Abstract
From August 2011 to February 2012, an outbreak caused by type 2 circulating vaccine-derived poliovirus (cVDPV) occurred in Aba County, Sichuan, China. During the outbreak, four type 2 VDPVs (≥0.6% nucleotide divergence in the VP1 region relative to the Sabin 2 strain) were isolated from 3 patients with acute flaccid paralysis (AFP) and one close contact. In addition, a type 2 pre-VDPV (0.3% to 0.5% divergence from Sabin 2) that was genetically related to these type 2 VDPVs was isolated from another AFP patient. These 4 patients were all unimmunized children 0.7 to 1.1 years old. Nucleotide sequencing revealed that the 4 VDPV isolates differed from Sabin 2 by 0.7% to 1.2% in nucleotides in the VP1 region and shared 5 nucleotide substitutions with the pre-VDPV. All 5 isolates were closely related, and all were S2/S3/S2/S3 recombinants sharing common recombination crossover sites. Although the two major determinants of attenuation and temperature sensitivity phenotype of Sabin 2 (A481 in the 5′ untranslated region and Ile143 in the VP1 protein) had reverted in all 5 isolates, one VDPV (strain CHN16017) still retained the temperature sensitivity phenotype. Phylogenetic analysis of the third coding position of the complete P1 coding region suggested that the cVDPVs circulated locally for about 7 months following the initiating oral poliovirus vaccine (OPV) dose. Our findings reinforce the point that cVDPVs can emerge and spread in isolated communities with immunity gaps and highlight the emergence risks of type 2 cVDPVs accompanying the trivalent OPV used. To solve this issue, it is recommended that type 2 OPV be removed from the trivalent OPV or that inactivated polio vaccine (IPV) be used instead.
INTRODUCTION
The use of attenuated oral poliovirus vaccine (OPV) for more than 6 decades has reduced the worldwide incidence of polio by >99%; type 2 wild poliovirus (WPV) was eliminated globally in 1999 (1), no type 3 WPV has been reported since 2012, and the transmission of type 1 WPV is now endemic only in Nigeria, Pakistan, and Afghanistan. But with the successful application of OPV immunization, the emergence of vaccine-derived polioviruses (VDPVs) has become a major public health concern, because VDPVs resemble WPVs biologically, can cause paralytic disease in humans, and have the capacity for sustained person-to-person circulation (2, 3). VDPVs were defined as having 1% to 15% nucleotide differences from their parental Sabin strains in the VP1 capsid region of polioviruses at the 17th Informal Consultation on the Global Polio Laboratory Network held in 2011. This arbitrary demarcation represents ∼1 year of poliovirus circulation after the initiating OPV dose (4), but it does not imply that poliovirus isolates having <1% nucleotide divergence would lack the capacity to cause paralytic illness in humans or sustain transmission in poorly immunized populations.
For polio eradication programs, the derivatives of the OPV-related polioviruses have been classified into 4 categories (2, 3): (i) vaccine-related polioviruses, which exhibit the closest sequence relationships to the ancestral Sabin OPV strains and include most of the polioviruses isolated from acute flaccid paralysis (AFP) surveillance systems in polio-free countries; (ii) circulating vaccine-derived polioviruses (cVDPVs), which are associated with sustained person-to-person transmission and have caused paralytic cases in which related but nonidentical viruses have been isolated (3); (iii) immunodeficiency vaccine-derived polioviruses (iVDPVs), which are known to be excreted for a prolonged period from the same immunodeficient patient (5, 6); and (iv) ambiguous VDPVs (aVDPVs), which include other VDPVs that cannot be classified into the above 2 VDPV categories. These are viruses that either have been isolated from a single patient without immunodeficiency or are environmental isolates with an unidentified source (2, 7).
The risk for emergence of cVDPVs may be the highest for poliovirus type 2 (8, 9) due to the following. (i) Compared with types 1 and 3, the type 2 OPV strain appears to spread most readily to unimmunized people, as shown by the much higher seroprevalence of poliovirus type 2 found among unvaccinated individuals (10, 11). (ii) Because paralytic attack rates for type 2 poliovirus infections are low, circulation of type 2 VDPVs is the most difficult to detect by AFP surveillance (3). (iii) Type 2 WPV has been eliminated worldwide, suggesting a decrease in the natural immunity of the population to type 2 polioviruses (2). Meanwhile, data from several countries indicated that type 2 vaccine-related viruses from different immunologically normal individuals clustered by serotype, time, or place and followed a common evolutionary pathway (sharing some mutations in the VP1 region) suggestive of type 2 vaccine-related virus circulation occurrence, yet their sequence divergence from the Sabin strain does not exceed 1% (9, 12). Consequently, for avoiding inadequacies of the current cVDPV definition used in the Global Polio Eradication Initiative, which may result in underestimates of the scope of some outbreaks, the World Health Organization (WHO) redefines type 2 VDPV as type 2 polioviruses which differ from Sabin 2 strain by >0.6% in the VP1 region, and all the polioviruses isolated after January 2010 which meet this new definition will be classified as type 2 VDPVs.
In this report, we describe the basic genomic and phenotypic characteristics of type 2 cVDPVs in Aba County, Sichuan, China, during August 2011 to February 2012. This was the first type 2 cVDPV outbreak under the new definition in China. This outbreak was localized and limited in its circulation extent and time.
MATERIALS AND METHODS
Ethics statement.
This study did not involve human participants or human experimentation; the only human materials used were stool samples collected from AFP patients and healthy children at the instigation of the Ministry of Health, People's Republic of China, for public health purposes, and written informed consent for the use of their clinical samples was obtained from the parents of the children involved in this study. This study was approved by the second session of the Ethics Review Committee of the National Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention.
Poliovirus isolate classification.
In this study, poliovirus isolates are classified as follows: VDPV, Sabin 2-related PV with ≥6 VP1 nucleotide substitutions (≥0.6% divergent); pre-VDPV, Sabin 2-related PV with 3 to 5 VP1 nucleotide substitutions (0.3 to 0.5% divergent), clustered with VDPV geographically and temporally and following the same evolutionary pathway as VDPV. All VDPV and pre-VDPV isolates were included in the description of the type 2 cVDPV outbreak.
Virus isolation and characterization.
Polioviruses were isolated by culture in RD cells (human rhabdomyosarcoma cells) and L20B cells (mouse L cells expressing the human CD155 poliovirus receptor [PVR]) and identified by microneutralization with type-specific antisera. Poliovirus isolates were subjected to intratypic differentiation directly using VP1 region sequencing. The complete genomes of five type 2 poliovirus isolates were sequenced as described previously (13).
Phylogenetic analysis.
VP1 sequence alignments were performed with MEGA software, and phylogenetic trees were constructed by the neighbor-joining method with Kimura's two-parameter method (version 5.0; Sudhir Kumar, Arizona State University, Tempe, AZ, USA) (14). The reliability of the tree was estimated with 1,000 bootstrap replicates.
Assay for temperature sensitivity.
Temperature sensitivities of five Sichuan type 2 cVDPV strains were assayed on monolayers of RD cells in 24-well plates as described previously (15). Briefly, the 24-well plates were inoculated with 50 μl of undiluted virus stocks (P2/Sabin and 5 Sichuan strains). Two different incubators were used; the temperature of one incubator was adjusted to 36°C (optimal temperature for virus propagation), while the other incubator was adjusted to 39.5°C (supraoptimal temperature for virus propagation). After absorption at 36°C or at 39.5°C for 1 h, the unabsorbed viruses were removed, 100 μl of maintenance medium was added to each well, and the plates were continually incubated at set temperatures separately. After 8 h and 24 h postinfection, the plates were harvested, and the 50% cell culture infectious dose (CCID50) was calculated by the endpoint dilution method on RD cell monolayers in 96-well plates at 36°C. Virus isolates showing more than 2-logarithm reductions in titers at different temperatures were considered to be temperature sensitive.
Estimation of the date of the initiating OPV dose.
The sequence relationships in the 3rd coding position (closely approximating synonymous substitutions) of the P1 coding region (VP1 to VP4 regions) of all five type 2 isolates were summarized in a phylogenetic tree constructed by Bayesian Markov chain Monte Carlo (MCMC) analysis using the BEAST program (version 1.6.1) (16). The tree was rooted to the Sabin 2 strain, and the time of the initiating OPV dose and divergence of different VDPV branches was estimated from the rate of 3rd-coding-position substitution in the P1 coding region inferred by the MCMC method. The phylogenetic tree was displayed and edited using Figtree software.
Nucleotide sequence accession numbers.
The complete genome sequences of the five type 2 Sichuan cVDPV strains described in this study have been deposited in the GenBank database under the accession numbers KJ419273 to KJ419277.
RESULTS
Epidemiologic and clinical background.
Circulation of indigenous wild poliovirus (WPV) ceased in China in 1994 (17); along with the other countries in the Western Pacific region, China was certified as free of indigenous WPV in 2000 (18). However, the polio-free status was interrupted by the importation of type 1 WPV from Pakistan in August 2011; the outbreak was stopped 1.5 months later (19).
In that same year, four type 2 cVDPVs occurred in Aba County of Sichuan, China (20). The first patient developed paralysis on 20 August 2011, and a type 2 pre-VDPV was isolated. During October 2011 to February 2012, 3 other AFP patients also yielded type 2 VDPV isolates. In addition, a type 2 VDPV was isolated from a 4-year-old contact (Table 1). All 4 case patients were unimmunized children 0.7 to 1.1 years old, and all cases were diagnosed as polio by an expert panel.
TABLE 1.
Acute flaccid paralysis and contact cases associated with type 2 cVDPV in Sichuan, China
| Case | Age (yr)/sex | Source | No. of doses of OPV | Date of onset | Date of stool specimen collection | Diagnosis | No. (%) of VP1 nucleotide substitutions | Virus type |
|---|---|---|---|---|---|---|---|---|
| CHN15261 | 0.7/male | AFP | 0 | 20 Aug 2011 | 20 Sep 2011 | Laboratory-confirmed polio case | 5 (0.6) | Pre-VDPV |
| CHN15284 | 0.9/female | AFP | 0 | 15 Oct 2011 | 18 Oct 2011 | Laboratory-confirmed polio case | 6 (0.7) | VDPV |
| CHN16003 | 1.1/female | AFP | 0 | 7 Jan 2012 | 19 Jan 2012 | Laboratory-confirmed polio case | 8 (0.9) | VDPV |
| CHN16017 | 0.8/male | AFP | 0 | 6 Feb 2012 | 23 Feb 2012 | Laboratory-confirmed polio case | 11 (1.2) | VDPV |
| CHN16019c | 4.0/male | Contact of case CHN16003 | Unknown | 8 Feb 2012 | 8 Feb 2012 | Non-AFP | 10 (1.1) | VDPV |
Aba County, located in the mountainous area of north Sichuan province, has a large area but a sparse population (83,426 square kilometers with a total population of 0.813 million), which limited the delivery of quality routine immunization services. The annual reported routine immunization coverage of trivalent OPV is more than 95% since 2008 in Aba County. Moreover, an immunization coverage survey of 210 children less than 5 years old in the county after the cVDPV outbreak found that 91% had received three or more doses of OPV. Therefore, immunity gaps may be an important reason for the type 2 cVDPV outbreak (20).
Nucleotide sequence and phylogenetic analysis.
Isolate CHN15261 is identified as pre-VDPV because it differs from Sabin 2 at 5 nucleotides in the VP1 region. The isolates CHN15284, CHN16003, CHN16017, and CHN16019c were classified as VDPVs because they differed from Sabin 2 by 0.7% to 1.2% (Table 1). All five isolates shared 5 nucleotide substitutions and were closely related, indicating that they were involved in the same transmission link.
Two nucleotide substitutions identified as key determinants of the attenuated phenotype of the Sabin 2 strain (21) (an A-to-G reversion at nucleotide [nt] 481 in the 5′ untranslated region and a U-to-C reversion at nt 2909 in the VP1 coding region, which caused an Ile143Thr substitution in VP1) had reverted in all five Sichuan type 2 cVDPV strains.
In order to elucidate the divergence and evolution of the Sichuan type 2 cVDPVs, the VP1 sequences of the following viruses were analyzed: 5 Sichuan type 2 cVDPV strains in this study, sporadic type 2 VDPV identified in China earlier (GenBank accession numbers AY948201 and HM107832–HM107835) (7, 22), Egypt type 2 cVDPVs (GenBank accession numbers AF448782 and AF448783) (23), Madagascar type 2 cVDPVs (GenBank accession numbers AM084223 and AM084225) (24), and Nigeria type 2 cVDPVs (GenBank accession numbers JX274980, JX274985, JX275162, and JX275380) (9).
The phylogenetic tree based on the entire VP1 coding region revealed that all five Sichuan type 2 cVDPV strains were much more closely related to each other than to other cVDPVs, could be grouped into a single cluster with pathways of divergence different from Sabin 2 strain, and showed limited divergence from Sabin 2, unlike other cVDPVs in the tree (Fig. 1).
FIG 1.

Neighbor-joining tree showing phylogenetic sequence relationships in the VP1 coding region of Sichuan type 2 cVDPVs. Circulating VDPVs isolates are from Egypt (AF448782 and AF448783), Madagascar (AM084223 and AM084225), and Nigeria (JX275128, JX275219, JX275329, and JX275370). Ambiguous type 2 VDPV isolates (AY948201 and HM107832 to HM107835) are from China. Bootstrap values of >80% for each cluster are shown at the branch nodes.
Genomic recombination structure of isolates.
Sequencing of the full-length genome of all five Sichuan type 2 cVDPV strains revealed that they were all S2/S3/S2/S3 recombinants sharing common recombination crossover sites, suggesting that these isolates were derived from a common recombinant ancestor strain. The first crossover site mapped to the middle of the 2C region (S2/S3, between nt 4504 and 4508), the second crossover site mapped to the 3′ end of the 2C region (S3/S2, between nt 4771 and 4772), and the third crossover site mapped to the 3′ end of the 3A region (S2/S3, between nt 5257 and 5270) (Fig. 2; also, see Fig. S1 in the supplemental material).
FIG 2.
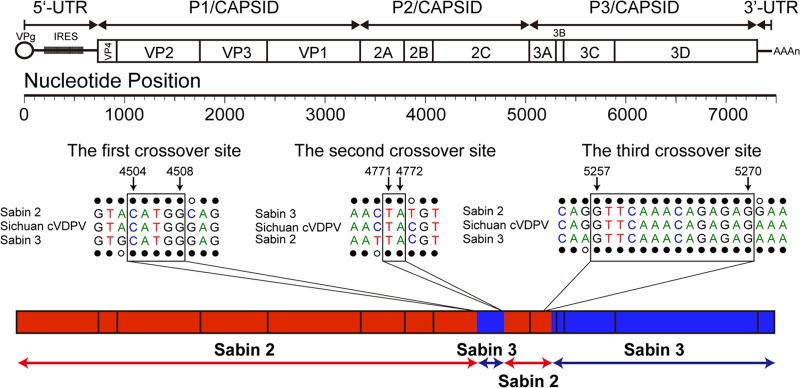
Schematic diagram of complete genome of poliovirus and the recombinant structure. The nucleotide positions of the 3 crossover sites are indicated.
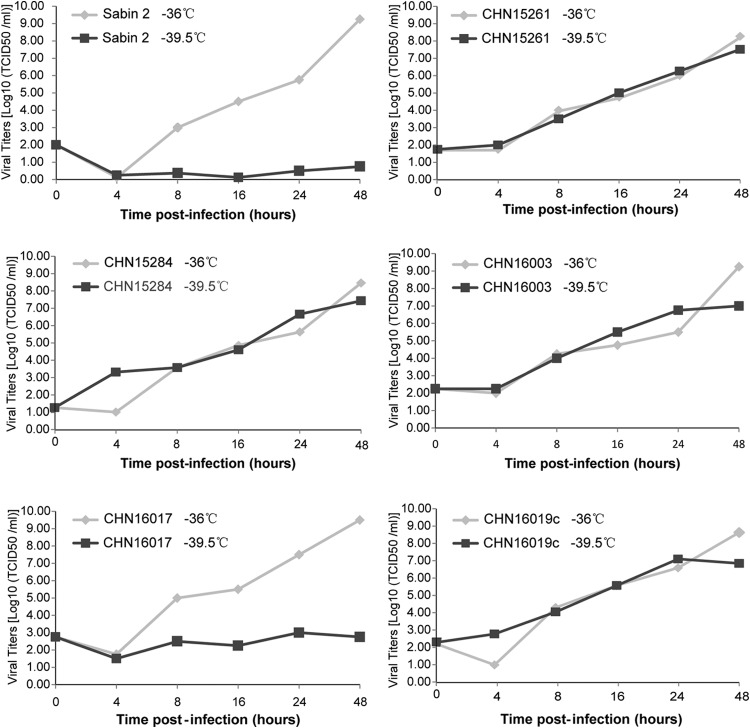
Temperature sensitivity properties of the cVDPV isolates.
All five Sichuan type 2 cVDPV strains were compared to Sabin 2 with regard to replication capacity at a supraoptimal temperature (39.5°C) and showed different temperature sensitivity properties. Sabin 2 was temperature sensitive as expected, with a titer reduction of more than 2 logarithms at 36°C versus 39.5°C, and four isolates showed a markedly lesser effect (titers reduced less than 2 logarithms at 36°C versus 39.5°C). This indicates that the replication efficiency of these isolates remains the same even at elevated temperatures and suggests that they had partially lost the temperature-sensitive phenotype (Fig. 3). The exception was VDPV strain CHN16017, which displayed a growth pattern similar to that of Sabin 2. This suggests that it had resumed the temperature sensitivity for growth at 39.5°C in RD cells (Fig. 3).
FIG 3.
Temperature sensitivity test curves of 5 Sichuan type 2 cVDPV isolates.
Properties in neutralizing antigenic sites.
The amino acid sequences within or near the predicted neutralizing antigenic (NAg) sites (25, 26) were aligned, including the five Sichuan type 2 cVDPVs, MEF-1, and representative strains of type 2 cVDPVs from outbreaks in Egypt, Madagascar, and Nigeria. All five Sichuan isolates showed the same amino acids in the NAg sites as Sabin 2 (see Fig. S2 in the supplemental material).
Estimated time of the initiating OPV dose.
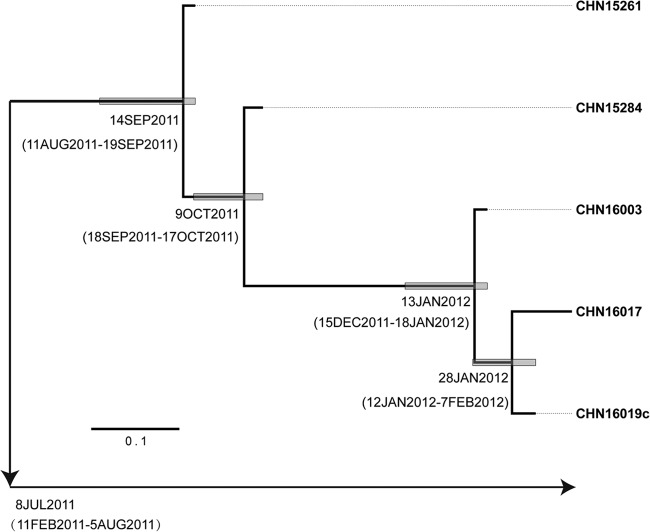
A Bayesian MCMC phylogenetic tree was constructed from the sequences at the 3rd coding position of the complete P1 region (2,637 nt) of five Sichuan type 2 cVDPV strains. The 3rd coding position sequences were compared because they closely approximate synonymous sites, thereby largely screening out selected changes. The complete P1 tree diverged from a common ancestral infection estimated to have occurred around 14 September 2011 (95% highest posterior density [HPD], 11 August 2011 to 19 September 2011) (Fig. 4). The rate of fixation of the 3rd-coding-position substitutions into the P1 region was estimated from the data set to be 5.106 × 10−2 3rd-coding-position substitution/site/year, similar to previous estimates for the poliovirus capsid region (27). Under the assumption of a strict molecular clock, we estimated that the initiating OPV dose was given on 8 July 2011 (Fig. 4). The HPD interval for the date of the initiating OPV dose, calculated from the variance in the evolution rate, was from 11 February 2011 to 5 August 2011.
FIG 4.
Bayesian Markov chain Monte Carlo tree based on the 3rd coding position of the complete P1 sequences of the 5 Sichuan type 2 cVDPV isolates (Table 1) rooted to the sequences of Sabin 2. The date of the initiating OPV dose and the times of divergence of different lineages were estimated by assuming a strict molecular clock.
MCMC phylogenetic trees based on the complete open reading frame (ORF) (nucleotides 748 to 7368) and the recombinant portions of the genome (type 3 from nucleotides 4504 to 4771, type 2 from nucleotides 4772 to 5257, and type 3 from nucleotides 5258 to 7368) of these viruses were also constructed. We found that the topology (the nodes and the branches) of the tree based on the complete ORF was quite similar to that of the tree based on the P1 capsid sequences, but the uncertainty (95% HPD) of the node dates of the initial OPV dose became wider in the complete ORF tree (data not shown). The topologies of the trees based on each recombinant interval were similar to that of the tree based on the P1 capsid sequences, except for the tree based on the first donor sequence (type 3 from nucleotide 4504 to 4771, which was too short and quite similar among these virus) (data not shown), suggesting that the recombination occurred soon after the initiating OPV dose, probably in the original trivalent-OPV recipient.
DISCUSSION
The VDPVs (both cVDPVs and iVDPVs) that have been identified recently worldwide pose a major challenge for the polio eradication program, especially in “polio-free” countries (3, 5), because their biological properties are indistinguishable from those of the wild polioviruses, including the capacity to cause paralytic disease in human beings, loss of the temperature sensitivity phenotype, and capability of person-to-person transmission. Several studies have been conducted to identify the determinants of attenuation for vaccine-associated strains, but the mechanism underlying the higher transmissibility of polioviruses remains unclear (8, 28).
From August 2011 to February 2012, under the new definition of type 2 VDPV, the first cVDPV outbreak occurred in China. One type 2 pre-VDPV and four type 2 VDPVs were isolated from the cases. These isolates were all newly emergent VDPVs and differed from Sabin 2 by 0.6% to 1.2% nucleotide differences in the VP1 region, and the phylogenetic analysis implied that the cVDPVs circulated for only less than 1 year following the initiating OPV dose. Hence, localization and limitation in circulation extent and time are the characteristics of the cVDPV outbreak in Sichuan which differed from those of earlier reported outbreaks in Egypt (23), Madagascar (24, 29), Democratic Republic of Congo (30), and Nigeria (9, 31), in which more cases were involved and the sequence divergences were larger.
The possibility of antigenic divergence increases along with the replication of OPV strains in human guts (32), and nearly all cVDPVs reported globally have been antigenic variants of OPV strains (9, 23, 33), so WHO adopted a fast intratypic differentiation method (real-time reverse transcription-PCR) to distinguish vaccine-related isolates from VDPV (34). Of the five Sichuan cVDPV isolates, four had partially lost the temperature-sensitive phenotype, but one of the VDPV isolates (strain CHN16017) retained the phenotype of temperature sensitivity, even though it was the most genetically divergent isolate from Sabin 2 and was associated with one of laboratory-confirmed polio cases, showing that key phenotypic properties may fluctuate during the early phase of cVDPV emergence. We assume that the existence of other unknown temperature sensitivity-related determinants could have contributed to this observation, although two known key determinants of the attenuated phenotype of strain CHN16017 reverted to those of the wild type as well as the other 4 isolates.
All five Sichuan type 2 cVDPV strains were vaccine/vaccine (S2/S3/S2/S3) recombinants sharing common recombination crossover sites. This observation addresses a key controversial point in the understanding of cVDPV emergence. Apart from cVDPVs detected in China, which exhibited no recombination of Guizhou cVDPVs (35), and the vaccine/vaccine recombination of Sichuan cVDPVs reported here, all cVDPVs previously described underwent recombination with species C enteroviruses (cVDPVs identified in Egypt, Hispaniola, and the Philippines) (23, 33, 35, 36), prompting the suggestion that recombination with species C enteroviruses is essential to cVDPV emergence and spread. The findings in this report cast some doubt on that assumption and indicate that cVDPVs detected soon after emergence may have properties more typical of vaccine-related strains and that the more extensive evolution (including greater opportunities for recombination) observed elsewhere may be a result of more extensive and prolonged cVDPV transmission. Further virological studies, such as reverse genetic studies, are needed to understand the role of nucleotide mutation and genetic recombination in poliovirus evolution and cVDPV emergence.
Although annual province-wide national immunization days (NIDs) have been conducted in Sichuan since 1996 and the annual reported OPV coverage is as high as 95% since 2008, the fact that none of the 4 patients in this cVDPV outbreak was immunized indicates that OPV coverage in some areas is declining and that gaps in population immunity existed which may have established local conditions favoring cVDPV emergence. However, overall population immunity appears to have been sufficiently high to restrict cVDPV transmission to a small extent temporally and geographically. In additional, maintenance of highly sensitive AFP case and virological surveillance permits early detection and a rapid response to interrupt VDPV circulation and restrict transmission.
As a part of the national plan to achieve and maintain a “polio-free” status, China has continued to support an active case-based surveillance system (AFP surveillance) and large-scale annual NIDs. Province-wide immunization in Sichuan that targets all children ages 0 to 3 years has been conducted every December and January since 1996. The cVDPV outbreak in Sichuan of China has important implications in the global initiative to eradicate polio: cVDPVs can emerge and spread in isolated communities with immunity gaps. The detection of the newly emergent cVDPVs in Sichuan highlights the significant role of sensitive field and laboratory surveillance and the risks of type 2 cVDPV emergence accompanying the use of trivalent OPV, and type 2 OPV should be removed from the trivalent OPV or IPV should be used, in order to solve this problem.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dustin Yang for his critical review of this article.
This study was funded by grants from the Key Technologies R&D Program of the National Ministry of Science (2012ZX10004215, 2013ZX10004-202, and 2012ZX10004201-003).
Footnotes
Published ahead of print 21 May 2014
REFERENCES
- 1.Centers for Disease Control and Prevention. 2001. Apparent global interruption of wild poliovirus type 2 transmission. MMWR Morb. Mortal. Wkly. Rep. 50:222–224 [PubMed] [Google Scholar]
- 2.Kew OM, Sutter RW, de Gourville EM, Dowdle WR, Pallansch MA. 2005. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu. Rev. Microbiol. 59:587–635. 10.1146/annurev.micro.58.030603.123625 [DOI] [PubMed] [Google Scholar]
- 3.Kew OM, Wright PF, Agol VI, Delpeyroux F, Shimizu H, Nathanson N, Pallansch MA. 2004. Circulating vaccine-derived polioviruses: current state of knowledge. Bull. World Health Organ. 82:16–23 [PMC free article] [PubMed] [Google Scholar]
- 4.Jorba J, Campagnoli R, De L, Kew O. 2008. Calibration of multiple poliovirus molecular clocks covering an extended evolutionary range. J. Virol. 82:4429–4440. 10.1128/JVI.02354-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kew OM, Sutter RW, Nottay BK, McDonough MJ, Prevots DR, Quick L, Pallansch MA. 1998. Prolonged replication of a type 1 vaccine-derived poliovirus in an immunodeficient patient. J. Clin. Microbiol. 36:2893–2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellmunt A, May G, Zell R, Pring-Akerblom P, Verhagen W, Heim A. 1999. Evolution of poliovirus type I during 5.5 years of prolonged enteral replication in an immunodeficient patient. Virology 265:178–184. 10.1006/viro.1999.0003 [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Yan D, Zhu S, Wen N, Li L, Wang H, Liu J, Ye X, Ding Z, Wang D, Zhu H, Chen L, Hou X, An H, Liang X, Luo H, Kew O, Xu W. 2010. Type 2 vaccine-derived poliovirus from patients with acute flaccid paralysis in China: current immunization strategy effectively prevented its sustained transmission. J. Infect. Dis. 202:1780–1788. 10.1086/657410 [DOI] [PubMed] [Google Scholar]
- 8.Fine PE, Carneiro IA. 1999. Transmissibility and persistence of oral polio vaccine viruses: implications for the global poliomyelitis eradication initiative. Am. J. Epidemiol. 150:1001–1021. 10.1093/oxfordjournals.aje.a009924 [DOI] [PubMed] [Google Scholar]
- 9.Burns CC, Shaw J, Jorba J, Bukbuk D, Adu F, Gumede N, Pate MA, Abanida EA, Gasasira A, Iber J, Chen Q, Vincent A, Chenoweth P, Henderson E, Wannemuehler K, Naeem A, Umami RN, Nishimura Y, Shimizu H, Baba M, Adeniji A, Williams AJ, Kilpatrick DR, Oberste MS, Wassilak SG, Tomori O, Pallansch MA, Kew O. 2013. Multiple independent emergences of type 2 vaccine-derived polioviruses during a large outbreak in northern Nigeria. J. Virol. 87:4907–4922. 10.1128/JVI.02954-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dowdle WR, De Gourville E, Kew OM, Pallansch MA, Wood DJ. 2003. Polio eradication: the OPV paradox. Rev. Med. Virol. 13:277–291. 10.1002/rmv.401 [DOI] [PubMed] [Google Scholar]
- 11.Chen RT, Hausinger S, Dajani AS, Hanfling M, Baughman AL, Pallansch MA, Patriarca PA. 1996. Seroprevalence of antibody against poliovirus in inner-city preschool children. Implications for vaccination policy in the United States. JAMA 275:1639–1645 [PubMed] [Google Scholar]
- 12.Kilpatrick DR, Ching K, Iber J, Campagnoli R, Freeman CJ, Mishrik N, Liu HM, Pallansch MA, Kew OM. 2004. Multiplex PCR method for identifying recombinant vaccine-related polioviruses. J. Clin. Microbiol. 42:4313–4315. 10.1128/JCM.42.9.4313-4315.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Zhu S, Yan D, Liu G, Bai R, Wang D, Chen L, Zhu H, An H, Kew O, Xu W. 2010. Natural type 3/type 2 intertypic vaccine-related poliovirus recombinants with the first crossover sites within the VP1 capsid coding region. PLoS One 5:e15300. 10.1371/journal.pone.0015300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Q, Zhang Y, Zhu S, Tian H, Huang G, Cui H, Li X, Yan D, Zhu Z, Li J, Zheng P, Jiang H, Zhang B, Tan X, Zhu H, An H, Xu W. 2013. Transmission of human enterovirus 85 recombinants containing new unknown serotype HEV-B donor sequences in Xinjiang Uighur autonomous region, China. PLoS One 8:e55480. 10.1371/journal.pone.0055480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drummond AJ, Rambaut A. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7:214. 10.1186/1471-2148-7-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang K, Zhang LB, Otten MW, Jr, Zhang XL, Yasuo C, Zhang RZ, Xu T, Liu X, Liu M, Li QL, Yu JJ, Wang Z. 1997. Status of the eradication of indigenous wild poliomyelitis in the People's Republic of China. J. Infect. Dis. 175(Suppl 1):S105–S112. 10.1093/infdis/175.Supplement_1.S105 [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. 2000. Certification of poliomyelitis eradication. WHO Western Pacific Region, October 2000. Wkly. Epidemiol. Rec. 75:399–400 [PubMed] [Google Scholar]
- 19.Luo HM, Zhang Y, Wang XQ, Yu WZ, Wen N, Yan DM, Wang HQ, Wushouer F, Wang HB, Xu AQ, Zheng JS, Li DX, Cui H, Wang JP, Zhu SL, Feng ZJ, Cui FQ, Ning J, Hao LX, Fan CX, Ning GJ, Yu HJ, Wang SW, Liu DW, Wang DY, Fu JP, Gou AL, Zhang GM, Huang GH, Chen YS, Mi SS, Liu YM, Yin DP, Zhu H, Fan XC, Li XL, Ji YX, Li KL, Tang HS, Xu WB, Wang Y, Yang WZ. 2013. Identification and control of a poliomyelitis outbreak in Xinjiang, China. N. Engl. J. Med. 369:1981–1990. 10.1056/NEJMoa1303368 [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Fang G, Du F, Tan ZY, Liu QL, Fang SM, Nian XH, Tong WB, Ma XZ, Chen N, Yang RP, Huang RN, Wang YM, Fu QP, Chen XC, Li YQ, Jing YL, Liu JJ. 2013. The epidemiologic investigation and analysis of circulating type 2 vaccine hypervariable poliovirus/vaccine-derived poliovirus event in Sichuan Province. Chin. J. Vaccines Immun. 19:407–412 [Google Scholar]
- 21.Macadam AJ, Pollard SR, Ferguson G, Skuce R, Wood D, Almond JW, Minor PD. 1993. Genetic basis of attenuation of the Sabin type 2 vaccine strain of poliovirus in primates. Virology 192:18–26. 10.1006/viro.1993.1003 [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Wang H, Zhu S, Li Y, Song L, Liu Y, Liu G, Nishimura Y, Chen L, Yan D, Wang D, An H, Shimizu H, Xu A, Xu W. 2010. Characterization of a rare natural intertypic type 2/type 3 penta-recombinant vaccine-derived poliovirus isolated from a child with acute flaccid paralysis. J. Gen. Virol. 91:421–429. 10.1099/vir.0.014258-0 [DOI] [PubMed] [Google Scholar]
- 23.Yang CF, Naguib T, Yang SJ, Nasr E, Jorba J, Ahmed N, Campagnoli R, van der Avoort H, Shimizu H, Yoneyama T, Miyamura T, Pallansch M, Kew O. 2003. Circulation of endemic type 2 vaccine-derived poliovirus in Egypt from 1983 to 1993. J. Virol. 77:8366–8377. 10.1128/JVI.77.15.8366-8377.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rousset D, Rakoto-Andrianarivelo M, Razafindratsimandresy R, Randriamanalina B, Guillot S, Balanant J, Mauclere P, Delpeyroux F. 2003. Recombinant vaccine-derived poliovirus in Madagascar. Emerg. Infect. Dis. 9:885–887. 10.3201/eid0907.020692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minor PD, Ferguson M, Evans DM, Almond JW, Icenogle JP. 1986. Antigenic structure of polioviruses of serotypes 1, 2 and 3. J. Gen. Virol. 67:1283–1291. 10.1099/0022-1317-67-7-1283 [DOI] [PubMed] [Google Scholar]
- 26.Wiegers K, Dernick R. 1992. Molecular basis of antigenic structures of poliovirus: implications for their evolution during morphogenesis. J. Virol. 66:4597–4600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan D, Li L, Zhu S, Zhang Y, An J, Wang D, Wen N, Jorba J, Liu W, Zhong G, Huang L, Kew O, Liang X, Xu W. 2010. Emergence and localized circulation of a vaccine-derived poliovirus in an isolated mountain community in Guangxi, China. J. Clin. Microbiol. 48:3274–3280. 10.1128/JCM.00712-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fine PE. 2000. Gaps in our knowledge about transmission of vaccine-derived polioviruses. Bull. World Health Organ. 78:358–359 [PMC free article] [PubMed] [Google Scholar]
- 29.Rakoto-Andrianarivelo M, Gumede N, Jegouic S, Balanant J, Andriamamonjy SN, Rabemanantsoa S, Birmingham M, Randriamanalina B, Nkolomoni L, Venter M, Schoub BD, Delpeyroux F, Reynes JM. 2008. Reemergence of recombinant vaccine-derived poliovirus outbreak in Madagascar. J. Infect. Dis. 197:1427–1435. 10.1086/587694 [DOI] [PubMed] [Google Scholar]
- 30.Gumede N, Lentsoane O, Burns CC, Pallansch M, de Gourville E, Yogolelo R, Muyembe-Tamfum JJ, Puren A, Schoub BD, Venter M. 2013. Emergence of vaccine-derived polioviruses, Democratic Republic of Congo, 2004–2011. Emerg. Infect. Dis. 19:1583–1589. 10.3201/eid1910.130028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wassilak S, Pate MA, Wannemuehler K, Jenks J, Burns C, Chenoweth P, Abanida EA, Adu F, Baba M, Gasasira A, Iber J, Mkanda P, Williams AJ, Shaw J, Pallansch M, Kew O. 2011. Outbreak of type 2 vaccine-derived poliovirus in Nigeria: emergence and widespread circulation in an underimmunized population. J. Infect. Dis. 203:898–909. 10.1093/infdis/jiq140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minor PD, Evans DM, Ferguson M, Schild GC, Westrop G, Almond JW. 1985. Principal and subsidiary antigenic sites of VP1 involved in the neutralization of poliovirus type 3. J. Gen. Virol. 66:1159–1165. 10.1099/0022-1317-66-5-1159 [DOI] [PubMed] [Google Scholar]
- 33.Shimizu H, Thorley B, Paladin FJ, Brussen KA, Stambos V, Yuen L, Utama A, Tano Y, Arita M, Yoshida H, Yoneyama T, Benegas A, Roesel S, Pallansch M, Kew O, Miyamura T. 2004. Circulation of type 1 vaccine-derived poliovirus in the Philippines in 2001. J. Virol. 78:13512–13521. 10.1128/JVI.78.24.13512-13521.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kilpatrick DR, Yang CF, Ching K, Vincent A, Iber J, Campagnoli R, Mandelbaum M, De L, Yang SJ, Nix A, Kew OM. 2009. Rapid group-, serotype-, and vaccine strain-specific identification of poliovirus isolates by real-time reverse transcription-PCR using degenerate primers and probes containing deoxyinosine residues. J. Clin. Microbiol. 47:1939–1941. 10.1128/JCM.00702-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang X, Zhang Y, Xu W, Wen N, Zuo S, Lee LA, Yu J. 2006. An outbreak of poliomyelitis caused by type 1 vaccine-derived poliovirus in China. J. Infect. Dis. 194:545–551. 10.1086/506359 [DOI] [PubMed] [Google Scholar]
- 36.Kew O, Morris-Glasgow V, Landaverde M, Burns C, Shaw J, Garib Z, Andre J, Blackman E, Freeman CJ, Jorba J, Sutter R, Tambini G, Venczel L, Pedreira C, Laender F, Shimizu H, Yoneyama T, Miyamura T, van Der Avoort H, Oberste MS, Kilpatrick D, Cochi S, Pallansch M, de Quadros C. 2002. Outbreak of poliomyelitis in Hispaniola associated with circulating type 1 vaccine-derived poliovirus. Science 296:356–359. 10.1126/science.1068284 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.