Abstract
Toxoplasma gondii can cause serious public health problems and economic losses worldwide. Calcium-dependent protein kinases (CDPKs) are key mediators of T. gondii signaling pathways and are implicated as important virulence factors. In the present study, we cloned a novel T. gondii CDPK gene, named TgCDPK5, and constructed the eukaryotic expression vector pVAX-CDPK5. Then, we evaluated the immune protection induced by pVAX-CDPK5 in Kunming mice. After injection of pVAX-CDPK5 intramuscularly, immune responses, determined with lymphoproliferative assays and cytokine and antibody measurements, were monitored, and mouse survival times and brain cyst formation were evaluated following challenges with the T. gondii RH strain (genotype I) and the PRU strain (genotype II). pVAX-CDPK5 effectively induced immune responses with increased specific antibodies, a predominance of IgG2a production, and a strong lymphocyte proliferative response. The levels of gamma interferon (IFN-γ), interleukin 2 (IL-2), and IL-12(p70) and the percentages of CD3+ CD4+ and CD3+ CD8+ cells in mice vaccinated with pVAX-CDPK5 were significantly increased. However, IL-4 and IL-10 were not produced in the vaccinated mice. These results demonstrate that pVAX-CDPK5 can elicit strong humoral and cellular Th1 immune responses. The survival time of immunized mice challenged with the T. gondii RH strain (8.67 ± 4.34 days) was slightly, but not significantly, longer than that in the control groups within 7 days (P > 0.05). The numbers of brain cysts in the mice in the pVAX-CDPK5 group were reduced by ∼40% compared with those in the control groups (P < 0.05), which provides a foundation for the further development of effective subunit vaccines against T. gondii.
INTRODUCTION
Infection with the obligate intracellular protozoan parasite Toxoplasma gondii has worldwide importance (1, 2, 3). The parasite can infect human beings and virtually all food production and companion animals and thus is able to cause serious public health problems (2, 4, 5, 6). T. gondii infection in fetuses and in immunodeficient individuals, including AIDS patients, can result in severe disease and even death (1, 7, 8). When the primary T. gondii infection occurs during pregnancy, it can lead to miscarriage, severe neonatal malformations, and ocular complications in the fetus (1, 9). T. gondii infection in animals not only is a veterinary problem causing abortion in sheep and goats but also represents a real risk for humans via ingestion of tissue cysts and oocysts (8, 10, 11).
Currently, no available drug treatments can eliminate T. gondii cysts from infected hosts. Furthermore, the chemotherapeutic agents can cause consumer concerns about chemical residues in meat and also the emergence of drug-resistant parasites following long-term use (12). So, immunoprophylaxis would be extremely valuable for prevention of human and animal toxoplasmosis (13, 14). However, after more than 20 years of effort, only one licensed vaccine (Toxovax) can be used to prevent abortion in sheep, and it is based on the live attenuated tachyzoites of strain S48 (14, 15).
In recent years, DNA vaccination has been demonstrated to elicit a predominantly Th1 immune response: inducing CD4+ T-lymphocyte and CD8+ cytotoxic T-lymphocyte (CTL) responses against the administered antigen (14, 16) and several T. gondii DNA vaccine candidates evaluated using this administration route (17, 18, 19, 20, 21, 22) have shown effective protection against T. gondii infection. Also, DNA vaccines are promising tools in the development of safe and effective vaccines against T. gondii infection in both humans and animals (14), and thus it would be valuable to identify novel T. gondii antigens for use in DNA vaccination.
As signaling mediators of calcium-related signaling pathways, calcium-dependent protein kinases (CDPK) can control a diverse array of functions in the life cycle of apicomplexans, including gliding motility, cell invasion and egress, and some other critical biological processes (23). Our unpublished data and previous studies showed that both TgCDPK1 and TgCDPK3 (22) are promising vaccine candidates that can elicit protective immunity against acute and chronic T. gondii infection, but the immunogenicity of other CDPK members is not yet known.
In the present study, we cloned a novel putative CDPK gene, named TgCDPK5, from the T. gondii RH strain and then constructed the eukaryotic expression vector pVAX-CDPK5. The aims of the present study were to evaluate the various immune responses induced by pVAX-CDPK5 in Kunming mice and to analyze the potential of TgCDPK5 as a vaccine candidate against infection with the virulent RH strain of T. gondii in Kunming mice.
MATERIALS AND METHODS
Mice and parasites.
Six- to 8-week-old specific-pathogen-free (SPF) female Kunming mice were purchased from the Center of Experimental Animals, Lanzhou Institute of Biological Products, Lanzhou, China. All mice were handled in strict accordance with the good animal practice requirements of the Animal Ethics Procedures and Guidelines of the People's Republic of China.
The virulent T. gondii RH strain and the brain cyst-forming PRU strain were used in this study. Tachyzoites of the T. gondii RH strain (type I) were propagated by serial intraperitoneal passage in Kunming mice. If needed, the peritoneal fluid of mice was centrifuged for 10 min at 1,000 × g at 4°C and then resuspended in sterile phosphate-buffered saline (PBS). The obtained tachyzoites were used for total RNA extraction following the instructions in the RNAprep pure tissue kit (Tiangen, China) manual and also to prepare Toxoplasma lysate antigen (TLA) as described in our previous study (24). Cysts of the PRU strain (genotype II) were maintained in the laboratory by oral passage of infectious brain homogenates in Kunming mice.
Expression and purification of TgCDPK5 protein in Escherichia coli.
Based on the corresponding sequence of the CDPK5 gene downloaded from GenBank (accession number XM_002366128), a pair of primers, pK5F (5′-GCGAATTCATGCCAGTCGCTGCAACAAAGA-3′) and pK5R (5′-GGGCGGCCGCTCACTCATGTTGCGACTCAC-3′), were designed, into which the EcoRI and NotI restriction sites (underlined) were introduced. The complete open reading frame (ORF) was amplified by reverse transcription (RT)-PCR, and the obtained products were inserted into the prokaryotic expression vector pGEX-4T-1 via the two restriction sites, to form pGEX-CDPK5. The recombinant plasmids were transformed into the E. coli BL21(DE3) strain and grown in Luria-Bertani (LB) agar containing kanamycin (25 μg/ml). The recombinant TgCDPK5 (rTgCDPK5) protein was expressed under the optimal conditions of 1 mmol/liter isopropyl-β-d-thiogalactopyranoside (IPTG) (Sangon, China), shaking for 6 h at 37°C. The resulting bacterial pellet containing the expressed protein was disintegrated by sonication and then centrifuged. The obtained inclusion bodies were solubilized in 8 mmol/liter urea and were visualized using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Construction of the eukaryotic expression plasmid.
To obtain the eukaryotic expression plasmid, the TgCDPK5 gene was amplified using primers eK5F (5′-GCGGGTACCATGCCAGTCGCTGCAACAA-3′) and eK5R (5′-GCGAATTCTCACTCATGTTGCGACTC-3′), into which the KpnI and EcoRI restriction sites (underlined) were introduced, and was inserted into pVAX I (Invitrogen). The recombinant plasmid was named pVAX-CDPK5, and the concentration was determined spectrophotometrically. The recombinant eukaryotic plasmids were diluted with sterile phosphate-buffered saline (PBS) to a final concentration of 1 μg/μl and stored at −20°C.
Expression of pVAX-CDPK5 in vitro.
HEK 293 cells, grown in 6-well plates, were transfected with pVAX-CDPK5 using Lipofectamine 2000 reagent (Invitrogen), according to the manufacturer's instructions. Forty-eight hours after transfection, the cells were fixed with 100% acetone and then incubated with goat anti-T. gondii tachyzoite polyclonal antibody (1:50 dilution in PBS containing 0.05% Tween 20 [PBST]). Then, fluorescein isothiocyanate (FITC)-labeled rabbit anti-mouse IgG antibody (1:2000; Sigma) was added into each well. The indirect immunofluorescence assay (IFA) results were obtained using a Zeiss Axioplan fluorescence microscope (Carl Zeiss, Germany). As a negative control, the HEK 293 cells were transfected with pVAX I.
Immunization and challenge.
A total of 100 mice were randomly divided into four groups (25 per group). The mice in groups I, II, and III received empty pVAX I vector, PBS, and a blank control, respectively; the mice in groups IV were immunized with 100 μl pVAX-CDPK5 by intramuscular injection and were given booster immunizations 2 and 4 weeks later. Blood samples were collected from the tail vein from 3 mice in each group at weeks 0, 2, 4, and 6 and were centrifuged at 3,000 × g for 10 min. The sera were stored at −20°C until enzyme-linked immunosorbent assays (ELISA).
Two weeks after the third immunization, 10 mice in each group were challenged intraperitoneally with 1 × 103 tachyzoites of the virulent T. gondii RH strain, and the survival periods were recorded daily until all mice died. Three mice of each group were instead inoculated orally with 10 PRU strain tissue cysts, and the brain cysts were counted at 4 weeks after the challenge.
Immunoblotting analysis of rTgCDPK5 protein.
The immunoreactivity of the rTgCDPK5 protein was detected by Western blotting using sera from immunized mice at week 6 of the immunization protocol (diluted 1:1,000). Bound antibodies were probed with horseradish peroxidase-conjugated goat anti-mouse IgG (1:5,000; Sigma, USA), and proteins were visualized with 4-chloro-1-naphthol (4-CN) (Sangon, China). As a negative control, sera from nonvaccinated mice were used.
Antibody assays.
The specific humoral immune response against TgCDPK5 was evaluated by an ELISA using 96-well microtiter plates. In each well, 100 μl rTgCDPK5 (10 μg/ml) was coated at 4°C overnight. After blocking of the nonspecific binding sites using 5% bovine serum albumin (BSA) for 1 h at 37°C, mouse sera were added to each well and incubated at 37°C for 1 h. Then, each well was incubated with 100 μl of horseradish peroxidase (HRP)-conjugated anti-mouse IgG diluted at 1:250 or anti-mouse IgG1 or IgG2a (1:500) for 1 h. After addition of 200 μl of substrate solution (0.1 g 3,3′,5,5′-tetramethylbenzidine, 0.03% H2O2) for 30 min, the reaction was stopped with 2 M H2SO4. All measurements were performed in triplicate. The results were read at 450 nm.
Lymphocyte proliferation assay by MTS.
Two weeks after the final immunization, three mice per group were euthanized to harvest their spleens. The splenocytes were aseptically collected through a wire mesh, and then red blood cells (RBC) were removed using RBC lysis solution (Sigma, USA). The purified lymphocytes from each group were then cultured in triplicate at a density of 2 × 105 cells/well in complete medium (Dulbecco's modified Eagle's medium [DMEM] + 10% fetal calf serum [FCS] + 100 U/ml penicillin-streptomycin). For positive controls, the cells were stimulated with TLA (10 μg/ml) or concanavalin A (ConA) (5 μg/ml; Sigma), and cells to which medium alone (M) was added served as negative controls. The proliferative activity was measured using the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) method (Promega, USA) after 4 days. The stimulation index (SI) was calculated by using the formula (OD570TLA/OD570M)/(OD570ConA/OD570M), where M stands for medium and OD570 is optical density at 570 nm.
Flow cytometry.
The percentages of CD4+ T cells and CD8+ T cells in spleens were determined by flow cytometry according to previously published methods (6). The specific antigen epitope of each T subclass was stained with phycoerythrin (PE)-labeled anti-mouse CD3 (eBioscience), allophycocyanin (APC)-labeled anti-mouse CD4 (eBioscience), and fluorescein isothiocyanate (FITC)-labeled anti-mouse CD8 (eBioscience) antibodies. The cells were then fixed with FACScan buffer (PBS containing 1% BSA and 0.1% sodium azide) and 2% paraformaldehyde. All the samples were analyzed for their fluorescence profiles on a FACScan flow cytometer (BD Biosciences) using System II software (Coulter).
Cytokine assays.
The splenocytes without RBC from each group were cocultured with rTgCDPK5 or medium alone (negative control) in 96-well microtiter plates as described in previous studies (6, 25). Culture supernatants were harvested at 24 h for determination of interleukin 2 (IL-2) and IL-4, at 72 h for IL-10, and at 96 h for gamma interferon (IFN-γ) and IL-12(p70) using commercial ELISA kits according to the manufacturer's instructions (BioLegend, USA). The analyses were performed in triplicate.
Statistical analysis.
All statistical analyses were performed using GraphPad Prism 5.0 and SAS (Statistical Analysis System, version 8.0). The differences for each measurement, including antibody responses, lymphoproliferation assays, cytokine production, and percentages of CD4+ and CD8+ T cells were compared by one-way analysis of variance (ANOVA). The level of significant difference in comparisons between groups was defined as a P value of <0.05.
RESULTS
Detection of the prokaryotic and eukaryotic expression of TgCDPK5.
The expression of recombinant plasmid pVAX-CDPK5 in vitro was detected by IFA, and the result showed specific green fluorescence of transfected HEK 293 cells. No green fluorescence was revealed in the cells transfected with the same quantity of empty pVAX I. These results revealed that the TgCDPK5 protein has been successfully expressed in HEK 293 cells.
E. coli BL21(DE3) lysates from cells transfected with pGEX-CDPK5 were separated by SDS-PAGE. After staining with Coomassie brilliant blue, the rTgCDPK5 protein was observed at approximately 100 kDa, which coincided with the theoretical value.
Western blotting and antibody responses.
Analysis of the immunogenicity of rTgCDPK5 protein by Western blotting indicated that the anti-CDPK5 antibody recognized the recombinant protein at ∼100 kDa, whereas the protein did not react with the negative sera.
Serum samples from 3 randomly chosen mice in each group were collected to detect specific anti-CDPK5 antibodies by ELISA. Two weeks after the third immunization, the specific IgG antibodies reached their highest levels in mice immunized with pVAX-CDPK5 (0.31 ± 0.05); these levels were significantly higher than those in the pVAX 1 (0.15 ± 0.08), PBS (0.14 ± 0.09), and blank control (0.15 ± 0.08) groups (P < 0.05) (Fig. 1A). In order to characterize the role of humoral immunity induced by pVAX-CDPK5, the subclasses of IgG (IgG1 and IgG2a) were evaluated individually in sera of vaccinated and control mice at 2 weeks after the last immunization. As shown in Fig. 1B, the vaccine induced significant IgG1 and IgG2a antibody responses (P < 0.05) with a higher level of the antigen-specific IgG2a antibody isotype, as determined by the antibody levels. These results indicate that pVAX-CDPK5 elicited both a specific humoral response and the Th1-type immune response.
FIG 1.
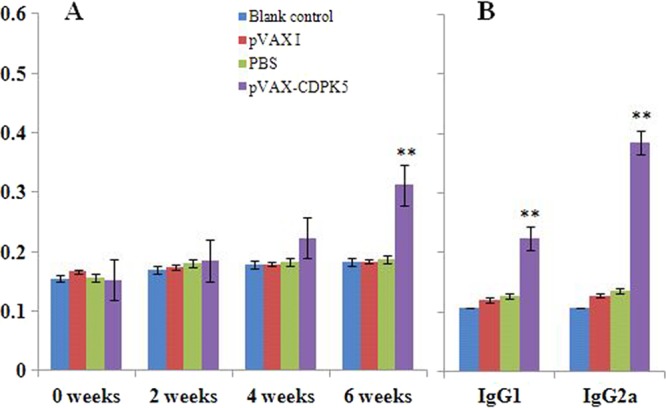
Determination of specific antibody responses induced by pVAX-CDPK5. (A) Determination of specific anti-CDPK5 IgG antibodies in the sera of Kunming mice at 0, 2, 4, and 6 weeks. (B) The specific anti-CDPK5 IgG subclass profile (IgG1 or IgG2a) in the sera of Kunming mice 2 weeks after the last immunization. Each bar represents the mean OD (± standard error [SE]; n = 3). **, statistically significant difference in OD values (P < 0.05).
Evaluation of splenocyte proliferation.
After 96 h of splenocyte/mitogen coculture, the proliferative response was identified by MTS. As shown in Fig. 2, the proliferation SI from the pVAX-CDPK5 vaccinated group (1.92 ± 0.07) was significantly increased compared with values from the three control groups, while the differences in splenocyte proliferation in the mice immunized with PBS (0.91 ± 0.01), pVAX I (0.89 ± 0.07), or the blank control (0.97 ± 0.01) were not statistically significant.
FIG 2.
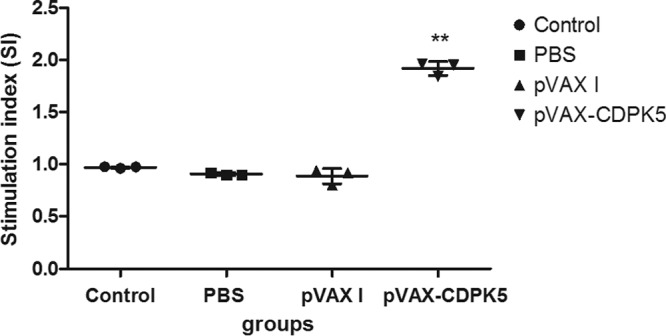
Splenocyte proliferative responses in immunized mice 2 weeks after the third immunization. **, statistically significant difference (P < 0.001).
Percentages of CD4+ and CD8+ T lymphocytes.
The levels of CD3+ CD4+ and CD3+ CD8+ T cells in each group are shown in Fig. 3. In the mice immunized with pVAX-CDPK5, the percentages of CD3+ CD4+ (17.83% ± 3.48%) (P > 0.05) and CD3+ CD8+ (8.43% ± 1.46%) (P < 0.05) T lymphocytes were higher than those in control mice. There was no difference in terms of the percentages of the two T-cell subtypes among the three control groups (P > 0.05).
FIG 3.
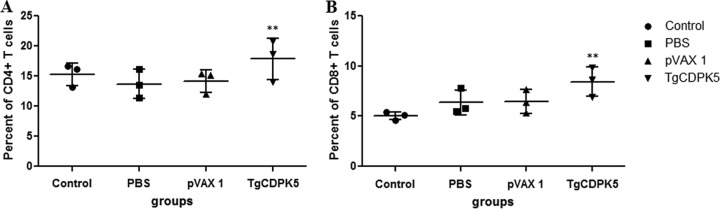
The percentages of T-cell subsets in immunized mice 2 weeks after the final immunization. **, statistically significant difference (P = 0.04).
Cytokine production by spleen cells.
Spleen cell suspensions from individual mice at 2 weeks after the third vaccination were stimulated in vitro and were tested by ELISAs. The IFN-γ, IL-12(p70), and IL-2 levels in spleen cell cultures from mice in the pVAX-CDPK5 group were significantly increased compared with those in the pVAX I, PBS, and blank control groups (P < 0.05) (Table 1). In contrast, the levels of IL-4 produced by pVAX-CDPK5-immunized mice were similar to those in mice from the three control groups (P > 0.05), and the production of IL-10 in the immunized group was significantly lower than that of the control groups (P < 0.05).
TABLE 1.
Cytokine production by splenocytes of immunized Kunming mice after stimulation with Toxoplasma lysate antigena
| Mouse group | Cytokine production (mean ± SD) (pg/ml) |
||||
|---|---|---|---|---|---|
| IFN-γ | IL-2 | IL-4 | IL-10 | IL-12(p70) | |
| pVAX-CDPK3 | 982.68 ± 378.22b | 266.04 ± 9.13b | <15 | 136.53 ± 71.63b | 96.84 ± 4.06b |
| pVAX I | 34.91 ± 11.23 | <15 | <15 | 322.50 ± 179.17 | 15.14 ± 7.63 |
| PBS | 36.34 ± 4.83 | <15 | <15 | 356.13 ± 175.27 | 22.31 ± 4.40 |
| Blank control | 47.28 ± 16.60 | <15 | <15 | 477.25 ± 87.53 | 25.29 ± 17.35 |
Splenocytes from mice were harvested 2 weeks after the final immunization.
Statistically significant difference (P < 0.05).
Protection of vaccinated mice.
To assess the protective immunity of pVAX-CDPK5, mice were challenged with 103 tachyzoites of the RH strain and 10 cysts of the PRU strain at 2 weeks after the final immunization. The average survival time of mice immunized with pVAX-CDPK5 (8.67 ± 4.34 days) was longer than that of the controls (Fig. 4), but the difference was not statistically significant from the control group survival. Mice immunized with pVAX-CDPK5 showed a 39.13% reduction in the numbers of brain cysts compared with those in the mice in the control groups (P < 0.05) (Fig. 5).
FIG 4.
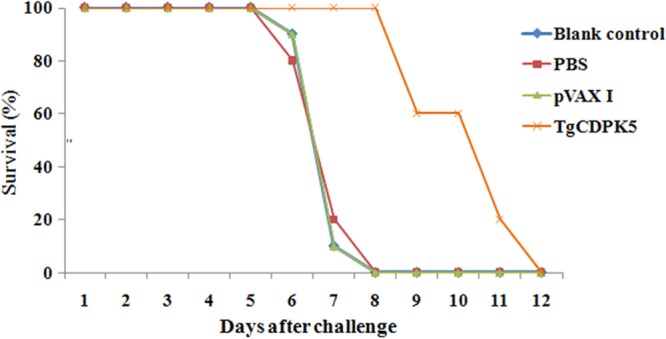
Survival rates of mice immunized with pVAX-CDPK5, pVAX I, and PBS and untreated mice followed by challenge with 1 × 103 tachyzoites 2 weeks after the final immunization.
FIG 5.
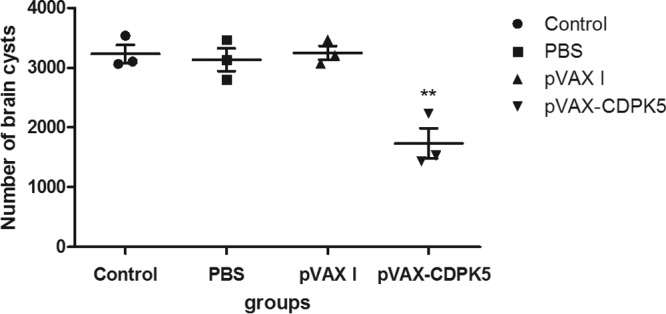
Protection against cerebral toxoplasmosis in pVAX-CDPK5-immunized mice and controls after challenge with 10 tissue cysts of the T. gondii PRU strain 2 weeks after the last booster immunization. **, statistically significant difference (P = 0.005).
DISCUSSION
The CDPK proteins were identified as key components of the signaling pathways that can regulate many crucial events in apicomplexan parasites. Previous studies showed that TgCDPK1 is involved in regulating micromere secretion, parasite motility, and host cell invasion and egress (26), TgCDPK3 is important in the calcium-dependent egress of parasites from host cells but not in motility or host cell invasion (27, 28), and TgCDPK7 was crucial for parasite division, growth, and proper maintenance of centrosome integrity (29). The comparative genomic and phylogenetic analyses revealed that T. gondii harbors 11 CDPK-like genes (30), but, for the most part, their immunogenicity has not been studied. In the present study, we cloned TgCDPK5 and then evaluated the gene as a potential DNA vaccine candidate against T. gondii infection for the first time. Although this DNA vaccine did not effectively protect mice from a lethal challenge, it did prolong the survival time (8.67 ± 4.34 days) and reduced the numbers of brain cysts in immunized mice (39.13%) in contrast with values for the control mice (P < 0.05).
Specific IgG antibodies against T. gondii can inhibit the parasite, facilitating its attachment to host cell receptors and promoting macrophages to kill intracellular parasites, which may be important in controlling T. gondii infection and preventing reactivation (31). Our results showed that the level of anti-T. gondii IgG antibodies in mice immunized with pVAX-CDPK5 was statistically significantly elevated compared with levels in the three control groups. However, antibody titers in sera from the immunized mice, following three immunizations, were only twice as high as those in control mice, an antibody level which is much lower than those elicited by eukaryotic initiation factor 4A (eIF4A), rhoptry protein 18 (ROP18), microneme protein 6 (MIC6), and other T. gondii vaccine candidates (18, 22, 24, 25, 32, 33). Because IgG-dependent phagocytosis, cytotoxicity, or complement-mediated lysis effects are crucial mechanisms for resistance to tachyzoites (31), the relatively low antibody levels resulting from DNA immunization with pVAX-CDPK5 failed to prevent acute infection.
T-cell-mediated adaptive immune responses are well known to determine the course of T. gondii infection (34, 35, 36). In the present study, a significant proliferative response of splenocytes was induced following DNA immunization with pVAX-CDPK5, indicating an activated cellular immune response. Furthermore, similar to the results from DNA vaccination with eIF4A (24) and ROP18 (37), the percentages of both CD4+ T and CD8+ T cells were also increased in pVAX-CDPK5 immunized mice, which may have contributed to a T. gondii-specific CTL response.
It has been established that the Th1-type immune response plays a critical role in protective immunity against T. gondii (38). In this study, we have further evaluated the Th1 and/or Th2 immune responses in mice immunized with pVAX-CDPK5. Thus, the high ratio of IgG2a to IgG1 antibody titers and Th1-biased cytokine production [IFN-γ, IL-12(p70) and IL-2] indicated that the DNA vaccine is capable of stimulating Th1-dominated immune responses. Furthermore, IL-4 and IL-10 cytokines, associated with Th2 responses, have been shown to play important roles during the early phase of acute T. gondii infection (39). However, the low levels of IL-4 and IL-10 in the immunized mice compared with those in the controls may not promote sufficient mast cell responses and B-cell proliferation, consistent with the relatively low level of specific IgG antibodies. These results may further explain the inability of pVAX-CDPK5 to protect against acute T. gondii infection.
Investigation of the expression profiles of CDPKs in three archetypal T. gondii lineages by microarray analysis showed higher TgCDPK5 gene expression in the PRU strain than in the RH strain (http://toxodb.org). Further analysis of cell cycle expression profiles based on the ME49 strain revealed that the expression of TgCDPK5 fluctuated in cells, being higher during the S phase and through cytokinetic periods (S/M phases) and then declining in the G1 period (40). The expression level of the gene increased 2.7-fold in intracellular bradyzoites (12 h) compared with that in cells at 0 h (40). These expression profiles further explained the reduced occurrence of brain cysts in the vaccinated group.
Taken together, our results have demonstrated, for the first time, that immunization with pVAX-CDPK5 can generate humoral and cellular Th1 immune responses, reduce brain cyst burden significantly, and also prolong the survival time (albeit in a limited fashion). These effects suggest that this gene is a potential vaccine candidate for inclusion in a multicomponent T. gondii vaccine against toxoplasmosis.
ACKNOWLEDGMENTS
Project support was provided by the National Natural Science Foundation of China (grants 31101812, 31172316, and 31230073) and the Science Fund for Creative Research Groups of Gansu Province (grant 1210RJIA006).
We thank Alasdair Nisbet of the Moredun Research Institute, Scotland, for his assistance in improving and copy-editing the manuscript.
Footnotes
Published ahead of print 30 April 2014
REFERENCES
- 1.Dubey JP. 2010. Toxoplasmosis of animals and humans, 2nd ed, p 1–313 CRC Press Inc., Boca Raton, FL [Google Scholar]
- 2.Robert-Gangneux F, Darde ML. 2012. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin. Microbiol. Rev. 25:264–296. 10.1128/CMR.05013-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou P, Chen N, Zhang RL, Lin RQ, Zhu XQ. 2008. Food-borne parasitic zoonoses in China: perspective for control. Trends Parasitol. 24:190–196. 10.1016/j.pt.2008.01.001 [DOI] [PubMed] [Google Scholar]
- 4.Gebremedhin EZ, Abebe AH, Tessema TS, Tullu KD, Medhin G, Vitale M, Di Marco V, Cox E, Dorny P. 2013. Seroepidemiology of Toxoplasma gondii infection in women of child-bearing age in central Ethiopia. BMC Infect. Dis. 13:101. 10.1186/1471-2334-13-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang HH, Huang SY, Zhou DH, Zhang XX, Su CL, Deng SZ, Zhu XQ. 2013. Genetic characterization of Toxoplasma gondii from pigs from different localities in China by PCR-RFLP. Parasit. Vectors 6:227. 10.1186/1756-3305-6-227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian YM, Dai FY, Huang SY, Deng ZH, Duan G, Zhou DH, Yang JF, Weng YB, Zhu XQ, Zou FC. 2012. First report of Toxoplasma gondii seroprevalence in peafowls in Yunnan Province, Southwestern China. Parasit. Vectors 5:205. 10.1186/1756-3305-5-205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xavier GA, Cademartori BG, Cunha Filho NA, Farias NA. 2013. Evaluation of seroepidemiological toxoplasmosis in HIV/AIDS patients in the south of Brazil. Rev. Inst. Med. Trop. Sao Paulo 55:25–30. 10.1590/S0036-46652013000100005 [DOI] [PubMed] [Google Scholar]
- 8.Zhou P, Chen ZG, Li HL, Zheng HH, He SY, Lin RQ, Zhu XQ. 2011. Toxoplasma gondii infection in humans in China. Parasit. Vectors 4:165. 10.1186/1756-3305-4-165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubey JP, Jones JL. 2008. Toxoplasma gondii infection in humans and animals in the United States. Int. J. Parasitol. 38:1257–1278. 10.1016/j.ijpara.2008.03.007 [DOI] [PubMed] [Google Scholar]
- 10.Cenci-Goga BT, Rossitto PV, Sechi P, McCrindle CM, Cullor JS. 2011. Toxoplasma in animals, food, and humans: an old parasite of new concern. Foodborne Pathog. Dis. 8:751–762. 10.1089/fpd.2010.0795 [DOI] [PubMed] [Google Scholar]
- 11.Elmore SA, Jones JL, Conrad PA, Patton S, Lindsay DS, Dubey JP. 2010. Toxoplasma gondii: epidemiology, feline clinical aspects, and prevention. Trends Parasitol. 26:190–196. 10.1016/j.pt.2010.01.009 [DOI] [PubMed] [Google Scholar]
- 12.Vercruysse J, Knox DP, Schetters TP, Willadsen P. 2004. Veterinary parasitic vaccines: pitfalls and future directions. Trends Parasitol. 20:488–492. 10.1016/j.pt.2004.07.009 [DOI] [PubMed] [Google Scholar]
- 13.Innes EA. 2010. Vaccination against Toxoplasma gondii: an increasing priority for collaborative research? Expert Rev. Vaccines 9:1117–1119. 10.1586/erv.10.113 [DOI] [PubMed] [Google Scholar]
- 14.Zhang NZ, Chen J, Wang M, Petersen E, Zhu XQ. 2013. Vaccines against Toxoplasma gondii: new developments and perspectives. Expert Rev. Vaccines 12:1287–1299. 10.1586/14760584.2013.844652 [DOI] [PubMed] [Google Scholar]
- 15.Buxton D, Innes EA. 1995. A commercial vaccine for ovine toxoplasmosis. Parasitology 110(suppl):S11–S16. 10.1017/S003118200000144X [DOI] [PubMed] [Google Scholar]
- 16.Jongert E, Roberts CW, Gargano N, Forster-Waldl E, Petersen E. 2009. Vaccines against Toxoplasma gondii: challenges and opportunities. Mem. Inst. Oswaldo Cruz 104:252–266. 10.1590/S0074-02762009000200019 [DOI] [PubMed] [Google Scholar]
- 17.Meng M, He S, Zhao G, Bai Y, Zhou H, Cong H, Lu G, Zhao Q, Zhu XQ. 2012. Evaluation of protective immune responses induced by DNA vaccines encoding Toxoplasma gondii surface antigen 1 (SAG1) and 14-3-3 protein in BALB/c mice. Parasit. Vectors 5:273. 10.1186/1756-3305-5-273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng GH, Yuan ZG, Zhou DH, He XH, Liu MM, Yan C, Yin CC, He Y, Lin RQ, Zhu XQ. 2009. Toxoplasma gondii microneme protein 6 (MIC6) is a potential vaccine candidate against toxoplasmosis in mice. Vaccine 27:6570–6574. 10.1016/j.vaccine.2009.08.043 [DOI] [PubMed] [Google Scholar]
- 19.Wang PY, Yuan ZG, Petersen E, Li J, Zhang XX, Li XZ, Li HX, Lv ZC, Cheng T, Ren D, Yuan ZG, Lin RQ, Zhu XQ. 2012. Protective efficacy of a Toxoplasma gondii Rhoptry protein 13 plasmid DNA vaccine in mice. Clin. Vaccine Immunol. 19:1916–1920. 10.1128/CVI.00397-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan HK, Yuan ZG, Song HQ, Petersen E, Zhou Y, Ren D, Zhou DH, Li HX, Lin RQ, Yang GL, Zhu XQ. 2012. Vaccination with a DNA vaccine coding for perforin-like protein 1 and MIC6 induces significant protective immunity against Toxoplasma gondii. Clin. Vaccine Immunol. 19:684–689. 10.1128/CVI.05578-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan ZG, Zhang XX, Lin RQ, Petersen E, He S, Yu M, He XH, Zhou DH, He Yong Li HX, Liao M, Zhu XQ. 2011. Protective effect against toxoplasmosis in mice induced by DNA immunization with gene encoding Toxoplasma gondii ROP18. Vaccine 29:6614–6619. 10.1016/j.vaccine.2011.06.110 [DOI] [PubMed] [Google Scholar]
- 22.Zhang NZ, Huang SY, Zhou DH, Chen J, Xu Y, Tian WP, Lu J, Zhu XQ. 2013. Protective immunity against Toxoplasma gondii induced by DNA immunization with the gene encoding a novel vaccine candidate: calcium-dependent protein kinase 3. BMC Infect. Dis. 13:512. 10.1186/1471-2334-13-512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sibley LD. 2011. Invasion and intracellular survival by protozoan parasites. Immunol. Rev. 240:72–91. 10.1111/j.1600-065X.2010.00990.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J, Huang SY, Li ZY, Yuan ZG, Zhou DH, Petersen E, Zhang NZ, Zhu XQ. 2013. Protective immunity induced by a DNA vaccine expressing eIF4A of Toxoplasma gondii against acute toxoplasmosis in mice. Vaccine 31:1734–1739. 10.1016/j.vaccine.2013.01.027 [DOI] [PubMed] [Google Scholar]
- 25.Li J, Han Q, Gong P, Yang T, Ren B, Li S, Zhang X. 2012. Toxoplasma gondii rhomboid protein 1 (TgROM1) is a potential vaccine candidate against toxoplasmosis. Vet. Parasitol. 184:154–160. 10.1016/j.vetpar.2011.08.014 [DOI] [PubMed] [Google Scholar]
- 26.Lourido S, Shuman J, Zhang C, Shokat KM, Hui R, Sibley LD. 2010. Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma. Nature 465:359–362. 10.1038/nature09022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCoy JM, Whitehead L, van Dooren GG, Tonkin CJ. 2012. TgCDPK3 regulates calcium-dependent egress of Toxoplasma gondii from host cells. PLoS Pathog. 8:e1003066. 10.1371/journal.ppat.1003066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garrison E, Treeck M, Ehret E, Butz H, Garbuz T, Oswald BP, Settles M, Boothroyd J, Arrizabalaga G. 2012. A forward genetic screen reveals that calcium-dependent protein kinase 3 regulates egress in Toxoplasma. PLoS Pathog. 8:e1003049. 10.1371/journal.ppat.1003049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morlon-Guyot J, Berry L, Chen CT, Gubbels MJ, Lebrun M, Daher W. 2014. The Toxoplasma gondii calcium-dependent protein kinase 7 is involved in early steps of parasite division and is crucial for parasite survival. Cell Microbiol. 16:95–114. 10.1111/cmi.12186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagamune K, Sibley LD. 2006. Comparative genomic and phylogenetic analyses of calcium ATPases and calcium-regulated proteins in the apicomplexa. Mol. Biol. Evol. 23:1613–1627. 10.1093/molbev/msl026 [DOI] [PubMed] [Google Scholar]
- 31.Sayles PC, Gibson GW, Johnson LL. 2000. B cells are essential for vaccination-induced resistance to virulent Toxoplasma gondii. Infect. Immun. 68:1026–1033. 10.1128/IAI.68.3.1026-1033.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui X, Lei T, Yang D, Hao P, Li B, Liu Q. 2012. Toxoplasma gondii immune mapped protein-1 (TgIMP1) is a novel vaccine candidate against toxoplasmosis. Vaccine 30:2282–2287. 10.1016/j.vaccine.2012.01.073 [DOI] [PubMed] [Google Scholar]
- 33.Liu MM, Yuan ZG, Peng GH, Zhou DH, He XH, Yan C, Yin CC, Lin RQ, Song HQ, Zhu XQ. 2010. Toxoplasma gondii microneme protein 8 (MIC8) is a potential vaccine candidate against toxoplasmosis. Parasitol. Res. 106:1079–1084. 10.1007/s00436-010-1742-0 [DOI] [PubMed] [Google Scholar]
- 34.Jordan KA, Hunter CA. 2010. Regulation of CD8+ T cell responses to infection with parasitic protozoa. Exp. Parasitol. 126:318–325. 10.1016/j.exppara.2010.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A. 1992. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J. Immunol. 149:175–180 [PubMed] [Google Scholar]
- 36.Brown CR, McLeod R. 1990. Class I MHC genes and CD8+ T cells determine cyst number in Toxoplasma gondii infection. J. Immunol. 145:3438–3441 [PubMed] [Google Scholar]
- 37.Yuan ZG, Zhang XX, He XH, Petersen E, Zhou DH, He Y, Lin RQ, Li XZ, Chen XL, Shi XR, Zhong XL, Zhang B, Zhu XQ. 2011. Protective immunity induced by Toxoplasma gondii rhoptry protein 16 against toxoplasmosis in mice. Clin. Vaccine Immunol. 18:119–124. 10.1128/CVI.00312-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sibley LD, Adams LB, Krahenbuhl JL. 1993. Macrophage interactions in toxoplasmosis. Res. Immunol. 144:38–40. 10.1016/S0923-2494(05)80095-1 [DOI] [PubMed] [Google Scholar]
- 39.Bessieres MH, Swierczynski B, Cassaing S, Miedouge M, Olle P, Sequela JP, Pipy B. 1997. Role of IFN-gamma, TNF-alpha, IL4 and IL10 in the regulation of experimental Toxoplasma gondii infection. J. Eukaryot. Microbiol. 44:87S. 10.1111/j.1550-7408.1997.tb05800.x [DOI] [PubMed] [Google Scholar]
- 40.Behnke MS, Wootton JC, Lehmann MM, Radke JB, Lucas O, Nawas J, Sibley LD, White MW. 2010. Coordinated progression through two subtranscriptomes underlies the tachyzoite cycle of Toxoplasma gondii. PLoS One 5:e12354. 10.1371/journal.pone.0012354 [DOI] [PMC free article] [PubMed] [Google Scholar]


