Abstract
Background
Centrifugal pumps are increasingly used for temporary mechanical support for the treatment of cardiogenic shock. However, centrifugal pumps can generate excessive negative pressure and are afterload-sensitive. A previously developed modified roller pump mitigates these limitations both in vitro and in preliminary animal experiments. We report the results of intermediate-term testing of our evolving pump technology, known as BioVAD.
Methods
The BioVAD was implanted in 6 adult male sheep (62.5 ± 3.9 kg), with drainage from the left atrium and reinfusion into the descending aorta. The sheep were monitored for 5 days. Heparin was given during the initial implantation, but no additional anti-coagulation was given. Data collected included hemodynamic status, pump flow and pressures, laboratory values to monitor end-organ function and hemolysis, pathologic specimens to evaluate for thromboembolic events and organ ischemia, and explanted pump evaluation.
Results
All animals survived the planned experimental duration and there were no pump malfunctions. Mean BioVAD flow was 3.57 ± 0.30 L/min (57.1 cc/kg/min) and mean inlet pressure was -30.51 ± 4.25 mmHg. Laboratory values, including plasma free hemoglobin, creatinine, lactate, and bilirubin levels, remained normal. Three animals had small renal cortical infarcts, but there were no additional thromboembolic events or other abnormalities seen on pathologic examination. No thrombus was identified in the BioVAD blood flow path.
Conclusions
The BioVAD performed well for five days in this animal model of temporary left ventricular assistance. Its potential advantages over centrifugal pumps may make it applicable for short-term mechanical circulatory support.
Introduction
Temporary cardiac support devices have become an important tool in the treatment of refractory cardiogenic shock. A variety of blood pumps have been used in this application, including roller, pneumatic, and now increasingly centrifugal designs. While generally safe, there are several disadvantages to centrifugal pumps. These pumps operate at a fixed rotational speed, requiring manual adjustments to accommodate the rapid fluctuations in intravascular volume status that can be seen in critically ill patients. They are sensitive to variations in afterload, resulting in potentially undesired changes in flow (1). There is also the concern for cavitation and hemolysis due to excessive negative pressure (2). In addition, rotary blood pumps are associated with acquired von Willebrand factor deficiency and may contribute to bleeding complications (3-7).
A non-occlusive peristaltic type pump (BioVAD, Michigan Critical Care Consultants, Ann Arbor, MI) has been developed which has the potential to mitigate the possible disadvantages of centrifugal pumps. Our group previously studied this device in vitro, demonstrating its intrinsic volume responsiveness, afterload insensitivity, and avoidance of excessive negative suction (8). The BioVAD was also tested in a 4-hour in vivo sheep model, and was found to have a favorable hemodynamic profile in the support of these animals (9). The objective of this study was to determine the performance and safety of the BioVAD pump in a 5-day sheep model, and to evaluate the effect of the pump on hemolysis, end-organ function, and thrombosis.
Material and Methods
Study Design
All animals received humane care in compliance with the 1996 “Guide for the Care and Use of Laboratory Animals” and the U.S. National Institutes of Health. The University of Michigan Committee on the Use and Care of Animals, protocol number 00004117, approved all experiments. The BioVAD device was implanted in six adult male sheep (62.5 ± 3.9 kg). Animals were supported for 5 days, during which routine vital signs, pump parameters, and laboratory tests were recorded. At the end of the experiments, the animals were euthanized and necropsy was performed along with evaluation of the pump.
BioVAD Design
The BioVAD device is shown in Figure 1, and can be modified for different sizes and flow demands. Details of the BioVAD pump have previously been published (10). Briefly, it consists of a collapsible polyurethane chamber stretched around three rollers and maintained in a sealed housing. Suction can be applied to the housing to generate vacuum-assisted drainage. A schematic of the pump chamber at different degrees of venous return is shown in Figure 2. The pump head is disposable and designed for single-use. It sits on a motor plate that is magnetically driven to a maximum speed of 150 RPM. For this animal model, the chosen pump chamber has a maximum volume of 65 cc and a maximum stroke volume of 55.5 cc per revolution. The pump is controlled by a console that displays the pump speed (in RPM), amount of suction generated (in mmHg), and the percent fill of the pump chamber (Figure 3). The console has a connection port for a 3/8” flow probe, and the amount of flow is shown on the console display.
Figure 1. BioVAD pump prototype.

Figure 2.
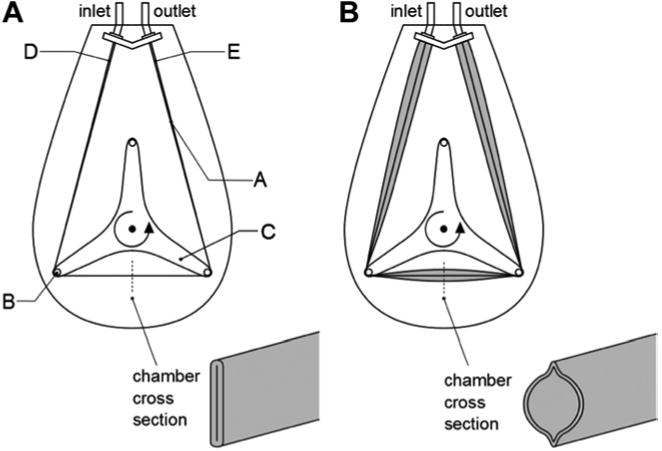
Depiction of BioVAD pump at different degrees of venous return. (a) Venous return is zero, resulting in flattening of pump chamber. (b) Venous return is sufficient for expansion of pump chamber. (A = pump chamber, B = roller, C = rotor, D = pump chamber inlet region, E = pump chamber outlet region)
Figure 3.
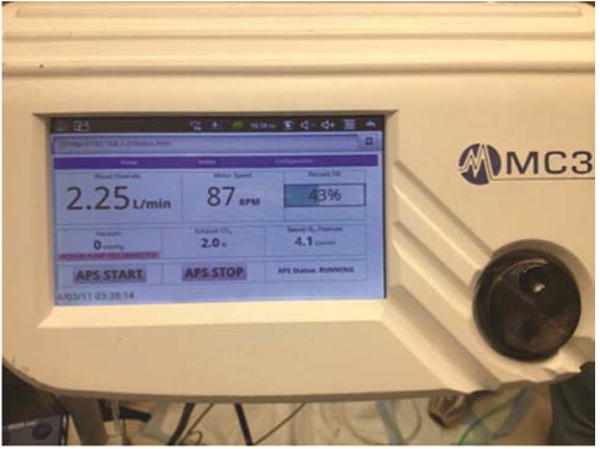
BioVAD console. The black dial in the lower right can be used to adjust the pump speed. The screen displays the pump speed in RPM, flow in liters per minute, amount of suction, and percent fill of the pump chamber.
Surgical Technique
Propofol (5 mg/kg) was used for anesthetic induction and Isoflurane (1-5%) was used for anesthetic maintenance. An arterial line was placed in the carotid artery, and a sheath introducer and pulmonary artery catheter were placed via the jugular vein. A left thoracotomy was performed and the pericardium was opened. Heparin was administered (100 IU/kg), and activated clotting times were measured with a goal greater than 300 seconds. After this initial heparinization, no further anticoagulation was administered during the operation or the post-operative course. A 10mm woven polyester graft bonded to 3/8” tubing was anastomosed to the descending aorta in an end to side fashion. A 28F right angle cannula (Medtronic DLP, Minneapolis, MN) was inserted into the left atrium and secured with double purse-string sutures. The cannula and graft were tunneled out of the chest. The chest was closed with a 32F thoracostomy tube in place. The pump and circuit were primed with 1 liter of lactated ringer's solution mixed with 50cc of 8.4% sodium bicarbonate. The cannula and graft were connected to the BioVAD device using standard barbed connectors and 3/8” polyvinyl chloride tubing.
Post-operative Care and Data Collection
The animals were extubated within hours following the surgery. The pump settings were maintained constant, with speed of 80 RPM and -60 mmHg of vacuum, settings which in previous in vivo studies produced a flow rate of approximately 60cc/kg/min. No adjustments were made to the pump settings in the post-operative period. Laboratory personnel observed the sheep for the entirety of the experiment for the purpose of collecting data, providing pain control, and ensuring the safety of the animals. Post-operative analgesia was achieved with buprenorphine (0.005-0.01 mg/kg), flunixin (2 mg/kg) and transdermal fentanyl (100mcg). Perioperative antibiotics were maintained for 24 hours following surgery (cefazolin 1g). Continuous monitoring of heart rate, systemic blood pressure, central venous pressure, and pulmonary artery pressure was performed (Maquet, Rastatt, Germany). Pump inlet and outlet pressures were measured from luer ports in the circuit immediately proximal and distal to the BioVAD device. Baseline laboratory tests were obtained, including complete blood count, comprehensive metabolic panel (including renal and liver function), coagulation panel, and plasma free hemoglobin, and repeated daily. Arterial blood gases were obtained to assess readiness for extubation, and on an as-needed basis to assist with acid-base and electrolyte management. Following necropsy, organs were submitted for gross and microscopic pathology, including lungs, heart, kidneys, spleen, and liver. The pump chambers were drained of blood and inspected for clot or signs of fatigue.
Analysis of variance (ANOVA) was performed to compare the means of the laboratory values for each day. Statistical analysis was performed using Stata version 12 (StataCorp LP, College Station, TX). Statistical significance was defined as p value <0.05.
Results
The BioVAD device was implanted successfully and without complication in each of the 6 animals. All animals survived 4-5 days without event (one animal was euthanized after 4 days for logistical reasons). There were no pump malfunctions and no setting manipulations throughout the duration of each experiment, with vacuum at -60 mmHg and pump speed set at 80 RPM. Mean pump flow remained stable and is shown in Figure 4. The overall mean flow was 3.57 ± 0.30 L/min (57.1 cc/kg/min). Mean inlet pressure was negative 30.51 ± 4.25 mmHg and was stable over the duration of support (Figure 5). Mean pulse pressure at baseline was 29.5 ± 4.1 mmHg, and decreased to 19.7 ± 3.4 mmHg after initiation of support.
Figure 4. Circuit flow through the BioVAD circulatory support device in six healthy sheep.
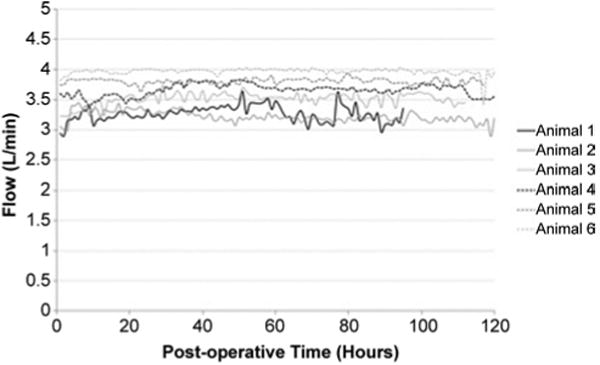
Figure 5. Inlet pressure values for six healthy sheep supported on the BioVAD circulatory support device.
Table 1 shows the mean laboratory values for each day of the experiments. There was a slight increase in plasma free hemoglobin levels in the two days following implantation, which remained well below clinically significant levels of hemolysis and returned to baseline levels soon after this. Likewise, platelet counts decreased in the two days following implantation, but returned to baseline levels by day 4. ANOVA showed significant variation in levels of plasma free hemoglobin, creatinine, and lactic acid, with the latter two decreasing from baseline.
Table 1.
Mean laboratory values for six sheep supported on the BioVAD circulatory support device. P value determined by ANOVA.
| Laboratory Value | Baseline | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | P value |
|---|---|---|---|---|---|---|---|
| Plasma free hemoglobin (mg/dL)a | 2.1 ± 1.6 | 4.4 ± 4.1 | 3.5 ± 2.7 | 2.4 ± 1.8 | 2.6 ± 1.9 | 1.2 ± 0.4 | 0.002 |
| Platelets (K/mm3)b | 223 ± 143 | 142 ± 85 | 108 ± 64 | 128 ± 72 | 182 ± 179 | 232 ± 245 | 0.05 |
| Lactic acid (mmol/L)c | 1.8 ± 0.9 | 1.2 ± 0.8 | 0.7 ± 0.3 | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.5 ± 0.3 | 0.002 |
| Creatinine (mg/dL)d | 1.0 ± 0.2 | 1.0 ± 0.3 | 0.9 ± 0.6 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | <0.001 |
| Total bilirubin (mg/dL)e | 0.1 ± 0.08 | 0.3 ± 0.16 | 0.2 ± 0.15 | 0.2 ± 0.11 | 0.2 ± 0.08 | 0.2 ± 0.08 | 0.46 |
| Hemoglobin (g/dL)f | 7.2 ± 0.7 | 6.9 ± 1.1 | 6.6 ± 0.7 | 6.7 ± 0.7 | 6.7 ± 0.8 | 6.3 ± 0.8 | 0.91 |
Reference ranges:
1.0-8.0 m/dL;
250-750 K/mm3;
0.5-2.2 mmol/L;
1.2-1.9 mg/dL;
0.1-0.5 mg/dL;
9.0-15.0 mg/dL
Following necropsy of the animal, the heart, lungs, liver, kidneys, and spleen were sent to pathology for gross and microscopic evaluation. There was no evidence of gross intestinal infarction. Three of the animals had small renal cortical infarctions, which appeared to be embolic in origin. There were no other signs of thromboembolism. The remaining 3 animals were free of any pathologic abnormalities.
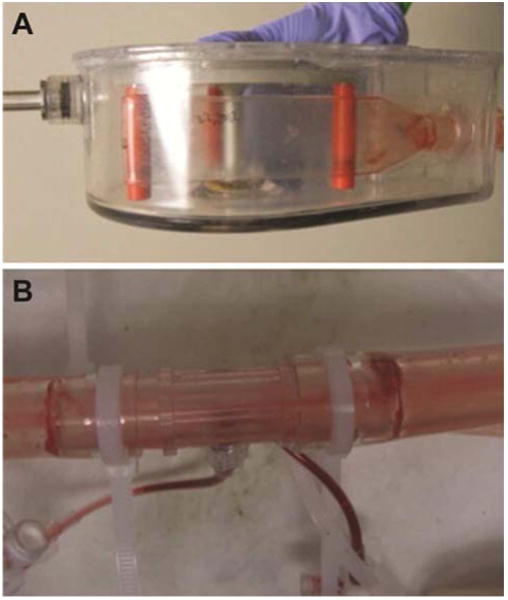
The circuit and the pump were inspected for thrombus formation or chamber disruption or fatigue. Selected photographs are shown in Figure 6. There was no evidence of thrombus formation in the pump chamber or the tubing from the pump to the animal. There was ring thrombus noted at the transition points of connectors in the circuit, as is typically seen in any prolonged use extracorporeal circuit.
Figure 6. Photographs of BioVAD pumphead (A) and tubing following necropsy (B).
Comment
The use of durable ventricular assist devices (VADs) for patients with cardiac failure as a bridge to transplantation or as destination therapy is increasing, with acceptable short and long-term survival (11-13). However, these devices are expensive and require specialized teams and protocols for implementation and long-term management. They are primarily utilized at tertiary care centers, and rarely implanted in patients with critical cardiogenic shock (14). Temporary mechanical circulatory support with simpler and less expensive systems allows resuscitation of end organs and provides time to select the most appropriate candidates for durable VAD implantation (15). This strategy may prevent overutilization of durable VADs, either in unsalvageable patients or in those in which significant cardiac recovery is possible. Furthermore, initiation of temporary mechanical support at community hospitals may stabilize critically ill shock patients prior to transfer to larger centers and prevent additional and potentially irreversible end organ injury (16, 17).
Several types of blood pumps have been used for temporary mechanical circulatory support (18-25). These include pneumatic systems such as the Abiomed BVS 5000 and the AB5000 (Abiomed, Inc., Danvers, MA). These pumps automatically adjust flow based on intravascular volume and avoid excessive negative pressure by precisely regulating vacuum applied to the filling chambers. However, the inflow and outflow valves result in zones of blood stagnation which can lead to thrombus formation and thromboembolic complications. Microaxial blood pumps like the Impella 2.5 and 5.0 (Abiomed, Inc.) require placement across the semilunar valves, using either fluoroscopy or echocardiography (26). These devices can easily become malpositioned, leading to inadequate support or hemolysis from impingement on the inflow or outflow blood flow paths (27). In addition, they can cause valvular injury during placement or removal (28). Centrifugal pumps, such as the Centrimag (Thoratec, Inc., Pleasanton, CA) and TandemHeart (Cardiac Assist, Inc., Pittsburgh, PA) can be used as short-term VADs either surgically or percutaneously, or used for Extracorporeal Membrane Oxygenation (ECMO). Centrifugal pumps operate at a fixed rotational speed and require manual adjustments for large changes in intravascular volume status. During periods of hemodynamic stability, centrifugal pumps do not require much manipulation and appear much less thrombogenic than their pulsatile predecessors. However, during abrupt reduction in intravascular volume, suction generated by centrifugal pumps can be substantial, manifested by “chattering,” and at high speeds can result in cavitation, hemolysis, and vascular injury. In addition, ventricular unloading may be less with continuous flow pumps than with pulsatile pumps that operate in a fill to empty cycle (29). This may have important implications in terms of creating the best conditions to allow myocardial recovery.
The ideal pump for short-term mechanical support should be easy to use, capable of functioning without a significant amount of manipulation, biocompatible with minimal thrombogenicity, intrinsically responsive to the patient's fluid status, and resistant to changes in afterload. The pump should also be able to function in an LVAD, RVAD, or BIVAD configuration, or as a component of an ECMO circuit. Priming and operation should be rapid and intuitive, facilitating use at centers who may apply the system infrequently.
The BioVAD described in this study could potentially fulfill each of these ideal qualities. The concept of the peristaltic non-occlusive roller type pump was originally formulated to create a safe pump for cardiopulmonary bypass perfusion systems. The original pump design was unsuitable for more prolonged circulatory assist, as it has a large footprint and requires gravity for venous drainage. The BioVAD was a result of modifications to this early pump which made it more compact with a sealed housing allowing vacuum-augmented drainage, and uses magnetic coupling to the motor, creating a cost effective disposable single use cartridge. The console provides a simple and intuitive user interface, with inputs of pump speed and vacuum strength. By reporting “percent pump chamber fill”, the bedside nurse can easily see if changes in pump speed are warranted. Alternatively, algorithms can easily be made to automatically adjust pump speed to maintain “percent fill” within a specified range. Our previous work has shown that the BioVAD pump, with these modifications, preserves the hemodynamic and safety advantages of the earlier pump (8).
In this study, 6 sheep were supported with the BioVAD pump with left atrial drainage and aortic reinfusion for 4-5 days without event. Pump flows and hemodynamic data remained stable throughout the experiments. There was minimal laboratory evidence of end-organ dysfunction, and there were minimal thromboembolic events. Our previous work with an animal model employed cannulation of the left ventricular apex for drainage into the pump. For this series of experiments, we chose to place the drainage cannula in the left atrium, due to concerns that contractility of the left ventricle artificially augmented flow through the pump. This study is also the first use of the pump for an extended period of time.
As stated above, one of the advantages of the BioVAD pump is fluid-responsiveness. As the venous pressure increases, the pump chamber expands and a greater percentage of the maximum volume capacity of the chamber is reached. This results in an increase in the flow generated by the pump. In our experiments, the flow remained largely stable throughout the postoperative course due to the fact that the sheep were previously healthy and the operations were performed efficiently with minimal blood loss. We previously demonstrated the intrinsic volume responsiveness of the BioVAD during simulated large volume shifts (8,9).
There are several limitations to this study. In this first recovery trial of the pump, we elected to use healthy animals for our study. The pump mechanics and hemodynamic data would likely be quite different in a cardiogenic shock model. Furthermore, the true effect of the pump on the failing heart cannot be assessed in this study, since the normal functioning heart may be compensating for pump deficiencies. There were also several renal thromboembolic events noted on post-necropsy pathology. After the initial heparin dose for graft and cannula placement, no additional anticoagulation was used. This was our first experience with a recovery model for this pump and we did not want the results to be clouded by potential bleeding complications. In addition, we wanted to determine the thrombogenicity of the device under a “worst case scenario” where anticoagulation was withheld. Future study will include the addition of anticoagulation as well as the use of coated surfaces to prevent thrombus formation. Temporary circulatory support is frequently used for longer than 5 days. While in vitro testing under accelerated conditions has been performed for 14 days, we plan to complete future in vivo studies for longer duration. In addition, we will also incorporate echocardiography in future studies to more thoroughly demonstrate the effect of the pump on cardiac function, particularly in a heart failure model.
This study demonstrates that the BioVAD pump performed well in a 5 day recovery implant model without anticoagulation. There was minimal negative pressure and hemolysis, and no need for pump setting adjustments. We feel that the BioVAD pump may be ideally suited for short-term mechanical support and could potentially be employed as a component of an LVAD, RVAD, or ECMO. Its simple concept and intuitive interface may be ideal for the community hospital which anticipates infrequent use of mechanical assist devices.
Acknowledgments
Supported by NIH grant # 5R42HL096168-3. The BioVAD term and patents related to the pump are owned by Michigan Critical Care Consultants, Inc. (Ann Arbor, MI). Three of the authors (EMJ, DEM, RHB) are either employees of Michigan Critical Care Consultants or equity stakeholders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Salamonsen RF, Mason DG, Ayre PJ. Response of rotary blood pumps to changes in preload and afterload at a fixed speed setting are unphysiological when compared with the natural heart. Artif Organs. 2011 Mar;35(3):E47–53. doi: 10.1111/j.1525-1594.2010.01168.x. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen TH, Videm V, Svennevig JL, Karlsen H, Ostbakk RW, Jensen O, et al. Extracorporeal membrane oxygenation using a centrifugal pump and a servo regulator to prevent negative inlet pressure. Ann Thorac Surg. 1997 May;63(5):1333–9. doi: 10.1016/s0003-4975(97)00098-2. [DOI] [PubMed] [Google Scholar]
- 3.Goda M, Jacobs S, Rega F, Peerlinck K, Jacquemin M, Droogne W, et al. Time course of acquired von Willebrand disease associated with two types of continuous-flow left ventricular assist devices: HeartMate II and CircuLite Synergy Pocket Micro-pump. J Heart Lung Transplant. 2013 May;32(5):539–45. doi: 10.1016/j.healun.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Uriel N, Pak SW, Jorde UP, Jude B, Susen S, Vincentelli A, et al. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol. 2010 Oct 5;56(15):1207–13. doi: 10.1016/j.jacc.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 5.Heilmann C, Geisen U, Beyersdorf F, Nakamura L, Trummer G, Berchtold-Herz M, et al. Acquired Von Willebrand syndrome is an early-onset problem in ventricular assist device patients. Eur J Cardiothorac Surg. 2011 Dec;40(6):1328–33. doi: 10.1016/j.ejcts.2011.03.021. discussion1233. [DOI] [PubMed] [Google Scholar]
- 6.Heilmann C, Geisen U, Beyersdorf F, Nakamura L, Benk C, Trummer G, et al. Acquired von Willebrand syndrome in patients with extracorporeal life support (ECLS) Intensive Care Med. 2012 Jan;38(1):62–8. doi: 10.1007/s00134-011-2370-6. [DOI] [PubMed] [Google Scholar]
- 7.Crow S, Chen D, Milano C, Thomas W, Joyce L, Piacentino V, et al. Acquired von Willebrand syndrome in continuous-flow ventricular assist device recipients. Ann Thorac Surg. 2010 Oct;90(4):1263–9. doi: 10.1016/j.athoracsur.2010.04.099. discussion1269. [DOI] [PubMed] [Google Scholar]
- 8.Spurlock DJ, Ranney DN, Fracz EM, Mazur DE, Bartlet RH, Haft JW. In vitro testing of a novel blood pump designed for temporary extracorporeal support. ASAIO J. 2012 Mar;58(2):109–14. doi: 10.1097/MAT.0b013e318245d356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spurlock DJ, Koch K, Mazur DE, Fracz EM, Bartlett RH, Haft JW. Preliminary in vivo testing of a novel pump for short-term extracorporeal life support. Ann Thorac Surg. 2012 Jan;93(1):141–6. doi: 10.1016/j.athoracsur.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montoya JP, Merz SI, Bartlett RH. Laboratory experience with a novel, non-occlusive, pressure-regulated peristaltic blood pump. ASAIO Journal. 1992 Jul;38(3):M406–11. doi: 10.1097/00002480-199207000-00065. [DOI] [PubMed] [Google Scholar]
- 11.Slaughter MS, Pagani FD, McGee EC, Birks EJ, Cotts WG, Gregoric I, et al. HeartWare ventricular assist system for bridge to transplant: Combined results of the bridge to transplant and continued access protocol trial. J Heart Lung Transplant. 2013 Jul;32(7):675–83. doi: 10.1016/j.healun.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Morgan JA, John R, Rao V, Weinberg AD, Lee BJ, Mazzeo PA, et al. Bridging to transplant with the HeartMate left ventricular assist device: The Columbia Presbyterian 12-year experience. J Thorac Cardiovasc Surg. 2004 May;127(5):1309–16. doi: 10.1016/j.jtcvs.2003.07.035. [DOI] [PubMed] [Google Scholar]
- 13.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001 Nov 15;345(20):1435–43. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 14.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, et al. Third INTERMACS Annual Report: the evolution of destination therapy in the United States. 2011:115–23. doi: 10.1016/j.healun.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Chen YS, Yu HY, Huang SC, Chiu KM, Lin TY, Lai LP, et al. Experience and result of extracorporeal membrane oxygenation in treating fulminant myocarditis with shock: what mechanical support should be considered first? J Heart Lung Transplant. 2005 Jan;24(1):81–7. doi: 10.1016/j.healun.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 16.Haft JWJ, Pagani FDF, Romano MAM, Leventhal CLC, Dyke DBD, Matthews JCJ. Short-and long-term survival of patients transferred to a tertiary care center on temporary extracorporeal circulatory support. Ann Thorac Surg. 2009 Sep 1;88(3):711–8. doi: 10.1016/j.athoracsur.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Stawinski GV, Chang ASY, Navia JL, Banbury MK, Buda T, Hoercher K, et al. Regional referral system for patients with acute mechanical support: experience at the Cleveland Clinic Foundation. ASAIO Journal. 2006 Jul;52(4):445–9. doi: 10.1097/01.mat.0000225265.11371.ed. [DOI] [PubMed] [Google Scholar]
- 18.Samuels LE, Holmes EC, Thomas MP, Entwistle JC, Morris RJ, Narula J, et al. Management of acute cardiac failure with mechanical assist: experience with the ABIOMED BVS 5000. Ann Thorac Surg. 2001 Mar;71(3 Suppl):S67–72. doi: 10.1016/s0003-4975(00)02644-8. discussionS82–5. [DOI] [PubMed] [Google Scholar]
- 19.Morgan JA, Stewart AS, Lee BJ, Oz MC, Naka Y. Role of the Abiomed BVS 5000 device for short-term support and bridge to transplantation. ASAIO Journal. 2004 Jul;50(4):360–3. doi: 10.1097/01.mat.0000130680.63196.7b. [DOI] [PubMed] [Google Scholar]
- 20.John R, Liao K, Lietz K, Kamdar F, Colvin-Adams M, Boyle A, et al. Experience with the Levitronix CentriMag circulatory support system as a bridge to decision in patients with refractory acute cardiogenic shock and multisystem organ failure. J Thorac Cardiovasc Surg. 2007 Aug;134(2):351–8. doi: 10.1016/j.jtcvs.2007.01.085. [DOI] [PubMed] [Google Scholar]
- 21.De Robertis F, Rogers P, Amrani M, Petrou M, Pepper JR, Bahrami T, et al. Bridge to decision using the Levitronix CentriMag short-term ventricular assist device. J Heart Lung Transplant. 2008 May;27(5):474–8. doi: 10.1016/j.healun.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 22.Pagani FD, Aaronson KD, Swaniker F, Bartlett RH. The use of extracorporeal life support in adult patients with primary cardiac failure as a bridge to implantable left ventricular assist device. Ann Thorac Surg. 2001 Mar;71(3 Suppl):S77–81. doi: 10.1016/s0003-4975(00)02620-5. discussionS82–5. [DOI] [PubMed] [Google Scholar]
- 23.Smedira NG, Moazami N, Golding CM, McCarthy PM, Apperson-Hansen C, Blackstone EH, et al. Clinical experience with 202 adults receiving extracorporeal membrane oxygenation for cardiac failure: survival at five years. J Thorac Cardiovasc Surg. 2001 Jul;122(1):92–102. doi: 10.1067/mtc.2001.114351. [DOI] [PubMed] [Google Scholar]
- 24.Kar B, Adkins LE, Civitello AB, Loyalka P, Palanichamy N, Gemmato CJ, et al. Clinical experience with the TandemHeart percutaneous ventricular assist device. Tex Heart Inst J. 2006;33(2):111–5. [PMC free article] [PubMed] [Google Scholar]
- 25.Bresson D, Sibellas F, Farhat F, Jegaden O, Kirkorian G, Bonnefoy E. Preliminary experience with Impella Recover(®) LP5.0 in nine patients with cardiogenic shock: a new circulatory support system in the intensive cardiac care unit. Arch Cardiovasc Dis. 2011 Aug;104(8-9):458–64. doi: 10.1016/j.acvd.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Patel KM, Sherwani SS, Baudo AM, Salvacion A, Herborn J, Soong W, et al. Echo rounds: the use of transesophageal echocardiography for confirmation of appropriate Impella 5.0 device placement. Anesth Analg. 2012 Jan;114(1):82–5. doi: 10.1213/ANE.0b013e3182367a7d. [DOI] [PubMed] [Google Scholar]
- 27.Griffith KE, Jenkins E. Abiomed Impella(®) 2.5 patient transport: lessons learned. Perfusion. 2010 Nov;25(6):381–6. doi: 10.1177/0267659110381450. [DOI] [PubMed] [Google Scholar]
- 28.Chandola R, Cusimano R, Osten M, Horlick E. Postcardiac transplant transcatheter core valve implantation for aortic insufficiency secondary to Impella device placement. Ann Thorac Surg. 2012 Jun;93(6):e155–7. doi: 10.1016/j.athoracsur.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 29.Klotz S, Deng MC, Stypmann J, Roetker J, Wilhelm MJ, Hammel D, et al. Left ventricular pressure and volume unloading during pulsatile versus nonpulsatile left ventricular assist device support. Ann Thorac Surg. 2004 Jan;77(1):143–9. doi: 10.1016/s0003-4975(03)01336-5. discussion149–50. [DOI] [PubMed] [Google Scholar]


