Abstract
Satellite-based tracking of migratory waterfowl is an important tool for understanding the potential role of wild birds in the long-distance transmission of highly pathogenic avian influenza. However, employing this technique on a continental scale is prohibitively expensive. This study explores the utility of stable isotope ratios in feathers in examining both the distances traveled by migratory birds and variation in migration behavior. We compared the satellite-derived movement data of 22 ducks from 8 species captured at wintering areas in Bangladesh, Turkey, and Hong Kong with deuterium ratios (δD) of these and other individuals captured at the same locations. We derived likely molting locations from the satellite tracking data and generated expected isotope ratios based on an interpolated map of δD in rainwater. Although δD was correlated with the distance between wintering and molting locations, surprisingly, measured δD values were not correlated with either expected values or latitudes of molting sites. However, population-level parameters derived from the satellite-tracking data, such as mean distance between wintering and molting locations and variation in migration distance, were reflected by means and variation of the stable isotope values. Our findings call into question the relevance of the rainfall isotope map for Asia for linking feather isotopes to molting locations, and underscore the need for extensive ground truthing in the form of feather-based isoscapes. Nevertheless, stable isotopes from feathers could inform disease models by characterizing the degree to which regional breeding populations interact at common wintering locations. Feather isotopes also could aid in surveying wintering locations to determine where high-resolution tracking techniques (e.g. satellite tracking) could most effectively be employed. Moreover, intrinsic markers such as stable isotopes offer the only means of inferring movement information from birds that have died as a result of infection. In the absence of feather based-isoscapes, we recommend a combination of isotope analysis and satellite-tracking as the best means of generating aggregate movement data for informing disease models.
Keywords: geographical indicators, connectivity, disease modeling, epidemiology, disease vector, waterfowl, deuterium
INTRODUCTION
The importance of migratory wild birds in the spread of H5N1 highly pathogenic avian influenza (HPAI) is a much-debated topic (Newman et al., 2012; Takekawa et al., 2010b). In areas commonly affected by HPAI, poultry trade is regarded as the primary cause for the persistence and spread of HPAI (Gauthier-Clerc et al., 2007), and long-distance transportation of poultry products along with unregulated practices at poultry markets have been linked to outbreaks in many parts of Asia (Amonsin et al., 2008; Liu et al., 2003; Shortridge et al., 1998; Wang et al., 2006; Yu et al., 2007). Waterbirds (i.e., Anatidae and Charadriidae) have been identified as primary reservoirs for low pathogenic influenza viruses (Stallknecht and Shane, 1988), but with our limited knowledge about the distances infected birds migrate and connectivity among populations, the importance of HPAI transmission by wild birds remains an open question (Gaidet et al., 2010; Takekawa et al., 2010b; van Gils et al., 2007; Weber and Stilianakis, 2007).
The potential of wild birds to spread HPAI is evident in the 2005 outbreak at Qinghai Lake in China that killed more than 6000 wild waterfowl. Poultry farming in this part of the Tibetan Plateau is rare which implies that migratory waterfowl were the likely source of the disease. Subsequent spread of HPAI into Russia, Western Europe, the Middle East, and Northern Africa, provides further evidence of transmission by wild birds (Gilbert et al., 2006b; Normile, 2005, 2006; Prosser et al., 2009), as do genetic similarities among the viruses from these disparate geographic areas (Prosser et al., 2011). In particular, related strains of HPAI along the eastern portion of the Central Asian Flyway provide what is perhaps the strongest evidence of wild bird involvement in HPAI transmission (Newman et al., 2012).
Areas where wild and domestic birds often come into contact have proven particularly prone to HPAI outbreaks, especially regions that feature extensive free-range duck production (Alexander and Brown, 2009; Gilbert et al., 2007; Hulse-Post et al., 2005). Within Thailand, hot spots for H5N1 HPAI outbreaks correspond closely with the density of free-ranging ducks, which serve as the disease reservoir but also often forage next to wild waterfowl known to carry the virus asymptomatically (Gilbert et al., 2006a; Gilbert et al., 2008; Songserm et al., 2006).
Although migration corridors and HPAI outbreaks may not be temporally correlated in some cases (Takekawa et al., 2010a), the potential for transmission (see Gaidet et al., 2010) and gaps in our knowledge about global migration routes warrant further investigation into regional connectivity. Considerable effort has been devoted to tracking waterfowl in areas affected by HPAI (e.g., Batbayar et al., 2011; Iverson et al., 2011; Newman et al., 2009; Prosser et al., 2011; Takekawa et al., 2010a). Although these efforts have generated significant insights into migration routes and the role of wild birds in HPAI transmission, they are limited in extent due to the expense associated with satellite tracking technology. The cost of a single satellite tracking device may exceed $4000 (USD) including hardware and data acquisition charges. In addition, satellite tags may stop transmitting within a few weeks of deployment, thus failing to convey tracking data for a full annual cycle (see methods and Cappelle et al., 2011).
Stable isotope ratios in feathers offer an alternative means of evaluating population connectivity in migratory birds (Hobson, 2005; Hobson et al., 2009b). Stable isotope ratios of hydrogen vary predictably across the landscape, and the keratin generated during feather growth typically reflects stable isotope ratios in the local environment (Hobson and Wassenaar, 2008). By analyzing feathers collected at a waterfowl wintering location, we can infer the geographic region where breeding and feather molt occurred during the previous fall. The resolution of this tracking technique is low and the inference from a single sample is often quite poor (Kelly et al., 2008; Wunder et al., 2005). However, a modest sample of feathers from 20 to 50 individuals can provide useful information about distances traveled as well as variability in migratory behavior. Although detailed migration routes are not obtainable from isotope data, this method is relatively inexpensive (most facilities charge less than $50 USD per sample), and it can be used for birds of any size. In contrast, the smallest satellite transmitters currently available can only be used on birds that weigh over 100 g (Bridge et al., 2011). Lastly, of particular importance for disease studies is that feather-isotopes can be quantified from samples collected from birds that have died as a result of an outbreak, allowing for potential inference of the provenance of the disease.
It should be noted that the meaning of “connectivity” differs among fields of study. In the context of migratory connectivity, a breeding population with high connectivity would have a tight spatial and temporal coupling with a particular wintering area, such that members of the population would rarely mix with members of other populations (Webster et al., 2002). In this study we focus primarily on population connectivity, wherein the categorization of high connectivity is conferred upon populations that undergo significant long-term contact with other populations. Our goal is to evaluate the utility of stable isotope ratios as a means of revealing varying degrees of population connectivity by comparing hydrogen stable-isotope ratios with satellite-tracking data from ducks captured at wintering locations in Bangladesh, Hong Kong, and Turkey. Deuterium in rainwater typically becomes more reduced the farther the distance travelled from the equator. Hence, we predicted that birds with more northerly breeding and molting grounds would have less deuterium in their feathers. Moreover, increased variation in northward migration flights should be associated with both increased population connectivity (i.e., more widespread intermingling of different breeding populations at the wintering site) and increased variation in hydrogen isotope signature. This study combines data from stable isotope analyses and from satellite-tracking studies collected from the same set of migratory birds, to provide a rare, but intuitively simple means of testing our predictions.
METHODS
Satellite transmitter deployment and feather collection
As part of efforts orchestrated by the United Nations Food and Agriculture Organization and the U. S. Geological Survey, hundreds of satellite tags were deployed in Asia, Eastern Europe, and Africa. This study is focused only on deployments in Hong Kong (Special Administrative Region, China; 22.5° N, 114.0° E), northeast Bangladesh (24.6° N, 92.1° E), and northern Turkey (41.7° N, 36.0° E), where at least five individuals marked on the wintering grounds carried a working tag to their breeding and molting locations. In Hong Kong feather sampling and tag deployment occurred on 9 December 2010 and consisted of 35 individuals sampled of which 7 were successfully tracked, For Bangladesh, birds were captured from 3 to 12 March, 2010, with 18 animals sampled and 10 animals tracked. In Turkey, field work occurred from 10 to 20 February, 2010 and resulted in 41 samples and 5 tracks. Generally, more than one species was tagged at each deployment location, and the species used at each location varied, resulting in a mixed sample from eight species. Species used for satellite tracking were Eurasian wigeon (Anas penelope, n = 5), Northern Pintail (Anas acuta, n = 3), Garganey (Anas querquedula, n = 2), Northern Shoveler (Anas clypeata, n = 3), Ruddy Shelduck (Tadorna ferruginea, n = 5), and Common Teal, (Anas crecca, n = 4). For stable isotopes, we sampled all individuals with satellite tracks, plus an additional 13 European Widgeon, 18 Northen Pintails, 3 Garganey, 2 Northern Shovelers, 4 Ruddy Shelducks, 29 Common Teal, 1 Ferruginous Duck (Aythya nyroca), 1 Mallard (Anas platyrhynchos), and 1 Common Pochard (Aythya ferina). Hence, there were 22 ducks with migration tracks and a total of 94 ducks sampled for isotopes. Although these species differ in various life-history characteristics, they have similar molting, breeding, and migration patterns (Cramp, 1980). Moreover, these species intermix on the wintering grounds where zoonotic diseases like HPAI could easily cross species boundaries. We assume that species differences with regard to life history characteristics (e.g. migration and molt) and capacity to transmit disease are negligible within the scope of this study.
Birds were captured with a variety of techniques, which are described elsewhere (Batbayar et al., 2011; Newman et al., 2009; Prosser et al., 2011; Takekawa et al., 2010a; Whitworth et al., 2007), and tags were attached with backpack harnesses made from Teflon ribbon (Bally Ribbon Mills, Bally, PA, USA). Depending on the size of the bird, we used 12, 18 and 30 g solar-powered transmitters. Satellite tags were a mixture of Argos transmitters, which operate on the Doppler-shift principle (Fancy et al., 1988), and geographic positioning system (GPS) tags (Microwave Telemetry, Inc., Columbia, MD, USA). Because this study requires only regional-scale accuracy from satellite tracking, we do not differentiate between data from GPS and Argos tags, and we do not consider tag location error in our analyses and conclusions.
A total of 58 ducks were tagged at the three sites, of which 22 yielded data that indicated a likely molting location. Molting locations were determined by examining daily locations during the post breeding period (mid-August to late October) and identifying a period exceeding three weeks during which movements were restricted to a single water body. In all cases, molting locations were within 300 km of an apparent breeding location characterized by a prolonged stationary period during the breeding season.
Isosope analysis
Feathers were sampled from tracked individuals and other ducks captured during the same field campaigns. Feathers sampled were either primaries, secondaries or primary coverts, all of which are replaced once each year after the breeding season as part of the pre-basic molt (see Humphrey and Parkes, 1959; Pyle, 2005), which generally takes place at or near the breeding grounds. Feathers were stored in paper envelopes and heat treated to disinfect them prior to transport.
We performed stable isotope analyses of the deuterium/hydrogen ratio (δD), which we express in standard delta notation, where δD = [(isotope ratiosample/isotope ratiostandard) − 1] × 1,000. Consequently, δD is shown in parts per thousand (‰) deviation from a standard (Vienna Standard Mean Ocean Water). Prior to stable-isotope analyses, all feathers were cleaned with dilute detergent (1:30 solution of Fisher Versa-Clean, Fisher Scientific, Pittsburgh, Pennsylvania; catalogue number 04-342) and then a 2:1 chloroform/methanol solution following Paritte and Kelly (2009). We then packed a 140–160 μg piece of the distal vane of each feather into a 3.5 × 5 mm silver capsule for insertion into an autosampling tray. Isotope ratio measurements were performed at the University of Oklahoma (Norman, Oklahoma, USA) with a ThermoFinnigan Delta V isotope-ratio mass spectrometer connected to a high-temperature pyrolysis elemental analyzer (TC/EA, Thermo-Finnigan, Bremen, Germany). We used three keratin standards in each group of feather samples analyzed, allowing us to estimate exchangeable hydrogen using the comparative equilibration method (Wassenaar and Hobson, 2003). Further details about our isotope measurements methods can be found in Kelly et al. (2009).
Comparing satellite tracks and isotope ratios
Stable isotopes alone cannot pinpoint the molting location of an individual bird. Most isotope-based studies of migratory birds, attempt to assign individual birds to general geographic categories or make geographic inferences based on averages from samples of several individuals. Similarly, variation in feather-isotope ratios from a population of birds can reveal the degree to which the birds differ with regard to their molt locations. If a sample of bird molt in a variety of locations spread out across the landscape, then we would expect a high degree of variation in stable isotope ratios. Our comparison of satellite tracking data and stable isotope ratios is aimed primarily at determining whether the two methods provide similar results with regard to the degree of geographic variation among molting locations. We focus only on hydrogen isotopes because this element is most commonly used as a means of inferring geographic locations for terrestrial birds. Isotope ratios for many other elements can be obtained from feathers, however, all other isotope are either very rarely used (e.g. strontium) or do no have predictable geographical gradients within the geographical scope of this study (e.g. carbon and nitrogen).
Feathers collected during tag deployment were grown prior to the acquisition of the tracking data. However, waterfowl typically demonstrate high fidelity to breeding areas among years (see Takekawa et al., 2011), and we examined the within-individual correspondence between migration tracks and feather isotopes under the assumption that general molting regions do not often change from year to year. To compare satellite data and isotope ratios, we downloaded raster data for a multi-year average of δD in rainfall during the month of August for Asia and Europe (www.waterisotopes.org; Bowen, 2011). This map was based on water isotope data from the Global Network for Isotopes in Precipitation (GNIP) database administered by the International Atomic Energy Association and World Meteorological Organization (IAEA/WMO 2001), which were collected discontinuously from ~1960 to 2004 (Bowen et al., 2005; IAEA/WMO, 2001). We estimated an expected isotope ratio for each molting location derived from satellite data. δD in bird feathers is typically reduced by about 25‰ relative to δD in the diet (Hobson and Wassenaar, 2008). Hence, we generated expected stable isotope ratios for feathers grown in a given location by subtracting 25‰ from the rainwater isotope value for that location. We then used R (v 2.15.2; R Development Core Team, 2004) to test for correlations between the observed feather-isotope values and 1) expected isotope values, 2) the latitude of the apparent molt location, and 3) the distance between the capture location and the apparent molt location.
Of particular interest for disease studies is the degree to which birds coming from different regions intermingle on breeding and wintering locations. By examining the variation in δD from birds sampled in the same wintering site, we can assess whether they all bred in the same general area or whether they represent multiple, widespread breeding populations. In this study, we compared variation in δD with variation in migration routes revealed by the satellite tracking data (specifically the distance between the capture location and the apparent molting location). Because relevant satellite tracking data are scarce, this study considered only three field sites, which limited opportunities for among-population comparison of δD and migration routes and precluded use of statistical methods. Hence, we do not attempt formal hypothesis testing, but we discuss the results in terms of the directionality of the apparent trends.
RESULTS
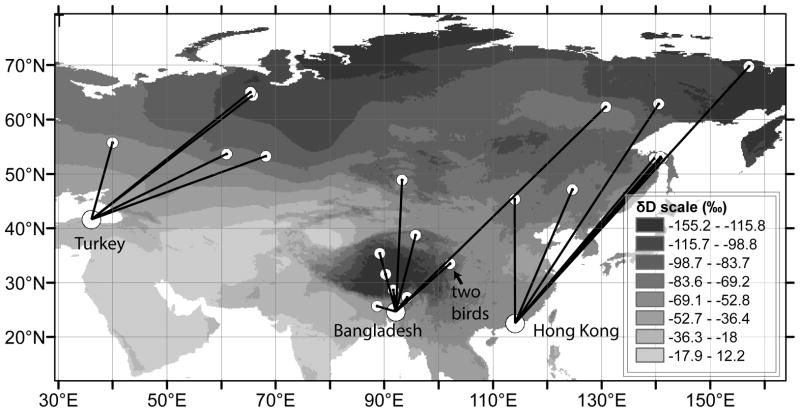
Satellite tracking data revealed notable differences among the migration behaviors of the ducks tagged in Hong Kong, Bangladesh, and Turkey (Fig 1). These data show that five birds tagged in Hong Kong generally migrated farther than birds at the other two sites with an average distance of 4075 ± 1070 km (mean ± one standard deviation) between the tagging location and the presumed molting location, and migration distances did not vary greatly among those individuals. The 10 ducks tagged in Bangladesh showed the highest degree of variability in their migration behavior with some birds moving very short distances (<500km), some traveling 1000 to 3000 km, and one bird that flew >5000 km from the capture location. For these birds, the average distance between tagging and molting locations was 1408 ± 1507 km. The five ducks from Turkey were the most uniform in terms of migration distance, averaging 2587 ± 667 km. An ANOVA indicated a significant difference among these distances (F2,19 = 9.28, P = 0.002), and a post-hoc test indicated that only the means from Bangladesh and Hong Kong were different (Tukey’s Contrasts, P = 0.001; Table 1). Variance in migration distance did not differ among the three groups (Levene’s test: F2,91= 0.62, P = 0.55), but there were differences among groups with regard to the geographical spread of molting locations as indicated by the mean pairwise distance within each group, with less spread among apparent molt locations in the birds from Turkey (based on 95% confidence limits, see Table 1).
Fig 1.
Map of Asia and Eastern Europe showing satellite tag deployment and feather collection locations (large circles), apparent molt locations (small circles), and the distribution of δD in rainwater during August (shading) based on data from Bowen (2011). Tagging and feather collection took place in late winter 2010 for Bangladesh and Turkey and in December 2010 for Hong Kong. Tagging locations and apparent molt locations are connected with straight lines and do not represent actual migration paths.
Table 1.
Sample sizes, means, and standard deviations for measured parameters in feather samples and satellite tracks for all three sampling locations. ΔD is shown for all feather samples and for those associated with tracked birds. For tracked individuals, ‘Distance’ shows the mean distance between the tagging (i.e., wintering) location and apparent molting location, and the rightmost column shows the mean pairwise great-circle distances between apparent molt locations within each group as an indicator of geographical spread. The 95% confidence interval for the mean pairwise neighbor distances are listed in parentheses.
| Feather samples
|
Birds tracked
|
|||||
|---|---|---|---|---|---|---|
| n | ΔD | n | ΔD | Distance | Mean pairwise neighbor distance | |
|
|
|
|||||
| Bangladesh | 18 | −143.4 ± 30.5 | 10 | −131.8 ± 30.3 | 1398 ± 1507 | 1744 ± 1422 (±292) |
| Hong Kong | 35 | −178.6± 23.1 | 7 | −172.9 ± 23.1 | 4075 ± 1070 | 1502 ± 963 (±284) |
| Turkey | 41 | −123.6 ±23.0 | 5 | −124.5 ± 23.6 | 2587 ± 829 | 1224 ± 539 (±236) |
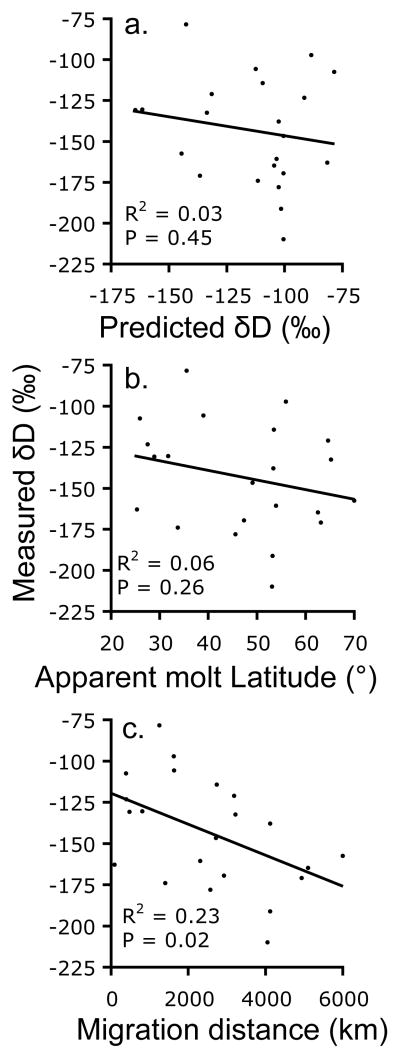
The three sites also differed in mean δD values (F2,92 = 47.6, P < 0.001), with the most negative mean associated with the Hong Kong group (Tukey’s Contrasts, P < 0.001), whose tracked individuals flew farther north than the other groups (Table 1). Although tracked birds from Bangladesh flew the shortest distance on average (Table 1), the mean δD value from the Bangladesh feather samples was intermediate with respect to Hong Kong and Turkey (Tukey’s Contrasts, P < 0.015). It is likely that the Gobi Desert had an influence on the isotope ratios of the Bangladesh birds, causing them to have a lower proportion of deuterium in their feathers than the birds from Turkey even though the birds from Bangladesh did not move as far north (Fig 1). The δD values in the feather samples from tracked individuals did not correlate with their respective predictions for δD based on the apparent molt locations indicated by satellite tracking data (R2 = 0.029, N = 22, P = 0.45; Fig 2a). Similarly there was no correlation between the latitude of apparent molting grounds and δD in feathers (R2 = 0.062, N = 22, P = 0.26; Fig 2b). There was an inverse relationship between δD in feathers and the distance between the apparent molting ground and the feather sampling location (R2 = 0.23, N = 22, P = 0.02; Fig 2c); birds that flew longer distances tended to have less deuterium in their feathers, which was consistent with our prediction.
Fig 2.
Comparisons of a) predicted δD, b) apparent molt latitude, and c) migration distance from 22 birds with satellite tags and δD from feather samples from the same individuals.
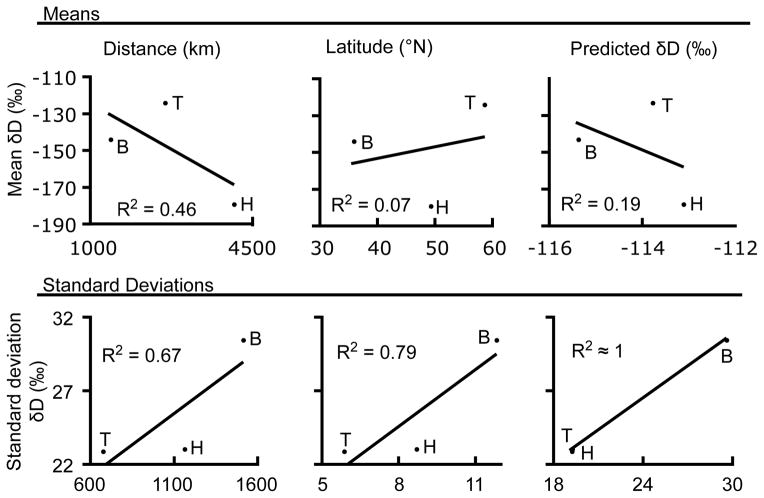
With regard to population-level comparisons, satellite data and isotopes provided similar indications of the degree of variation in migratory behavior. We evaluated correlations among the study sites of the mean and standard deviation of feather-isotope ratios from all samples compared with distance to molting site, latitude of molting site, and predicted feather isotope ratios from tracked individuals (Figure 3). Although, interpretation of these analyses was limited due to the fact that only three wintering populations were sampled in this study, correlations among means were in the expected direction with regard to migration distances and higher latitudes (i.e. δD was more negative in association with more northward movement, Fig 2b). However, we did not observe the anticipated positive correlation between predicted and observed δD (Fig 2a). The standard deviation of predicted and measured δD were very highly correlated (R2> 0.99), and correlations coefficients associated with standard deviations in distance and latitude were all positive (R2 > 0.67; see Fig 3), but difficult to interpret because of the limited number of study sites.
Fig. 3.
Correlations comparing means and standard deviations of measured feather-isotope values to great-circle distance to apparent molting grounds, apparent molting latitude, and predicted isotope values based on satellite transmitter locations. Points on the graphs are labled H for Hong Kong (nfeathers = 35 ntracks= 7) “B” for Bangladesh (n tracks = 10), and “T” for Turkey (ntracks = 5).
DISCUSSION
We observed general agreement among population-level connectivity inferred from stable isotopes and from satellite tracking. Based on stable-isotope data alone, Bangladesh would emerge as an area where transmission among different breeding populations is likely by virtue of the high degree of variation in δD in this area (Table 1). Hong Kong would be regarded as an area where long-distance transmission could occur (based on the more negative δD of the feathers from that site), and Turkey would be ranked among the three sites as having the lowest degree of population connectivity as indicated by the greatest mean δD (indicating the shortest migration distances) and the lowest variance in δD (indicating less geographic variation in molting areas). These are the same qualitative conclusions supported by the satellite tracking data.
Although migration distances of individual ducks were correlated with δD in their feathers, individual-level correspondence between satellite-based data and isotopes was considerably lower than expected. The often large discrepancies between observed and predicted δD as well as observed δD and latitude call into question the accuracy of the rainfall isotope map for Asia. The map is the result of an interpolation model that merges relatively sparse sampling of rainwater from throughout the region with temperature-driven rainout effects and regional patterns of vapor sourcing and delivery (see Bowen and Revenaugh, 2003; Bowen and Wilkinson, 2002). Moreover, the δD estimates for rainfall may not correspond directly to feather isotope ratios. Although Peréz et al. (2010) reported good correspondence between observed δD in feathers and expected δD in rainwater in a different suite of waterbirds sampled in Mongolia, birds that use landlocked water sources (which applies to most of the birds in this study) may be exposed to runoff waters that differ in their values from local rainfall. This disconnect between rainwater and water bodies used by the molting ducks could account for the discrepancies between expected and measured δD. Lastly, the rainwater isoscape in Asia and western Europe is somewhat more varied than the evenly clinal pattern of δD in other regions (e.g., Eastern North America, see Bowen and Revenaugh, 2003). Landscape features such as the Himalayan Mountains and the Gobi Desert introduce irregularities in the isoscape which disrupt the usual relationship between δD in rainwater and latitude (see Fig 1). This uneven isoscape probably contributed to our failure to correlate δD and latitude.
The analyses used in this study are relatively crude compared with newly generated modeling techniques for ascribing geographic data to animals based on stable isotope ratios (Wunder, 2010; Wunder and Norris, 2008a; Wunder and Norris, 2008b). These assignment tests often make use of all available data (e.g. known distributions) and attempt to determine whether a feather (or other tissue) was most likely generated in one of two or more discrete areas. Our use of more sophisticated approaches was hampered by a general lack of environmental sampling throughout our study region and in particular for the years associated with sample collection. Moreover, our goal was not to attempt to identify molt latitude, but to compare results from two different means of assessing connectivity. Therefore, isotope modeling was not used in this study, but it could be employed as a means of improving and quantifying isotopic inference in other situations. In addition, combining isotopes with geographically informative genetic data may allow for even finer scale discernment of molting and breeding regions based solely on intrinsic biomarkers (Clegg et al., 2003; Kelly et al., 2005; Robinson et al., 2010). Application of stable isotope tracking can further be improved by knowledge of the species under examination. Because empirically derived discrimination factors are lacking for the animals sampled, we applied a single discrimination factor and did not attempt to account for species differences in our analyses. This is a likely source of error that could be avoided in other studies that sample from a single species.
Although the use of stable isotopes cannot serve as a direct substitute for the detailed information obtained through satellite tracking, our results suggest that stable isotopes can provide general information about migration distances and variation in migration behavior. Given the cost associated with satellite tracking equipment and data acquisition, we see potential for stable isotope analysis of feather samples to serve as a supplementary method for characterizing wintering bird populations (or areas) in terms of disease-related connectivity. Perhaps the most advantageous approach is a combination of satellite tracking and stable isotope analyses, wherein satellite data provide detailed information about movement routes and sedentary periods from a limited number of birds, and isotope data expand the inference of the study by significantly increasing the sample size. This combined approach would be particularly suitable for populations that have suffered fatal outbreaks, as stable-isotope analyses could be employed on feathers from dead birds and satellite tags could be applied to survivors. Stable isotopes could also serve as part of a screening process in which potential high-connectivity areas are identified based on feather samples. Populations believed to be key nodes in a connectivity matrix (i.e. populations with highly variable isotope signatures) can then be investigated further by collecting detailed data on bird communities, habitat use, and migratory movements to fully characterize disease-transmission potential.
The use of stable isotopes in bird feathers to infer disease origins has been discussed previously (e.g. Horacek, 2011; Peterson and Williams, 2008; Whitworth et al., 2007), but there have been few attempts to evaluate the utility of this method. Yohannes, et al. (2008) found that they could predict the prevalence of malaria in Great Reed Warblers (Acrocephalus arundinaceus) based on feather isotopes. Aside from this work we know of no other attempts to test the utility of isotopes in disease connectivity studies. Our results indicate that the method has the potential to make significant contributions to disease modeling efforts. However, our findings also imply that there is a need for detailed ground-truthing and the generation of continental-scale feather-based isoscapes for isotope-tracking to attain its full potential (see Kelly and Finch, 1998; Sellick et al., 2009).
Feather isoscapes have been generated for some regions (e.g. Mexico; Hobson et al., 2009a), but the effort and expense of such an undertaking in Asia and Eastern Europe would be considerable, requiring the acquisition and analysis of hundreds or even thousands of feathers. Some of the background data may be gleaned from other work with feather isotopes in the region (e.g. Pérez et al., 2010). However, useful samples are those for which there is a high degree of certainty about where a feather was grown. Hence, feather samples collected for traditional isotope tracking studies (i.e. studies that seek to identify molting areas based on samples from a population of birds), cannot contribute to isoscapes. Unfortunately, funding required for active collection of feather samples and subsequent analyses would be substantial, which counters the argument that stable isotope studies are inexpensive.
In the absence of feather-based isoscapes we can still make general population-level inferences about the extent and variation of migration behavior within a population. However, a feather isoscape would greatly enhance our capacity to link birds to regional molting locations. The cost of continental-scale isoscapes could be greatly reduced if archived feathers were available. Hence, we encourage all scientists who handle live birds to save feathers (particularly feathers of known origin) and provide them to an appropriate archive (see Smith et al., 2003), and we encourage curators of ornithology collections to consider allowing sampling of museum specimens for stable isotope analyses. These ‘passive’ sampling schemes may generate sufficient background data to improve inference from stable isotopes for studies of migratory connectivity and disease transmission.
Acknowledgments
Isotope analyses and manuscript generation was supported by an NIH/NSF Ecology and Evolution of Infectious Diseases award from the Fogarty International Center of the National Institutes of Health (3R01-TW005869) with an ARRA U.S. Postdoctoral Scientist Administrative Supplement. Field work was supported by the United Nations Food and Agriculture Organization, the U. S. Geological Survey Western Ecological Research Center and Avian Influenza Program, and Erasmus University. The work was conducted in the context of Zoonotic Influenza Collaborative Network, led by the Fogarty International Center, and supported by International Influenza Funds from the Office of the Secretary of the Department of Health and Human Services. Research on animals was conducted with the approval the Animal Care and Use Committees of the USGS Western Ecological Center, the Patuxent Wildlife Research Center, The University of Maryland, Baltimore County (Protocol EE070200710), and the University of Oklahoma (Animal Use Statement R09-019). We thank the following people and organizations for their aid in organizing and executing work at the various field sites: C. Ozsemir, S. Inak, N. Yavuz, N. Tubbs, J. Epstein, J. Desmond, P. Sathyiaselvam, J. Allcock, K. Leung, M. Peiris, S. Luby, Y. Baris, M. Leven, A. Mikolon, and the Bangladesh Bird Club. We also thank Eben Paxton, Susan Jones, and two anonymous scientists for their helpful reviews. The use of trade names in this document is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- Alexander DJ, Brown IH. History of highly pathogenic avian influenza. Revue Scientifique Et Technique-Office International Des Epizooties. 2009;28:19–38. doi: 10.20506/rst.28.1.1856. [DOI] [PubMed] [Google Scholar]
- Amonsin A, Choatralkol C, Lapkuntod J, Tantilertcharoen R, Thanawongnuwech R, Suradhat S, Suwannakarn K, Theamboonlers A, Poovorawan Y. Influenza virus (H5N1) in live bird markets and food markets, Thailand. Emerging Infect Dis. 2008;14:1739–1742. doi: 10.3201/eid1411.080683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batbayar N, Takekawa JY, Newman S, Prosser DJ, Natsagdorj T, Xiao X. Migration strategies of Swan Geese Anser cygnoides from northeast Mongolia. Wildfowl. 2011;61:90–109. [Google Scholar]
- Bowen GJ. Gridded maps of the isotopic composition of meteoric waters. 2011 http://www.waterisotopes.org.
- Bowen GJ, Revenaugh J. Interpolating the isotopic composition of modern meteoric precipitation. Water Resour Res. 2003;39:1299–1311. [Google Scholar]
- Bowen GJ, Wassenaar LI, Hobson KA. Global application of stable hydrogen and oxygen isotopes to wildlife forensics. Oecologia. 2005;143:337–348. doi: 10.1007/s00442-004-1813-y. [DOI] [PubMed] [Google Scholar]
- Bowen GJ, Wilkinson B. Spatial distribution of delta O-18 in meteoric precipitation. Geology. 2002;30:315–318. [Google Scholar]
- Bridge ES, Thorup K, Bowlin MS, Chilson PB, Diehl RH, Fleron RW, Hartl P, Kays R, Kelly JF, Robinson WD, Wikelski M. Technology on the Move: Recent and Forthcoming Innovations for Tracking Migratory Birds. Bioscience. 2011;61:689–698. [Google Scholar]
- Cappelle J, Iverson SA, Takekawa JY, Newman SH, Dodman T, Gaidet N. Implementing telemetry on new species in remote areas: recommendations from a large-scale satellite tracking study of African waterfowl. Ostrich. 2011;82:17–26. [Google Scholar]
- Clegg SM, Kelly JF, Kimura M, Smith TB. Combining genetic markers and stable isotopes to reveal population connectivity and migration patterns in a Neotropical migrant, Wilson’s warbler (Wilsonia pusilla) Mol Ecol. 2003;12:819–830. doi: 10.1046/j.1365-294x.2003.01757.x. [DOI] [PubMed] [Google Scholar]
- Cramp S. Handbook of the birds of Europe, the Middle East and North Africa: the birds of the Western Palearctic, Vol 1: Ostrich to Ducks. Oxford University Press, Oxford Eng; New York: 1980. [Google Scholar]
- Fancy SG, Pank LF, Douglas DC, Curby CH, Garner GW, Amstrup SC, Regelin WL. Satellite telemetry: A new tool for wildlife research and management. U.S. Fish and Wildlife Service Publication. 1988;172:1–54. [Google Scholar]
- Gaidet N, Cappelle J, Takekawa JY, Prosser DJ, Iverson SA, Douglas DC, Perry WM, Mundkur T, Newman SH. Potential spread of highly pathogenic avian influenza H5N1 by wildfowl: dispersal ranges and rates determined from large-scale satellite telemetry. J Appl Ecol. 2010;47:1147–1157. [Google Scholar]
- Gauthier-Clerc M, Lebarbenchon C, Thomas F. Recent expansion of highly pathogenic avian influenza H5N1: a critical review. Ibis. 2007;149:202–214. [Google Scholar]
- Gilbert M, Chaitaweesub P, Parakarnawongsa T, Premashthira S, Tiensin T, Kalpravidh W, Wagner H, Slingenbergh J. Free-grazing ducks and highly pathogenic avian influenza, Thailand. Emerging Infect Dis. 2006a;12:227–234. doi: 10.3201/eid1202.050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M, Xiao XM, Chaitaweesub P, Kalpravidh W, Premashthira S, Boles S, Slingenbergh J. Avian influenza, domestic ducks and rice agriculture in Thailand. Agric, Ecosyst Environ. 2007;119:409–415. doi: 10.1016/j.agee.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M, Xiao XM, Domenech J, Lubroth J, Martin V, Slingenbergh J. Anatidae migration in the western palearctic and spread of highly pathogenic avian influenza H5N1 virus. Emerging Infect Dis. 2006b;12:1650–1656. doi: 10.3201/eid1211.060223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M, Xiao XM, Pfeiffer DU, Epprecht M, Boles S, Czarnecki C, Chaitaweesub P, Kalpravidh W, Minh PQ, Otte MJ, Martin V, Slingenbergh J. Mapping H5N1 highly pathogenic avian influenza risk in Southeast Asia. Proc Natl Acad Sci USA. 2008;105:4769–4774. doi: 10.1073/pnas.0710581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson KA, Van Wilgenburg SL, Larson K, Wassenaar LI. A feather hydrogen isoscape for Mexico. J Geochem Explor. 2009a;102:63–70. [Google Scholar]
- Hobson KA. Stable isotopes and the determination of avian migratory connectivity and seasonal interactions. Auk. 2005;122:1037–1048. [Google Scholar]
- Hobson KA, Lormee H, Van Wilgenburg SL, Wassenaar LI, Boutin JM. Stable isotopes (delta D) delineate the origins and migratory connectivity of harvested animals: the case of European woodpigeons. J Appl Ecol. 2009b;46:572–581. [Google Scholar]
- Hobson KA, Wassenaar LI. Tracking animal migration with stable isotopes. Elsevier; New York: 2008. [Google Scholar]
- Horacek M. Backtracking the movements of a migratory bird: a case study of a white-fronted goose (Anser albifrons) Rapid Commun Mass Spectrom. 2011;25:3146–3150. doi: 10.1002/rcm.5209. [DOI] [PubMed] [Google Scholar]
- Hulse-Post DJ, Sturm-Ramirez KM, Humberd J, Seiler P, Govorkova EA, Krauss S, Scholtissek C, Puthavathana P, Buranathai C, Nguyen TD, Long HT, Naipospos TSP, Chen H, Ellis TM, Guan Y, Peiris JSM, Webster RG. Role of domestic ducks in the propagation and biological evolution of highly pathogenic H5N1 influenza viruses in Asia. Proc Natl Acad Sci USA. 2005;102:10682–10687. doi: 10.1073/pnas.0504662102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey PS, Parkes KC. An approach to the study of molts and plumages. Auk. 1959;76:1–31. [Google Scholar]
- IAEA/WMO. Global network for isotopes in precipitation. the GNIP database 2001 [Google Scholar]
- Iverson SA, Gavrilov A, Katzner TE, Takekawa JY, Miller TA, Hagemeijer W, Mundkur T, Sivananinthaperumal B, DeMattos CC, Ahmed LS, Newman SH. Migratory movements of waterfowl in Central Asia and avian influenza emergence: sporadic transmission of H5N1 from east to west. Ibis. 2011;153:279–292. [Google Scholar]
- Kelly JF, Bridge ES, Fudickar AM, Wassenaar LI. A test of comparative equilibration for determining non-exchangeable stable hydrogen isotope values in complex organic materials. Rapid Commun Mass Spectrom. 2009;23:2316–2320. doi: 10.1002/rcm.4150. [DOI] [PubMed] [Google Scholar]
- Kelly JF, Finch DM. Tracking migrant songbirds with stable isotopes. Trends Ecol Evol. 1998;13:48–49. doi: 10.1016/s0169-5347(97)01299-8. [DOI] [PubMed] [Google Scholar]
- Kelly JF, Johnson MJ, Langridge S, Whitfield M. Efficacy of stable isotope ratios in assigning endangered migrants to breeding and wintering sites. Ecol Appl. 2008;18:568–576. doi: 10.1890/07-0027.1. [DOI] [PubMed] [Google Scholar]
- Kelly JF, Ruegg KC, Smith TB. Combining isotopic and genetic markers to identify breeding origins of migrant birds. Ecol Appl. 2005;15:1487–1494. [Google Scholar]
- Liu M, He SQ, Walker D, Zhou NN, Perez DR, Mo B, Li F, Huang XT, Webster RG, Webby RJ. The influenza virus gene pool in a poultry market in South Central China. Virology. 2003;305:267–275. doi: 10.1006/viro.2002.1762. [DOI] [PubMed] [Google Scholar]
- Newman SH, Hill NJ, Spragens KA, Janies D, Voronkin IO, Prosser DJ, Yan BP, Lei FM, Batbayar N, Natsagdorj T, Bishop CM, Butler PJ, Wikelski M, Balachandran S, Mundkur T, Douglas DC, Takekawa JY. An Eco-virological approach for assessing the role of wild birds in the spread of avian influenza H5N1 along the Central Asian Flyway. PLoS One. 2012;7:e30636. doi: 10.1371/journal.pone.0030636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SH, Iverson SA, Takekawa JY, Gilbert M, Prosser DJ, Batbayar N, Natsagdorjin T, Douglas DC. Migration of whooper swans and outbreaks of highly pathogenic avian influenza H5N1 virus in eastern Asia. Plos One. 2009;4:e5729. doi: 10.1371/journal.pone.0005729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normile D. Avian influenza - Are wild birds to blame? Science. 2005;310:426–428. doi: 10.1126/science.310.5747.426. [DOI] [PubMed] [Google Scholar]
- Normile D. Avian influenza - Evidence points to migratory birds in H5N1 spread. Science. 2006;311:1225–1225. doi: 10.1126/science.311.5765.1225. [DOI] [PubMed] [Google Scholar]
- Paritte JM, Kelly JF. Effect of cleaning regime on stable-isotope ratios of feathers in Japanese Quail (Coturnix japonica) Auk. 2009;126:165–174. [Google Scholar]
- Perez GE, Hobson KA, Garde EJ, Gilbert M. Deuterium (δD) in feathers of mongolian waterbirds uncovers migratory movements. Waterbirds. 2010;33:438–443. [Google Scholar]
- Peterson AT, Williams RAJ. Risk mapping of highly pathogenic avian influenza distribution and spread. Ecol Soc. 2008;13 [Google Scholar]
- Prosser DJ, Cui P, Takekawa JY, Tang MJ, Hou YS, Collins BM, Yan BP, Hill NJ, Li TX, Li YD, Lei FM, Guo S, Xing Z, He YB, Zhou YC, Douglas DC, Perry WM, Newman SH. Wild Bird Migration across the Qinghai-Tibetan Plateau: A Transmission Route for Highly Pathogenic H5N1. PLoS One. 2011;6 doi: 10.1371/journal.pone.0017622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser DJ, Takekawa JY, Newman SH, Yan B, Douglas DC, Hou Y, Xing Z, Zhang D, Li T, Li Y, Zhao D, Perry WM, Palm EC. Satellite-marked waterfowl reveal migratory connection between H5N1 outbreak areas in China and Mongolia. Ibis. 2009;151:568–576. [Google Scholar]
- Pyle P. Molts and plumages of ducks (anatinae) Waterbirds. 2005;28:208–219. [Google Scholar]
- R Development Core Team; Computing, R.F.f.S, editor. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2004. [Google Scholar]
- Robinson WD, Bowlin MS, Bisson IA, Shamoun-Baranes J, Thorup K, Diehl RH, Kunz TH, Mabey SE, Winkler DW. Integrating concepts and technologies to advance the study of bird migration. Front Ecol Environ. 2010;8:354–361. [Google Scholar]
- Sellick MJ, Kyser TK, Wunder MB, Chipley D, Norris DR. Geographic Variation of Strontium and Hydrogen Isotopes in Avian Tissue: Implications for Tracking Migration and Dispersal. PLoS One. 2009;4 doi: 10.1371/journal.pone.0004735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortridge KF, Zhou NN, Guan Y, Gao P, Ito T, Kawaoka Y, Kodihalli S, Krauss S, Markwell D, Murti KG, Norwood M, Senne D, Sims L, Takada A, Webster RG. Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virology. 1998;252:331–342. doi: 10.1006/viro.1998.9488. [DOI] [PubMed] [Google Scholar]
- Smith TB, Marra PP, Webster MS, Lovette I, Gibbs HL, Holmes RT, Hobson KA, Rohwer S. A call for feather sampling. Auk. 2003;120:218–221. [Google Scholar]
- Songserm T, Jam-on R, Sae-Heng N, Meemak N, Hulse-Post DJ, Sturm-Ramirez KM, Webster RG. Domestic ducks and H5N1 influenza epidemic, Thailand. Emerging Infect Dis. 2006;12:575–581. doi: 10.3201/eid1204.051614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallknecht DE, Shane SM. Host range of avian influenza-virus in free-living birds. Vet Res Commun. 1988;12:125–141. doi: 10.1007/BF00362792. [DOI] [PubMed] [Google Scholar]
- Takekawa JY, Del La Cruz SW, Wilson MT, Palm EC, Yee J, Nysewander DR, Evenson JR, Eadie JM, Esler D, Boyd WS, HWD . Breeding distribution and ecology of Pacific coast Surf Scoters. In: Wells JV, editor. Boreal birds of North America. Studies in Avian Biology. 41. University of California Press; Berkeley, CA: 2011. pp. 41–64. [Google Scholar]
- Takekawa JY, Newman SH, Xiao X, Prosser DJ, Spragens KA, Palm EC, Yan B, Li T, Lei F, Zhao D, Douglas DC, Muzaffar SB, Ji W. Migration of Waterfowl in the East Asian Flyway and Spatial Relationship to HPAI H5N1 Outbreaks. Avian Dis. 2010a;54:466–476. doi: 10.1637/8914-043009-Reg.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takekawa JY, Prosser DJ, Newman SH, Bin Muzaffar S, Hill NJ, Yan BP, Xiao XM, Lei FM, Li TX, Schwarzbach SE, Howell JA. Victims and vectors: highly pathogenic avian influenza H5N1 and the ecology of wild birds. Avian Biol Res. 2010b;3:51–73. [Google Scholar]
- van Gils JA, Munster VJ, Radersma R, Liefhebber D, Fouchier RAM, Klaassen M. Hampered foraging and migratory performance in swans infected with low-pathogenic avian influenza A virus. Plos One. 2007;2 doi: 10.1371/journal.pone.0000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Di B, Zhou DH, Zheng BJ, Jing HQ, Lin YP, Liu YF, Wu XW, Qin PZ, Wang YL, Jian LY, Li XZ, Xu JX, Lu EJ, Li TG, Xu JG. Food markets with live birds as source of avian influenza. Emerging Infect Dis. 2006;12:1773–1775. doi: 10.3201/eid1211.060675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenaar LI, Hobson KA. Comparative equilibration and online technique for determination of non-exchangeable hydrogen of keratins for use in animal migration studies. Isotopes Environ Health Stud. 2003;39:211–217. doi: 10.1080/1025601031000096781. [DOI] [PubMed] [Google Scholar]
- Weber TP, Stilianakis NI. Ecologic immunology of avian influenza (H5N1) in migratory birds. Emerging Infect Dis. 2007;13:1139–1143. doi: 10.3201/eid1308.070319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MS, Marra PP, Haig SM, Bensch S, Holmes RT. Links between worlds: unraveling migratory connectivity. Trends Ecol Evol. 2002;17:76–83. [Google Scholar]
- Whitworth D, Newman S, Mundkur T, Harris P. Wild Birds and Avian Influenza: An Introduction to Applied Field Research and Disease Sampling Techniques. Food and Agriculture Organization of the United Nations; Rome, Italy: 2007. [Google Scholar]
- Wunder MB. Using isoscapes to model probability surfaces for determining geographical origins. In: West JB, Bowen GJ, Dawson TE, Tu KP, editors. Isoscapes: Understanding Movements, Pattern, and Process on Earth through Isotope Mapping. Springer; New York: 2010. pp. 251–272. [Google Scholar]
- Wunder MB, Kester CL, Knopf FL, Rye RO. A test of geographic assignment using isotope tracers in feathers of known origin. Oecologia. 2005;144:607–617. doi: 10.1007/s00442-005-0071-y. [DOI] [PubMed] [Google Scholar]
- Wunder MB, Norris DR. Analysis and design for isotope-based studies of migratory animals, Tracking Animal Migration with Stable Isotopes. Elsevier; New York: 2008a. pp. 107–128. [Google Scholar]
- Wunder MB, Norris DR. Improved estimates of certainty in stable-isotope-based methods for tracking migratory animals. Ecol Appl. 2008b;18:549–559. doi: 10.1890/07-0058.1. [DOI] [PubMed] [Google Scholar]
- Yohannes E, Hansson B, Lee RW, Waldenstrom J, Westerdahl H, Akesson M, Hasselquist D, Bensch S. Isotope signatures in winter moulted feathers predict malaria prevalence in a breeding avian host. Oecologia. 2008;158:299–306. doi: 10.1007/s00442-008-1138-3. [DOI] [PubMed] [Google Scholar]
- Yu ZJ, Song YF, Zhou HB, Xu XJ, Hu QY, Wu HY, Zhang AD, Zhou YJ, Chen JF, Dan HB, Luo QP, Li XM, Chen HC, Jin ML. Avian influenza (H5N1) virus in waterfowl and chickens, central China. Emerging Infect Dis. 2007;13:772–775. doi: 10.3201/eid1305.061209. [DOI] [PMC free article] [PubMed] [Google Scholar]