Abstract
Background: Cancer of the larynx accounts for 1% to 2.5% of all human neoplasms and is the most common malignancy of the Head and Neck region. The purpose of this study is to analyze epidemiological data of patients with laryngeal cancer and to point out the geographical variations.
Methods: This is the first systematic recording of the laryngeal cancer epidemiological data in Northern Greece. During the period 1992-2010 1,638 patients were diagnosed with and treated for malignant head and neck tumors. One thousand one hundred and four cases (67.4%) were malignant laryngeal tumors, 98.4% of which (1,088 cases) were squamous cell carcinomas (SCC). Only 16 patients (1.5%) presented with other types of malignancies.
Results: The average age of the SCC patients was 62.1 years. Only 35 patients were women (3.2%). More than 60% of the patients were farmers or labor workers, 86.9%, were smokers, 43.2% were consuming alcohol on a daily basis and 36.1% had a positive family history of malignancy. Concerning tumor location, 60.2% were glottic cancers. T staging revealed that 1.2% of the cases were carcinomas in situ, 28% T1 tumors, 19% T2, 32 % T3, and 20% T4. Tumor grading showed that 43% of the cases were G1, 42.1% were G2, and 11.8% were G3.
Conclusions: The pathogenesis of laryngeal carcinoma is the result of the combined action of endogenous and environmental factors. The recording and analysis of the epidemiology of the disease is important for its better study and understanding.
Keywords: Laryngeal cancer, epidemiology, tobacco, alcohol, family history, staging, grading
Introduction
Cancer of the larynx is an important entity of oncology. According to international data it accounts for 30% to 40% of all malignant head and neck tumors and 1% to 2.5% of all malignant neoplasms in the human body1,2. In terms of histopathology, 95% to 98% of cancer of the larynx is of squamous cell origin3. The disease is much more common in the male gender. The highest incidence of laryngeal cancer occurs between the fifth and seventh decade of life4,5. As far as pathogenesis is concerned, several predisposing factors have been reported, including smoking and alcohol consumption which are the most important ones. Other possible risk factors are exposure to carcinogens in the work environment, nutrition, viral infections with HPV and EBV, radiation, gastroesophageal reflux disease and heredity. The progress of molecular biology in the field of the analysis and decoding of DNA proved that a number of genes, called oncogenes, are involved in the mechanism of carcinogenesis in the larynx6,7.
The purpose of this study is to present the epidemiological data of patients diagnosed with cancer of the larynx in our department during the last 19 years. At the same time, we review the current international literature and compare our results with those from similar series abroad.
Materials and methods
The data of this study originate from the oncology records of the 1st University Otolaryngology Department of Aristotle University of Thessaloniki, in the AHEPA Teaching hospital and refer to the period 1992-2010.
During this period, 1,638 malignant head and neck tumors were diagnosed and treated. Among these, 1,104 (67.4%) were situated in the larynx. In 98.5% of these cases (1,088 patients) the histological type of the tumor was squamous cell carcinoma of the larynx, whereas only 1.5% (16 patients) were diagnosed with other rare tumors.
Parameters related to the epidemiology and the possible pathogenesis of laryngeal carcinoma were studied. In particular, we analyzed patient data such as gender, age, occupation, tobacco use, alcohol consumption and family history. Furthermore, we studied the characteristics of tumors, mainly in terms of location, staging, presence of lymph node metastasis and the degree of differentiation.
For the transfer of data and statistical processing we used the program SPSS for Windows version 10.0 (SPSS Inc., Chicago, IL, USA).
Results
Age of patients
The average age of our patients was 62.1 (± 9.4) years, with a range from 31 to 90 years. About 70% of our patients were at the 6th and 7th decade of life, whereas only 12 patients (1.1%) were younger than 40 years of age at the time of diagnosis.
Gender
From 1088 patients with carcinoma of the larynx 1,053 (96.8%) were male and 35 (3.2%) female with a men to women ratio of 30:1. Further analysis of our data showed an increasing trend in the proportion of female patients in the recent years. During the period 1992 to 1994 no female was treated for cancer of the larynx. The first woman suffering from laryngeal cancer presented in 1994. Hence the percentage of female patients rose to 1% and gradually increased to 7% in 2010 (male: female ratio 15:1).
Occupation
In terms of occupation, 356 of our patients (32.7%) were farmers and 298 (27.4%) were workers. According to the General Secretariat of the National Statistical Service of Greece, in Macedonia and Thrace, where the majority of our patients come from, the percentage of farmers in the total population is 17.5% whereas that of workers is 12.3%.
Tobacco Use
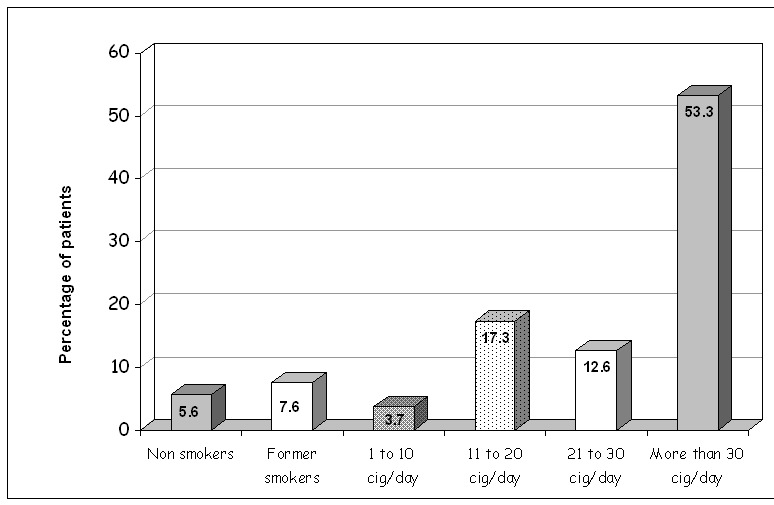
In total, 945 patients (86.9%) were active smokers, 82 (7.6%) were ex-smokers and only 61 patients (5.6%) were non smokers. More specifically, from the group of smokers, 40 patients (3.7%) smoked 1 to 10 cigarettes daily, 188 (17.3%) from 11 to 20 cigarettes, 137 (12.6%) from 21 to 30 cigarettes and finally 580 patients (53.3%) smoked more than 30 cigarettes per day (Figure 1). According to the WHO, 47% of adult men and 29% of adult women in Greece are active smokers8.
Figure 1. Use of tobacco in 1,088 laryngeal squamous cell carcinoma patients.
Alcohol Consumption
Daily alcohol consumption was reported by 470 patients (43.2%), of which 167 (15.4%) were heavy users. According to the US National Institute on Alcohol Abuse and Alcoholism (NIAAA) the definition for “heavy” alcohol use is over 5-6 drinks (12 grams of alcohol) per day for men and over 3-4 drinks per day for women. Of the remaining patients, 293 (27%) reported no consumption.
Family History
Positive oncological family history of at least one family member was noted in 312 patients (28.7%). Among these, 80 patients (7.4% of total number of patients) reported positive history in several family members. The recording of positive family history of oncological diseases applied to blood relatives up to 2nd degree (parents, children and siblings).
The lung was the most common location of malignant tumor in the relatives of our patients and concerned 17.7% of the tumors recorded.
It appears that the percentage of positive family history is higher in patients who do not smoke. More specifically, positive oncological history of at least one family member in non-smokers was documented in 45.8% of patients, whereas in smokers the percentage was 27.8% and in ex-smokers 25.8%.
Location
Regarding the location of the tumor, we recorded 633 (60.2%) cases of glottic neoplasms, 345 (32.8%) cases of supraglottic neoplasms, 62 patients (5.9%) with transglottic tumors and only 11 patients (1.1%) with purely subglottic tumors. In addition, we studied the relation between the location of the tumor and the consumption of alcohol. The incidence of supraglottic tumors showed a gradual increase in proportion to the amount of alcohol consumed. Thus, in patients with laryngeal cancer who did not consume any alcohol at all, the frequency of supraglottic tumors was 35%. The corresponding figure for patients who were classified as heavy drinkers was 50%.
Staging
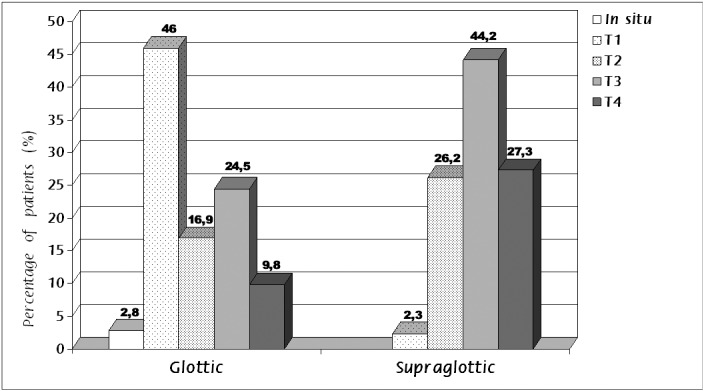
The staging of the tumor according to the TNM system, regarding the local extension of the tumor at the time of diagnosis were as follows: carcinoma in situ in 13 patients (1.2%), stage T1 in 300 patients (28%), stage T2 in 200 (19%), stage T3 in 341 (32%), and finally, stage T4 in 216 (20%). More specifically, in patients with carcinoma of the glottis 18 (2.8%) were diagnosed at stage Tis (in situ carcinoma), 291 (46%) at stage T1, 107 (16.9%) at stage T2, 155 (24.5%) at stage T3 and 62 (9.8%) at stage T4. From patients with supraglottic tumor, 8 (2.2%) were diagnosed at stage T1, 90 (26.2%) at T2, 152 (44.2%) at T3 and 95 (27.2%) at T4 (Figure 2).
Figure 2. Percentage of T stage of laryngeal squamous cell carcinomas, according to the site of the tumor.
Lymph Node Metastasis
The presence of cervical lymph node metastasis was recorded in 145 (13.3%) of 1088 patients. Sixty five individuals (45%) were staged N1, 65 (45%) N2 and 15 (10%) N3. Regarding N2 sub-staging, 38 patients were characterised as N2a, 15 as N2b and 12 as N2c.
The location of the tumor proved to influence decisively the rates of lymph node metastasis. Among the patients with glottic neoplasms, 24 (3.8%) showed the presence of metastatic lymphadenopathy at the time of diagnosis. The corresponding rates for patients with supraglottic and transglottic tumors were 28.8% and 27.9% respectively.
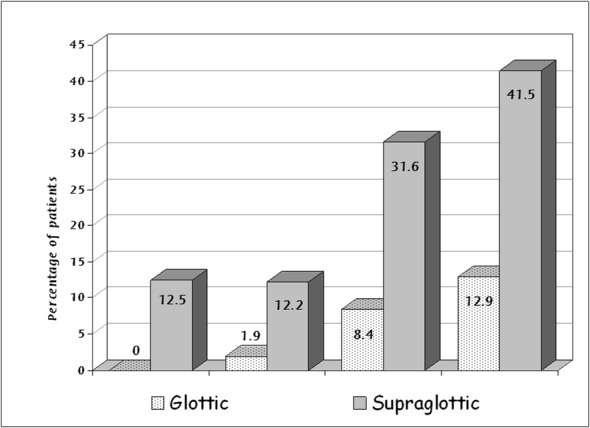
The probability of lymph node metastasis was significantly higher in tumors of advanced local stage (T3 and T4) compared to early-stage tumors (T1 and T2). This difference was more significant in supraglottic neoplasms. The presence of lymph node metastasis in glottic carcinomas was 0% for stage T1 tumors, 1.9% for T2 tumors, 8.4% for tumors T3 and 12.9% for stage T4 tumors. The corresponding rate for supraglottic carcinomas was 12.5% for T1, 12.2% for T2, 31.6% for T3 and 41.5% for stage T4 tumors (Figure 3).
Figure 3. Percentage of lymph node metastasis according to T stage and site of the tumor.
Differentiation
Study results concerning tumor differentiation were as follows: 468 tumors (43%) with good differentiation (stage G1), 458 tumors (42.1%) with moderate differentiation (stage G2) and 128 tumors (11.8%) with poor differentiation (stage G3). The remaining 34 patients (3.1%) had tumors that were carcinomas in situ, microinvasive and verrucous.
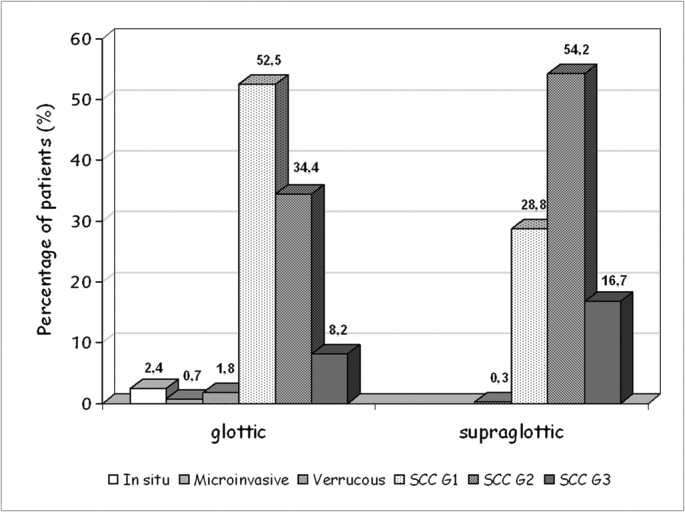
Analysis of the data showed that the location of the tumor showed a significant correlation with the degree of histological differentiation. Thus, the majority (52.5%) of glottic tumors were well differentiated (G1) while the majority of supraglottic tumors showed moderate (54.2%) and poor (16.7%) differentiation (Figure 4).
Figure 4. Degree of differentiation of carcinomas of the larynx in relation to the site of the tumor. SCC: squamous cell carcinoma, G1: well differentiated, G2: moderately differentiated, G3: poorly differentiated.
We also studied the degree of tumor differentiation in relation to the local extension of the tumor (T stage) and the presence of cervical lymph node metastasis (N stage). Among the well-differentiated tumors (G1) 39.2% were in stage T1, 16.2% in T2, 27.2% in T3 and finally, 16.4% in stage T4. On the contrary, poorly differentiated (G3) tumors were diagnosed at a more advanced stage, since 9.5% of those were diagnosed at stage T1, 24.8% at stage T2, 35.2% at T3 and 30.5% at T4.
Finally, as regards the existence of lymph node metastasis the percentage was 4.9% for neoplasms of good differentiation, 18.3% for tumors of moderate and 31.4% for tumors of poor differentiation.
Distant metastasis and Second Primary Neoplasms
Only 11 patients (1%) were diagnosed with distant metastasis, (M1). The presence of second primary neoplasms was established in 89 patients (8.18%), 17 (1.56%) having synchronous and 72 (6.62%) metachronous lesions. Respiratory and upper gastrointestinal sites were primarily affected and staging of the primary was found to be irrelevant to the incidence of second primaries.
Discussion
The last fifty years we have witnessed a significant increase in the incidence of laryngeal cancer, especially in certain countries, as well as a decrease in the rate difference between men and women. For example, studies of the past decade indicate that, in Texas the frequency in men increased from 5.6 to 9.0 per 100,000 inhabitants per year and in women from 0.5 to 1.5 per 100,000 inhabitants per year9. In Italy cancer rates were doubled in both genders10.In Poland the frequency was increased from 7.4 to 11.4 per 100,000 inhabitants per year, while in Australia no increase was observed5. In the Greek population and according to the results of this study, cancer of the larynx is the most common head and neck tumor, accounting for 67.4% of all malignant neoplasms in the anatomic region.
Cancer of the larynx is a predominantly male disease. The male to female ratio varies internationally from 30:1 to 5:1. In Europe, the ratio is 7:11,7. In recent years however, we have noted a significant increase in the percentage of women with laryngeal cancer. In the U.S. the ratio of men to women was 15:1 in 1950 and it was reduced to 4:1 in the late nineties. Wynder et al observed a reduction in the ratio of men to women between the 60s and 80s from 14.9:1 to 4.6:1 as well as an increasing frequency of supraglottic carcinomas in women3. The increasing proportion of women is mainly attributed to the increased tobacco and alcohol consumption. In our study the male to female ratio is significantly higher (30:1). Further analysis of the epidemiological data of our study, per year, shows a clear upward trend in the proportion of women with the disease. Thus, in 1994, the percentage of women in our series was only 1% to increase to 7% in 2005. This difference may be explained by the fact that in previous decades, pathogenetic factors of laryngeal carcinoma, such as tobacco and alcohol consumption were not as widespread in the Greek female population compared to the rest of Europe. It is also possible that gender hormones may play a significant role in the male predominance of cancer of the larynx11.
Tobacco is evidently the most important risk factor for the development of laryngeal carcinoma. It is commonly accepted that the risk is associated with the duration of exposure to this habit, but also the total intake dose of tobacco12. According to data from the international literature, smoking is associated with 95% of carcinomas of the larynx and is especially related to glottic cancer5. In our study, from a total of 1,088 patients with laryngeal cancer, 86.8% were smokers, 5.6% non-smokers and 7.6% ex-smokers. From the histopathological point of view, it has been proved that derivatives of the burning cigarette, and particularly nitrosamines and polycyclic aromatic hydrocarbons act as carcinogens in the laryngeal epithelium. In particular, they cause mutations in the DNA and disrupt the normal division and proliferation of cells, triggering the mechanism of carcinogenesis3. A study by Tuyns et al concerning central Europe showed that the risk for developing cancer of the larynx is 16 times greater in heavy smokers than in non-smokers13. Smoking of black (air-cured) tobacco has been associated with greater risk due to the higher exposure to carcinogens14.
Alcohol has been implicated as well, as an important risk factor in the pathogenesis of laryngeal cancer. Indeed, the relation between alcohol consumption and the development of laryngeal carcinoma is proportional to the dose and duration of exposure15. Chronic inflammation of the lining of the larynx from ethanol causes a series of mutations at gene level that disturb cell proliferation and promote carcinogenesis. The precise mechanism of cancer development is not fully defined.Ethyl alcohol is not considered a carcinogen, but acts as a co-factor which affects the local or systemic carcinogenesis through different mechanisms and at different stages, especially during initiation or promotion of carcinogenesis16. Furthermore, chronic alcoholism is associated with poor diet, changes in the immunoglobulins levels and vitamin deficiencies, particularly vitamin A and E, which are known to be important antioxidant anticancer agents3,17,18. A german study reports that from all patients with cancer of the larynx, daily alcohol consumption was noted in 85.1%, while another study shows that 76.3% of the patients consumed more than 25 gr of ethanol daily6. In our study, in a total of 1088 patients with laryngeal cancer, daily alcohol consumption was observed in 43.2% of the patients. Alcohol consumption is mainly related to the development of cancer in the supraglottic part of the larynx and the hypopharynx. A study on the population of New York for the period 1985 -1990 indicates that the risk for developing supraglottic cancer in heavy drinkers (more than 207 ml of ethanol a day) is 10 times greater than that of developing a glottic cancer19. In our series the frequency of supraglottic tumors was 35% in patients who did not consume alcohol, whereas this percentage was 50% in heavy drinkers.
Smoking and alcohol consumption are considered to act synergistically. The exact mechanism of this action has yet to be clarified5. According to data from published studies, the reduction of tobacco and alcohol use will contribute to the prevention of cancer of the larynx and their cessation will reduce the disease by 90%12.
Several epidemiological studies indicate a correlation between malignancy in the larynx and the socioconomic profile of patients. It is considered that the incidence of the disease is higher in lower-income and low educational level population. The majority of patients with cancer of the larynx are unemployed or unskilled workers and farmers without basic education20-22. A study from Germany, stated that from a total of 162 patients with cancer of the larynx 10.1% had basic education, 8.2% had higher and university education, while the remaining 81.7 were illiterate or had elementary skills training. Furthermore, among these, 20.3% lived below the poverty line6. The increased incidence of tobacco use and alcohol consumption, poor nutritional diet, lack of preventive strategies and poor sanitation are among the factors that characterize the low social level population and justify the high rates of laryngeal cancer6,22-24. In accordance with the above the majority of our patients were farmers and unskilled workers, of low educational and socioeconomic level.
Cancer of the larynx has been associated with inhaled carcinogens in the workplace, but this is not completely documented as with other cancer locations in the head and neck area. The majority of patients with cancer of the larynx are workers in chemical industries and farmers. Cement dust, fertilizers, petroleum derivatives and polycyclic aromatic hydrocarbons, wood and metal dust, varnish etc have been implicated as risk factors at work environment5,19,25. A german study indicated that 23.2% of patients with this disease have worked in manufacturing industries with the above materials for a period of more than 10 years6. The results from a recent large multicenter study for southern Europe show that exposure to formaldehyde, asbestos dust and chemical solvents in the workplace is associated with increased risk for developing laryngeal carcinoma. More specifically, exposure to chemical solvents doubles the risk for laryngeal tumor and even acts synergistically with other pathogenic factors such as smoking and alcohol25.
According to the epidemiology of cancer of the larynx, a small number of patients (5%), develop the tumor with no history of exposure to any of the known exogenous pathogenetic factors, thereby, other endogenous mechanisms such as inheritance are incriminated. Studies show that there is an inherited predisposition to laryngeal cancer26,27. The risk for this neoplasm is higher when there is a family history of cancer in a first-degree relative and is increased when the tumor is located in the head and neck region27. These patients were found to have specific histocompatibility antigens, metabolic changes and chromosomal abnormalities such as duplications or extinctions of parts of chromosomes, thus reinforcing the hypothesis of the influence of heredity26. Our study shows that from the total of 1088 patients with laryngeal carcinoma, 312 patients had positive oncology inheritance history in at least one family member (28.7%). Furthermore, a result of this study that is worthy of remark, is the high proportion of non-smoking patients with positive history of malignancy. It seems, therefore, that about half of our non-smoking patients, who lacked a significant risk factor such as smoking, had another one: heredity.
Significant differences are observed in the percentage of the anatomical position of the site of cancer in the larynx. The data of the international literature indicate the glottis as the most frequent location, while tumors found in the subglottis are considered extremely rare1,3,7. The frequency of the location however, varies considerably among countries. In a study in Uruguay, one of the countries with the highest rates in tumors of the larynx, the percentage of the supraglottic carcinomas was 60% versus 30% of the glottic ones28. In Italy the percentage is 50% for the glottis and 50% for the supraglottis. On the contrary, in Scandinavian countries and France there is a clear preponderance of glottic carcinomas at rates 85% and 65% respectively. Finally, in U.S.A., the rate of glottic tumors is 30-40%3,7. According to our data, the percentage of glottic carcinomas is significantly higher in Greece, almost double from that of supraglottic ones (60.2 to 32.8). Linking glottic carcinomas with smoking and supraglottic ones with the combination of alcohol and smoking may partly justify these differences, depending on the habits in each region.
It is also known that, depending on the degree of differentiation of cancer cells, carcinoma of the larynx is classified as highly, moderately and poorly differentiated. Tumors with lower differentiation have poorer prognosis, because of the greater risk of recurrence or metastasis28,29. This is demonstrated in our study, by the fact that the neoplasms with good differentiation had a lower percentage of cervical metastasis (4.9%) compared with the tumors with low degree of differentiation, where the percentage was 31.4%.
The mortality from laryngeal cancer also varies depending on the region, with an increasing trend worldwide. For the year 2004, 89,000 deaths from cancer of the larynx in men and 12,000 in women are reported worldwide. In southern Europe the incidence is 6.5 to 7.5 deaths per 100,000 persons per year. High mortality rates also occur in South America and particularly in Uruguay and southern Brazil1,2,7. However, in Italy, while an increase of 22% in cancer of the larynx has been observed in the last 30 years, mortality rates from the disease have been reduced by 13% for the same period. This was attributed mainly to the increased awareness and prevention measures, thus diagnosing the disease at an earlier stage10.
Although the incidence of cancer of the larynx and mortality rates are increasing, the percentage of healing is still one of the highest. Regardless of the stage, the type of treatment and the location, five-year survival is around 60%. In particular, in Europe the past 20 years the five-year survival was increased from 58% to 63%28-30. Conditions associated with decreased survival are regional or distant metastasis, advanced local disease, old age of the patient and supraglottic or subglottic location of the tumor. Depending on the stage of the disease it seems that five-year survival rates for T1 and T2 tumors are approaching 90%, while in advanced stages, they are decreasing to 30% to 50%29,30. It is proven by numerous studies that prognosis of supraglottic and subglottic carcinomas are clearly worse than the glottic ones. This is mainly due to late diagnosis and the early metastatic potential, especially in the neck28-31. More specifically, with regard to the glottic carcinomas five-year survival rates for T1 and T2 tumors is 85% to 95%, while the presence of lymph node metastasis is associated with reduction of this percentage to 60%. For T3 tumors the five-year survival rate is 65% and is reduced to 50% with the presence of lymph node metastasis. Finally, for the glottic T4 tumors that percentage is 40% and decreases to 10-30% in the presence of metastatic lymph nodes30,32. In supraglottic carcinomas five-year survival rates are substantially lower. In T1 and T2 tumors, the rate is 75%, in T3 50% and in T4 less than 30%. It is also characteristic that at the time of diagnosis 40% of patients have already metastases in the neck30,31. Jakobsen et al reported in their study that the rate of lymph node metastasis at the time of diagnosis for the glottic and supraglottic carcinomas were 1% and 29% respectively33. In accordance with that, the results of our study show that the majority of patients with glottic tumors were diagnosed at early stages: 65.7% of these patients were in stage Tis, T1 and T2, in contrast with the patients who had supraglottic tumorsof whom 71.4% belonged to stages T3 and T4. Moreover, the percentage of lymph node metastasis in the neck was 3.8% for the glottic tumors while it was 28.8% for the supraglottic ones. Finally, in relation to the biological behavior of tumor, most of the glottic carcinomas were well differentiated, while the majority of the supraglottic tumors showed moderate or poor differentiation.
Conclusions
The pathogenesis of laryngeal carcinoma is multifactorial. More specifically, it is the result of the combined action of endogenous and environmental factors. The recording and analysis of the epidemiology of a disease is important for its better study and understanding, particularly for laryngeal cancer.
Conflict of interest
None of the authors has any conflict of interest.
References
- 1.Bray F, Ferlay J, Parkin DM, Pisani P. International Agency for Research on Cancer. GLOBOCAN 2000 : Cancer incidence, mortality and prevalence worldwide. International Agency for Research on Cancer, Lyon. 2001 [Google Scholar]
- 2.Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer. 1999;80:827–841. doi: 10.1002/(sici)1097-0215(19990315)80:6<827::aid-ijc6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 3.Ballenger JJ, Snow JB. Otorhinolaryngology: head and neck surgery. 15th ed. Media, Williams & Wilkins, PA, USA. 1996 [Google Scholar]
- 4.Markou KD, Vlachtsis KC, Nikolaou AC, Petridis DG, Kouloulas AI, Daniilidis IC. Incidence and predisposing factors of pharyngocutaneous fistula formation after total laryngectomy. Is there a relationship with tumor recurrence? Eur Arch Otorhinolaryngol. 2004;261:61–67. doi: 10.1007/s00405-003-0643-6. [DOI] [PubMed] [Google Scholar]
- 5.Cattaruzza MS, Maisonneuve P, Boyle P. Epidemiology of laryngeal cancer. Eur J Cancer B Oral Oncol. 1996;32B:293–305. doi: 10.1016/0964-1955(96)00002-4. [DOI] [PubMed] [Google Scholar]
- 6.Maier H, Tisch M. Epidemiology of laryngeal cancer: results of the Heidelberg case-control study. Acta Otolaryngol Suppl. 1997;527:160–164. doi: 10.3109/00016489709124063. [DOI] [PubMed] [Google Scholar]
- 7.Wünsch Filho V. The epidemiology of laryngeal cancer in Brazil. Sao Paulo Med J. 2004;122:188–194. doi: 10.1590/S1516-31802004000500002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Regional Office for Europe. Highlights on health in Greece 2004. WHO Regional Office for Europe, Copenhagen. 2006 [Google Scholar]
- 9.DeRienzo DP, Greenberg SD, Fraire AE. Carcinoma of the larynx. Changing incidence in women. Arch Otolaryngol Head Neck Surg. 1991;117:681–684. doi: 10.1001/archotol.1991.01870180117023. [DOI] [PubMed] [Google Scholar]
- 10.Capocaccia R, Micheli A, Berrino F, Gatta G, Sant M, Ruzza MR, et al. Time trends of lung and larynx cancers in Italy. Int J Cancer. 1994;57:154–161. doi: 10.1002/ijc.2910570204. [DOI] [PubMed] [Google Scholar]
- 11.Mattox DE, Von Hoff DD, McGuire WL. Androgen receptors and antiandrogen therapy for laryngeal carcinoma. Arch Otolaryngol. 1984;110:721–724. doi: 10.1001/archotol.1984.00800370023005. [DOI] [PubMed] [Google Scholar]
- 12.Ramroth H, Dietz A, Becher H. Interaction effects and population-attributable risks for smoking and alcohol on laryngeal cancer and its subsites. A case-control study from Germany. Methods Inf Med. 2004;43:499–504. [PubMed] [Google Scholar]
- 13.Tuyns AJ, Estève J, Raymond L, Berrino F, Benhamou E, Blanchet F, et al. Cancer of the larynx/hypopharynx, tobacco and alcohol: IARC international case-control study in Turin and Varese (Italy), Zaragoza and Navarra (Spain), Geneva (Switzerland) and Calvados (France) Int J Cancer. 1988;41:483–491. doi: 10.1002/ijc.2910410403. [DOI] [PubMed] [Google Scholar]
- 14.Sancho-Garnier H, Theobald S. Black (air-cured) and blond (flue-cured) tobacco and cancer risk II: Pharynx and larynx cancer. Eur J Cancer. 1993;29A:273–276. doi: 10.1016/0959-8049(93)90192-i. [DOI] [PubMed] [Google Scholar]
- 15.Rothman KJ. The effect of alcohol consumption on risk of cancer of the head and neck. Laryngoscope. 1978;88:51–55. [PubMed] [Google Scholar]
- 16.Seitz HK, Simanowski UA. Alcohol and carcinogenesis. Annu Rev Nutr. 1988;8:99–119. doi: 10.1146/annurev.nu.08.070188.000531. [DOI] [PubMed] [Google Scholar]
- 17.Riboli E, Kaaks R, Estève J. Nutrition and laryngeal cancer. Cancer Causes Control. 1996;7:147–156. doi: 10.1007/BF00115645. [DOI] [PubMed] [Google Scholar]
- 18.Raitiola HS, Pukander JS. Etiological factors of laryngeal cancer. Acta Otolaryngol Suppl. 1997;529:215–217. doi: 10.3109/00016489709124126. [DOI] [PubMed] [Google Scholar]
- 19.Muscat JE, Wynder EL. Tobacco, alcohol, asbestos, and occupational risk factors for laryngeal cancer. Cancer. 1992;69:2244–2251. doi: 10.1002/1097-0142(19920501)69:9<2244::aid-cncr2820690906>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.Lefebvre JL, Lartigau E, Kara A, Sarini J, Dr. Sobin LH. Oral Cavity, Pharynx, and Larynx Cancer. TNM Online, John Wiley & Sons, Inc. 2003 [Google Scholar]
- 21.Boffetta P, Merletti F, Faggiano F, Migliaretti G, Ferro G, Zanetti R, et al. Prognostic factors and survival of laryngeal cancer patients from Turin, Italy. A population-based study. Am J Epidemiol. 1997;145:1100–1105. doi: 10.1093/oxfordjournals.aje.a009072. [DOI] [PubMed] [Google Scholar]
- 22.Menvielle G, Luce D, Goldberg P, Leclerc A. Smoking, alcohol drinking, occupational exposures and social inequalities in hypopharyngeal and laryngeal cancer. Int J Epidemiol. 2004;33:799–806. doi: 10.1093/ije/dyh090. [DOI] [PubMed] [Google Scholar]
- 23.Olsen J, Sabroe S. Occupational causes of laryngeal cancer. J Epidemiol Community Health. 1984;38:117–121. doi: 10.1136/jech.38.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elwood JM, Pearson JC, Skippen DH, Jackson SM. Alcohol, smoking, social and occupational factors in the aetiology of cancer of the oral cavity, pharynx and larynx. Int J Cancer. 1984;34:603–612. doi: 10.1002/ijc.2910340504. [DOI] [PubMed] [Google Scholar]
- 25.Boffetta P, Richiardi L, Berrino F, Estève J, Pisani P, Crosignani P, et al. Occupation and larynx and hypopharynx cancer: an international case-control study in France, Italy, Spain, and Switzerland. Cancer Causes Control. 2003;14:203–212. doi: 10.1023/a:1023699717598. [DOI] [PubMed] [Google Scholar]
- 26.Agudelo D, Quer M, León X, Díez S, Burgués J. Laryngeal carcinoma in patients without a history of tobacco and alcohol use. Head Neck. 1997;19:200–204. doi: 10.1002/(sici)1097-0347(199705)19:3<200::aid-hed6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 27.Wünsch-Filho V, Boffetta P, Colin D, Moncau JE. Familial cancer aggregation and the risk of lung cancer. Sao Paulo Med J. 2002;120:38–44. doi: 10.1590/S1516-31802002000200003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Stefani E, Boffetta P, Deneo-Pellegrini H, Brennan P, Correa P, Oreggia F, et al. Supraglottic and glottic carcinomas: epidemiologically distinct entities? Int J Cancer. 2004;112:1065–1071. doi: 10.1002/ijc.20501. [DOI] [PubMed] [Google Scholar]
- 29.Myers EN, Suen JY, Myers JN, Hanna EY. Cancer of the head and neck. 4th ed. Saunders, Philadelphia. 2003;340 [Google Scholar]
- 30.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 31.Teppo H, Koivunen P, Sipilä S, Jokinen K, Hyrynkangas K, Läärä E, et al. Decreasing incidence and improved survival of laryngeal cancer in Finland. Acta Oncol. 2001;40:791–795. doi: 10.1080/02841860152703391. [DOI] [PubMed] [Google Scholar]
- 32.Sasaki CT, Jassin B. Cancer of the pharynx and larynx. Am J Med. 2001;111 Suppl 8A:118S–123S. doi: 10.1016/s0002-9343(01)00850-6. [DOI] [PubMed] [Google Scholar]
- 33.Jakobsen J, Hansen O, Jørgensen KE, Bastholt L. Lymph node metastases from laryngeal and pharyngeal carcinomas--calculation of burden of metastasis and its impact on prognosis. Acta Oncol. 1998;37:489–493. doi: 10.1080/028418698430467. [DOI] [PubMed] [Google Scholar]


