Abstract
Lipid peroxidation generates reactive aldehydes, most notably hydroxynonenal (HNE), which covalently binds amino acid residue side chains leading to protein inactivation and insolubility. Specific adducts of lipid peroxidation have been demonstrated to be intimately associated with pathological lesions of Alzheimer’s disease (AD), suggesting that oxidative stress is a major component in the disease. Here, we examined the HNE-cross-linking modifications by using an antibody specific for a lysine–lysine cross-link. Since in a prior study we noted no immunolabeling of neuritic plaques or neurofibrillary tangles but instead found strong labeling of axons, we focused this study on axons. Axonal labeling was examined in mouse sciatic nerve, and immunoblotting showed the cross-link was restricted to neurofilament heavy and medium subunits, which while altering migration, did not indicate larger NF aggregates, indicative of intermolecular cross-links. Examination of mice at various ages showed the extent of modification remaining relatively constant through the life span. These findings demonstrate lipid-cross-linking peroxidation primarily involves lysine-rich neurofilaments and is restricted to intramolecular cross-links.
Keywords: Alzheimer disease, axon, cytoskeleton, lipid peroxidation, neurofibrillary tangle, oxidative stress
Introduction
Increased oxidative stress marks the earliest transition from normal aging to the onset of Alzheimer’ s disease (AD) [1,2]. Oxidative damage to all categories of macro-molecules has been identified, with the greatest number of studies involving carbonyl modification stemming from lipid or sugar-derived oxidized metabolites [3-8]. Adduction of these products modifies the side chains of proteins changing solubility, hydrophobicity, and molecular weight if intermolecular cross-links are formed. Among these, the latter has been shown to be the most critical, as carbonyl-mediated cross-links are powerful inhibitors of protein degradation [9-11]. The best-studied reactive carbonyl is hydroxynonenal (HNE) [8] and one of its defined products is a fluorescent cross-link (HNE-fluorophore) between two lysines [12]. In AD, antibodies specific to HNE-fluorophore show its accumulation in the degradation pathway and granulovacuolar degeneration (GVD) in vulnerable neurons [13]. Additionally, HNE cross-links are seen in axons of AD and controls, as well as non-cross-linking HNE modifications [14].
In this study of the mouse sciatic nerve, we explore the molecular targets of HNE cross-linking, specifically the neurofilament heavy (NFH) subunit. Surprisingly, we found NFH molecular weight was not associated with high molecular weight aggregates by the formation of HNE-fluorophore, indicating that the majority of the cross-links are intramolecular. Further, we found that the extent of modification is constant over the life span.
Methods
Tissue
Spinal cord collected from C57BL6 mice (3–21 months of age) was fixed by immersion in methacarn, embedded in paraffin, and sectioned at 6 μm. Immunocytochemistry was developed as previously described [13]. Sciatic nerve from B6C3F1 mice (3–33 months of age, n = 3 per age group) was collected for immunoblot analysis. Mice were obtained from the National Institute on Aging colony at Charles River and maintained at the Case Western Reserve University Animal Facility under an approved protocol for 7–10 days before sacrifice. Euthanasia was induced by an overdose of pentobarbital before dissection. Upon death, animals were refrigerated immediately and maintained on ice during dissection. Under a stereomicroscope (Zeiss), the entire sciatic nerve was collected, beginning within the spinal column and extending to the soleus muscle. Samples were prepared as previously described [14].
Antibodies
Antiserum to HNE-fluorophore and HNE-Michael was used as described [12-14]. SMI-34 (Sternberger/Meyer Incorporated) monoclonal antibody to phosphorylated NFH was used to identify axons and NFH protein on blots.
Immunoblotting
In previous studies using antibodies to non-cross-linking HNE modifications, we have found specific labeling of NFH throughout the life span [14]. Blots of the cytoskeleton fraction from mouse sciatic nerve, prepared as described previously [14], were probed with the HNE-fluorophore antisera as well as with an antibody to a Michael adduction product of HNE-Michael [14], and the levels of HNE adduction to NFH were quantified using one-way ANOVA. Care was taken to analyze the insoluble axonal material not entering the gel, but rather retaining it in the well of the stacking gel.
Results
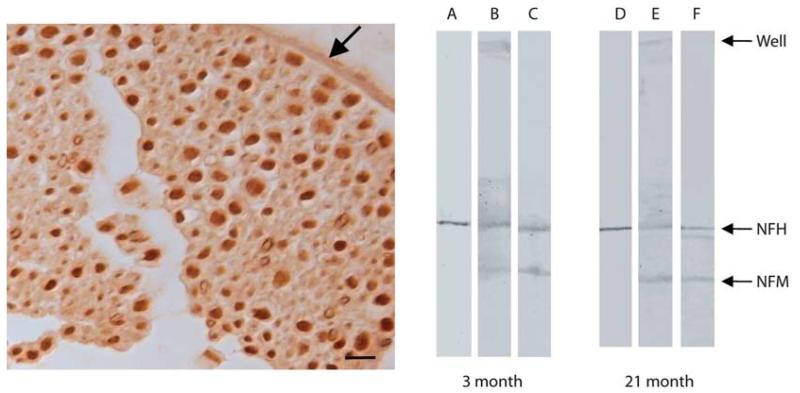
Sections of mouse sciatic nerve showed intense labeling by HNE-fluorophore corresponding to axons (Figure 1) labeled by SMI-34 (not shown). There was little recognition of the myelin covering and weak recognition of the connective covering of the nerve (arrow). Immunoblots of sciatic nerve protein showed only bands corresponding to NFH and NFM recognized by the HNE-fluorophore antisera (Figure 2) and additional recognition of material remaining in the stacking gel for HNE-Michael but not detectable for HNE-fluorophore. The majority of NFH and NFM molecular weight was unchanged by modification. Importantly, neither the HNE-fluorophore or antibody nor NFH antibody recognized material remaining in the stacking gel well.
Figure 1.
HNE-fluorophore modifications are readily detected in axons in mouse spinal cord tissue, consistent with our findings of the presence of other HNE modifications in the same site [14] (left panel). Also recognized is connective tissue of the nerve sheath (arrow). Scale bar = 20 μm. The same axons are labeled with SMI-34, a monoclonal antibody directed to phosphorylated NFH (not shown). In blots of mouse sciatic nerve, fluorophore modifications recognize a band near 200 kD (lanes C and F), corresponding to NFH stained with SMI-34 (lanes A and D) as well as a band corresponding to NFM. Both NFH and NFM are also recognized with an antibody specific for HNE-Michael adducts (lanes B and E). High molecular weight proteins remaining in the well and unable to enter the gel contain HNE-Michael adducts. Similar results seen in mice at 3 and 21 months of mice.
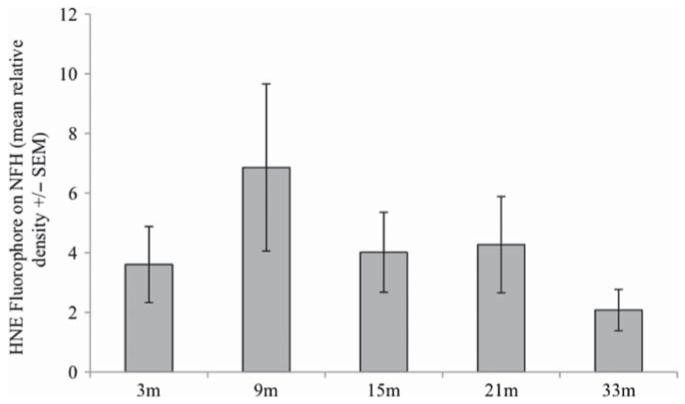
Figure 2.
Quantitative densitometry of HNE-fluorophore modified axonal NFH showed no age-dependent increase, but surprisingly showed a statistically insignificant maximum in midlife. Bars correspond to +/− SEM with n = 3 for each age.
We quantitatively examined the NFH band over the mouse life span. There was no age-related accumulation of HNE-fluorophore-modified NFH with aging (Figure 2), but rather an insignificant increase in midlife similar to what we previously reported for other HNE modifications of NFH [14].
Discussion
The antibody used in this study recognizes the 2-hydroxy-3-imino-1,2-dihydropyrrol structural feature of fluorophores produced by the reaction of primary amines (usually lysine) with HNE [12]. The same epitope has been demonstrated to form following the reaction of specific proteins (e.g., glucose-6-phosphate dehydrogenase) with HNE and is associated with fluorescence and with inactivation of the multicatalytic protease, providing direct evidence for HNE–protein cross-links. We undertook this study to define the biochemical target(s) of modification in the nervous system.
In AD, HNE-based cross-links were found to accumulate in GVD, a structure often considered to be linked to a lysosomal/proteasomal pathway [13]. Importantly, GVD were not found to be the specific site of accumulation of the other HNE adducts which accumulated diffusely within the cytoplasm of affected neurons [8]. These findings in AD suggest that lipid peroxidation and subsequent HNE modification of lysine residues target proteins for degradation in AD and accumulate in the lysosomal/proteasomal pathway.
It was noted that axons are primary sites for HNE modifications, and therefore in a prior study, we determined two HNE adducts that specifically target the lysine-rich high neurofilament subunit, NFH, and neurofilament medium subunit, NFM. HNE modifications were found to follow NFH/M levels in the nervous system at the earliest ages and do not accumulate with further aging. This surprising result suggests HNE adduct levels are regulated. Phosphorylation of NFH and NFM plays a critical role in NFH/M susceptibility to HNE adductions, but there were no indications that proteolysis regulated levels. In this study of HNE cross-link modification, we again find specific lysine involvement (because the HNE-fluorophore is lysine specific) and no signs of age-related accumulation. Surprisingly, although HNE-fluorophore is a cross-link modification, most NFH molecular weight remains at the original molecular weight, indicating the cross-links are intramolecular. Similarly, the materials remaining in the well of the stacking gel are recognized by the HNE-Michael antibody but poorly by HNE-fluorophore and are not recognized by the NFH antibody, suggesting they represent other proteins adducted by HNE. That HNE-fluorophore is physiologically formed in axons throughout life suggests it, together with other HNE products, plays a role in neuronal homeostasis, possibly protecting neurons from carbonyl-mediated stress [14].
Our relative inability to detect intermolecular cross-links suggests that NFH/M molecular structure does not place lysine in sufficiently close juxtaposition with lysines of other proteins, including other NFH subunits to span a cross-link. Functionally, lack of intermolecular cross-link may reduce the effect of HNE-fluorophore to inhibit proteolysis.
Conclusions
NFH and NFM are major targets of carbonyl adduction in the nervous system, including HNE cross-links between lysines. In NFH and NFM, primarily intramolecular cross-links were detected. The level of NFH and NFM cross-linking adducts remained constant over the life span of mice.
Acknowledgments
Work in the authors’ laboratories is supported by the Alzheimer’s Association (IIRG-10-173471) and the National Institute on Minority Health and Health Disparities (G12MD007591) from the National Institutes of Health.
Footnotes
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- [1].Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, et al. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- [2].Nunomura A, Tamaoki T, Motohashi N, Nakamura M, McKeel DW, Jr, Tabaton M, et al. The earliest stage of cognitive impairment in transition from normal aging to Alzheimer disease is marked by prominent RNA oxidation in vulnerable neurons. J Neuropathol Exp Neurol. 2012;71:233–241. doi: 10.1097/NEN.0b013e318248e614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sultana R, Butterfield DA. Oxidative modifi cation of brain proteins in Alzheimer’s disease: perspective on future studies based on results of redox proteomics studies. J Alzheimers Dis. 2012 doi: 10.3233/JAD-2012-129018. doi:10.3233/JAD-2012-129018. [DOI] [PubMed] [Google Scholar]
- [4].Uchida K, Kanematsu M, Sakai K, Matsuda T, Hattori N, Mizuno Y, et al. Protein-bound acrolein: potential markers for oxidative stress. Proc Natl Acad Sci U S A. 1998;95:4882–4887. doi: 10.1073/pnas.95.9.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Castellani RJ, Harris PL, Sayre LM, Fujii J, Taniguchi N, Vitek MP, et al. Active glycation in neurofi brillary pathology of Alzheimer disease: N(epsilon)-(carboxymethyl) lysine and hexitol-lysine. Free Radic Biol Med. 2001;31:175–180. doi: 10.1016/s0891-5849(01)00570-6. [DOI] [PubMed] [Google Scholar]
- [6].Yan SD, Chen X, Schmidt AM, Brett J, Godman G, Zou YS, et al. Glycated tau protein in Alzheimer disease: a mechanism for induction of oxidant stress. Proc Natl Acad Sci U S A. 1994;91:7787–7791. doi: 10.1073/pnas.91.16.7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Moreira PI, Siedlak SL, Wang X, Santos MS, Oliveira CR, Tabaton M, et al. Autophagocytosis of mitochondria is prominent in Alzheimer disease. J Neuropathol Exp Neurol. 2007;66:525–532. doi: 10.1097/01.jnen.0000240476.73532.b0. [DOI] [PubMed] [Google Scholar]
- [8].Sayre LM, Zelasko DA, Harris PL, Perry G, Salomon RG, Smith MA. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer’s disease. J Neurochem. 1997;68:2092–2097. doi: 10.1046/j.1471-4159.1997.68052092.x. [DOI] [PubMed] [Google Scholar]
- [9].Friguet B, Stadtman ER, Szweda LI. Modification of glucose-6-phosphate dehydrogenase by 4-hydroxy-2-nonenal. Formation of cross-linked protein that inhibits the multicatalytic protease. J Biol Chem. 1994;269:21639–21643. [PubMed] [Google Scholar]
- [10].Szweda LI. Age-related increase in liver retinyl palmitate. Relationship to lipofuscin. J Biol Chem. 1994;269:8712–8715. [PubMed] [Google Scholar]
- [11].Szweda LI, Uchida K, Tsai L, Stadtman ER. Inactivation of glucose-6-phosphate dehydrogenase by 4-hydroxy-2-nonenal. Selective modifi cation of an active-site lysine. J Biol Chem. 1993;268:3342–3347. [PubMed] [Google Scholar]
- [12].Tsai L, Szweda PA, Vinogradova O, Szweda LI. Structural characterization and immunochemical detection of a fluorophore derived from 4-hydroxy-2-nonenal and lysine. Proc Natl Acad Sci U S A. 1998;95:7975–7980. doi: 10.1073/pnas.95.14.7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhu X, Castellani RJ, Moreira PI, Aliev G, Shenk JC, Siedlak SL, et al. Hydroxynonenal-generated crosslinking fluorophore accumulation in Alzheimer disease reveals a dichotomy of protein turnover. Free Radic Biol Med. 2012;52:699–704. doi: 10.1016/j.freeradbiomed.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wataya T, Nunomura A, Smith MA, Siedlak SL, Harris PL, Shimohama S, et al. High molecular weight neurofi lament proteins are physiological substrates of adduction by the lipid peroxidation product hydroxynonenal. J Biol Chem. 2002;277:4644–4648. doi: 10.1074/jbc.M110913200. [DOI] [PubMed] [Google Scholar]