Abstract
The Escherichia coli lineage sequence type 131 (ST131)-O25b:H4 is a globally spread multidrug-resistant clone responsible for a great proportion of extraintestinal infections. Driven by the significant medical needs associated with this successful pathogenic lineage, we generated murine monoclonal antibodies (MAbs) against its lipopolysaccharide (LPS) O25b antigen in order to develop quick diagnostic tests. Murine monoclonal antibodies were generated by immunizing mice with whole killed nonencapsulated ST131-O25b E. coli cells and screening hybridoma supernatants for binding to purified LPS molecules obtained from an E. coli ST131-O25b clinical isolate. The MAbs selected for further study bound to the surface of live E. coli O25b strains irrespective of the capsular type expressed, while they did not bind to bacteria or purified LPS from other serotypes, including the related classical O25 antigen (O25a). Using these specific MAbs, we developed a latex bead-based agglutination assay that has greater specificity and is quicker and simpler than the currently available typing methods. The high specificities of these MAbs can be explained by the novel structure of the O25b repeating unit elucidated in this article. Based on comparative analysis by nuclear magnetic resonance (NMR) and mass spectrometry, the N-acetyl-fucose in the O25a O-antigen had been replaced by O-acetyl-rhamnose in the O25b repeating unit. The genetic determinants responsible for this structural variation were identified by aligning the corresponding genetic loci and were confirmed by trans-complementation of a rough mutant by the subserotype-specific fragments of the rfb operons.
INTRODUCTION
Infections by Gram-negative multidrug-resistant (MDR) bacteria represent an increasing health care problem worldwide. While there are still some new antibiotics with some degree of efficacy against Gram-positive bacteria, the pipeline of novel drugs being developed to treat Gram-negative pathogens is essentially empty. The potential spread of MDR pathogens therefore poses a threat, which might result in levels of morbidity and mortality similar to those associated with infectious diseases in the preantibiotic era. A particular concern is the recent emergence of clonal lineages that can balance the normally mutually exclusive phenotypic properties of being MDR with the retention of high virulence potential, a feature that is generally unusual in MDR strains.
Escherichia coli sequence type 131 (ST131)-O25b:H4 is a well-characterized multidrug-resistant clonal lineage that has spread globally (1–3) in the last few years since it was first described in 2008 (4). This clone alone is responsible for >10% of all extraintestinal E. coli infections and accounts for the greatest majority of E. coli strains that are resistant to clinically relevant antibiotics (5). The vast majority of ST131-O25b isolates are resistant to fluoroquinolones. Moreover, approximately 50% of the isolates producing an extended-spectrum β-lactamase (ESBL) that confers resistance to all β-lactam antibiotics, except the carbapenems, originate from this clone. Even more alarming, there are several recent reports that describe representative strains of this lineage expressing various carbapenemases (6–8). Consequently, infections by ST131-O25b:H4 strains are a growing concern, with very limited therapeutic options.
The obvious pathogenic success of this lineage is conferred by the MDR phenotype and retained virulence potential. However, the range of factors contributing to its virulence still has to be fully elucidated (9). In a murine model of ascending urinary tract infection, a representative ST131 strain was shown to outcompete prototype uropathogenic E. coli (UPEC) isolates (10). Conversely, other studies have reported that ST131 isolates are not more virulent (11) or even less virulent (12) in various animal models than fully susceptible E. coli isolates. This might be explained by the lower number of average virulence factors expressed in ST131 isolates than in non-MDR strains (11). However, compared with other lineages of ESBL-producing E. coli isolates, ST131 strains were shown to carry a significantly higher number of virulence genes (9, 13, 14). The high metabolic potential of this lineage was recently suggested to contribute to its overall success (10, 15). Finally, the substantial reduction in core genome recombination events shown recently for this clone (16) results in a phylogenetically distinct and stable pathogenic clone that is expected to remain an important extraintestinal pathogenic E. coli (ExPEC) lineage.
In spite of the great medical importance, the detection of this specific clone among clinical isolates of E. coli is not routinely performed. This is partly due to the lack of reliable and rapid diagnostic assays. For epidemiological studies, ST131-O25b isolates are identified by multilocus sequence typing and the detection of the specific lipopolysaccharide (LPS) O-antigen repeating units (RUs). For the identification of the RUs, two methods are used: (i) agglutination with O25 rabbit typing serum and (ii) detection of a serotype-specific gene segment within the rfb locus encoding O-antigen synthesis by PCR. The sensitivity and specificity of the immune assay are suboptimal, and the PCR-based method is not practical for routine clinical microbiology testing.
In this article, we describe the discovery of MAbs with specificity toward a sugar epitope that is unique to the O25b O-antigen carried by ST131 strains. We demonstrate that these MAbs function as reliable diagnostic tools in a convenient agglutination assay that is more sensitive and more easily applicable to routine use than are the currently available typing methods.
MATERIALS AND METHODS
Bacteria and growth conditions.
Two representative and genotypically and phenotypically confirmed ST131-O25b:H4 clinical isolates (17) were used for the in vitro studies. For the agglutination assay, a larger panel (n = 44) of ST131-O25b isolates, as well as nonrelated (i.e., expressing O-antigens other than O25b) E. coli strains were used, which were kindly provided by Agnes Sonnevend (Al Ain, United Arab Emirates [UAE]), Aranzazu Valverde (Madrid, Spain), Franz-Josef Schmitz (Minden, Germany), or obtained from commercial strain collections (ATCC, NCTC, and the Polish Collection of Microbes [PCM]). The prototype sequenced O25a strain E. coli E47a was obtained from the NCTC. E. coli strain 509A is a human fecal isolate (18), which was confirmed by O-typing (Hungarian Epidemiology Center) to express the O2 antigen. The mouse immunization experiments (see below) were performed with an isogenic knockout mutant of the representative ST131 strain E. coli 81009 that was generated by deleting the whole kps cluster encoding capsular synthesis. A rough derivative of 81009 was generated by deleting the gene encoding O-antigen ligase (waaL). E47aΔrfb3 is a rough derivative of E47a lacking the 3′ end of the rfbO25a locus. All mutants were generated by the Red recombinase method (19) using the oligonucleotides listed in Table S1 in the supplemental material.
The bacteria were grown in Luria-Bertani (LB) broth (Fisher Scientific) or on agar plates. When appropriate, selective media were used containing ampicillin (100 μg/ml), kanamycin (100 μg/ml), or chloramphenicol (25 μg/ml).
LPS purification.
The LPS of E. coli strain 81009 was isolated by the hot phenol/water method and purified by dialysis, proteinase K digestion, and ultracentrifugation. All other LPS molecules were purified using a commercial kit (LPS extraction kit; Intron).
Molecular cloning.
The approximately 7- and 3-kb fragments of the O25a and O25b rfb loci, respectively, were amplified (Phusion PCR mix; New England BioLabs) using primers O25 control-forw and O25 control-rev and O25b-spec1 and O25b-spec2 (see Table S1 in the supplemental material) and were directly cloned into the high-copy-number expression vector pJET1.2 (Fermentas), giving rise to p3O25a and p3O25b, respectively. The identical orientation (negative strand with respect to the T7 promoter) was confirmed by PCR and sequencing. Plasmids were purified and transformed according to standard protocols.
Immunizations and hybridoma generation.
Six- to 8-week-old female BALB/c mice were immunized subcutaneously with approximately 108 CFU of formalin-killed cells of strain 81009Δkps 3 times at 2-week intervals. Four days following a final intravenous boost, the splenocytes of selected mice were isolated and subjected to hybridoma fusion. Fusion and subculturing of hybridomas were performed in the Medical University of Vienna (Austria) Monoclonal Antibody Facility (MAF). The culture supernatants of hybridoma clones were tested by an enzyme-linked immunosorbent assay (ELISA), flow cytometry, and immunoblots, based on which specific clones were isolated. The isotypes of the purified MAbs obtained from the selected clones were determined using the IsoQuick mouse isotyping kit (Sigma-Aldrich).
Immunoblotting.
Purified LPS (1 μg) was separated in 12% polyacrylamide gels (Bio-Rad) at a constant 35-mA current. LPS was transferred to 0.2 μm polyvinylidene difluoride (PVDF) membranes using the Trans-Blot Turbo blotting system (Bio-Rad) with a high-molecular-weight (MW) program (1.3 A up to 25 V for 10 min). Following overnight blocking in 5% bovine serum albumin (BSA) (PAA, Austria), the membranes were reacted with 1 μg/ml monoclonal antibody (MAb) or hyperimmune rabbit serum (E. coli O25; Statens Serum Institut [SSI]) in a 1:1,000 dilution for 1 h at room temperature (RT). Following 3 washes in 0.05% Tween 20 (Fisher Scientific)-Tris-buffered saline (TBS) buffer for 10 min, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (goat anti-mouse IgG [SouthernBiotech] at a 1:20,000 dilution or anti-rabbit IgG [SouthernBiotech] at a 1:5,000 dilution) for 1 h at RT. Following repeated washing, the membranes were developed with ECL Prime Western blotting detection reagent (GE Healthcare).
Silver staining.
The LPS samples were separated as described above for immunoblotting. Silver staining was performed as published previously (20). Briefly, following overnight fixation in a 25% isopropanol and 7% acetic acid (Fisher Scientific) solution, the gel was oxidized by 0.7% periodic acid (Sigma-Aldrich) in 40% ethanol and 5% acetic acid. Following repeated washing in distilled water, the gel was stained with 0.8% silver nitrate (Sigma-Aldrich) in 1.4% ammonium hydroxide and 200 mM sodium hydroxide solution and developed with 0.019% formaldehyde in 0.005% citric acid buffer. Fifty millimolar EDTA was used to stop the reaction.
Flow cytometry.
For surface staining, the bacteria inoculated from overnight cultures in LB medium were grown to mid-log phase (optical density at 600 nm [OD600], ≈0.5), washed twice, and resuspended in Hanks' balanced salt solution (HBSS) (Gibco Life Technologies) without Ca2+ and Mg2+. The bacteria (106 CFU) were stained with the indicated hybridoma supernatants diluted 2.5-fold in HBSS buffer supplemented with 0.5% BSA and 0.01% sodium azide and incubated for 30 min on ice. Following two washing steps in HBSS buffer with BSA (PAA, Austria) and sodium azide (Sigma-Aldrich), the bacteria were stained with 3 μg/ml of Alexa Fluor 488-conjugated goat F(ab′)2 secondary antibody against mouse IgG (Jackson ImmunoResearch) for 30 min on ice. Next, the bacteria were washed twice, resuspended in HBSS buffer containing 5 μM SYTO-62 nucleic acid stain (Life Technologies), and incubated for 10 min at room temperature. The samples were measured by an Eclipse flow cytometer (i-Cyt/Sony Biotechnology), and the list mode data were analyzed using the FCS Express software version 4 (De Novo Software).
ELISA.
An ELISA was performed using 96-well plates coated with lysates of E. coli with different O-serotypes (O1, O4, O7, O12, O15, O16, O17, O18, O25, O75, O105, and O157). The bacteria were cultured in LB medium overnight at 37°C. After a wash with phosphate-buffered saline (PBS) containing Ca2+ and Mg2+ (PAA, Austria), the bacteria were lysed at 100°C for 1 h. The plates were coated with lysates of bacteria (108 CFU/well) in PBS with Ca2+ and Mg2+ overnight at 4°C, followed by blocking with 2% BSA (PAA) in PBS for 1 h at RT. After washing 3 times with 0.05% Tween 20 (Fisher Scientific)-PBS, the plates were reacted with O25b-specific mouse MAb (1 μg/ml) or with mAb9004 (1 μg/ml; Glycobiotech, Germany) for 1 h at RT and then washed 3 times. As a secondary antibody, HRP-conjugated goat anti-mouse IgG (SouthernBiotech) was used at a 1:10,000 dilution. ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] solution (Novex; Life Technologies) was added to the washed plates, incubated for 30 min in the dark, and the absorbance was measured at 405 nm with the Synergy HT reader (Bio-Tek).
Agglutination assays.
O-serotyping was performed with rabbit serum specific to E. coli O25 (SSI), according to the manufacturer's instructions. The O25b-specific MAb was coupled to latex beads with passive absorption. One hundred microliters of a 2.5% slurry of red polystyrene beads with 1-μm diameter (Polysciences) was washed and incubated with 100 μg antibody for 30 min at 37°C, with shaking at 400 rpm. After washing and centrifugation, the beads were resuspended in PBS containing 0.1% BSA and 0.05% Tween 20 to obtain a 1% bead suspension. Agglutination with live bacteria was tested with freshly prepared antibody-coated beads; a 5- to 10-μl bead suspension was mixed with the bacterial mass of a single colony. Agglutination (clumping of the beads and clearance of the background) was read by the naked eye within 30 s.
Sequencing.
The genomic DNA of strain 81009 was purified using the Wizard Genomic DNA purification kit (Promega). The rfb operon was amplified with KlenTaq LA DNA polymerase (Sigma-Aldrich) with the corresponding primers listed in Table S1 in the supplemental material, sequenced with the primer walk technique at Microsynth AG, and assembled with CLC Main Workbench 6.7.1.
Isolation of poly- and oligosaccharides.
Two hundred milligrams LPS was degraded by treatment with 1.5% acetic acid at 100°C for 30 min, frozen at −20°C, and then hydrolysis was continued for 20 min. Soluble polysaccharides (PS) and oligosaccharides (OS) were fractionated on a column (1.6 cm by 100 cm) of Bio-Gel P-10 equilibrated with 0.05 M pyridine-acetic acid buffer at pH 5.6. Selected fractions were analyzed by 1H nuclear magnetic resonance (NMR) spectroscopy, electrospray ionization (ESI), and matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS).
Compositional analysis.
Methylation of PS and OS was performed according to the method described by Ciucanu and Kerek (21). Alditol acetates and partially methylated alditol acetates were analyzed by gas chromatography (GC)-MS with the Thermo Scientific TSQ system using an RX5 fused-silica capillary column (0.2 mm by 30 m) and a temperature program of 150 to 270°C at 12°C/min. The absolute configuration of sugar residues was assigned according to the method of Gerwig et al. (22). Silylated butyl glycosides of l and d sugars were analyzed with a temperature program of 100 to 270°C at 5°C/min.
NMR spectroscopy.
All NMR spectra were recorded on a Bruker Avance III 600 MHz spectrometer. The NMR spectra of the PS and OS samples were obtained for 2H2O solutions at 25°C using acetone (δH, 2.225; δC, 31.05) as an internal reference. The samples were first repeatedly exchanged with 2H2O (99%). The data were acquired and processed using standard Bruker software. The processed spectra were assigned with the help of SPARKY (T. D. Goddard and D. G. Kneller, SPARKY 3; University of California, San Francisco). The signals were assigned by one- and two-dimensional experiments (correlation spectroscopy [COSY], double quantum filter [DQF]-COSY, cross-relaxation compensated total correlation spectroscopy [Clean TOCSY], nuclear Overhauser effect spectroscopy [NOESY], rotating frame nuclear Overhauser effect spectroscopy [ROESY], heteronuclear multiple-bond correlation [HMBC], heteronuclear single quantum coherence-distortionless enhancement by polarization transfer [HSQC-DEPT], HSQC, HSQC-TOCSY, and 1H, 13C, and 31P HMBC). In the Clean TOCSY experiments, mixing times of 30, 60, and 100 ms were used. The delay time in the HMBC was 60 ms, and the mixing time in the NOESY and ROESY experiments was 200 ms.
Mass spectrometry.
Negative ion mode MALDI-TOF MS of PS and OS was carried out on a Bruker Reflex III time of flight instrument. As the matrix, 10 mg/ml 2,5-dihydroxybenzoic acid (acetonitrile to water ratio, 1:1) was used. The spectra were scanned in the range of m/z 800 to 6,000. External calibration in the negative ion mode was applied using the Peptide calibration standard II (Bruker Daltonics, Germany). ESI-MS experiments were carried out on a micrOTOF-Q II spectrometer (Bruker Daltonics) in the positive ion mode. The samples were dissolved in a 50-μg/ml acetonitrile-water-formic acid solution (50:50:0.5 [vol/vol/vol]). The source parameters were as follows: sample flow, 3 μl/min; ion source temperature, 180°C; nitrogen flow, 4 liters/min; and pressure, 0.4 bar. The spectra were scanned in the m/z 50 to 3,000 range. External calibration in positive ion mode was applied using the ESI Low Tuning Mix (Agilent) in quadratic regression mode. All structures were drawn and their molecular weights were calculated with GlycoWorkbench software (23).
Nucleotide sequence accession number.
The O-antigen biosynthesis cluster of strain 81009 has been deposited in GenBank under the accession no. KF277146.
RESULTS
Generation of O25b-specific MAbs.
Murine MAbs specific for the E. coli O25b LPS antigen were generated by a standard hybridoma technique using the spleens of mice immunized with formalin-killed bacterial cells, as described in Materials and Methods. The selection of antibodies beneficial for serotype determination (i.e., diagnostics) was based on their capacity to bind to the surface of live E. coli ST131 cells. The flow cytometry-based staining confirmed the accessibility of the epitope recognized by three different antibodies (MAbs 6D1-1B2, 8D5-1G10, and 8A1-1G8) using two representative E. coli ST131 strains (81009 and 80503) expressing different capsular polysaccharides (Fig. 1). E. coli strains expressing non-O25 antigens (data not shown) and the related O25a (strain E47a) antigen were not labeled by these MAbs (Fig. 1). The specificity of the MAbs was further confirmed by immunoblot analysis using purified LPS molecules (Fig. 2). The murine MAbs recognized the LPS molecules purified from ST131 strains containing the O25b antigen; however, they did not react with those containing the O25a or other O-antigens (Fig. 2B). Furthermore, LPS purified from an isogenic mutant of strain 81009 lacking the O-antigen ligase WaaL, and hence expressing no O-antigen on the surface (rough [R] mutant), was not detected by these antibodies (Fig. 2B). Immunoblot staining with the commercial O25 typing serum confirmed cross-reactivity between O25a and O25b antigens, although with significantly lower reactivity to O25b (Fig. 2C).
FIG 1.
Surface staining. Binding of 3 different O25b-specific murine MAbs to different ST131-O25b strains expressing various capsular polysaccharides (K5 and K2), as well as control strain E47a (O25a). As a control, an irrelevant isotype-matched murine antibody was used.
FIG 2.
Immunoreactivity of purified LPS molecules to various anti-O25 reagents. LPS samples were separated by SDS-PAGE and silver stained (A), blotted and developed by O25b-specific MAb 8D5-1G10 (1 μg/ml) (B), or blotted and developed by commercial O25 typing serum (SSI, 1:1,000 dilution) (C). Lane 1, 81009 (O25b); lane 2, 80503 (O25b); lane 3, 81009ΔwaaL (rough); lane 4, E47a (O25a); lane 5, H54 (O25a); lane 6, 509A (O2); lane M, molecular weight marker.
Similarly, in an ELISA using bacterial lysates for coating, only strains expressing O25b antigen were detected, while none of the common extraintestinal pathogenic E. coli (ExPEC) serotypes investigated reacted with the O25b-specific MAb (Fig. 3A to C). Nevertheless, all strains reacted strongly with the inner core-specific (i.e., cross-reactive) mAb9004 (Glycobiotech, Germany), confirming the availability of LPS antigens in the assay (Fig. 3D).
FIG 3.
ELISA reactivity of O25b MAbs to bacterial lysates of different serotypes. The reactivities of different dilutions of O25b-specific MAbs 6D1-1B2 (A), 8A1-1G8 (B), 8D5-1G10 (C), as well as the cross-reactive E. coli MAb 9004 as a control (D) were determined to heat-killed lysates of E. coli cells expressing various LPS O-antigens.
These data suggest that the MAbs are highly specific to the O25b antigen. Moreover, our data provide experimental evidence that the structure of O25b antigen indeed differs from that of classical O25 (termed O25a) antigen (24).
Structural analysis of O25b antigen.
The novel structure of the O25b O-antigen repeating unit (RU) was elucidated, and the K-12 type of the core oligosaccharide was identified by 1H and 13C NMR spectroscopy, MALDI-TOF MS, ESI mass spectrometry, and sugar and methylation analyses. The LPS of E. coli O25b (strain 81009) was isolated by the hot phenol-water method with a yield of 2.6%. The O-specific PS and OS were released by mild acidic hydrolysis of the LPS and isolated by gel filtration. Four fractions were obtained (fractions 1 to 4), and due to the complexity of fraction 1, fractions 2, 3, and 4 were used for structural analysis. ESI and/or MALDI-TOF MS showed that fraction 4 consisted of unsubstituted core OS (a heptasaccharide; see Fig. S1 in the supplemental material), fraction 3 consisted of the core OS substituted with one repeating unit (RU) of the O-specific PS (Fig. 4B; see also Fig. S2 in the supplemental material), and fraction 2 consisted of the core OS substituted with 1 to 4 RUs (Fig. 4C).
FIG 4.
Structural analysis of the fraction 3 isolated from LPS ST131. (A) Structure of the fraction 3 built of the K-12 type core OS substituted with O25 RU (framed with a solid line). The uppercase letters refer to carbohydrate residues identified by NMR analysis. Residue D discriminates O25b RU (gray shaded box) from O25a RU (α-l-FucpNAc). Single asterisks indicate nonstoichiometric substituents; double asterisks indicate that residue C is 3,4,6-substituted in fractions 2 and 1, and a subsequent RU is placed at position 4. (B) Positive ion mode ESI-MS2 of the fraction 3 glycoform represented by the ion at m/z 1,338.886 (inset structure). The interpretation of the observed fragment ions is presented in the inset structures and is based on the nomenclature of Domon and Costello (27). Pound signs indicate noninterpreted ions. (C) Negative ion mode MALDI-TOF mass spectrum of fraction 2. m/z values represent monoisotopic masses.
The structure of the RU of the O25b antigen and its linkage to the core OS were determined with the use of NMR spectroscopy. The complete assignment of fraction 3 1H and 13C resonances (see Table S2 in the supplemental material) was assigned by one- and two-dimensional NMR experiments. The interresidue connections between the adjacent sugar residues were observed by NOESY and HMBC experiments (see Table S3 in the supplemental material). Additionally, all residues, besides 3-deoxy-d-manno-octulosonic acid (Kdo) and phosphate-substituted monosaccharides, were identified by basic sugar and methylation analyses (data not shown). As the core oligosaccharide region of fraction 3 was identified as a known K-12 type, the glycoform assignments were compared with published data (25). The spectra indicated a tetradecasaccharide structure for fraction 3 (Fig. 4A; see also Tables S1 and S2 in the supplemental material) containing a pentasaccharide RU built of terminal β-d-Glcp (residue A), terminal α-l-Rhap (residue B), →3,6)-α-d-Glcp (residue C), 3-substituted α-l-Rhap2OAc (residue D), and →3)-β-d-GlcpNAc (residue E). An analysis of fraction 2 allowed for the identification of the biological repeating unit of the O-specific PS, which is a pentasaccharide with →3)-β-GlcpNAc (residue E) as an RU constituent substituting the first residue of the core OS, →7)-α-l,d-Hepp (residue F). Methylation analysis performed on fractions 1 and 2 indicated the presence of 3,4,6-substituted-Glcp, which indicates the position C4 of residue C as a place of substitution of a subsequent RU of the O-specific PS. Thus, the structure of the O25b RU of ST131 LPS differs from the O25a RU by a single sugar residue: →3)-α-l-FucpNAc (in O25a) is replaced by →3)-α-l-Rhap2OAc (in O25b) (Fig. 4A).
The sequences of the identified tetradecasaccharide (core + RU) and unsubstituted core OS glycoform (fraction 4) were confirmed with the use of electrospray ionization mass spectrometry (ESI-MSn) (Fig. 4B; see also Fig. S1C in the supplemental material). All fragment ions were interpreted on the basis of the herein-elucidated structure of the O25b RU, the previously identified glycoforms of K-12 core OS (25, 26), and according to the nomenclature of Domon and Costello (27). It was shown that the purified LPS molecules contained two main alternative core OS glycoforms. The type of glycoform is dependent on the presence or absence of the O-specific PS. The prevailing glycoform of the unsubstituted core OS is a truncated version of the K-12 core oligosaccharide, which is devoid of the outer core region →7)-α-Hepp-(1→6)-α-Glcp disaccharide.
The molecular weight of the identified O25b RU was confirmed for fraction 2. The MALDI-TOF MS spectrum (Fig. 4C) showed clusters of ions with the following prevailing ions: m/z 2,797.2, m/z 3,658.8, m/z 4,520.8, and m/z 5,382.6 attributed to core OS (with P and/or pyrophosphoryl-ethanolamine [PPEtN]) substituted with 1, 2, 3, and 4 RU, respectively. The average mass difference among these ions was 861.9 Da, which matched the calculated monoisotopic mass of the O-specific PS repeating unit (861.3 Da, RU-H2O).
Genetic analysis of rfbO25b.
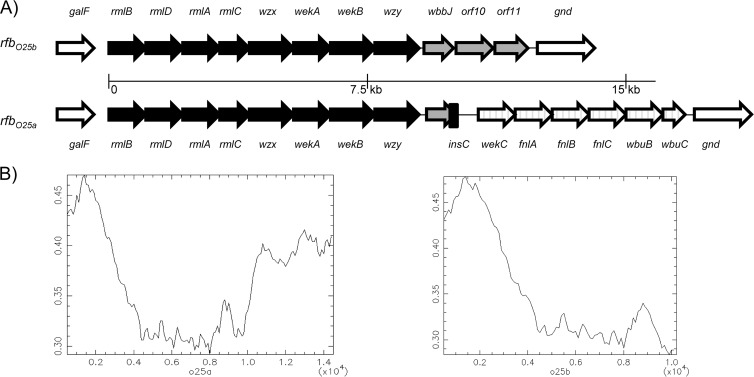
To determine the genetic basis of the structural differences observed between the O25a and O25b O-antigen RUs, we analyzed the rfb gene clusters responsible for their synthesis. We sequenced the complete rfbO25b locus from ST131-O25b isolate 81009 (GenBank accession no. KF277146) and compared it to the corresponding rfbO25a locus from O25a strain E47a (GenBank accession no. GU014554). We found that the approximately 9-kb-long 5′ regions encompassing 8 genes are highly homologous, while the 3′ ends downstream of the wzy genes are dissimilar (Fig. 5). In case of O25a, the unique region includes 7 genes predicted to be involved in N-acetyl-fucose synthesis and transfer. This region is completely replaced by 3 putative genes in the O25b operon. The first gene, wbbJ, has a potential O-acetyltransferase function, and the two downstream genes show homology with putative glycosyltransferases of E. coli O16 (GenBank accession no. U00096). The 5′ 533 bp of the putative O-acetyltransferase gene is also present in the O25a rfb operon; however, due to a frameshift mutation, a premature stop codon was introduced at the 8th codon. Furthermore, the 3′ end of the gene is truncated (73-bp deletion) due to the insertion sequence insC, which might have been involved in the lateral transfer of the downstream O25a-specific genes. Interestingly, the O25b-specific region displays a GC content that is similar to that of the shared immediate upstream region, while that in the O25a-specific part is significantly higher (Fig. 5B), implying a more recent recombination event within rfbO25a.
FIG 5.
Comparison of genetic loci encoding O25a and O25b subunits. (A) Schematic representation (not drawn to scale) of the gene compositions of the different rfb operons. The genes flanking the rfb loci (galF and gnd) are represented by the empty arrows. (B) GC/AT content across the rfb operons encoding O25a and O25b RUs.
In order to prove that the observed differences within the rfb operons are the sole determinants of the structural differences in the O-antigen subunits, complementation studies were performed. The approximately 3- and 7-kb-long 3′ serotype-specific fragments were cloned into an expression vector giving rise to p3O25b and p3O25a, respectively. These plasmids were used for complementing E47aΔrfb3, an O25a mutant with a deletion of the 3′ variable end of the rfb operon. The mutant exhibited a rough LPS phenotype, as shown by the lack of agglutination by O25 serum and by silver staining of purified LPS (Fig. 6A). As expected, complementation with the homologous region encoded on p3O25a restored the smooth O25a phenotype. In contrast, complementation of the same mutant with p3O25b resulted in the expression of the polymer of O25b repeat units, as confirmed by agglutination and reactivity in immunoblots with O25b-specific MAb (Fig. 6B). These data corroborate that the distinct 3′ region within the rfbO25b cluster is the exclusive determinant of the observed serological difference.
FIG 6.
Immunoblot analysis of purified LPS molecules from recombinant E. coli strains. LPS samples were separated by SDS-PAGE and silver stained (A), blotted and developed by O25b-specific MAb 8D5-1G10 (1 μg/ml) (B), or blotted and developed by commercial O25 typing serum (SSI, 1:1,000 dilution) (C). Lane 1, E47a (O25a); lane 2, E47aΔrfb3 (rough [R]); lane 3, E47Δrfb3/p3O25a clone no. 1; lane 4, E47aΔrfb3/p3O25a clone no. 2; lane 5, E47Δrfb3/p3O25b clone no. 1; lane 6, E47Δrfb3/p3O25b clone no. 2; lane 7, 81009 (O25b).
Diagnostic assay with O25b-specific MAbs.
To explore the applicability of the O25b-specific MAbs for a clinically useful diagnostic assay, MAb 6D1-1B2 was coupled to latex beads and tested for its ability to agglutinate E. coli strains belonging to the ST131-O25b lineage (Table 1). We observed a highly specific reaction with all ST131 O25b strains tested and no visible agglutination with any other strains, such as those expressing the O25a, O2, O4, and O16 antigens (Fig. 7).
TABLE 1.
Validation of the O25b latex bead assay with ST131 isolates collected from various geographical regions
| E. coli strain (reference) | Clinical specimen/infection source | Origin | ESBL | Ciprofloxacin resistancea | Resultsb from: |
Other identifying informationc | ||
|---|---|---|---|---|---|---|---|---|
| O25b PCR | Agglutination with O25 typing serum | Agglutination with latex beads coupled O25b MAb | ||||||
| FJS 020 | Blood | Germany | + | R | + | + | + | |
| FJS 024 | Blood | Germany | + | R | + | + | + | |
| FJS 053 | Urine | Germany | + | R | + | + | + | |
| FJS 059 | Blood | Germany | − | R | + | + | + | |
| FJS 072 | Blood | Germany | + | R | + | + | + | |
| FJS 095 | VAPd | Germany | + | R | + | + | + | |
| FJS 098 | VAP | Germany | + | R | + | + | + | |
| 80503 | Urine | UAEe | + | R | + | + | + | |
| 80505 | Urine | UAE | + | R | + | + | + | |
| 80703 | Urine | UAE | + | R | − | − | − | O16 ST131 |
| 80907 | Urine | UAE | + | R | + | + | + | |
| 81010 | Urine | UAE | + | R | + | − | − | Rough |
| 81012 | Urine | UAE | + | R | + | + | + | |
| 81102 | Urine | UAE | + | R | + | + | + | |
| 90103 | Urine | UAE | + | R | + | − | + | |
| 90105 | Urine | UAE | + | R | + | − | + | |
| 90108 | Urine | UAE | + | R | + | − | + | |
| 90306 | Urine | UAE | + | R | + | + | + | |
| 90309 | Urine | UAE | + | R | + | + | + | |
| 90310 | Urine | UAE | + | R | + | + | + | |
| 90405 | Urine | UAE | + | R | + | + | + | |
| 90409 | Urine | UAE | + | R | + | + | + | |
| 90416 | Urine | UAE | + | R | + | + | + | |
| 306-0838 | Urine | UAE | − | S | + | − | + | |
| B15 | Urine | Hungary | − | R | + | + | + | |
| DE22404 | Blood | Hungary | − | S | + | + | + | |
| DE8881 | Blood | Hungary | − | R | + | + | + | |
| SE40 | Blood | Hungary | − | S | − | − | − | O16 ST131 |
| SE42 | Blood | Hungary | − | R | + | + | + | |
| SE6 | Blood | Hungary | + | R | + | + | + | |
| 8 JN (33) | Wound | Spain | + | ND | + | + | + | |
| 2N (33) | Urine | Spain | + | ND | + | + | + | |
| 5JN (33) | Urine | Spain | + | ND | + | + | + | |
| 10AR (33) | Urine | Spain | + | ND | + | + | + | |
| 7O (33) | Urine | Spain | + | ND | + | + | + | |
| 3O (33) | Blood | Spain | + | ND | + | + | + | |
| 1N (33) | Blood | Spain | + | ND | + | + | + | |
| 10JN (33) | Urine | Spain | + | ND | + | + | + | |
| 3MR (33) | Urine | Spain | + | ND | + | + | + | |
| 10J (33) | Urine | Spain | + | ND | + | + | + | |
| 8MI (33) | Urine | Spain | + | ND | + | + | + | |
| 6MI (33) | Exudate | Spain | + | ND | + | + | + | |
| FEC250 (34) | Stool | Spain | + | ND | + | + | + | Phylogenetic group D |
| C70 | Urine | Spain | + | ND | + | + | + | Phylogenetic group D |
R, resistant; S, susceptible; ND, not determined.
+, positive result; −, negative result.
All isolates were found to be of the K-12 core type.
VAP, ventilator-associated pneumonia.
UAE, United Arab Emirates.
FIG 7.

Latex agglutination. Detection of O25b antigen expressing E. coli strains with agglutination assay using MAb 6D1-1B2 coupled to latex beads. A loopful of bacteria was mixed with 10 μl of a 1% bead solution in PBS. The reactions were developed by gentle agitation for about 10 s.
We compared the specificity and sensitivity of this assay to those of the currently used state-of-the-art typing assays, i.e., PCR detection of a specific region within rfbO25b (28) and agglutination with O25-specific rabbit serum. Agglutination with commercial typing serum requires heat treatment of the E. coli cells in order to expose the target O-antigen and to denature potential reactive protein targets. No such pretreatment was necessary to enable positive agglutination with the MAb-coupled beads (Table 1). Even in the case of heat-killed cells, the sensitivity of the MAb coupled-bead assay was found superior to that of the serum-based typing.
Interestingly, one strain that was found to be positive with the O25b-specific PCR did not agglutinate with the O25b MAb-coated beads. We determined that this particular strain displayed a rough LPS phenotype by silver staining (data not shown) due to a yet-unidentified mutation (strain 81010 in Table 1).
DISCUSSION
Infections by E. coli strains from the ST131-O25b lineage represent an increasingly significant medical concern due to their extended multidrug-resistant phenotype. By definition, all strains from this clone express the O25b LPS repeating unit. Therefore, we aimed to generate MAbs against this specific antigen to develop improved diagnostics. To obtain specific MAbs, mice were immunized with whole killed cells of an isogenic nonencapsulated mutant of an ST131-O25b clinical isolate in order to avoid generating antibodies against the bulky capsular polysaccharide. We selected three MAbs with unique complementarity-determining region (CDR) sequences that specifically recognized the O25b antigen expressed on the surface of the multidrug-resistant ST131 clone but that showed no cross-reactivity with other E. coli strains expressing different O-antigens. Specificity was further demonstrated by the lack of binding to LPS molecules obtained from an isogenic rough mutant as well as to other smooth LPS molecules from several E. coli serotypes, including the related O25a antigen. The fact that O25a polyclonal serum is cross-reactive with O25b antigens (representing one of the state-of-the-art detection methods for O25b strains) while these MAbs do not cross-react implies that these MAbs recognize a unique epitope present in the O25b RU, one that is not available, however, within the O25a antigen.
In order to support this notion, the structure of the O25b repeating unit was elucidated. As expected, the chemical composition of the O25b antigen was found to be different from that of the related O25a antigen. The main alteration was the presence of l-Rhap2OAc versus l-FucpNAc in the central position of the repeating units. Since O-acetylated carbohydrate structures are considered to be immunodominant, it is not surprising that all 3 MAbs selected from the hybridoma fusion were highly specific to the O25b antigen (i.e., do not cross-react with O25a). Next, we tested the reactivity of these MAbs to E. coli cells expressing O-antigens with a similar sugar composition, i.e., those containing an O-acetylated rhamnose residue (29). None of these antigens or any of the common ExPEC O-types were detected by any of the 3 MAbs, corroborating that their epitope is restricted to the unique O25b antigen structure presented in this article.
The distinctiveness of the O25b structure is supported by the genetic background. We sequenced the entire rfb cluster of the clinical isolate that had been used for the immunization, and we completed the LPS structural analysis. The rfb locus was found to be essentially identical to that of ST131-O25b strain E. coli EC958 reported earlier (30). A comparison of the rfb clusters from O25a and O25b, however, revealed completely dissimilar regions at the 3′ end of the operons following a shared approximately 9-kb-long 5′ region. The unique rfbO25b region consists of 3 genes, including 2 with putative glycosyltransferase functions. The third gene encodes a putative O-acetyltransferase, which is in good agreement with the structure described above. As O25a is a common serotype of E. coli (among both ExPEC and intestinal pathogenic strains) (31), it is postulated that the O25b variant has evolved in order to evade preexisting immunity that is prevalent in the community. However, genetic analysis implies the opposite order of genetic rearrangement. In rfbO25a, the gene encoding the putative O-acetyltransferase is truncated and flanked by a completely different 3′ region, adjacent to an insertion sequence, suggesting a potential site for recombination events. Therefore, we hypothesize that the locus encoding O25a RU evolved by integration of a different 3′ region within the archetype O25 rfb operon (currently known as rfbO25b), which was also supported by analyzing the GC contents of the determinants. Interestingly, the O25a-specific region encoding FucNAc synthesis and transfer shows high homology to the determinants of other O types (e.g., O4 and O26) also containing this sugar; hence, those serotypes might have served as the source of the O25a-specific genes.
Since it is known that phage-derived sequences carried outside the rfb operon can modify O-antigen backbone structures (e.g., with O-acetyl groups) in enterobacterial pathogens, we considered it important to show that the 3 specific genes present within rfbO25b are the sole determinants of the unique O25b RU structure. This was shown by creating an isogenic mutant from E. coli O25a whose O-antigen synthesis was lost upon deletion of the 3′ end of its rfb locus (i.e., the O25a-specific portion). Subsequently, this mutant (E47aΔ3rfb) was trans-complemented with either the O25a- or O25b-specific region of the corresponding operon, both of which retained the expression of smooth LPS. Nevertheless, unlike p3O25a, the carriage of p3O25b in an O25a genetic background elicited reactivity to O25b-specific MAbs, corroborating the exclusivity of this region in the expression of the specific epitope.
Next, we tested the diagnostic potentials of the specific MAbs in comparison to those assays currently used to detect ST131-O25b strains. We collected a panel of isolates from various geographical regions, which were confirmed to belong to ST131-O25b. All of these strains gave a clear positive result with the agglutination assay using O25b-specific murine IgG3. Agglutination by MAbs coupled to commercial latex beads (i.e., the assay described in this article) appeared to be superior to the currently used agglutination test with O25 hyperimmune rabbit serum with respect to specificity and sensitivity. Furthermore, in case this assay is aimed to be used as a companion diagnostic tool for a prospective immunotherapy targeting the O25b antigen, it is essential that only the isolates that do express the antigen be detected. In this respect, the agglutination assay has a clear advantage over PCR-based detection of O25b genes, which is illustrated by the PCR-positive but rough strain we identified. The frequency of such mutants carrying the rfbO25b operon but not expressing the antigen is unknown and requires further investigation. Still, such strains identified by PCR would give a false-positive indication for O25b-targeting therapy. Besides these scientific considerations, the convenience, resources, and time required for the latex agglutination assay described are clearly superior to those of any of the currently used tools for the detection of ST131-O25b strains. Interestingly, environmental isolates belonging to the ST69-D clonal group were shown to express O25b antigen, as suggested by rfb-specific PCR positivity (32). In case these strains in fact express the O25b antigen, the phenotypic test described above would also detect them. Nevertheless, the low-virulence-gene content within this clone (32) predicts a rare association of this clone with human infections. Recently, one clinical isolate from Denmark was identified (13) as ST69-D-O25:H4; however, it is uncertain which variant of the O25 antigen was expressed by this isolate.
Having shown the accessibility of O25b epitopes on the surface of encapsulated ST131-O25b isolates, it is tempting to speculate that humanized MAbs with such specificities might be developed as efficacious novel immune therapeutics against this MDR clone. In this case, the described rapid and reliable agglutination assays might serve as an invaluable companion diagnostic tool for the identification of relevant infections.
Supplementary Material
ACKNOWLEDGMENTS
We thank Srijib Banerjee and Pallavi Banerjee for their technical assistance with MAb purification and molecular typing of strains, respectively. We also thank Agnes Sonnevend (Al Ain, United Arab Emirates), Aranzazu Valverde (Madrid, Spain), and Franz-Josef Schmitz (Minden, Germany) for providing clinical isolates for this study, and we thank Fraser Leslie for critical reading of the manuscript.
The research work of the Vienna Team was substantially supported by the General Programme of the Austrian Research Promotion Agency (FFG).
V.S., Z.M., E.N., and G.N. are employees of Arsanis Biosciences GmbH, a privately owned biotechnology company, and they own shares of the company.
Footnotes
Published ahead of print 30 April 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00685-13.
REFERENCES
- 1.Peirano G, Pitout JD. 2010. Molecular epidemiology of Escherichia coli producing CTX-M beta-lactamases: the worldwide emergence of clone ST131 O25:H4. Int. J. Antimicrob. Agents 35:316–321. 10.1016/j.ijantimicag.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 2.Rogers BA, Sidjabat HE, Paterson DL. 2011. Escherichia coli O25b-ST131: a pandemic, multiresistant, community-associated strain. J. Antimicrob. Chemother. 66:1–14. 10.1093/jac/dkq415 [DOI] [PubMed] [Google Scholar]
- 3.Woodford N, Turton JF, Livermore DM. 2011. Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol. Rev. 35:736–755. 10.1111/j.1574-6976.2011.00268.x [DOI] [PubMed] [Google Scholar]
- 4.Nicolas-Chanoine MH, Blanco J, Leflon-Guibout V, Demarty R, Alonso MP, Caniça MM, Park YJ, Lavigne JP, Pitout J, Johnson JR. 2008. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. J. Antimicrob. Chemother. 61:273–281. 10.1093/jac/dkm464 [DOI] [PubMed] [Google Scholar]
- 5.Johnson JR, Johnston B, Clabots C, Kuskowski MA, Castanheira M. 2010. Escherichia coli sequence type ST131 as the major cause of serious multidrug-resistant E. coli infections in the United States. Clin. Infect. Dis. 51:286–294. 10.1086/653932 [DOI] [PubMed] [Google Scholar]
- 6.Bonnin RA, Poirel L, Carattoli A, Nordmann P. 2012. Characterization of an IncFII plasmid encoding NDM-1 from Escherichia coli ST131. PLoS One 7:e34752. 10.1371/journal.pone.0034752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dimou V, Dhanji H, Pike R, Livermore DM, Woodford N. 2012. Characterization of Enterobacteriaceae producing OXA-48-like carbapenemases in the UK. J. Antimicrob. Chemother. 67:1660–1665. 10.1093/jac/dks124 [DOI] [PubMed] [Google Scholar]
- 8.Morris D, Boyle F, Ludden C, Condon I, Hale J, O'Connell N, Power L, Boo TW, Dhanji H, Lavallee C, Woodford N, Cormican M. 2011. Production of KPC-2 carbapenemase by an Escherichia coli clinical isolate belonging to the international ST131 clone. Antimicrob. Agents Chemother. 55:4935–4936. 10.1128/AAC.05127-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peirano G, Mulvey GL, Armstrong GD, Pitout JD. 2013. Virulence potential and adherence properties of Escherichia coli that produce CTX-M and NDM β-lactamases. J. Med. Microbiol. 62:525–530. 10.1099/jmm.0.048983-0 [DOI] [PubMed] [Google Scholar]
- 10.Vimont S, Boyd A, Bleibtreu A, Bens M, Goujon JM, Garry L, Clermont O, Denamur E, Arlet G, Vandewalle A. 2012. The CTX-M-15-producing Escherichia coli clone O25b: H4-ST131 has high intestine colonization and urinary tract infection abilities. PLoS One 7:e46547. 10.1371/journal.pone.0046547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson JR, Porter SB, Zhanel G, Kuskowski MA, Denamur E. 2012. Virulence of Escherichia coli clinical isolates in a murine sepsis model in relation to sequence type ST131 status, fluoroquinolone resistance, and virulence genotype. Infect. Immun. 80:1554–1562. 10.1128/IAI.06388-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lavigne JP, Vergunst AC, Goret L, Sotto A, Combescure C, Blanco J, O'Callaghan D, Nicolas-Chanoine MH. 2012. Virulence potential and genomic mapping of the worldwide clone Escherichia coli ST131. PLoS One 7:e34294. 10.1371/journal.pone.0034294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olesen B, Hansen DS, Nilsson F, Frimodt-Møller J, Leihof RF, Struve C, Scheutz F, Johnston B, Krogfelt KA, Johnson JR. 2013. Prevalence and characteristics of the epidemic multiresistant Escherichia coli ST131 clonal group among extended-spectrum beta-lactamase-producing E. coli isolates in Copenhagen, Denmark. J. Clin. Microbiol. 51:1779–1785. 10.1128/JCM.00346-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van der Bij AK, Peirano G, Pitondo-Silva A, Pitout JD. 2012. The presence of genes encoding for different virulence factors in clonally related Escherichia coli that produce CTX-Ms. Diagn. Microbiol. Infect. Dis. 72:297–302. 10.1016/j.diagmicrobio.2011.12.011 [DOI] [PubMed] [Google Scholar]
- 15.Gibreel TM, Dodgson AR, Cheesbrough J, Bolton FJ, Fox AJ, Upton M. 2012. High metabolic potential may contribute to the success of ST131 uropathogenic Escherichia coli. J. Clin. Microbiol. 50:3202–3207. 10.1128/JCM.01423-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNally A, Cheng L, Harris SR, Corander J. 2013. The evolutionary path to extraintestinal pathogenic, drug-resistant Escherichia coli is marked by drastic reduction in detectable recombination within the core genome. Genome Biol. Evol. 5:699–710. 10.1093/gbe/evt038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szijártó V, Pal T, Nagy G, Nagy E, Ghazawi A, al-Haj M, El Kurdi S, Sonnevend A. 2012. The rapidly emerging ESBL-producing Escherichia coli O25-ST131 clone carries LPS core synthesis genes of the K-12 type. FEMS Microbiol. Lett. 332:131–136. 10.1111/j.1574-6968.2012.02585.x [DOI] [PubMed] [Google Scholar]
- 18.Benckert J, Schmolka N, Kreschel C, Zoller MJ, Sturm A, Wiedenmann B, Wardemann H. 2011. The majority of intestinal IgA+ and IgG+ plasmablasts in the human gut are antigen-specific. J. Clin. Invest. 121:1946–1955. 10.1172/JCI44447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645. 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson D, Neill W, Poxton IR. 1990. A comparison of immunoblotting, flow cytometry and ELISA to monitor the binding of anti-lipopolysaccharide monoclonal antibodies. J. Immunol. Methods 133:227–233. 10.1016/0022-1759(90)90363-Z [DOI] [PubMed] [Google Scholar]
- 21.Ciucanu I, Kerek F. 1984. A simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 131:209–217. 10.1016/0008-6215(84)85242-8 [DOI] [Google Scholar]
- 22.Gerwig GJ, Kamerling JP, Vliegenthart JFG. 1978. Determination of the d and l configuration of neutral monosaccharides by high-resolution capillary g.l.c. Carbohydr. Res. 62:349–357. 10.1016/S0008-6215(00)80881-2 [DOI] [Google Scholar]
- 23.Ceroni A, Maass K, Geyer H, Geyer R, Dell A, Haslam SM. 2008. GlycoWorkbench: a tool for the computer-assisted annotation of mass spectra of glycans. J. Proteome Res. 7:1650–1659. 10.1021/pr7008252 [DOI] [PubMed] [Google Scholar]
- 24.Kenne L, Lindberg B, Madden JK, Lindberg AA, Gemski P., Jr 1983. Structural studies of the Escherichia coli O-antigen 25. Carbohydr. Res. 122:249–256. 10.1016/0008-6215(83)88336-0 [DOI] [PubMed] [Google Scholar]
- 25.Müller-Loennies S, Lindner B, Brade H. 2003. Structural analysis of oligosaccharides from lipopolysaccharide (LPS) of Escherichia coli K12 strain W3100 reveals a link between inner and outer core LPS biosynthesis. J. Biol. Chem. 278:34090–34101. 10.1074/jbc.M303985200 [DOI] [PubMed] [Google Scholar]
- 26.Duda KA, Lindner B, Brade H, Leimbach A, Brzuszkiewicz E, Dobrindt U, Holst O. 2011. The lipopolysaccharide of the mastitis isolate Escherichia coli strain 1303 comprises a novel O-antigen and the rare K-12 core type. Microbiology 157:1750–1760. 10.1099/mic.0.046912-0 [DOI] [PubMed] [Google Scholar]
- 27.Domon B, Costello CE. 1988. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconjugate J. 5:397–409 [Google Scholar]
- 28.Clermont O, Dhanji H, Upton M, Gibreel T, Fox A, Boyd D, Mulvey MR, Nordmann P, Ruppé E, Sarthou JL, Frank T, Vimont S, Arlet G, Branger C, Woodford N, Denamur E. 2009. Rapid detection of the O25b-ST131 clone of Escherichia coli encompassing the CTX-M-15-producing strains. J. Antimicrob. Chemother. 64:274–277. 10.1093/jac/dkp194 [DOI] [PubMed] [Google Scholar]
- 29.Stenutz R, Weintraub A, Widmalm G. 2006. The structures of Escherichia coli O-polysaccharide antigens. FEMS Microbiol. Rev. 30:382–403. 10.1111/j.1574-6976.2006.00016.x [DOI] [PubMed] [Google Scholar]
- 30.Totsika M, Beatson SA, Sarkar S, Phan MD, Petty NK, Bachmann N, Szubert M, Sidjabat HE, Paterson DL, Upton M, Schembri MA. 2011. Insights into a multidrug resistant Escherichia coli pathogen of the globally disseminated ST131 lineage: genome analysis and virulence mechanisms. PLoS One 6:e26578. 10.1371/journal.pone.0026578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clermont O, Johnson JR, Menard M, Denamur E. 2007. Determination of Escherichia coli O types by allele-specific polymerase chain reaction: application to the O types involved in human septicemia. Diagn. Microbiol. Infect. Dis. 57:129–136. 10.1016/j.diagmicrobio.2006.08.007 [DOI] [PubMed] [Google Scholar]
- 32.Colomer-Lluch M, Mora A, López C, Mamani R, Dahbi G, Marzoa J, Herrera A, Viso S, Blanco JE, Blanco M, Alonso MP, Jofre J, Muniesa M, Blanco J. 2013. Detection of quinolone-resistant Escherichia coli isolates belonging to clonal groups O25b:H4-B2-ST131 and O25b:H4-D-ST69 in raw sewage and river water in Barcelona, Spain. J. Antimicrob. Chemother. 68:758–765. 10.1093/jac/dks477 [DOI] [PubMed] [Google Scholar]
- 33.Novais Å, Viana D, Baquero F, Martínez-Botas J, Cantón R, Coque TM. 2012. Contribution of IncFII and broad-host IncA/C and IncN plasmids to the local expansion and diversification of phylogroup B2 Escherichia coli ST131 clones carrying blaCTX-M-15 and qnrS1 genes. Antimicrob. Agents Chemother. 56:2763–2766. 10.1128/AAC.06001-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valverde A, Coque TM, Sánchez-Moreno MP, Rollán A, Baquero F, Cantón R. 2004. Dramatic increase in prevalence of fecal carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae during nonoutbreak situations in Spain. J. Clin. Microbiol. 42:4769–4775. 10.1128/JCM.42.10.4769-4775.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.