Abstract
Viral and cellular oncogenes converge in targeting critical protein interaction networks to reprogram the cellular DNA and protein replication machinery for pathological replication. In this issue, Thai et al. (2014) show that adenovirus E4ORF1 activates MYC glycolytic targets to induce a Warburg-like effect that converts glucose into nucleotides for viral replication.
Adenovirus is a small double-stranded DNA virus (36 kb) that expresses ‘early’ (E) E1 and E4 proteins that hijack cellular growth regulatory networks to replicate the viral genome and proteins. Many of these early proteins are oncoproteins and have led to the discovery of key cellular targets and mechanisms that are also deregulated in cancer, including E2F and Rb tumor suppressor proteins (Ou et al., 2011). One of the hallmarks of cancer is a tendency to ferment glucose to lactose even when sufficient oxygen is available to support mitochondrial oxidative phosphorylation (Ward and Thompson, 2012). This is known as the Warburg effect, which is less efficient in terms of ATP generation (2 vs 36 ATPs) but enables glucose to be used as a carbon source for the synthesis of nucleotides, amino acids and lipids in cell growth and division. In this issue, Thai et al. (2014) reveals adenovirus infection as a novel model to identify cellular mechanisms that reprogram metabolism. They show that adenovirus infection decreases cellular respiration and induces a rapid Warburg-like shift to glycolysis, providing a powerful genetic system to reveal the dynamics and key network interactions that reprogram metabolism in a single cell cycle.
Countless generations of propagation and selective pressure have driven the evolution of both minimal adenovirus genomes and ‘early’ proteins that attack critical cellular hubs to elicit pathological replication (Ou et al., 2011). Thai et al exploit both the genetic and biochemical power of viruses to reveal new insights into molecular mechanisms that reprogram cellular metabolism. In contrast to wild type Ad5, ΔE4 adenovirus mutants are defective for inducing glycolysis and decreased respiration. They show that the gene product of adenovirus E4ORF1 enhances glycolytic flux but not decreased oxygen consumption. E4ORF1 is thought to have evolved from dUTPpyrophosphatases, losing enzymatic activity along the way and acquiring novel transforming properties (Chung et al., 2007). This includes a PDZ binding motif that binds to cellular PDZ-containing proteins, such as DLG and MUPP1, and is required for the activation of PI-3 kinase (PI-3K). PI-3K activity is also targeted by growth factor pathway mutations in cancer and upregulates glucose transporter expression and hexokinase activity, rendering cells dependent on glucose flux (Ward and Thompson, 2012). However, defying a seemingly obvious mechanistic connection, E4ORF1 ΔPDZ mutants still induce the glycolytic shift.
E4ORF1 also localizes to the nucleus but its functions there have been unclear. In the search for a link to metabolism, Thai et al. show that E4ORF1 increases MYC levels in the nucleus and induces global gene expression changes that are enriched for a MYC signature. MYC is a multifunctional transcription factor that is the driving oncogenic translocation in Burkitt’s lymphoma and amplified or upregulated in many tumors (Dang, 2012). MYC binds to E-box sequences and can potentially modulate up to a third of the human transcriptome. MYC levels are normally tightly regulated through rapid mRNA and protein turnover (Farrell and Sears, 2014). E4ORF1 and E4ORF6 co-immunoprecipitate with MYC in the nucleus, potentially stabilizing MYC through direct interactions. The authors show that E4ORF1 D68A mutants that are defective for PI-3K activation and cellular transformation (Chung et al., 2007) also exhibit reduced binding to MYC and E4ORF6. These data indicate that D68A mutants are hypomorphic, perhaps due to protein aggregation. WT E4ORF1, but not D68A mutants, induces MYC activation of a subset of glycolytic targets, including HK2 and PFKM (Fig. 1). Although the effects on HK2 mRNA levels are modest, it may be rate-limiting, and the overexpression of HK2 is sufficient to enhance glycolysis to a similar extent as E4ORF1. MYC shRNA inhibits the glycolytic shift and virus replication. However, MYC also cooperates with E2F to drive DNA replication. Therefore, it will be important to determine the specific contribution of MYC metabolic targets, such as HK2, in viral replication.
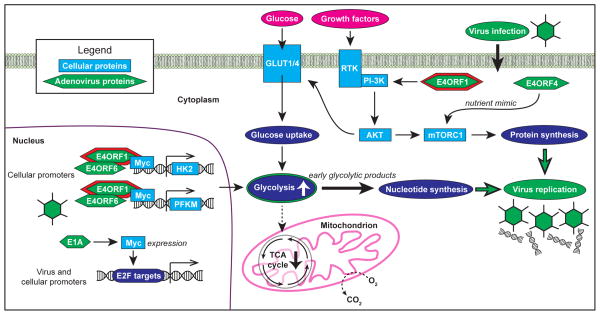
Figure 1. Adenovirus oncoproteins reprogram cellular metabolism at multiple signaling and transcriptional nodes to induce a glycolytic shift that converts glucose into the molecular building blocks required for viral replication.
Adenovirus E4ORF1 binds to MYC in the nucleus to activate the transcription of a subset of glycolytic target genes, HK2 and PFKM, that promote a Warburg-like shift to aerobic glycolysis. The activation of MYC, PI-3K and mTOR by E4-ORF1 and additional adenovirus oncoproteins could have emergent functions in reprogramming cellular metabolism to synthesize proteins and nucleic acids required for viral replication.
In an elegant series of experiments, Thai et al use positional glucose tracers to demonstrate that glycolysis generates metabolites for increased nucleotide biosynthesis in adenovirus-infected cells. An E4ORF1 D68A mutant adenovirus that fails to induce the glycolytic shift is defective for replication. However, E4ORF1 D68A mutant viruses also fail to activate PI-3K. Therefore, to determine the specific contribution of metabolic reprogramming in viral replication will require E4ORF1 mutations that selectively uncouple its functions in MYC induced glycolysis from its other cellular activities and targets in infection.
The mechanism through which E4ORF1 enhances MYC activity at a subset of glycolytic target genes remains unknown. E1A, the master adenovirus oncogene, also induces and stabilizes MYC (Chakraborty and Tansey, 2009) (Kadeppagari et al., 2009). However, E1A is not sufficient to induce MYC metabolic targets in E4 mutant virus infected cells. Thus, E1A and E4ORF1 may directly specify MYC activated transcription of cell cycle and metabolism genes, respectively, or target its recruitment through distinct collaborating cofactors. Alternatively, the temporal kinetics of E1A and E4ORF1 expression in infection could induce different MYC levels that determine the activation of cell cycle and metabolic promoters. Distinguishing between these possibilities is critical for the development of strategies that selectively target MYC’s oncogenic effects in tumor metabolism and growth. As such, adenovirus infection could prove a powerful system to reconcile the raging contemporary debate as to whether MYC amplifies rather than specifies transcriptional reprogramming (Lin et al., 2012).
These studies reinforce the profound overlap that exists between the cellular targets and phenotypes of tumor and viral replication (Ou et al., 2011). Both viral and cellular oncogenes divert glucose into energetically costly anabolic pathways to generate macromolecular building blocks for replication. Similar to other DNA viruses, adenovirus increases glycolysis, mirroring the Warburg effect in cancer. E4ORF1 has evolved independent mechanisms to usurp two of the major metabolic nodes targeted by tumor pathway mutations, PI-3K and MYC. Although these E4ORF1 interactions may be independent, they could have synergistic effects in reprogramming metabolism. PI-3K induced activation of AKT can prevent GSK3β-induced phosphorylation of MYC at Thr58, thereby stabilizing MYC (Farrell and Sears, 2014). In addition, adenovirus E4ORF4 phenocopies glucose in activating mTOR (O’Shea et al., 2005). The activation of MYC, PI-3K and mTOR could have emergent functions in reprogramming metabolism to synthesize proteins and nucleic acids for viral replication (Fig. 1). It will be intriguing to determine if the functions of Ad5 E4ORF1 in activating MYC and glycolysis are conserved across all human adenoviruses. Interestingly, Ad36 infection and E4ORF1 has been reported to improve glycemic control, which has been suggested as a possible approach to treat hepatic steatosis and diabetes (Dhurandhar et al., 2012). One thing is certain; metabolism is going viral, revealing exciting new insights into the dynamics of cellular network interactions that are rewired to elicit pathological replication.
Acknowledgments
S.J.M. is supported by NIH T32 GM007240-35. This work was supported by NCI grants (R01CA137094 and P30CA014195) and the Leona M. and Harry B. Helmsley Charitable Trust grant #2012-PG-MED002.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chakraborty AA, Tansey WP. Adenoviral E1A function through Myc. Cancer Res. 2009;69:6–9. doi: 10.1158/0008-5472.CAN-08-3026. [DOI] [PubMed] [Google Scholar]
- Chung SH, Frese KK, Weiss RS, Prasad BV, Javier RT. A new crucial protein interaction element that targets the adenovirus E4-ORF1 oncoprotein to membrane vesicles. J Virol. 2007;81:4787–4797. doi: 10.1128/JVI.02855-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang CV. MYC on the path to cancer. Cell. 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhurandhar EJ, Krishnapuram R, Hegde V, Dubuisson O, Tao R, Dong XC, Ye J, Dhurandhar NV. E4orf1 improves lipid and glucose metabolism in hepatocytes: a template to improve steatosis& hyperglycemia. PLoS One. 2012;7:e47813. doi: 10.1371/journal.pone.0047813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell AS, Sears RC. MYC Degradation. Cold Spring Harbor perspectives in medicine. 2014;4:1–15. doi: 10.1101/cshperspect.a014365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadeppagari RK, Sankar N, Thimmapaya B. Adenovirus transforming protein E1A induces c-Myc in quiescent cells by a novel mechanism. J Virol. 2009;83:4810–4822. doi: 10.1128/JVI.02145-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Loven J, Rahl PB, Paranal RM, Burge CB, Bradner JE, Lee TI, Young RA. Transcriptional amplification in tumor cells with elevated c-Myc. Cell. 2012;151:56–67. doi: 10.1016/j.cell.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea C, Klupsch K, Choi S, Bagus B, Soria C, Shen J, McCormick F, Stokoe D. Adenoviral proteins mimic nutrient/growth signals to activate the mTOR pathway for viral replication. EMBO J. 2005;24:1211–1221. doi: 10.1038/sj.emboj.7600597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou HD, May AP, O’Shea CC. The critical protein interactions and structures that elicit growth deregulation in cancer and viral replication. Wiley interdisciplinary reviews. Systems biology and medicine. 2011;3:48–73. doi: 10.1002/wsbm.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai M, Graham NA, Braas D, Nehil M, Komisopoulou E, Kurdistani SK, McCormick F, Graeber TG, Christofk HR. Adenovirus E4ORF1-induced MYC activation promotes host cell anabolic glucose metabolism and virus replication. Cell Metabolism. 2014;#:#–#. doi: 10.1016/j.cmet.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]