Abstract
Belliveau JW, Kwong KK, Kennedy DN, Baker JR, Stern CE, Benson R, Chesler DA, Weisskoff RM, Cohen MS, Tootell RBH, Fox PT, Brady TJ, Rosen BR. Magnetic resonance imaging mapping of brain function: human visual cortex. Invest Radiol 1992;27:SS9–S65.
Magnetic resonance imaging (MRI) studies of human brain activity are described. Task-induced changes in brain cognitive state were measured using high-speed MRI techniques sensitive to changes in cerebral blood volume (CBV), blood flow (CBF), and blood oxygenation. These techniques were used to generate the first functional MRI maps of human task activation, by using a visual stimulus paradigm. The methodology of MRI brain mapping and results from the investigation of the functional organization and frequency response of human primary visual cortex (Vl) are presented.
Keywords: brain function, cerebral blood flow, magnetic resonance imaging.
Assessment of individual pathophysiology and characterization of distinctly human functions such as language and memory require noninvasive functional measurement of the human brain. This has been accomplished through the thesis that functional activity in the brain is directly related to local alterations in cerebral metabolism and blood supply, as first presented by Roy and Sherrington in 1890. Today , physiologic, anatomic, and cognitive psychophysical studies indicate that the brain possesses anatomically distinct processing regions. 1-4 A complete understanding of brain function requires determination of the location of these sites, the operations performed, and the organization of distributed processing. 5 During cognitive task performance, local alterations in neuronal activity induce local changes in cerebral metabolism and cerebral per-fusion (blood flow , blood volume, and blood oxygenation).6-13 These physiologic changes can be used to map the functional loci of component mental operations.2,14,15
These functional maps have relied on radionuclide techniques (primarily positron emission tomography [PET]) that typically suffer from limited spatial and temporal resolution. In comparison, nuclear magnetic resonance (NMR) imaging (or magnetic resonance imaging [MRI]) is a high-resolution in vivo method for studying human cerebral anatomy. Recent advances have yielded high-speed functional MRI techniques that are sensitive to changes in cerebral blood volume (CBV) , blood flow (CBF) , and blood oxygenation.12,13
Two tomographic MRI approaches for measuring brain activity have been developed. Over the last 6 years, our laboratory has investigated the use of paramagnetic contrast agents to measure changes in CBV. 12,16-20 More recently, we have developed MRI techniques that use no exogenous contrast agents at all, relying instead on intrinsic changes in blood contrast due to changes in CBF and blood oxygenation. 13 The latter is completely noninvasive and provides temporal resolution on the order of the hemodynamic response time. Data summarizing the basis of the MRI techniques and their application to task activation mapping in humans are presented. Cerebral blood volume mapping with MRI contrast agents is described first, followed by our most recent noncontrast studies of CBF and blood oxygenation.
Magnetic resonance imaging CBY measurement relies on high-speed imaging to resolve the cerebral transit (seconds) of a paramagnetic contrast agent bolus , using a mechanism of image contrast based on microscopic changes in tissue magnetic susceptibility. 17, 18, 20, 21 Cerebral blood volume maps with a voxel size of 1.5 × 1.5 × 7 mm are routinely acquired. This technique was used to obtain the first MRI maps of human brain activation. 12 Compared with PET CBY maps, which are heavily weighted by the venous system, we note that spin-echo MRI CBV maps reflect the microvascular blood volume. 21-23
One of the most exciting advances in NMR imaging is the development of completely noninvasive techniques, intrinsically sensitive to changes in cerebral blood flow and the oxygenation state of hemoglobin13 (deoxyhemoglobin is paramagnetic; oxyhemoglobin is diamagnetic24). Conventional MRI examinations provide high spatial resolution anatomic images primarily based on contrast derived from the tissue relaxation parameters T1 and T2. Several investigators have demonstrated in animals that brain tissue relaxation is influenced by the oxygenation state of hemoglobin (T2* effect, modulated by the local blood volume)24-31 and intrinsic tissue perfusion (T1 effect). 32-34 Fluctuations in blood oxygenation and tissue perfusion occur during mental activity.8,10 Thus, MRI techniques sensitive to these relaxation phenomena can be used to generate noninvasive tomographic images of human brain activity. 13
Since our original demonstration, at the 1991 Society of Magnetic Resonance in Medicine annual meeting, of completely noninvasive magnetic resonance imaging of human brain activity (based on either blood flow or blood oxygenation change), 13,35 at least eight other laboratories have obtained similar results, including the National Institutes of Health,36,37 Medical College of Wisconsin,38 University of Minnesota, and AT&T Bell Laboratories, 39 Los Alamos National Laboratory ,40 Royal College of Surgeons, and Hammersmith Hospital (England),41 Max-Planck-Institut Biophysikalische Chemie (Germany),42 Carnegie-Mellon University,43 and Yale University.44 Measurement of blood oxygenation change has been accomplished using standard gradient-echo (T2*-weighted) imaging sequences at both 1.5 and 4 Tesla. The basic methodology of MRI brain mapping is described using examples from the human visual system. The visual system was explored first because of its robust activation and our prior knowledge of its functional organization derived from PET and nonhuman primate studies.
Materials and Methods
In our laboratory, normal subjects underwent dynamic NMR imaging using both a conventional and a prototype high-speed echo planar imaging device (1.5 Tesla GE Signa, modified by Advanced NMR Systems, Inc., Wilmington, MA).45 Approval for these studies was obtained from the MGH Subcommittee on Human Studies. Subject motion was minimized by using a head holder. Either a conventional head coil or a radio frequency receive-only surface coil over the occipital pole was used. A parasagittal slice from a three-dimensional T !-weighted data set (1 × 1 × 1.5 mm) was used to identify the plane of the calcarine fissures bilaterally.12 Tl-weighted anatomic images were used to correlate functional images to the underlying neural substrate, allowing for precise determination of gray–white matter boundaries and activated–nonactivated borders. Functional and anatomic data sets were translated into proportionately measured stereotactic coordinates relating to the line between the anterior and posterior commissures (AC–PC line or bicommissuralline).46 This translation allows direct correlation to reported standardized PET maps of the visual cortex.
Visual stimulation was provided by either light-proof stimulating goggles (Grass S10VS) placed over the subject's eyes, or with an LCD projection television (Sharp Electronics). The photic stimulus rate was varied to investigate the frequency response of visual cortex47 Full-field and hemifield stimulation (black and white checkerboard, counterphased at 8 Hz) was used to investigate the retinotopic organization of primary visual cortex.48 The general mapping of retinal projections to striate cortex is well known. Central retina projects caudally, in the vicinity of the occipital pole, whereas peripheral retina projects rostrally. Lower visual field projects above the calcarine fissure and superior visual field projects beneath it. Left visual field goes to the right hemisphere and vice versa.49
Dynamic susceptibility-contrast NMR imaging of an intravenously administered paramagnetic contrast agent (0.5 mol/L, Gd-DTPA2–, ie, Magnevist) was used to produce regional CBV maps of the human brain during resting and activated states.12 Two doses of 0.1 mmol/kg Gd-DTPA2– (1 during darkness and 1 during visual stimulation) were administered by antecubital vein bolus injection (4 seconds, using a Medrad power injector). Cerebral blood volume maps were derived from a series of images oriented in the plane of the calcarine fi ssures, collected at 750-msecond intervals using an echo planar lipid-suppressed spin echo pulse sequence (TE = 100 mseconds, TR = 750 mseconds, 32–64 msecond image acquisition window) before, during, and after contrast agent injection. Changes in brain signal intensity occurring during cerebral transit of the high magnetic susceptibility Gd-DTPA2– were converted into contrast agent concentration–time curves. The area under the concentration–time curve, corrected for recirculation by gamma-variate fitting, is proportional to the local CBV.17 These calculations were performed on a voxel-by-voxel basis to generate images of relative CBV . The resulting resting and activated functional CBY images were subtracted to show areas involved in cognitive processing of the task.
Changes in blood oxygenation were detected by using a gradient-echo imaging sequence sensitive to the paramagnetic state of deoxygenated hemoglobin.13 Long echo times of 30 to 50 mseconds provide sensitivity to variations in T2*. Blood flow changes were evaluated by a spin-echo inversion recovery, T1-sensitive pulse sequence (TI = 1100 mseconds, TR = 3500 mseconds, TE = 42 mseconds). 13 We have used both conventional and echo planar gradient-echo imaging sequences. The latter is capable of acquiring complete two-dimensional images in less than 100 mseconds, permitting real-time monitoring of brain activity. A slice thickness of 4 to 10 mm was used, with in-plane resolution of either 1.5 × 3 or 3 × 3 mm. A series of images was acquired continuously in time, like a movie. To visualize subtle signal changes, we employed a sequential task activation paradigm, alternating between resting and stimulated states. Activated cortical areas are shown by subtraction of averaged baseline (resting) images from all subsequent images. Cine display of subtraction images (activated minus baseline) directly demonstrates activity-induced changes in brain MRI signal.
Results
During visual stimulation in all of our subjects, we detected a significant increase in CBV, CBF, and blood oxygenation occurring within the anatomically defined primary visual cortex (Vl). The greatest changes were observed in the medial-posterior regions of the occipital lobes along the calcarine fissures. We also observed areas outside of area 17 that showed a significant activation in several subjects, however. Some of these loci are extrastriate visual areas.
Patterned-flash photic stimulation elicited a significant increase in regional blood volume within VI (paired Student's t test, P<.001), with an increase(± standard deviation) in CBV of 32 ± 10%. 12 In addition to direct intra-subject correlation of active areas to their underlying anatomy (Fig. 1), stereotactic results also demonstrate that NMR-determined regions of activity fall within PET-determined center-of-mass coordinates of the primary visual system. Because of increased spatial resolution, estimates of the extent (area) of activated cortex could be made. We observed a good correspondence between the extents of activation and the extent of underlying anatomy (ie, calcarine fissures).12 Moreover, demarcation of the activated region corresponds well to the anatomically determined gray/white borders. This result is in agreement with previous autoradiographic results in animals, which show sharp boundaries between functionally distinct regions.
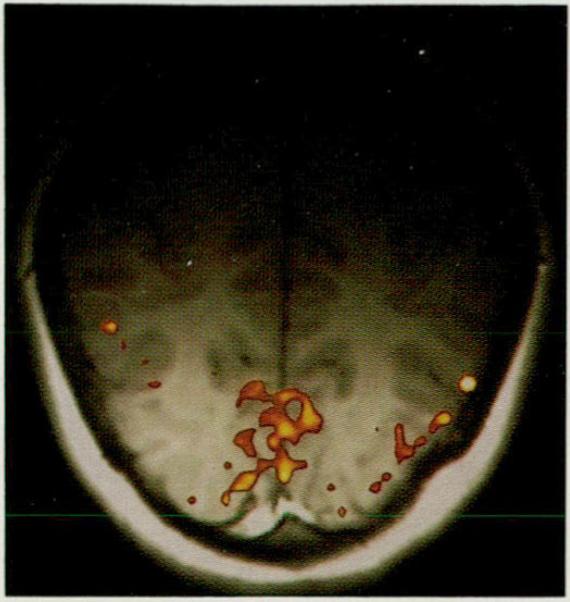
Fig. 1.
Magnetic resonance functional/anatomic map of the brain during 8-Hz patterned-flash visual stimulation . Image intensity (color) is proportional to CBV, superimposed onto an anatomic (T1 -weighted) image . Image is aligned along the calcarine fissure with the occipital pole at the bottom. During photic stimulation lo cal increases in blood volume are detected in the medial-posterior regions of the occipital lobes along the banks of the calcarine fissures. A linear color scale was used , with red equivalent to greatest activity. A marked area (approximately 600 mm2) of increased blood volume (approximately 24%) is localized in the anatomically defined primary visual cortex. This CBV image was acquired using a 3 × 3 × 10-mm voxel.
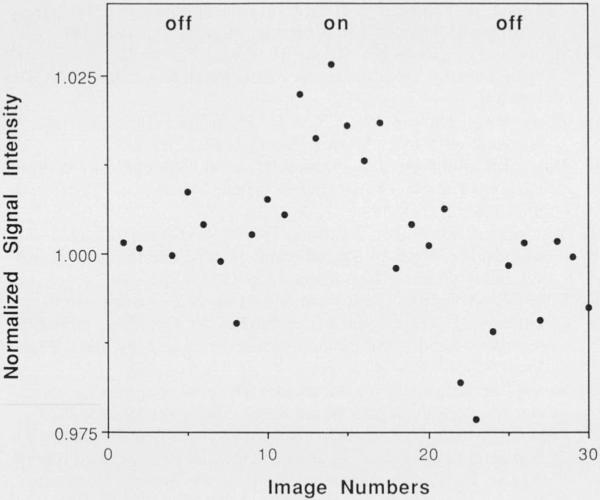
Dynamic changes in cortical activity have been followed completely noninvasively, using sequences sensitive to changes in either blood oxygenation or blood flow. Figure 2 shows signal intensity changes as a function of time for a region of interest (ROI) within V1 using an echo planar blood oxygenation sensitive (T2*) imaging technique. The onset of visual cortex activation is observed within 1 second subsequent to visual stimulation. Imaging time resolution of 250 mseconds between frames has been achieved. During 8-Hz patterned-flash visual stimulation, a significant increase in signal intensity (paired Student's t test, P <. 001) of 1.8 ± 0.8% (gradient-echo sequence) and 1.8 ± 0.9% (inversion recovery sequence) was observed in the primary visual cortex (V1) of seven normal volunteers. 13
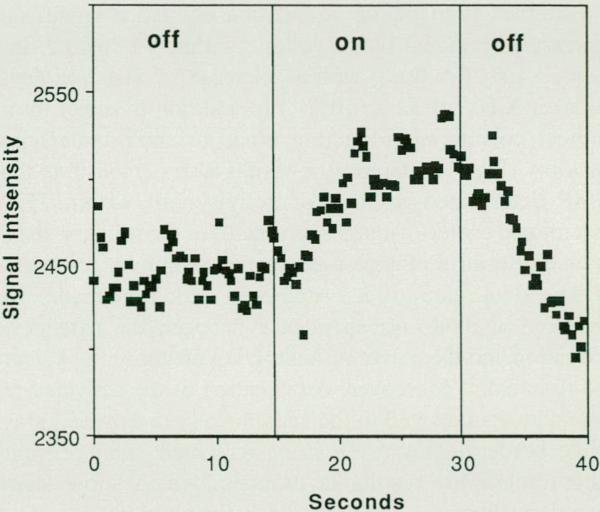
Fig. 2.
Real-time magnetic resonance imaging mapping of V1 activation during visual stimulation . Signal intensity changes for a region of interest (approximately 50 mm2) within the visual cortex during darkness and during 8-Hz patterned-flash visual stimulation. An echo planar gradient echo technique (TEITR, 50/250 mseconds; rf angle = 40) was used , sensitive to changes in the oxygenation state of hemoglobin. Hemodynamic activity was detected with a temporal resolution of 250 mseconds. The mean rise-time constant of the signal change was approximately 4 seconds.
Full-field and hemifield stimulation was used to investigate the retinotopic organization of primary visual cortex. Figure 3 shows a hemifield experiment used to produce activation in either the left or right V1. Single-slice activation maps (based on changes in blood oxygenation) are shown superimposed on a T1-weighted anatomic image, from an experiment where a half-circle checkerboard stimulus was alternated between the left and right visual fields every 20 seconds (Fig. 3A). The time course of activity for an ROI within the left hemisphere V1 and another ROI within the right hemisphere V1 is shown in Figure 3B. Several complete cycles of activation were acquired within a single experiment. In this subject there was a 2% to 3% change in signal intensity with a 1% variation for the left and right ROis within Vl during a single, unaveraged sampling interval. The observed significance for activation in the selected regions within V1 is P = .03 and the corresponding power is greater than .9 from an analysis of the activation map computed from an average of two time samples. With this fairly robust activation task, only two intra-subject averages (4 seconds of data) were required to obtain significance. Figure 3A shows asymmetric contralateral activation within the primary visual cortex (V1). The observed activation asymmetry is due to the calcarine fissures not being symmetrically sampled using a single slice. Parasagittal views clearly show large bilateral differences in the length and orientation of the calcarine fissures in this subject. Therefore, three-dimensional time-resolved imaging is required for proper and complete assessment of cerebral activity, and for detection and characterization of distributed parallel processing.
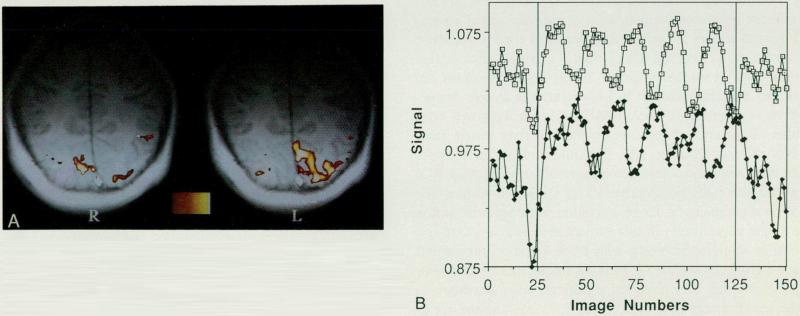
Figs. 3A and 3B.
(A) Alternating hemifield activation. The stimulus was a black and white counterphased (8 Hz) semiannular checkerboard. Left and right visual fields were stimulated, alternating every 20 seconds. Dynamic changes in cortical activation (left and right V1) exactly follow the reversal frequency of the visual field. Images were acquired using an echo planar gradient echo (TR = 2 seconds, TE = 50 mseconds) blood oxygenation-sensitive sequence. Hemifield activation maps are superimposed on the corresponding T1-weighted anatomic image. The response to stimulation of the left hemifield is shown on the left and the response to stimulation of the right hemifield is shown on the right. Diminished cortical response in the right hemisphere is due to anatomic asymmetry. (B) Dynamic hemifield experiment time course. Echo planar gradient echo (TR = 2 seconds, TE = 50 mseconds) signal intensity from a region of interest in either the left (open squares with dots) or right (closed diamonds) V1. V1 cortical activation turns on and off, following closely the switching of the stimulus between the left and right hemifields every 20 seconds.
Changes in cortical activation depend on the frequency of stimulation. The frequency response of striate cortex was measured using MRI CBF and blood oxygenation images (Fig. 4). 13 Superimposed onto our MRI data is a reproduction of the original Fox and Raichle PET result, showing PET CBF response as a function of visual stimulation frequency peaking at 8 Hz. 7,47 Excellent correspondence is observed.
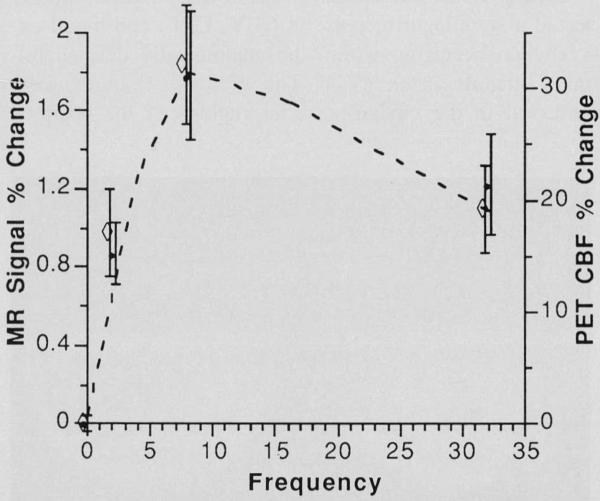
Fig. 4.
Magnetic resonance imaging gradient echo ( ◇ ) and inversion recovery (•) signal response in striate cortex as a function of the frequency (Hz) of light stimulus (0 Hz = darkness). Signal response is expressed as percent change from the baseline unstimulated level. The magnitude of the change depends on the exact choice of pulse sequence parameters, static fie ld strength, etc. The largest observed response occurred at 8 Hz for both techniques. Error bars indicate ± 1 standard error of the mean (n = 5 for 2 and 32 Hz, n = 7 for 8 Hz). For comparison, CBF % change data(--) were obtained using positron emission tomography (PET) and the identical stimulation paradigm (adapted from Fox PT, Raichle ME. Ann Neuro/1985;17:303) at 0, 1, 4, 8, 16, and 32 Hz are superimposed on the magnetic resonance imaging data.
Although echo planar techniques may be needed for ultimate temporal resolution, conventional imaging at 1.5 T appears adequate to detect blood oxygenation changes occurring during task activation. We and our colleagues have observed oxygenation changes using conventional gradient-echo imaging sequences on several standard clinical instruments.40-43 Figure 5 shows signal intensity changes as a function of time for an ROI within VI using a conventional gradient-echo imaging sequence on a standard GE 1.5-T Signa, during photic stimulation.
Fig. 5.
Brain activity changes observed using a conventional 1.5-T magnetic resonance imaging machine. Signal intensity changes for a region of interest (approximately 50 mm2) within the visual cortex during darkness and during 8-Hz patterned-flash visual stimulation. A conventional (spoiled grass) gradient echo technique (TE/TR, 30/50 mseconds; rf angle = 30) was used, sensitive to changes in the oxygenation state of hemoglobin.
Discussion
Activated brain areas determined by MRI agree well with previous PET results. However, MRI permits direct correlation of functional and anatomic data within a single imaging modality. Functional images exhibit the appropriate sensitivity to stimulation frequency. Hemifield experiments at different left- right reversal rates show that the cortical response tracks the stimulus presentation. Such studies can show the latency of the hemodynamic response time, which sets the ultimate limit on temporal resolution for this technique.
Our noncontrast MRI data demonstrate that the hemodynamic alterations that accompany neuronal activation lead to subtle, but readily detectable changes on T 1- and T2*-weighted MR images. Flow-sensitive images show increased perfusion with activation, whereas our susceptibility-sensitive images show changes consistent with an increase in venous blood oxygenation. This finding confirms previous PET results regarding focal changes in CBF, CBV, and nonoxidative glucose consumption. 9,10 Although the precise biophysical mechanisms responsible for the signal changes have yet to be completely elucidated, good hypotheses exist to account for our observations. 13,50
Noncontrast MRI human brain studies are intrinsically different from all previous work. Using echo planar imaging, the temporal window offered by this technique is almost two orders of magnitude shorter than any previous PET or MRI studies. The only existing techniques with similar or better temporal resolution suitable for human use are electroencephalography or magnetoencephalography based, neither of which provide tomographic images. Our data demonstrate sensitivity to hemodynamic changes on the order of a second. Because MRI measurement of CBF and blood oxygenation changes is completely noninvasive, imaging can be repeated within the same subject, allowing for intrasubject averaging. Repeatability in the same subject is restricted with radionuclide techniques such as PET, in which human subject regulations limit multiple longitudinal or repeated studies across single subjects because of buildup of radiation exposure. Many activation paradigms require averaging to achieve statistical significance. The ability to repeat studies in a single subject offers the hope of understanding and quantifying individual variations in location and extent of activation for tasks too subtle to be studied with a single experiment. Given the inherent regional asymmetry of both anatomy and function, 12 intersubject averaging as used in PET imaging is thus fundamentally more limiting than intrasubject averaging.
Although we have been able to collect data using a 1.5-T instrument, an increase in static field strength to 4 T should improve one's ability to detect changes in blood oxygenation by severalfold. This is a consequence of the way magnetic susceptibility effects scale with static field strength. Our colleagues at the National Institutes of Health and the University of Minnesota, using a 4-T magnet, have observed the expected improvement in brain signal change. Using both echo planar36,37 and conventional gradient-echo39 sequences at 4 T, investigators have observed task-induced changes in human brain signal intensity of approximately 10% (through deoxyhemoglobin effects).
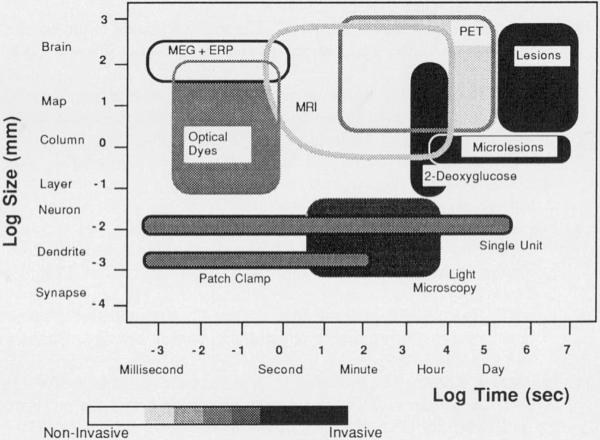
Magnetic resonance imaging now can be counted among the “functional” brain techniques (Fig. 6). Our new MRl methods can be repeatedly applied in normal subjects with complete safety, thereby expanding the spatial-temporal window of in vivo brain investigation. In conjunction with other functional measures, particularly PET and magnetoencephalography, the development of a standardized functional neuroanatomy will broaden our understanding of the unique structure–function correlates involved in cognitive processing. Three-dimensional MRI movies of the brain in health and disease soon may become routine.
Fig . 6.
Spatial resolution, temporal resolution, and invasiveness of available techniques for the study of brain function (adapted from Churchland PS, Sejnowski TJ. Science 1988;242:741). Spatial resolving power is shown on the vertical axis and temporal resolution is plotted on the horizontal axis. Invasiveness, defined loosely as the risk of significant disruption of normal function or health of the subject, is plotted by gray shading. Dynamic magnetic resonance imaging (MRI) gathers functional information related to cerebral hemodynamics and is appropriate for the study of a variety of cognitive functions. MEG : magnetoencephalography; ERP : evoked response potential (electroencephalography); PET: positron emission tomography; 2-deoxyglucose : autoradiography.
References
- 1.Zeki S, Shipp S. The functional logic of cortical connections. Nature. 1988;335:311–317. doi: 10.1038/335311a0. [DOI] [PubMed] [Google Scholar]
- 2.Zeki S, Watson JDG, Lueck CJ, Friston KJ, Kennard C, Frackowiak RSJ. A direct demonstration of functional specialization in human visual cortex. J Neurosci. 1991;11(3):641–649. doi: 10.1523/JNEUROSCI.11-03-00641.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kosslyn SM. Aspects of a cognitive neuroscience of mental imagery. Science. 1988;240:1621–1626. doi: 10.1126/science.3289115. [DOI] [PubMed] [Google Scholar]
- 4.Born RT, Tootell RBH. Segregation of global and local motion processing in primate middle temporal visual area. Nature. 1992;357:497–499. doi: 10.1038/357497a0. [DOI] [PubMed] [Google Scholar]
- 5.Churchland PS, Sejnowski TJ. Perspectives on cognitive neuroscience. Science. 1988;242:741–745. doi: 10.1126/science.3055294. [DOI] [PubMed] [Google Scholar]
- 6.Phelps ME, Kuhl DE, Mazziotta JC. Metabolic mapping of the brain’s response to visual stimulation: studies in humans. Science. 1981;211:14450–1448. doi: 10.1126/science.6970412. [DOI] [PubMed] [Google Scholar]
- 7.Fox PT, Raichle ME. Stimulus rate dependence of regional cerebral blood flow in human striate cortex demonstrated by positron emission tomography. J Neurophysiol. 1984;51:1109–1120. doi: 10.1152/jn.1984.51.5.1109. [DOI] [PubMed] [Google Scholar]
- 8.Fox PT, Mintun MA, Raichle ME, Miezin FM, Allman JM, Van Essen DC. Mapping human visual cortex with positron emission tomography. Nature. 1986;323:806–809. doi: 10.1038/323806a0. [DOI] [PubMed] [Google Scholar]
- 9.Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci U S A. 1986;83:1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- 11.Prichard J, Rothman D, Novotny E, et al. Lactate rise detected by 1HNMR in human visual cortex during physiologic stimulation. Proc Natl Acad Sci USA. 1991;88:5829–5831. doi: 10.1073/pnas.88.13.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belliveau JW, Kennedy DN, McKinstry RC, et al. Functional mapping of the human visual cortex by magnetic resonance imaging. Science. 1991;254:716–719. doi: 10.1126/science.1948051. [DOI] [PubMed] [Google Scholar]
- 13.Kwong KK, Belliveau JW, Chesler DA, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Posner MI, Petersen SE, Fox PT, Rajchle ME. Localization of cognitive operations in the human brain. Science. 1988;240:1627–1631. doi: 10.1126/science.3289116. [DOI] [PubMed] [Google Scholar]
- 15.Peterson SE, Fox PT, Snyder AZ, Raichle ME. Activation of extrastriate and frontal cortical areas by visual words and word-like stimuli. Science. 1990;249:1041–1044. doi: 10.1126/science.2396097. [DOI] [PubMed] [Google Scholar]
- 16.Belliveau JW, Villringer A, Rosen BR, et al. Magnetic susceptibility induced signal attenuation changes in rat brain caused by hypercapnia.. Proceedings of the Fifth Annual Meeting of the Society of Magnetic Resonance in Medicine; Montreal, Canada. 1986. p. 273. [Google Scholar]
- 17.Belliveau JW, Rosen BR, Kantor HL, et al. Functional cerebral imaging by susceptibility-contrast NMR. Magn Reson Med. 1990;14:538–546. doi: 10.1002/mrm.1910140311. [DOI] [PubMed] [Google Scholar]
- 18.Rosen BR, Belliveau JW, Chien D. Perfusion imaging by nuclear magnetic resonance. Magn Reson Q. 1989;5:263–281. [PubMed] [Google Scholar]
- 19.Rosen BR, Belliveau JW, Aronen HJ, et al. Susceptibility contrast imaging of cerebral blood volume: human experience. Magn Reson Med. 1991;22:293–299. doi: 10.1002/mrm.1910220227. [DOI] [PubMed] [Google Scholar]
- 20.Villringer A, Rosen BR, Belliveau JW, et al. Dynamic imaging with lanthanide chelates in normal brain: contrast due to magnetic susceptibility effects. Magn Reson Med. 1988;6:164–174. doi: 10.1002/mrm.1910060205. [DOI] [PubMed] [Google Scholar]
- 21.Fisel CR, Ackerman JL, Buxton RB, et al. MR contrast due to microscopically heterogeneous magnetic susceptibility: numerical simulations and applications to cerebral physiology. Magn Reson Med. 1991;17:336–347. doi: 10.1002/mrm.1910170206. [DOI] [PubMed] [Google Scholar]
- 22.Rosen BR, Belliveau JW, Buchbinder BR, et al. Contrast agents and cerebral hemodynamics. Magn Reson Med. 1991;19:285–292. doi: 10.1002/mrm.1910190216. [DOI] [PubMed] [Google Scholar]
- 23.Fisel CR, Moore JR, Garrido L, Ackerman JL, Rosen BR, Brady TJ. A general model for susceptibility-based MR contrast.. Proceedings of the Eighth Annual Meeting of the Society of Magnetic Resonance in Medicine; Amsterdam, The Netherlands. 1989. p. 324. [Google Scholar]
- 24.Thulborn KR, Waterton JC, Matthews PM, Radda GK. Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field. Biochim Biophys Acta. 1982;714:265–270. doi: 10.1016/0304-4165(82)90333-6. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990;14:68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- 26.Ogawa S, Lee TM. Magnetic resonance imaging of blood vessels at high fields: in vivo and in vitro measurements and image simulation. Magn Reson Med. 1990;16:9–18. doi: 10.1002/mrm.1910160103. [DOI] [PubMed] [Google Scholar]
- 27.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation . Proc Natl Acad Sci U S A. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner R, Le Bihan D, Moonen CTW, Frank J. Echo-planar imaging of deoxygenation episodes in cat brain at 2 T. J Magn Reson Imaging. 1991;1(2):227. [Google Scholar]
- 29.Turner R, Le Bihan D, Moonen CTW, Despres D, Frank J. Echo-planar time course MRI of cat brain oxygenation changes. Magn Reson Med. 1991;22:159–166. doi: 10.1002/mrm.1910220117. [DOI] [PubMed] [Google Scholar]
- 30.Hoppel BE, Weisskoff RM, Thulborn KR, Moore JB, Rosen B. Measurement of regional brain oxygenation state using echo planar linewidth mapping.. Proceedings of the Tenth Annual Meeting of the Society of Magnetic Resonance in Medicine; San Francisco, CA. 1991. p. 308. [Google Scholar]
- 31.Kwong KK, Hoppel BE, Weisskoff RM, et al. Regional cerebral tissue oxygenation studied with EPI at clinical field strengths. J Magn Reson Imaging. 1992;2(P):44. Abstract. [Google Scholar]
- 32.Detre JA, Leigh JS, Williams DS, Korestsky AP. Quantitative NMR imaging of perfusion in rat brain; Proceedings of the Ninth Annual Meeting of the Society of Magnetic Resonance in Medicine; New York, NY. 1990. p. 1289. Abstract. [Google Scholar]
- 33.Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med. 1992;23:37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W, Williams DS, Detre JA, Koretsky AP. Measurement of brain perfusion by volume-localized NMR spectroscopy using inversion of arterial water spins: accounting for transit time and cross-relaxation. Magn Reson Med. 1992;25:362–371. doi: 10.1002/mrm.1910250216. [DOI] [PubMed] [Google Scholar]
- 35.Brady TJ. Future prospects for MR imaging.. Proceedings of the Tenth Annual Meeting of the Society of Magnetic Resonance in Medicine; San Francisco, CA. 1991. p. 2. [Google Scholar]
- 36.Turner R, Jezzard P, Wen H, et al. Functional mapping of the human visual cortex at 4 Tesla and 1.5 Tesla using deoxygenation contrast EPI. Magn Reson Med. doi: 10.1002/mrm.1910290221. in press. [DOI] [PubMed] [Google Scholar]
- 37.Turner R. Magnetic resonance imaging of brain function. Am J Physiol Imaging. 1992 in press. [PubMed] [Google Scholar]
- 38.Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS. Time course EPI of human brain function during task activation. Magn Reson Med. 1992;25:390–397. doi: 10.1002/mrm.1910250220. [DOI] [PubMed] [Google Scholar]
- 39.Ogawa S, Tank OW, Menon R, et al. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping using MRI. Proc Natl Acad Sci U S A. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.George JS, Sanders J, Belliveau JW, et al. Proceedings of the Society for Neuroscience. Anaheim, CA: 1992. Functional MRI studies of human vision on a clinical imager. p. 1393. [Google Scholar]
- 41.Gadian DG, Frackowiak RSJ. (personal communication)
- 42.Frahm J, Bruhn H, Merboldt K-D, Hanicke W. Dynamic MRI of human brain oxygenation during rest and photic stimulation. J Magn Reson Imaging. 1992;2:501–505. doi: 10.1002/jmri.1880020505. [DOI] [PubMed] [Google Scholar]
- 43.Schneider W, Cohen D. (personal communication)
- 44.Blamire AM, McCarthy G, Gruetter R, et al. Echo planar imaging of the left inferior frontal lobe during word generation.. Proceedings of the Eleventh Annual Meeting of the Society of Magnetic Resonance in Medicine; Berlin, Germany. 1992. p. 1834. [Google Scholar]
- 45.Cohen MS, Weisskoff RM. Ultra-fast imaging. Magn Reson Imaging. 1991;9:1–37. doi: 10.1016/0730-725x(91)90094-3. [DOI] [PubMed] [Google Scholar]
- 46.Talairach J, Toumoux P. Co-planar stereotaxic atlas of the human brain. Thieme Medical Publishers; New York, NY: 1988. [Google Scholar]
- 47.Fox PT, Raichle ME. Stimulus rate determines regional brain blood flow in striate cortex . Ann Neurol. 1985;17:303–305. doi: 10.1002/ana.410170315. [DOI] [PubMed] [Google Scholar]
- 48.Fox PT, Miezin FM, Allman JM, Van Essen DC, Raichle ME. Retinotopic organization of human visual cortex mapped with positron-emission tomography. J Neurosci. 1987;7:913–922. doi: 10.1523/JNEUROSCI.07-03-00913.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horton JC, Hoyt WF. The representation of the visual field in human striate cortex. Arch Ophthalmol. 1991;109:816–824. doi: 10.1001/archopht.1991.01080060080030. [DOI] [PubMed] [Google Scholar]
- 50.Lassen NA, Ingvar DH, Raichle ME, Friberg L, editors. Alfred Benzon Symposium 31. Mosby-Year Book; Chicago, IL: 1991. Brain work and mental activity. pp. 68–77. [Google Scholar]