Abstract
This study assessed the potential antinociceptive effects of liquiritigenin, a plant-derived compound with transient receptor potential melastatin 3 blocking activity in a rat model of persistent neuropathic pain. Chronic constriction injury (CCI) to the sciatic nerve was induced in male Sprague-Dawley rats to model human peripheral neuropathic pain. Liquiritigenin (1, 3, or 9 mg/kg) was administered intraperitoneally to examine the effects on mechanical, thermal, and cold hyperalgesia using the von Frey test, plantar test, and cold plate test, respectively. A rotarod test was also conducted to examine motor function. Liquiritigenin dose dependently alleviated mechanical, thermal and cold hyperalgesia. In addition, daily repeated treatment with liquiritigenin did not demonstrate significant antinociceptive tolerance in the measures of hyperalgesia. Within the doses studied, liquiritigenin did not significantly affect motor performance. These results suggest that liquiritigenin may be potentially useful novel treatments for neuropathic pain.
In the general population, the prevalence rate of chronic pain in France is 31.7% and that of chronic pain with neuropathic characteristics is 6.9%1, which clearly shows that chronic pain, particularly with neuropathic characteristics, is a significant health challenge. Unlike other painful conditions such as inflammatory pain, neuropathic pain is a pathological disorder of the somatosensory system2 with peripheral nervous system and central nervous system (CNS) origins3. Neuropathic pain can be caused by peripheral or central lesions but does not have to have apparent nerve tissue damage, and the nature and severity of neuropathic pain is not always proportional to the injury4. Decades of research has suggested that neuropathic pain often involves ion channel disorders. For example, drugs that block sodium channels such as carbamazepine and lamotrigine are considered efficacious pharmacotherapeutic treatment for intractable neuropathic pain conditions such as diabetic neuropathy and trigeminal neuralgia5,6. In preclinical studies, animal models are often used to mimic human neuropathic pain conditions, many of which involve surgical lesion of peripheral nerves of the animals. For example, the chronic constriction injury (CCI) model is a widely used and well-characterized animal model of peripheral mononeuropathic pain. In this model, the sciatic nerve of rats is ligated with four 4-0 chromic gut ligatures, spaced 1 mm apart, to induce hyperalgesia, allodynia, and spontaneous pain7. Although the exact mechanisms regarding CCI-induced neuropathic pain-related behavior is unclear, it is suggested that the pain is at least partially caused by the chemical toxicity induced by the interactions between the chromic gut and sciatic and sympathetic nerves, and these changes then lead to peripheral and/or central sensitization8. Transient receptor potential melastatin 3 (TRPM 3) is a calcium-permeable nonselective cation channel that is expressed in a subset of dorsal root (DRG) and trigeminal ganglia sensory neurons. Because these neurons actively involve pain processing, drugs that block TRPM 3 may have antinociceptive activity9,10.
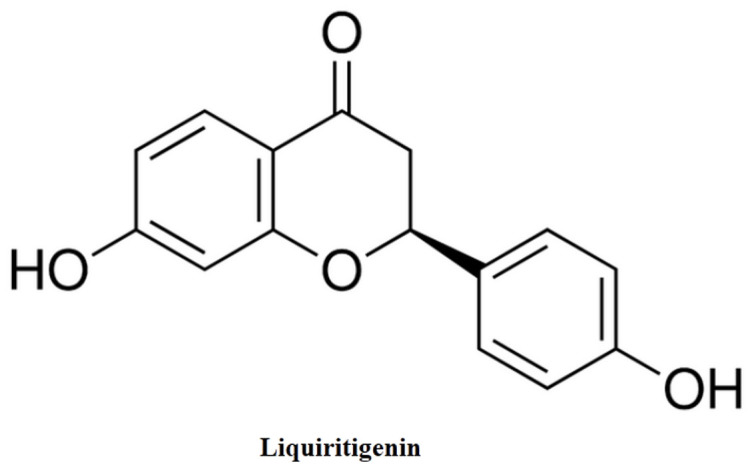
In this report, we demonstrated potent antinociceptive effects of a natural product and TRPM 3 blocker, liquiritigenin ((2S)-7-Hydroxy-2-(4-hydroxyphenyl)-2, 3-dihydro-4H-chromen-4-one, Fig. 1), in a rat model of CCI-induced neuropathic pain. We found that liquiritigenin demonstrated significant antinociceptive activities in tests of mechanical, thermal and cold hyperalgesia at doses that did not impair coordination performance.
Figure 1. Chemical structure of liquiritigenin.
Methods
Animals
In this study, male Sprague-Dawley rats weighing 250–300 g (Weitong Lihua, Beijing, China) were used. Animals were acclimated to the temperature, humidity and lighting (12 h light/dark cycle, lights on at 7:00 AM) controlled housing rooms and were singly housed for at least one week before behavioral studies were initiated. The animals had free access to regular rodent chow and water except during the behavioral test sessions. All animal experimental protocols were approved by the Institutional Animal Care and Use Committee, Suzhou Institute for Food and Drug Control. Animals were maintained in accordance with the Guide for the Care and Use of Laboratory Animals (8th edition, Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences, Washington DC). All efforts were made to minimize animal suffering and to reduce the number of animals used.
Drugs
Liquiritigenin was purchased from Sigma-Aldrich (St. Louis, MO, USA) and was suspended in 5% DMSO. All injections were given intraperitoneally in a volume of 1 ml/kg of body weight.
CCI surgery
Peripheral neuropathy was induced using the procedure described previously with minor modification7. Briefly, rats were anesthetized with pentobarbital sodium (60 mg/kg, intraperitoneal), after which the left sciatic nerve was exposed at mid-thigh level. At the proximal end to the sciatic trifurcation, four loose 4-0 silk ligatures were tied around the nerve at 1-mm intervals. To confirm the consequence of nerve injury, a sham operation was performed with exposure of the left sciatic nerve without ligation. The CCI rats were tested six days after the operation, unless otherwise stated. After the experiments, the rats were euthanized using an injection of urethane (3 g/kg, intraperitoneal).
Behavioral assessment
For the establishment of chronic pain, a constriction injury was applied to the sciatic nerve, which induced neuropathic pain characterized by hyperalgesia and allodynia. The electronic von Frey test, the plantar test, and cold plate test were performed to assess mechanical, thermal, and cold hyperalgesia, respectively. The rotarod test was used to examine potential motor dysfunction. In studies that examine the drug duration of actions, baseline measurement was immediately followed by an injection of the drug liquiritigenin, and the paw withdrawal threshold was then measured every 10 min until the drug effect dissipated to a level that the paw withdrawal threshold was not significantly different from the control level.
Von Frey filament test
To evaluate the magnitude of mechanical allodynia, a Von Frey filament method was used. For this method, filaments of varying forces (0.07–300 g) were applied to the mid-plantar surface of the right hind paw, with each application held until curved for 6 s using the up-down method11. The mechanical allodynia was assessed prior to and daily after 5 days of liquiritigenin treatment. During the tests, rats were placed in individual Plexiglas chambers on top of a wire meshed floor suspended 50 cm above the laboratory bench top and acclimated to the environment for 30 min prior to each test session.
Plantar test
To evaluate the magnitude of thermal hyperalgesia, plantar test (model 7360, Ugo Basile, Comerio, Italy) was used to measure hind paw withdrawal latency in response to radiant heat according to the method described elsewhere12. Each rat was placed into an acrylic compartment on a clear glass surface. A moveable heat source was then put under the plantar surface of the hind paw and activated with a light beam. The intensity of the light beam was adjusted such that the withdrawal latencies in sham-operated rats was around 8–10 s. The built-in digital timer automatically recorded the response latency for paw withdrawal to the nearest 0.1 s. A cutoff time of 20 s was used to prevent tissue damage when a rat failed to respond after 20 s. The mean withdrawal latencies(s) for the left hind paw were determined from the average of two trials separated by a 5-min interval to prevent thermal sensitization.
Cold plate test
Cold hyperalgesia was assessed in CCI rats using a Hot/Cold Plate (model 35100, Ugo Basile) on the 14th day after the operation. Rats were placed on a cold stainless steel plate maintained at 21°C and the latency(s) to the first lifting or shaking of the CCI hind paw was measured. A cutoff time of 180 s was imposed to prevent tissue damage.
Rotarod test
The effect on motor performance was evaluated using an accelerating rotarod (model 47700, Ugo Basile) in which normal rats were placed on a rotating drum with the speed increasing from 4 to 40 rpm over 5 min, forcing them to walk forward to avoid falling. The time(s) to falling was measured. Training sessions were carried out 1 and 2 days prior to the experiments, with three trials on each day. On the experimental day, a baseline response was obtained and the effects on motor performance of liquiritigenin or its vehicle administered were assessed 20 min post-injection.
Data analyses
Data were presented as paw withdrawal threshold (gram) or latency (s) plotted as a function of time (min or days), respectively. The final data were analyzed using two-way repeated measures analysis of variance (ANOVA) (time × treatment) followed by post hoc Bonferroni test. When two data points were compared, student's t test was used. For the rotarod test, data were analyzed with one-way ANOVA followed by post hoc Bonferroni test.
Results
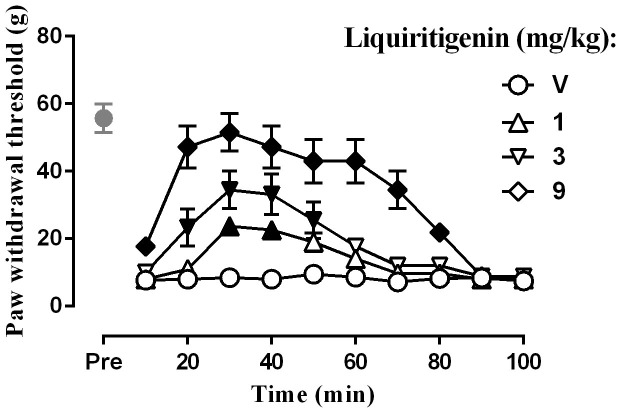
The effect on mechanical hyperalgesia was examined using the von Frey test. After surgery, the left paw withdrawal threshold in the CCI rats was 10.6 ± 1.2 g (n = 32), whereas the withdrawal threshold in the sham-operated rats was 55.8 ± 5.9 g (n = 8) (Fig. 2). Student's two-sample t-test revealed that the withdrawal threshold in the CCI rats was significantly lower than that in the sham-operated rats [t (38) = 31.78, P < 0.0001].Two-way ANOVA indicated a significant dose main effect for liquiritigenin in the CCI rats [F (7,224) = 120.39, P < 0.0001]. The Bonferroni post hoc test revealed that liquiritigenin increased the paw withdrawal threshold. Significant increases were observed at all the three doses. The effect of the highest dose (9 mg) persisted for 80 min.
Figure 2. Effect of liquiritigenin on the paw withdrawal thresholds as tested by von Frey test (n = 8 per group).
Filled symbols indicated data significantly different from vehicle-treated group.
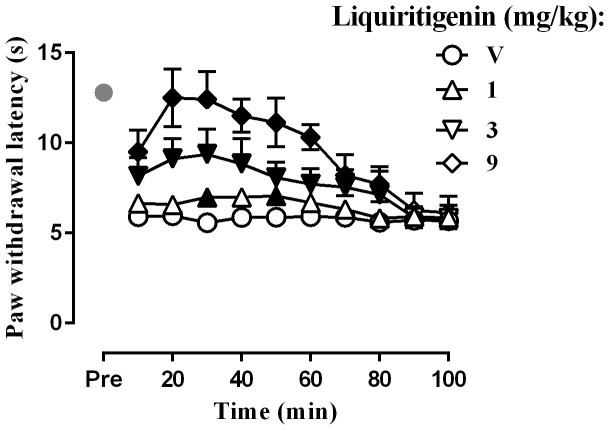
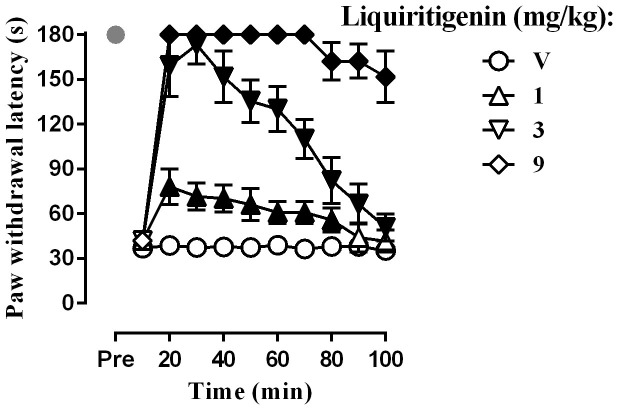
To determine whether liquiritigenin affects thermal hyperalgesia, the plantar test was performed. The baseline level of the paw withdrawal latency after CCI surgery was 5.9 ± 0.6 s (n = 32), whereas the paw withdrawal latency after sham surgery was 12.8 ± 0.9 s(n = 8) (Fig. 3). Student's two-sample t-test indicated that the withdrawal threshold in the CCI rats was significantly shorter than that in the sham-operated rats [t (38) = 28.47, P < 0.0001]. Two-way ANOVA revealed significant dose main effect for liquiritigenin in the CCI rats [(F (3,210) = 44.33, P < 0.0001)]. Liquiritigenin completely reversed the thermal hyperalgesia at 9 mg/kg. Similarly, CCI produced cold hyperalgesia, as observed in the cold plate test. None of the sham-operated rats displayed lifting or shaking of the CCI hind paw over180 s (n = 10), whereas the baseline level of withdrawal latency of the CCI hind paw was 37.0 ± 6.5 s (n = 32) (Fig. 4). Student's two-sample t-test indicated that withdrawal latency of the CCI rats was significantly shortened than that of the sham-operated rats (t (38) = 23.93, P < 0.0001). Two-way ANOVA indicated a significant dose main effect for liquiritigenin in the CCI rats [F (3,210) = 2404, P < 0.0001].The Bonferroni post hoc test revealed that CGA increased the withdrawal latencies in the cold plate test. Significant increases were observed at all doses and the effect of the highest dose of 9 mg/kg lasted for at least 100 min.
Figure 3. Effect of liquiritigenin on the paw withdrawal latency as tested by plantar test (n = 8 per group).
Filled symbols indicated data significantly different from vehicle-treated group.
Figure 4. Effect of liquiritigenin on the paw withdrawal latency as tested by cold plate test (n = 8 per group).
Filled symbols indicated data significantly different from vehicle-treated group.
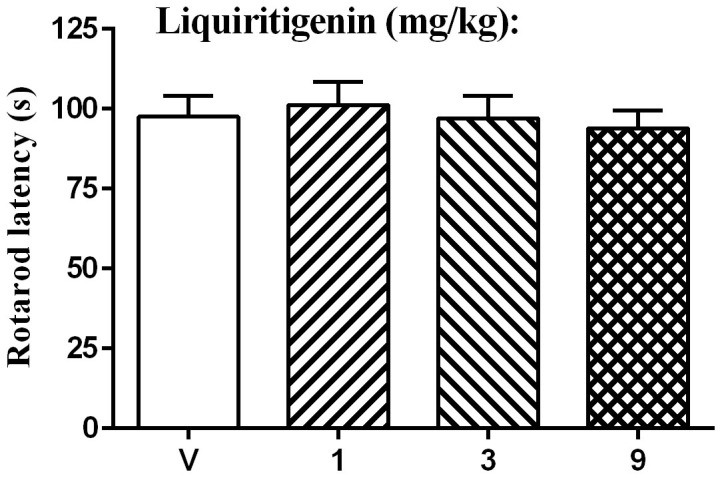
The effect on motor activity in the CCI rats was determined using the rotarod test. Baseline latency was 95.5 ± 7.8 s. One-way ANOVA indicated no significant dose effect [F (3, 28) = 0.21, P > 0.05]. The Bonferroni post hoc test revealed that liquiritigenin produced no significant change in the rotarod latency at all doses (Fig. 5).
Figure 5. Effect of liquiritigenin on the rotarod performance in rats (n = 8 per group).
Discussion
In this study, we reported that a plant-derived compound and TRPM 3 blocker, liquiritigenin, produced robust anti-hyperalgesic effect in a rat model of peripheral neuropathic pain. Importantly, the effect was behaviorally specific and did not seem to be due to general behavioral suppression. These results increased the current knowledge of the involvement of TRPM 3 in the pain processing and modulation and call for increasing effort to better understand liquiritigenin, which may well serve as a potential novel analgesic for the treatment of chronic neuropathic pain and other chronic pain conditions.
Due to the low potency and to the known off-target effects, previously described TRPM3 blockers such as the peroxisome proliferator-activated receptor-γ-agonistic antidiabetic drug rosiglitazone13 or the nonsteroidal anti-inflammatory drug mefenamic acid14 have not been used extensively to study TRPM3 functions in vivo. Recently, it was found that liquiritigenin is a selective TRPM 3 inhibitor10. Liquiritigenin strongly and reversibly inhibited both recombinant TRPM3 and TRPM3-related [Ca2+]i signals and ionic currents in DRG neurons while other sensory TRP channels or TRPM1, the closest relative of TRPM3, remains largely unaffected at concentrations that fully blocks TRPM310. In this study, we found that liquiritigenin was extremely efficacious in attenuating the thermal, mechanical and cold hyperalgesia in rats with CCI-induced neuropathic pain. This study greatly extended a previous report that TRPM 3 blockers are effective modulators of pain hypersensitivity10.
Until now little is known of the exact physiological and pathophysiological roles of TRPM3. The reported existence of different splice variants of TRPM3 that block the Ca2+ permeability of the pore (e.g., the α1 splicing event)15 or even eliminate a functional pore16 make the understanding of TRPM3 more difficult. Studies employing genetically modified animals begin to shed light on the role of TRPM3 in pain modulation. For instance, in TRPM3-deficient transgenic mice, animals demonstrated a behavioral phenotypic alteration of thermal pain sensation9, which is reminiscent of the effects of pharmacologic blockade of the channel10. Because both TRPV1 and TRPM3 are expressed in DRG neurons, it seems unusually complicated that both heat-sensing sensory channels contribute to thermal pain sensation and even seems somewhat redundant. Pharmacologically, inhibition of either TRPV1 or TRPM3 produces similar antinociceptive effects in animals. Although up till now a cross-talk between both channels has not been established, such a possibility is tantalizing. The discovery and development of TRPV1 blockers as analgesics has been hampered by the severe side effect of hyperthermia related to TRPV 1 blockade and enthusiasm of targeting TRPV1 for the management of pain has been dampened. In contrast to TRPV1 blockers, current data suggest that systemic administration of TRPM 3 blockers do not induce significant alteration in the body temperature10, suggesting the promising possibility that TRPM3 may be a superior analgesic drug target without the adverse effects of TRPV1 blockers. More in vivo pharmacology studies are needed on liquiritigenin to understand its safety profile.
In summary, this study for the first time reported a systematic study on the antinociceptive effects of a selective TRPM 3 blocker liquiritigenin. Overall, liquiritigenin demonstrated an impressive antinociceptive activity in a well-validated rat model of peripheral neuropathic pain. Strikingly, liquiritigenin was not only efficacious for dampening temperature nociception, it was also effective against mechanical hyperalgesia, which reveals a broad spectrum activity of antinociception for neuropathic pain. Although these findings are preliminary and more studies are certainly needed to examine the generality of these findings, the current data do suggest that liquiritigenin could be a potential novel analgesic for pain management. Future studies should further examine the antinociceptive effects, the safety pharmacology of liquiritigenin and explore the potential of developing liquiritigenin as a novel analgesic with novel mechanisms of action.
Author Contributions
L.C. designed the research; L.C., W.C., X.Q., Y.F. and N.Z. conducted the studies; L.C. analyzed the data and prepared the manuscript; all authors read and approved the manuscript.
Acknowledgments
We thank Dr. Feihong Chen and Dr. Qinsheng Dai for helpful discussions and comments on this manuscript.
References
- Bouhassira D., Lanteri-Minet M., Attal N., Laurent B. & Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 136, 380–387 (2008). [DOI] [PubMed] [Google Scholar]
- Loeser J. D. & Treede R. D. The Kyoto protocol of IASP Basic Pain Terminology. Pain 137, 473–477 (2008). [DOI] [PubMed] [Google Scholar]
- Treede R. D. et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology 70, 1630–1635 (2008). [DOI] [PubMed] [Google Scholar]
- Attal N. et al. Neuropathic pain: are there distinct subtypes depending on the aetiology or anatomical lesion? Pain 138, 343–353 (2008). [DOI] [PubMed] [Google Scholar]
- Attal N. et al. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol 13, 1153–1169 (2006). [DOI] [PubMed] [Google Scholar]
- Attal N. et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol 17, 1113–e1188 (2010). [DOI] [PubMed] [Google Scholar]
- Bennett G. J. & Xie Y. K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33, 87–107 (1988). [DOI] [PubMed] [Google Scholar]
- Maves T. J., Pechman P. S., Gebhart G. F. & Meller S. T. Possible chemical contribution from chromic gut sutures produces disorders of pain sensation like those seen in man. Pain 54, 57–69 (1993). [DOI] [PubMed] [Google Scholar]
- Vriens J. et al. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 70, 482–494 (2011). [DOI] [PubMed] [Google Scholar]
- Straub I. et al. Flavanones that selectively inhibit TRPM3 attenuate thermal nociception in vivo. Mol Pharmacol 84, 736–750 (2013). [DOI] [PubMed] [Google Scholar]
- Dixon W. J. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 20, 441–462 (1980). [DOI] [PubMed] [Google Scholar]
- Hargreaves K., Dubner R., Brown F., Flores C. & Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32, 77–88 (1988). [DOI] [PubMed] [Google Scholar]
- Majeed Y. et al. Rapid and contrasting effects of rosiglitazone on transient receptor potential TRPM3 and TRPC5 channels. Mol Pharmacol 79, 1023–1030 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose C. et al. Fenamates as TRP channel blockers: mefenamic acid selectively blocks TRPM3. Br J Pharmacol 162, 1757–1769 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberwinkler J., Lis A., Giehl K. M., Flockerzi V. & Philipp S. E. Alternative splicing switches the divalent cation selectivity of TRPM3 channels. J Biol Chem 280, 22540–22548 (2005). [DOI] [PubMed] [Google Scholar]
- Fruhwald J. et al. Alternative splicing of a protein domain indispensable for function of transient receptor potential melastatin 3 (TRPM3) ion channels. J Biol Chem 287, 36663–36672 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]