of all major classes of protease in MS events, ongoing understanding of the physiologic consequences of alterations in the MS degradome will offer insight into novel therapeutic approaches.
Acknowledgments
This work was supported in part by a grant from the National Multiple Sclerosis Society RG 3367-B-4 and the Craig H. Neilsen Foundation.
References
- 1.Abraham M, Shapiro S, Lahat N, Miller A. The role of IL-18 and IL-12 in the modulation of matrix metalloproteinases and their tissue inhibitors in monocytic cells. Int Immunol. 2002;14:1449–1457. doi: 10.1093/intimm/dxf108. [DOI] [PubMed] [Google Scholar]
- 2.Akassoglou K, Kombrinck KW, Degen JL, Strickland S. Tissue plasminogen activator-mediated fibrinolysis protects against axonal degeneration and demyelination after sciatic nerve injury. J Cell Biol. 2000;149:1157–1166. doi: 10.1083/jcb.149.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akassoglou K, Adams RA, Bauer J, Mercado P, Tseveleki V, Lassmann H, Probert L, Strickland S. Fibrin depletion decreases inflammation and delays the onset of demyelination in a tumor necrosis factor transgenic mouse model for multiple sclerosis. Proc Natl Acad Sci USA. 2004;101:6698–6703. doi: 10.1073/pnas.0303859101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akenami FO, Koskiniemi M, Farkkila M, Vaheri A. Cerebrospinal fluid plasminogen activator inhibitor-1 in patients with neurological disease. J Clin Pathol. 1997;50:157–160. doi: 10.1136/jcp.50.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angelo PF, Lima AR, Alves FM, Blaber SI, Scarisbrick IA, Blaber M, Juliano L, Juliano MA. Substrate specificity of human kallikrein 6: salt and glycosaminoglycan activation effects. J Biol Chem. 2006;281:3116–3126. doi: 10.1074/jbc.M510096200. [DOI] [PubMed] [Google Scholar]
- 6.Anthony DC, Ferguson B, Matyzak MK, Miller KM, Esiri MM, Perry VH. Differential matrix metalloproteinase expression in cases of multiple sclerosis and stroke. Neuropathol Appl Neurobiol. 1997;23:406–415. [PubMed] [Google Scholar]
- 7.Balabanov R, Lisak D, Beaumont T, Lisak RP, Dore-Duffy P. Expression of urokinase plasminogen activator receptor on monocytes from patients with relapsing-remitting multiple sclerosis: effect of glatiramer acetate (copolymer 1) Clin Diagn Lab Immunol. 2001;8:1196–1203. doi: 10.1128/CDLI.8.6.1196-1203.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balaji KN, Schaschke N, Machleidt W, Catalfamo M, Henkart PA. Surface cathepsin B protects cytotoxic lymphocytes from self-destruction after degranulation. J Exp Med. 2002;196:493–503. doi: 10.1084/jem.20011836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bando Y, Ito S, Nagai Y, Terayama R, Kishibe M, Jiang YP, Mitrovic B, Takahashi T, Yoshida S. Implications of protease M/neurosin in myelination during experimental demyelination and remyelination. Neurosci Lett. 2006;405:175–180. doi: 10.1016/j.neulet.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 10.Banik NL, McAlhaney WW, Hogan EL. Calcium-stimulated proteolysis in myelin: evidence for a Ca2+-activated neutral proteinase associated with purified myelin of rat CNS. J Neurochem. 1985;45:581–588. doi: 10.1111/j.1471-4159.1985.tb04026.x. [DOI] [PubMed] [Google Scholar]
- 11.Banik NL. Pathogenesis of myelin breakdown in demyelinating diseases: role of proteolytic enzymes. Crit Rev Neurobiol. 1992;6:257–271. [PubMed] [Google Scholar]
- 12.Baramova EN, Bajou K, Remacle A, L’Hoir C, Krell HW, Weidle UH, Noel A, Foidart JM. Involvement of PA/plasmin system in the processing of pro-MMP-9 and in the second step of pro-MMP-2 activation. FEBS Lett. 1997;405:157–162. doi: 10.1016/s0014-5793(97)00175-0. [DOI] [PubMed] [Google Scholar]
- 13.Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol. 2004;55:458–468. doi: 10.1002/ana.20016. [DOI] [PubMed] [Google Scholar]
- 14.Bar-Or A, Nuttall RK, Duddy M, Alter A, Kim HJ, Ifergan I, Pennington C, Bourgoin P, Edwards DR, Yong VW. Analyses of all matrix metalloproteinase members in leukocytes emphasize monocytes as major inflammatory mediators in multiple sclerosis. Brain. 2003;126:2738–2749. doi: 10.1093/brain/awg285. [DOI] [PubMed] [Google Scholar]
- 15.Bates IR, Boggs JM, Feix JB, Harauz G. Membrane-anchoring and charge effects in the interaction of myelin basic protein with lipid bilayers studied by site-directed spin labeling. J Biol Chem. 2003;278:29041–29047. doi: 10.1074/jbc.M302766200. [DOI] [PubMed] [Google Scholar]
- 16.Baumhackl U, Kappos L, Radue EW, Freitag P, Guseo A, Daumer M, Mertin J. A randomized, double-blind, placebo-controlled study of oral hydrolytic enzymes in relapsing multiple sclerosis. Mult Scler. 2005;11:166–168. doi: 10.1191/1352458505ms1132oa. [DOI] [PubMed] [Google Scholar]
- 17.Beers C, Honey K, Fink S, Forbush K, Rudensky A. Differential regulation of cathepsin S and cathepsin L in interferon gamma-treated macrophages. J Exp Med. 2003;197:169–179. doi: 10.1084/jem.20020978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beilin O, Karussis DM, Korczyn AD, Gurwitz D, Aronovich R, Hantai D, Grigoriadis N, Mizrachi-Kol R, Chapman J. Increased thrombin inhibition in experimental autoimmune encephalomyelitis. J Neurosci Res. 2005;798:351–359. doi: 10.1002/jnr.20270. [DOI] [PubMed] [Google Scholar]
- 19.Benjamins JA, Nedelkoska L, George EB. Protection of mature oligodendrocytes by inhibitors of caspases and calpains. Neurochem Res. 2003;28:143–152. doi: 10.1023/a:1021612615554. [DOI] [PubMed] [Google Scholar]
- 20.Berahovich RD, Miao Z, Wang Y, Premack B, Howard MC, Schall TJ. Proteolytic activation of alternative CCR1 ligands in inflammation. J Immunol. 2005;174:7341–7351. doi: 10.4049/jimmunol.174.11.7341. [DOI] [PubMed] [Google Scholar]
- 21.Bernett MJ, Blaber SI, Scarisbrick IA, Dhanarajan P, Thompson SM, Blaber M. Crystal structure and biochemical characterization of human kallikrein 6 reveals a trypsin-like kallikrein is expressed in the central nervous system. J Biol Chem. 2002;277:24562–24570. doi: 10.1074/jbc.M202392200. [DOI] [PubMed] [Google Scholar]
- 22.Bever CT, Jr, Panitch HS, Johnson KP. Increased cathepsin B activity in peripheral blood mononuclear cells of multiple sclerosis patients. Neurology. 1994;44:745–748. doi: 10.1212/wnl.44.4.745. [DOI] [PubMed] [Google Scholar]
- 23.Bever CT, Jr, Garver DW. Increased cathepsin B activity in multiple sclerosis brain. J Neurol Sci. 1995;131:71–73. doi: 10.1016/0022-510x(95)00039-5. [DOI] [PubMed] [Google Scholar]
- 24.Bjartmar C, Kinkel RP, Kidd G, Rudick RA, Trapp BD. Axonal loss in normal-appearing white matter in a patient with acute MS. Neurology. 2001;57:1248–1252. doi: 10.1212/wnl.57.7.1248. [DOI] [PubMed] [Google Scholar]
- 25.Blaber S, Heysook Y, Scarisbrick IA, Juliani L, Blaber M. The autolytic regulation of human kallkrein 6. Biochemistry. 2007 doi: 10.1021/bi6025006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blaber SI, Scarisbrick IA, Bernett MJ, Dhanarajan P, Seavy MA, Jin Y, Schwartz MA, Rodriguez M, Blaber M. Enzymatic properties of rat myelencephalon specific protease. Biochemistry. 2002;41:1165–1173. doi: 10.1021/bi015781a. [DOI] [PubMed] [Google Scholar]
- 27.Blaber SI, Ciric B, Christophi GP, Bernett MJ, Blaber M, Rodriguez M, Scarisbrick IA. Targeting kallikrein 6-proteolysis attenuates CNS inflammatory disease. FASEB J. 2004;19:920–922. doi: 10.1096/fj.03-1212fje. [DOI] [PubMed] [Google Scholar]
- 28.Bo L, Vedeler CA, Nyland H, Trapp BD, Mork SJ. Intracortical multiple sclerosis lesions are not associated with increased lymphocyte infiltration. Mult Scler. 2003;9:323–331. doi: 10.1191/1352458503ms917oa. [DOI] [PubMed] [Google Scholar]
- 29.Boehme DH, Marks N. Mitigation of experimental allergic encephalomyelitis by cathepsin D inhibition. Adv Exp Med Biol. 1979;121B:317–323. doi: 10.1007/978-1-4684-8914-9_30. [DOI] [PubMed] [Google Scholar]
- 30.Borgono CA, Miacovos MP, Diamandis EP. Human tissue kallikreins: physiologic roles and applications in cancer. Mol Cancer Res. 2004;2:257–280. [PubMed] [Google Scholar]
- 31.Brundula V, Rewcastle NB, Metz LM, Bernard CC, Yong VW. Targeting leukocyte MMPs and transmigration: minocycline as a potential therapy for multiple sclerosis. Brain. 2002;125:1297–1308. doi: 10.1093/brain/awf133. [DOI] [PubMed] [Google Scholar]
- 32.Busso N, Frasnelli M, Feifel R, Cenni B, Steinhoff M, Hamilton J, So A. Evaluation of protease-activated receptor 2 in murine models of arthritis. Arthritis Rheum. 2007;56:101–107. doi: 10.1002/art.22312. [DOI] [PubMed] [Google Scholar]
- 33.Califano F, Giovanniello T, Pantone P, Campana E, Parlapiano C, Alegiani F, Vincentelli GM, Turchetti P. Clinical importance of thrombomodulin serum levels. Eur Rev Med Pharmacol Sci. 2000;4:59–66. [PubMed] [Google Scholar]
- 34.Cammer W, Bloom BR, Norton WT, Gordon S. Degradation of basic protein in myelin by neutral proteases secreted by stimulated macrophages: a possible mechanism of inflammatory demyelination. Proc Natl Acad Sci USA. 1978;75:1554–1558. doi: 10.1073/pnas.75.3.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao L, Goodin R, Wood D, Moscarello MA, Whitaker JN. Rapid release and unusual stability of immunodominant peptide 45–89 from citrullinated myelin basic protein. Biochemistry. 1999;38:6157–6163. doi: 10.1021/bi982960s. [DOI] [PubMed] [Google Scholar]
- 36.Cassim B, Mody G, Bhoola KD. Kallikrein cascade and cytokines in inflammed joints. Pharmacol Ther. 2002;94:1–34. doi: 10.1016/s0163-7258(02)00166-3. [DOI] [PubMed] [Google Scholar]
- 37.Centonze D, Napolitano M, Saulle E, Gubellini P, Picconi B, Martorana A, Pisani A, Gulino A, Bernardi G, Calabresi P. Tissue plasminogen activator is required for corticostriatal long-term potentiation. Eur J Neurosci. 2002;16:713–721. doi: 10.1046/j.1460-9568.2002.02106.x. [DOI] [PubMed] [Google Scholar]
- 38.Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Van Ness K, Greenstreet TA, March CJ, Kronheim SR, Druck T, Cannizzaro LA, et al. Molecular cloning of the interleukin-1 beta converting enzyme. Science. 1992;256:97–100. doi: 10.1126/science.1373520. [DOI] [PubMed] [Google Scholar]
- 39.Chabas D, Baranzini SE, Mitchell D, Bernard CC, Rittling SR, Denhardt DT, Sobel RA, Lock C, Karpuj M, Pedotti R, Heller R, Oksenberg JR, Steinman L. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science. 2001;294:1731–1735. doi: 10.1126/science.1062960. [DOI] [PubMed] [Google Scholar]
- 40.Chandler S, Coates R, Gearing A, Lury J, Wells G, Bone E. Matrix metalloproteinases degrade myelin basic protein. Neurosci Lett. 1995;201:223–226. doi: 10.1016/0304-3940(95)12173-0. [DOI] [PubMed] [Google Scholar]
- 41.Chandler S, Cossins J, Lury J, Wells G. Macrophage metalloelastase degrades matrix and myelin proteins and processes a tumour necrosis factor-alpha fusion protein. Biochem Biophys Res Commun. 1996;228:421–429. doi: 10.1006/bbrc.1996.1677. [DOI] [PubMed] [Google Scholar]
- 42.Chang C, Werb Z. The many faces of metalloproteases: cell growth, invasion, angiogenesis and metastasis. Trends Cell Biol. 2001;11:S37–S43. doi: 10.1016/s0962-8924(01)02122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chapman J. Thrombin in inflammatory brain diseases. Autoimmun Rev. 2006;5:528–531. doi: 10.1016/j.autrev.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 44.Chou YK, Burrows GG, LaTocha D, Wang C, Subramanian S, Bourdette DN, Vandenbark AA. CD4 T-cell epitopes of human alpha B-crystallin. J Neurosci Res. 2004;75:516–523. doi: 10.1002/jnr.20000. [DOI] [PubMed] [Google Scholar]
- 45.Christophi GP, Isackson PJ, Blaber SI, Blaber M, Rodriguez M, Scarisbrick IA. Distinct promoters regulate tissue-specific and differential expression of kallikrein 6 in CNS demyelinating disease. J Neurochem. 2004;91:1439–1449. doi: 10.1111/j.1471-4159.2004.02826.x. [DOI] [PubMed] [Google Scholar]
- 46.Cirino G, Cicala C, Bucci MR, Sorrentino L, Maraganore JM, Stone SR. Thrombin functions as an inflammatory mediator through activation of its receptor. J Exp Med. 1996;183:821–827. doi: 10.1084/jem.183.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Claudio L, Raine CS, Brosnan CF. Evidence of persistent blood brain barrier abnormalities in chromic-progressive multiple sclerosis. Acta Neuropathol (Berl) 1995;90:228–238. doi: 10.1007/BF00296505. [DOI] [PubMed] [Google Scholar]
- 48.Clements JM, Cossins JA, Wells GM, Corkill DJ, Helfrich K, Wood LM, Pigott R, Stabler G, Ward GA, Gearing AJ, Miller KM. Matrix metalloproteinase expression during experimental autoimmune encephalomyelitis and effects of a combined matrix metalloproteinase and tumour necrosis factor-alpha inhibitor. J Neuroimmunol. 1997;74:85–94. doi: 10.1016/s0165-5728(96)00210-x. [DOI] [PubMed] [Google Scholar]
- 49.Colton CA, Keri JE, Chen WT, Monsky WL. Protease production by cultured microglia: substrate gel analysis and immobilized matrix degradation. J Neurosci Res. 1993;35:297–304. doi: 10.1002/jnr.490350309. [DOI] [PubMed] [Google Scholar]
- 50.Comabella M, Romera C, Camina M, Perkal H, Moro MA, Leza JC, Lizasoain I, Castillo M, Montalban X. TNF-alpha converting enzyme (TACE) protein expression in different clinical subtypes of multiple sclerosis. J Neurol. 2006;253:701–706. doi: 10.1007/s00415-006-0090-6. [DOI] [PubMed] [Google Scholar]