Abstract
The EcfG-type sigma factor RpoE2 is the regulator of the general stress response in Sinorhizobium meliloti. RpoE2 activity is negatively regulated by two NepR-type anti-sigma factors (RsiA1/A2), themselves under the control of two anti-anti-sigma factors (RsiB1/B2) belonging to the PhyR family of response regulators. The current model of RpoE2 activation suggests that in response to stress, RsiB1/B2 are activated by phosphorylation of an aspartate residue in their receiver domain. Once activated, RsiB1/B2 become able to interact with the anti-sigma factors and release RpoE2, which can then associate with the RNA polymerase to transcribe its target genes. The purpose of this work was to identify and characterize proteins involved in controlling the phosphorylation status of RsiB1/B2. Using in vivo approaches, we show that the putative histidine kinase encoded by the rsiC gene (SMc01507), located downstream from rpoE2, is able to both positively and negatively regulate the general stress response. In addition, our data suggest that the negative action of RsiC results from inhibition of RsiB1/B2 phosphorylation. From these observations, we propose that RsiC is a bifunctional histidine kinase/phosphatase responsible for RsiB1/B2 phosphorylation or dephosphorylation in the presence or absence of stress, respectively. Two proteins were previously proposed to control PhyR phosphorylation in Caulobacter crescentus and Sphingomonas sp. strain FR1. However, these proteins contain a Pfam:HisKA_2 domain of dimerization and histidine phosphotransfer, whereas S. meliloti RsiC harbors a Pfam:HWE_HK domain instead. Therefore, this is the first report of an HWE_HK-containing protein controlling the general stress response in Alphaproteobacteria.
INTRODUCTION
Bacteria naturally live in constantly changing environments, where they are exposed to many stressful conditions, including nutrient limitation and biotic or abiotic stresses. The capacity to sense and adapt to these stresses is essential for the survival of the bacteria, which have evolved various types of stress responses. A number of these responses function by eliminating the inducing stress and/or repairing the associated cell damage. In parallel to these stress-specific responses, a so-called general stress response is activated under numerous different stress conditions and confers multiple stress resistances to the bacteria.
It has been known for a long time that sigma factors play a central role in the control of the general stress response of both Gram-positive and Gram-negative bacteria. In Bacillus subtilis and other firmicutes, this response is controlled by σB (1, 2), whereas in Escherichia coli and related Gammaproteobacteria, as well as in several Beta- and Deltaproteobacteria, it is controlled by σS (3, 4). However, in the alphaproteobacterial group, the prominent role of extracytoplasmic-function sigma factors was uncovered recently with the finding that RpoE2 controls a general stress response in Sinorhizobium meliloti, the nitrogen-fixing symbiont of alfalfa (5). RpoE2 is activated under a number of stress and starvation conditions and controls the transcription of >100 genes, including several involved in stress resistance (5–10). Accordingly, rpoE2 mutants have been found to be more sensitive than the wild-type strain to desiccation and osmotic stress, as well as heat and oxidative stress in the stationary phase (6–8, 11). RpoE2 orthologues, collectively called EcfG or ECF15 sigma factors (12), are widely distributed among Alphaproteobacteria, and several of them have been described as activated under stress or starvation conditions and to play various roles in stress resistance and/or host colonization (13–22).
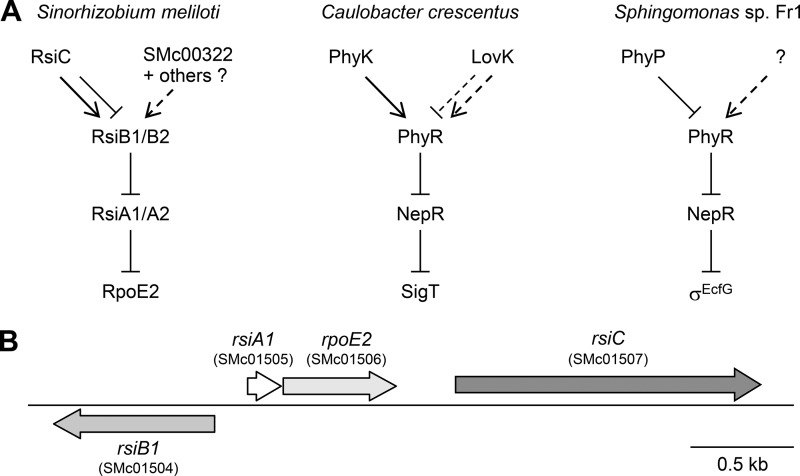
The mechanisms of activation of EcfG sigma factors in response to stress have been studied in several bacteria, including Methylobacterium extorquens, Bradyrhizobium japonicum, S. meliloti, Caulobacter crescentus, Sphingomonas sp. strain Fr1, Brucella abortus, and Bartonella quintana (15, 16, 18, 19, 21, 23–27). These mechanisms appear to be conserved, with some species-specific variations, and the current common model can be summarized as follows (Fig. 1A): under nonstress conditions, the sigma factor is kept inactive by interaction with one or several anti-sigma factors (called RsiA or NepR); following stress exposure, one or several anti-anti-sigma factors (called RsiB or PhyR) become activated, thereby enabling the interaction with the anti-sigma factor(s) and relieving sigma factor inhibition.
FIG 1.
(A) Current models of EcfG sigma factor regulation in S. meliloti, C. crescentus, and Sphingomonas sp. strain Fr1. Arrows and T lines stand for positive and negative regulations, respectively. Dotted lines indicate hypothetical regulations or regulations only revealed in mutant backgrounds whose relevance under wild-type conditions is not clear. In the absence of stress, the sigma factors (RpoE2/SigT/σEcfG) are kept inactive by interaction with anti-sigma factors (RsiA/NepR). Under stress or starvation conditions, anti-anti-sigma factors (RsiB/PhyR) are activated by phosphorylation and relieve sigma factor inhibition by interacting with the anti-sigma factors. The various actors controlling the phosphorylation status of anti-anti-sigma factors are indicated at the top. These models were drawn according to the literature (5, 18, 23, 24, 35, 36) and the present work. (B) Schematic representation of the S. meliloti chromosomal region encoding RpoE2 and its regulators RsiA1, RsiB1, and RsiC.
Interestingly, the RsiB/PhyR anti-anti-sigma factors behave as response regulators of two-component regulatory systems, as they are activated by phosphorylation of the aspartate residue in a conserved phospho-receiver domain. This suggests that one or several histidine kinases are involved in their phosphorylation in response to stress. However, much less is understood about this step of the model. It was noted early on that putative histidine kinase-encoding genes are located in the sigma factor-encoding genomic regions of most alphaproteobacterial species (19), suggesting that the corresponding enzymes (here referred to as cis-encoded kinases) are involved in stress perception, autophosphorylation, and phosphotransfer to RsiB/PhyR response regulators. These cis-encoded histidine kinases are atypical in that they do not contain the usual domain of dimerization and histidine phosphotransfer found in classical histidine kinases (Pfam:HisKA) but instead harbor either a Pfam:HWE_HK or, less frequently (33%), a Pfam:HisKA_2 domain (here referred to as HW- or HK-type kinases, respectively) (28). Two studies have reported on the involvement of such cis-encoded kinases in the general stress response of Alphaproteobacteria. In the first study, the PhyK kinase of C. crescentus has been shown to be essential in vivo for PhyR phosphorylation and activation of SigT in response to stress (24). In a second study, PhyP of Sphingomonas sp. strain Fr1 has been suggested to act not as a kinase but solely as a phosphatase to dephosphorylate PhyR under nonstress conditions (18), the origin of the phosphate being unknown in this case. Both C. crescentus PhyK and Sphingomonas PhyP belong to the HK type, and there is no report to date on the role(s) played by cis-encoded kinases of the HW type in the general stress response of Alphaproteobacteria in spite of their more widespread occurrence (28).
In S. meliloti, a putative cytoplasmic HW-type histidine kinase, which we called RsiC, is encoded by SMc01507, located just downstream from rpoE2 (Fig. 1B). In this paper, we investigate the function of this protein in the general stress response of S. meliloti and report in vivo data which strongly suggest that it acts as a bifunctional kinase/phosphatase to control the anti-anti-sigma factor phosphorylation status and, as a result, the RpoE2-dependent general stress response.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains used in this study are listed in Table 1. Escherichia coli strains were grown in Luria-Bertani (LB) medium at 37°C. S. meliloti strains were grown at 28°C, either in LB medium supplemented with 2.5 mM CaCl2 and 2.5 mM MgCl2 (LBMC; used for strain constructions and precultures), in TY medium supplemented with 6 mM CaCl2 (TYC), or in Vincent minimal medium (VMM; 7.35 mM KH2PO4, 5.74 mM K2HPO4, 1 mM MgSO4, 456 μM CaCl2, 35 μM FeCl3, 4 μM biotin, 48.5 μM H3BO3, 10 μM MnSO4, 1 μM ZnSO4, 0.5 μM CuSO4, 0.27 μM CoCl2, 0.5 μM NaMoO4; pH 7) containing as carbon and nitrogen sources either 10 mM sodium succinate and 18.7 mM NH4Cl (VMMS medium), 10 mM galactose and 10 mM sodium aspartate (VMMGAS medium), or 55 mM mannitol and 18.7 mM NH4Cl (VMMM medium). When required, antibiotics were added at the following final concentrations: 100 to 300 μg ml−1 streptomycin (Sm), 10 μg ml−1 tetracycline (Tet), 40 μg ml−1 gentamicin (Gm), 50 to 100 μg ml−1 trimethoprim (Tmp), 40 μg ml−1 hygromycin (Hyg), or 50 μg ml−1 carbenicillin (Cb).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Sinorhizobium meliloti | ||
| Rm1021 | Wild-type strain (Smr) | 46 |
| GMI11495 | Wild-type strain (Smr), Rm2011 background | 9, 47 |
| 2011mTn5STM.2.04.C11 | GMI11495 SMb20515::mTn5 | 47 |
| 2011mTn5STM.3.10.F07 | GMI11495 SMa0113::mTn5 | 47 |
| 2011mTn5STM.3.08.E09 | GMI11495 SMb20933::mTn5 | 47 |
| 2011mTn5STM.4.06.A07 | GMI11495 SMa1696::mTn5 | 47 |
| CBT208 | Rm1021 rpoE2::hph (Hygr) | 5 |
| CBT430 | Rm1021 ΔrsiB1 ΔrsiB2 | 23 |
| CBT785 | Rm1021 ΔrsiC | This work |
| CBT862 | Rm1021 ΔrsiC ΔSMa1001 | This work |
| CBT866 | Rm1021 ΔrsiC ΔSMa2063 | This work |
| CBT1051 | Rm1021 ΔrsiC ΔrsiB1 ΔrsiB2 | This work |
| CBT1129 | Rm1021 ΔSMc00322 | This work |
| CBT1169 | Rm1021 ΔrsiC ΔSMc00322 | This work |
| CBT1702 | GMI11495 ΔrsiC SMa0113::mTn5 | This work |
| CBT1704 | GMI11495 ΔrsiC SMb20515::mTn5 | This work |
| CBT1706 | GMI11495 ΔrsiC SMa1696::mTn5 | This work |
| CBT1708 | GMI11495 ΔrsiC SMb20933::mTn5 | This work |
| CBT1710 | GMI11495 ΔrsiC | This work |
| Escherichia coli | ||
| DH5α | supE44 ΔlacU169 Φ80dlacZ ΔM15 hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | Invitrogen |
| Plasmids | ||
| pGEM-T | Cloning vector (Ampr) | Promega |
| pJQ200mp19 | Gene replacement vector (Gmr) | 48 |
| pRK2013 | Helper plasmid for triparental matings (Kanr) | 49 |
| pMP220-885 | pMP220-PSMc00885-lacZ fusion (Tetr) | 23 |
| pMLBAD | Expression vector, inducible by arabinose (Tmpr) | 50 |
| pMLBAD-rsiB1 | pMLBAD derivative expressing rsiB1 | 23 |
| pMLBAD-rsiB2 | pMLBAD derivative expressing rsiB2 | 23 |
| pMLBAD-rsiB1-D191A | pMLBAD derivative expressing rsiB1-D191A | 23 |
| pMLBAD-rsiB2-D191A | pMLBAD derivative expressing rsiB2-D191A | This work |
| pMLBAD-rsiB1-strep | pMLBAD derivative expressing rsiB1-strep | This work |
| pMLBAD-rsiC | pMLBAD derivative expressing rsiC | This work |
| pMLBAD-rsiC-H318K | pMLBAD derivative expressing rsiC-H318K | This work |
| pLS100-17 | pJQ200mp19 derivative for rsiC deletion | This work |
| pLS166-5 | pJQ200mp19 derivative for SMc00322 deletion | This work |
| pLS124-1 | pJQ200mp19 derivative for SMa1001 deletion | This work |
| pLS125-1 | pJQ200mp19 derivative for SMa2063 deletion | This work |
Stress sensitivity assays.
To test salt sensitivity, cells grown overnight to saturation in LBMC supplemented with Sm were collected and washed in VMMGAS before being diluted to an optical density at 600 nm (OD600) of 0.025 in VMMGAS with or without 0.5 M NaCl, and growth was monitored by measuring the OD600.
To test desiccation sensitivity, precultures saturated overnight in LBMC supplemented with Sm were diluted to an OD600 of 0.1 in VMMM and grown to saturation for 24 h. The cultures were then 10-fold serially diluted in VMM salts, and 5-μl aliquots of dilutions were spotted on wet sterile nitrocellulose membranes put on the surface of VMMM agar plates. After spot evaporation, membranes were removed under sterile conditions and allowed to dry in the dark at room temperature in a closed jar (∼2 liters) maintained at ∼23% relative humidity by the presence of an oversaturated solution of potassium acetate (100 ml). At time intervals, membranes were removed from the jar, put on the surface of TYC plates supplemented with Sm, and incubated at 28°C to allow surviving bacteria to form colonies.
Strain and plasmid constructions.
All plasmid constructions were performed in E. coli DH5α. The absence of mutations in all constructs was checked by DNA sequencing. Open reading frames (ORFs) or ORF-flanking DNA fragments were amplified by PCR using S. meliloti Rm1021 genomic DNA as the template and the oligonucleotides listed in Table S1 in the supplemental material as primers and then were cloned into pGEM-T.
pMLBAD-rsiC was constructed by subcloning in pMLBAD an EcoRI/XmaI fragment from pGEMT-rsiC. To construct pMLBAD-rsiC-H318K, two internal rsiC fragments flanking the mutation were generated by PCR using OCB1035-OCB1031 and OCB1032-OCB985, the overlapping OCB1031 and OCB1032 primers generating the CAC→AAA mutation and an MluI site. These fragments were separately cloned into pGEM-T and then subsequently juxtaposed as NcoI-MluI and MluI-AhdI fragments into NcoI-AhdI-digested pMLBAD-rsiC. To construct pMLBAD-rsiB2-D191A, a PCR fragment containing the 5′ coding region of rsiB2 was generated using OCB684 and OCB938 (which generates the GAT→GCT mutation), cloned into pGEM-T, and juxtaposed as an EcoRI-PvuII fragment to the PvuII-XmaI fragment from pMLBAD-rsiB2 into EcoRI-XmaI-cut pMLBAD. pMLBAD-rsiB1-strep was derived from a plasmid designed to express the strep-tagged C-terminal part of RsiC, obtained by cloning in pMLBAD an OCB984-OCB985 PCR fragment cut by EcoRI-XbaI. The rsiC sequence of this plasmid was exchanged with the coding sequence of rsiB1, cloned as an EcoRI-XmaI digest of a PCR fragment generated using OCB668-OCB986.
Gene deletions were performed using pJQ200mp19 derivatives containing ∼400- to 500-bp regions flanking the gene to be deleted (rsiC, SMc00322, SMa1001, or SMa2063). The flanking regions individually cloned into pGEM-T were subsequently juxtaposed as BamHI-SpeI and SpeI-SacI fragments into BamHI-SacI-cut pJQ200mp19 (ΔrsiC), as XhoI-BamHI and BamHI-SacI fragments into XhoI-SacI-digested pJQ200mp19 (ΔSMa1001), and as SalI-BamHI and BamHI-SacI fragments into SalI-SacI-digested pJQ200mp19 (ΔSMc00322 and ΔSMa2063).
Plasmids, either integrative or replicative, were introduced in S. meliloti by triparental mating (29) using pRK2013 as a helper plasmid with subsequent selection for antibiotic resistance. For the construction of deletion mutants, single-crossover genomic integration of the corresponding pJQ200 derivatives was generated by selecting for Gm resistance. The resulting strains were then propagated in the absence of antibiotic, and cells having lost the plasmid by a second recombination event were selected by plating on LBMC supplemented with 5% sucrose (Suc). Sucr Gms colonies were screened by PCR analysis using as primers OCB516-OCB714, OCB962-OCB963, OCB857-OCB858, or OCB859-OCB860 for deletion of rsiC, SMc00322, SMa1001, or SMa2063, respectively.
rsiC single-deletion mutants were constructed in both Rm1021 and Rm2011 backgrounds. Double kinase mutants were constructed either by introducing the rsiC deletion in each of the four kinase mutants (SMa0113, SMa1696, SMb20515, and SMb20933) already available as Tn5 insertions in the Rm2011 background or by introducing the SMa1001, SMa2063, or SMc00322 deletion into the ΔrsiC mutant in the Rm1021 background.
Measurement of RpoE2 activity.
To measure RpoE2 activity in S. meliloti, the following procedure generally was used. Five to 10 ml overnight precultures of strains carrying the reporter plasmid pMP220-885 were diluted to an OD600 of 0.1 in 5 ml of fresh VMMS and grown for ∼6 to 8 h. Cultures were then diluted once more in 20 to 25 ml in order to reach an OD600 of ∼0.1 to 0.2 the day after. After overnight growth, cultures were divided into two flasks; one was kept at 28°C, and the other was shifted to 40°C. After 1 h, 100 μl of each culture was collected, frozen in liquid nitrogen, and stored at −20°C. The culture at 28°C was allowed to reach the stationary phase and kept for 48 h before 100 μl of culture was collected (stationary-phase samples). For the experiments described in Fig. 4, arabinose was first added to the overnight culture at a final concentration of 2%, and cultures were allowed to grow for 2 h before being divided in two halves as described above. β-Galactosidase assays were performed on the thawed samples as described previously (30). Measurements were performed on at least three independent experiments. Statistical analyses were performed using GraphPad Prism software v5.03 for Windows.
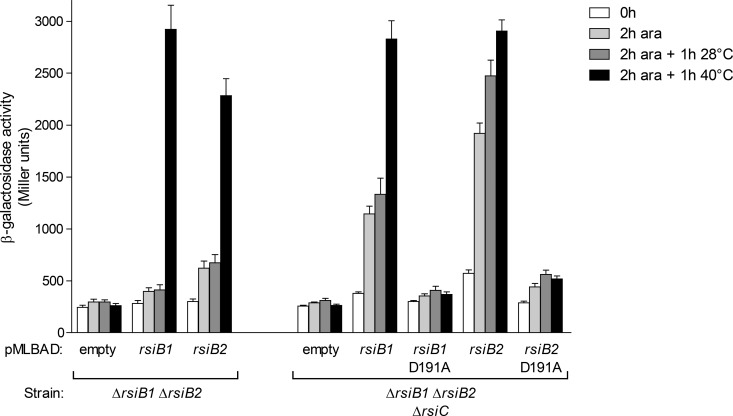
FIG 4.
RsiC negatively controls both RsiB1 and RsiB2. The transcription level of the PSMc00885-lacZ fusion carried on plasmid pMP220-885, used as a reporter of RpoE2 activity, was measured in the S. meliloti strain CBT430 (ΔrsiB1 ΔrsiB2) or CBT1051 (ΔrsiB1 ΔrsiB2 ΔrsiC) containing either the empty vector pMLBAD or pMLBAD derivatives expressing either rsiB1 or rsiB2 or mutated forms thereof (D191A), as indicated below the graphs. β-Galactosidase activity was measured on aliquots of cultures grown to exponential phase before (0 h; white bars) and after incubation for 2 h in the presence of 2% arabinose (2 h ara; pale gray bars) and after a further 1 h of incubation at either 28°C (medium gray bars) or 40°C (black bars), as described in Materials and Methods. β-Galactosidase activities are means and standard errors from at least three independent experiments.
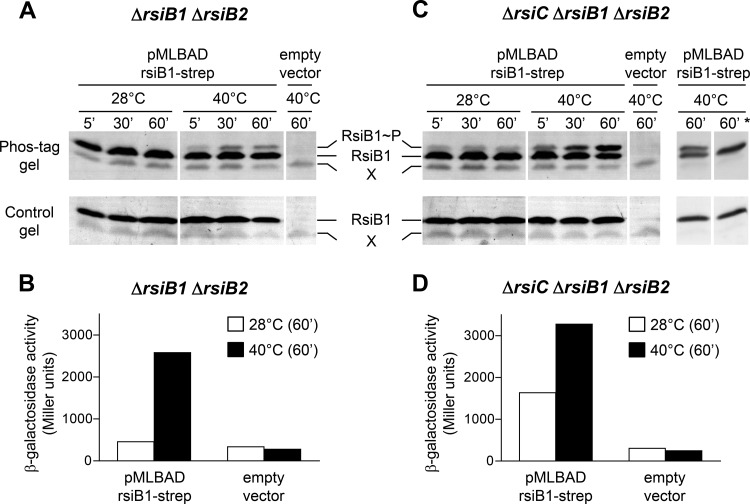
RsiB1 phosphorylation in vivo.
Strains carrying pMP220-885 (a plasmid belonging to the IncP incompatibility group) and either pMLBAD-rsiB1-strep or the empty vector pMLBAD (which do not belong to the IncP group [31]) were grown overnight in LBMC medium. Cells were diluted to an OD600 of 0.1 in 5 ml of fresh VMMS and grown for ∼6 to 8 h. Cultures were diluted once more in 50 ml of the same medium in order to reach an OD600 of ∼0.3 the day after and then were treated with 2% arabinose in order to induce overexpression of rsiB1-strep. After 2 h, the cultures were divided into two flasks; one was kept at 28°C, and the other was shifted to 40°C. After 5, 30, and 60 min of incubation, 1.5-ml aliquots (≥0.4 OD600 units) were collected by centrifugation and frozen in liquid nitrogen. Cells subsequently were thawed and lysed at 4°C in BugBuster master mix (80 μl for 1 OD600 unit; Novagen) containing antiproteases (Complete, Mini, EDTA-free; Roche) and antiphosphatases (5 mM NaF, 1 mM Na3VO4). Lysates were centrifuged (20 min at 20,000 × g), and supernatants were loaded without heat denaturation (unless otherwise indicated) on 12% SDS-polyacrylamide gels left unsupplemented or supplemented with 25 μM Phos-tag acrylamide (NARD Chemicals, Hiroshima, Japan) and 50 μM MnCl2 and electrophoresed at 4°C. After separation, proteins were transferred onto a Protran BA85 nitrocellulose membrane (GE Healthcare Life Sciences, Germany), and the strep-tagged RsiB1 protein was detected using Strep-Tactin AP conjugate (IBA GmbH, Gottingen, Germany).
RESULTS
RsiC (SMc01507) acts as a negative regulator of RpoE2.
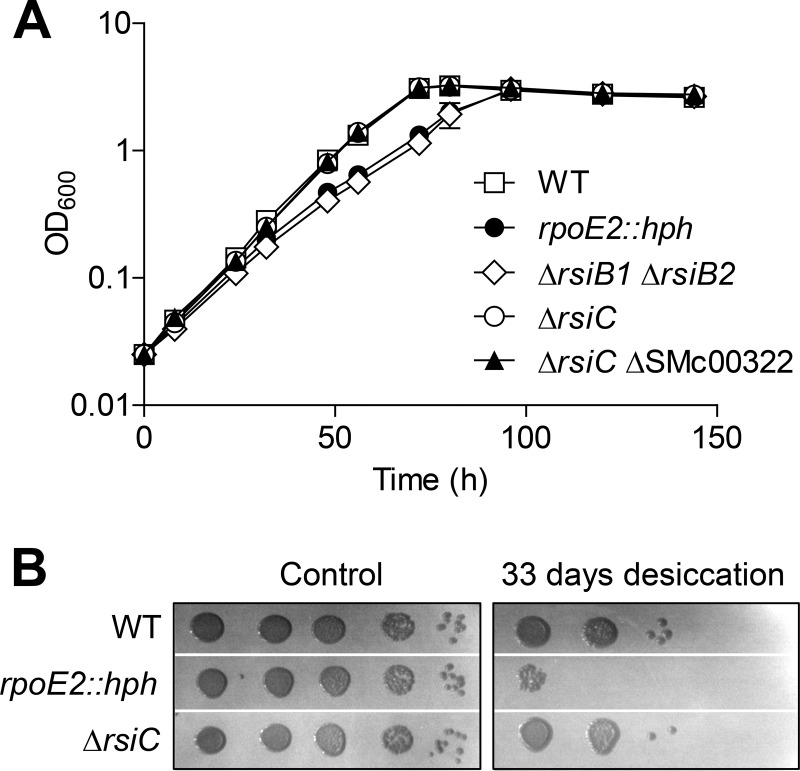
SMc01507, located just downstream from rpoE2 on the S. meliloti chromosome (Fig. 1B), encodes a putative HW-type histidine kinase that we named RsiC. To know whether RsiC is involved in the RpoE2 transduction cascade, we constructed an rsiC deletion mutant and tested RpoE2 activation in this strain under stress conditions, using as a reporter the RpoE2-dependent PSMc00885-lacZ transcriptional fusion (23). The expression of the fusion was clearly inducible in the ΔrsiC background, either following a heat shock or in stationary phase, two RpoE2-activating conditions (Fig. 2A) (5, 23). Equivalent results were obtained using two additional RpoE2-dependent lacZ fusions (to the rsiA1 and rsiB1 promoters; not shown), indicating that the observations apply to the whole RpoE2 regulon. Similar observations were also made in a ΔrsiC mutant constructed in a different genetic background (Rm2011; see Fig. S1A and B in the supplemental material). To confirm that the RpoE2-dependent general stress response is still functionally active in the ΔrsiC mutant, we tested its stress resistance phenotypes compared to those of the wild-type and rpoE2 strains. As previously described (7), the rpoE2 mutant was sensitive to osmotic stress, as it grew more slowly than the wild-type strain in the presence of 0.5 M NaCl (Fig. 3A). The double ΔrsiB1 ΔrsiB2 mutant displayed a similar phenotype (Fig. 3A), which confirms that the RpoE2 response is also completely abolished in this strain, as previously proposed (23). In contrast, growth of the ΔrsiC mutant was unaffected compared to that of the wild-type strain (Fig. 3A). Similarly, the rpoE2 mutant was more sensitive than the wild-type strain to desiccation stress, as previously described (11), whereas the ΔrsiC mutant was as resistant as the wild-type strain (Fig. 3B). Taken together, these expression data and phenotypic analyses indicate that RsiC is not essential for RpoE2 activation under the conditions tested.
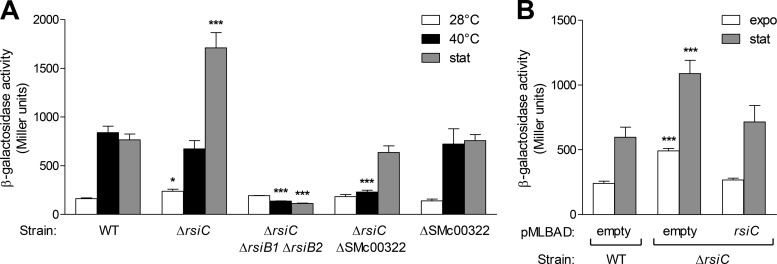
FIG 2.
Induction of the RpoE2-dependent transcriptional response in various genetic backgrounds. The transcription level of the PSMc00885-lacZ fusion carried on plasmid pMP220-885, used as a reporter of RpoE2 activity, was measured in the S. meliloti strains Rm1021 (WT), CBT785 (ΔrsiC), CBT1051 (ΔrsiC ΔrsiB1 ΔrsiB2), CBT1169 (ΔrsiC ΔSMc00322), and CBT1129 (ΔSMc00322) (A) or in the strains Rm1021 (WT) and CBT785 (ΔrsiC) containing the empty vector pMLBAD or the pMLBAD derivative expressing rsiC (B) as indicated below the graphs. β-Galactosidase activity was measured on aliquots of cultures grown to exponential phase at 28°C (expo; white bars), after 1 h at 40°C (black bars), or after growth for 48 h in stationary phase (stat; gray bars). β-Galactosidase activities are means and standard errors from at least three independent experiments. Statistical analyses were performed on log-transformed data using a one-way analysis of variance (P < 0.0001). The Bonferroni posttest was used to compare each of the mutant strains to the WT grown under the same culture conditions (*, P < 0.05; ***, P < 0.001).
FIG 3.
RpoE2 response is still functionally active in the absence of RsiC. (A) Strains Rm1021 (WT), CBT208 (rpoE2::hph), CBT430 (ΔrsiB1 ΔrsiB2), CBT785 (ΔrsiC), and CBT1169 (ΔrsiC ΔSMc00322) were grown overnight to saturation in rich medium (LBMC), washed in the minimal medium VMMGAS, and then diluted to an OD600 of 0.025 in VMMGAS supplemented with 0.5 M NaCl. The growth was monitored by measuring the OD600 over several days. Results shown are means and standard errors from at least three independent experiments (error bars are not visible in most cases because of weak variations). All five strains grew at similar rates in the absence of NaCl (not shown). (B) Strains Rm1021 (WT), CBT208 (rpoE2::hph), and CBT785 (ΔrsiC) were grown overnight to saturation in minimal medium (VMMM), and 10-fold serial dilutions were spotted on wet nitrocellulose membranes (from left to right, starting from 10−3) and allowed to dry at room temperature under 23% relative humidity (see Materials and Methods). Either before (control) or after 33 days of desiccation, the membranes were put on rich medium plates and surviving bacteria were allowed to form colonies at 28°C for at least 3 days. The experiment was performed twice independently with equivalent results.
However, two lines of evidence indicated that RsiC plays a role in the regulation of RpoE2 activity. First, the basal expression level of the PSMc00885-lacZ fusion was slightly but reproducibly higher in ΔrsiC mutants than in isogenic wild-type strains (Fig. 2A and B; also see Fig. S1A and B in the supplemental material). Second, even though the expression of the PSMc00885-lacZ fusion was upregulated in stationary phase in both the wild-type and the ΔrsiC mutant strains, it reached a 2-fold or higher level in the mutant than in the wild-type cells (Fig. 2A and B; also see Fig. S1B). Both basal and stationary-phase expression could be restored to wild-type levels by complementing with an rsiC-expressing plasmid (Fig. 2B), showing that the observed effects were indeed due to the loss of RsiC. Therefore, we conclude from these observations that although not essential for activating the RpoE2 response, RsiC can act as a negative regulator of RpoE2 activity.
RsiC negatively regulates RpoE2 by decreasing RsiB1/B2 phosphorylation in vivo.
To explain the negative regulation of RpoE2 by RsiC, the simplest hypothesis is to assume that, like many histidine kinases, RsiC displays a phosphatase activity. To test whether RsiC acts as a phosphatase on the RsiB1 (SMc01504) and RsiB2 (SMc00794) response regulators, we intended to perform in vitro activity tests of RsiC. However, despite several attempts and although RsiC was predicted not to contain any transmembrane domains, we were unable to purify either the full-length RsiC protein or the isolated C-terminal catalytic domain in soluble forms from either E. coli or S. meliloti strains overproducing various tagged versions of these proteins (data not shown).
Therefore, we conducted several in vivo approaches. First, in a ΔrsiB1 ΔrsiB2 background, the rsiC deletion did not lead to increased RpoE2 activity under either nonstress or stress conditions (Fig. 2A and 4). This confirms that the RpoE2 activation described above for the rsiC mutant (either basal or in stationary phase) was dependent on the known RpoE2 transduction cascade and suggests that RsiC exerts its negative effects upstream from RsiB1/B2. Complementation with rsiB1- or rsiB2-overexpressing plasmids led to a strong activation of RpoE2, even in the absence of stress, whereas the effect of this overexpression was much less pronounced in the RsiC-proficient background (Fig. 4) (23). In contrast, almost no effect was observed upon overexpression of mutated versions of rsiB1 and rsiB2, which encode proteins whose phosphorylatable aspartate residue has been converted to an alanine (D191A) (Fig. 4). Therefore, these results support the hypothesis that RsiC inhibits RsiB1 and RsiB2 by preventing their phosphorylation.
To get direct evidence that the phosphorylation status of the response regulators is modulated by RsiC, we assessed the level of phosphorylated RsiB1 in vivo in either wild-type or ΔrsiC mutant backgrounds. Experiments were performed in ΔrsiB1 ΔrsiB2 cells complemented with a plasmid-encoded strep-tagged RsiB1 protein. The strains also contained the PSMc00885-lacZ transcriptional fusion to assess RpoE2 activity in parallel. To detect phosphorylated RsiB1, soluble protein extracts from cells grown at 28°C or 40°C were separated by either normal or Phos-tag SDS-PAGE (32) and transferred to a membrane, and RsiB1 was detected using the Streptactin-AP conjugate. As expected, no RsiB1∼P was detected in RsiC-proficient cells in the absence of stress (Fig. 5A), which is consistent with the low RpoE2 activity (Fig. 5B). In contrast, incubation at 40°C led to RpoE2 activation (Fig. 5B), and a retarded band corresponding to RsiB1∼P became clearly visible in the Phos-tag gel as soon as 5 min after the temperature shift (Fig. 5A). Therefore, this experiment confirms that RsiB1 is phosphorylated under stress conditions (23). In the ΔrsiC background, RsiB1∼P was already visible in unstressed conditions (Fig. 5C), in agreement with the already-high RpoE2 activity (Fig. 5D), and its level increased at 40°C to reach values higher than those in the RsiC-proficient background (Fig. 5C). Therefore, these data confirm that RsiC is not essential for RsiB1 phosphorylation. More importantly, they suggest that RsiC, directly or indirectly, inhibits RsiB1 phosphorylation in the absence of stress and limits RsiB1 phosphorylation under heat stress conditions. Altogether, these results show that RsiC negatively regulates the general stress response by inhibiting the phosphorylation of RsiB1/2, suggesting that it acts as a phosphatase on the response regulators.
FIG 5.
RsiC inhibits RsiB1 phosphorylation in vivo. (A and C) The level of RsiB1 phosphorylation was quantified in vivo in S. meliloti strain CBT430 (ΔrsiB1 ΔrsiB2) or CBT1051 (ΔrsiC ΔrsiB1 ΔrsiB2) containing either the pMLBAD derivative expressing rsiB1-strep or the empty vector pMLBAD. Cells were grown to exponential phase in VMMS, treated for 2 h with 2% arabinose to induce rsiB1-strep overexpression, and incubated at either 28°C or 40°C. Aliquots of the cultures were collected after 5, 30, and 60 min of incubation. Equivalent amounts of soluble proteins were loaded on SDS-polyacrylamide gels left unsupplemented (bottom) or supplemented (top) with 25 μM Phos-tag and 50 μM MnCl2. All samples were loaded on both gels without heat denaturation, except in the last well (noted with an asterisk), where the indicated sample was preheated for 10 min at 95°C. After electrophoresis at 4°C, proteins were transferred onto nitrocellulose membranes and RsiB1 was detected using Streptactin-AP conjugate. That the retarded band corresponds to the phosphorylated form of RsiB1 (RsiB1∼P) is shown by its specific presence on the Phos-tag gel (32) and its absence when proteins are preheated before loading (*), consistent with the known heat lability of aspartyl-phosphate bonds (32, 45). X corresponds to a protein cross-reacting with the Streptactin-AP conjugate, as attested by its presence in the empty vector-containing strains. (B and D) As the strains also contained the reporter plasmid pMP220-885, RpoE2 activity was assessed by measuring β-galactosidase activity. The entire experiment was performed twice independently with equivalent results.
At least two HW kinases, RsiC and SMc00322, positively regulate RpoE2.
Although the results presented above strongly suggested that RsiC could act as a phosphatase on RsiB1/B2, it was not possible to draw conclusions about the implication of RsiC in the phosphorylation of the response regulators. Moreover, the results suggested that one or several other unknown histidine kinases were involved, at least in the absence of RsiC, in activating the cascade under heat stress or upon entry into stationary phase.
In addition to RsiC, seven putative HW histidine kinases are encoded by the S. meliloti genome (SMa0113, SMa1001, SMa1696, SMa2063, SMb20515, SMb20933, and SMc00322) (28, 33, 34). In order to test whether one of these proteins is involved in RpoE2 activation in the absence of RsiC, seven double mutant strains were constructed (see Materials and Methods). RpoE2 activation was then estimated following heat shock or in stationary phase using the PSMc00885-lacZ fusion in these strains. RpoE2 could still be activated in each of these double mutants except in the ΔrsiC ΔSMc00322 strain, where it was no longer activated at 40°C and was much less active in stationary phase than in the single rsiC mutant (Fig. 2A; also see Fig. S1 in the supplemental material). This suggested that SMc00322 is involved in RpoE2 activation in the ΔrsiC background. Nevertheless, we do not exclude the possible involvement of the other HW kinases, as some of the mutations are Tn5 insertions rather than deletions and may only alter regulatory activity rather than knocking out activity. In addition, these mutations were tested in the Rm2011 background only (see Fig. S1).
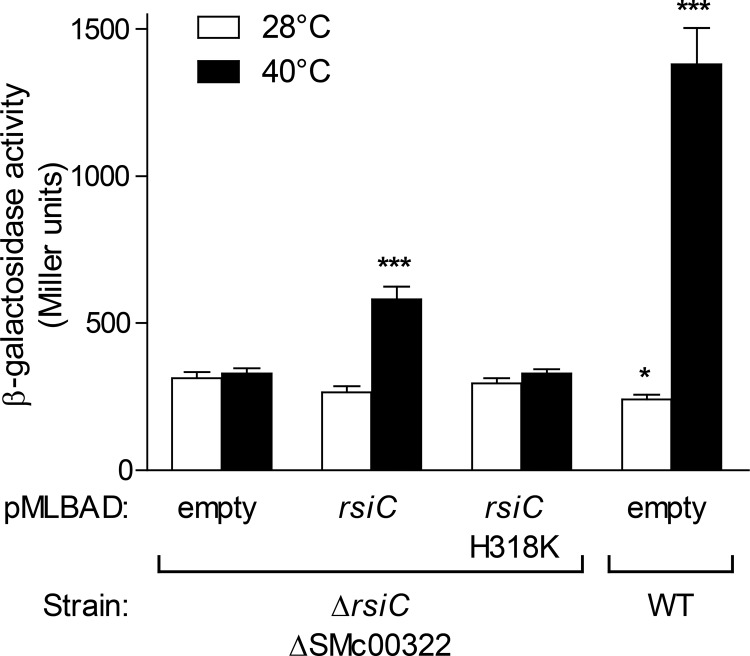
However, in spite of the strong reduction of RpoE2 activity in the ΔrsiC ΔSMc00322 double mutant, RpoE2 activation was found to not be significantly affected in the single ΔSMc00322 mutant in comparison to the wild-type strain (Fig. 2A). This finding indicated that RsiC can also act as a positive regulator of RpoE2 activity. Indeed, complementation of the ΔrsiC ΔSMc00322 double mutant strain with a plasmid expressing rsiC partially restored the capacity to activate RpoE2 by a heat stress (Fig. 6). In contrast, no complementation was observed with a mutant version of RsiC whose predicted catalytic histidine residue has been converted to a lysine (H318K) (Fig. 6). This suggested that RsiC acts as a histidine kinase to activate RpoE2, most likely by phosphorylating RsiB1/B2.
FIG 6.
RsiC positively controls RpoE2 activity. The transcription level of the PSMc00885-lacZ fusion carried on plasmid pMP220-885 was measured in the S. meliloti strain CBT1169 (ΔrsiC ΔSMc00322) containing the empty vector pMLBAD or the pMLBAD derivatives expressing rsiC or a mutated version thereof (H318K), as indicated below the graph. Results obtained with the Rm1021 strain (WT) carrying the empty vector are included as a control. β-Galactosidase activity was measured on aliquots of cultures grown to exponential phase at 28°C (white bars) or 40°C (black bars). β-Galactosidase activities are means and standard errors from at least three independent experiments. Statistical analyses were performed on log-transformed data using a one-way analysis of variance (P < 0.0001). The Bonferroni posttest was used to compare each strain to the ΔrsiC ΔSMc00322 strain carrying the empty vector grown under identical culture conditions (*, P < 0.05; ***, P < 0.001).
Nevertheless, a significant RpoE2 activity was still visible in the double ΔrsiC ΔSMc00322 mutant in stationary phase (Fig. 2A), showing that the RpoE2 response was not completely abolished in this strain. This was confirmed by showing that the ΔrsiC ΔSMc00322 mutant was as resistant to osmotic stress as the wild-type strain (Fig. 3A). We assume that phosphorylation of RsiB1/B2 in this strain is achieved by other, unknown histidine kinases or by alternative phosphodonors.
DISCUSSION
The activity of the EcfG sigma factor RpoE2, the regulator of the general stress response in S. meliloti, is controlled by the two anti-sigma factors RsiA1 (SMc01505) and RsiA2 (SMc04884), themselves under the control of the two anti-anti-sigma factors, RsiB1 (SMc01504) and RsiB2 (SMc00794), belonging to the PhyR family of response regulators (5, 23). In response to stress, RsiB1 and RsiB2 are activated via phosphorylation of an aspartate residue in their receiver domain. The objective of this work was to characterize proteins involved in the control of the RsiB1/B2 phosphorylation status.
Putative histidine kinases are often encoded in the same locus as EcfG sigma factors in Alphaproteobacteria (19). These proteins are unusual because their putative dimerization and histidine phosphotransfer domain is not a Pfam:HisKA domain, as in classical histidine kinases, but instead either a Pfam:HWE_HK or, less frequently, a Pfam:HisKA_2 domain (28) (referred to as HW- or HK-type kinases, respectively). Two HK-type proteins were previously found to be involved in EcfG control, namely, PhyK in C. crescentus and PhyP in Sphingomonas sp. strain FR1 (18, 24, 35, 36), but nothing was known about kinases of the HW type. In S. meliloti, a putative HW-type kinase is encoded by the rsiC gene (SMc01507) located downstream from rpoE2. We found that RsiC plays both positive and negative regulatory roles on RpoE2 activity in vivo. Therefore, this is the first demonstration that HW-type kinases encoded at the ecfG loci can be implicated in the control of the general stress response of Alphaproteobacteria.
As we were not able to purify the protein to test its autokinase and phosphotransfer activities in vitro, we could not prove that RsiC specifically acts as a kinase on the RsiB1/B2 response regulators. Such evidence is also lacking for PhyK, the presumed histidine kinase of PhyR in C. crescentus (24). Nevertheless, we obtained in vivo indications that RsiC acts upstream from RsiB1 and RsiB2 in the cascade and exerts its negative effects through dephosphorylation of the response regulators. As many histidine kinases display both kinase and phosphatase activities on their cognate response regulators (37), we assume that RsiC is the cognate histidine kinase/phosphatase of RsiB1/2. In C. crescentus, whether or not a phosphatase activity is associated with the PhyK kinase has not been tested yet (24, 35). In contrast, in Sphingomonas sp. strain FR1, it has been suggested that PhyP acts only as a phosphatase on PhyR, with the mechanism of phosphorylation unknown (18). To the best of our knowledge, S. meliloti is the first alphaproteobacterial species in which the ecfG cis-encoded histidine kinase is suggested to be bifunctional, with both kinase and phosphatase activities toward PhyR-type response regulators. Of course, direct in vitro demonstration would be required to confirm this model.
In the absence of RsiC, the basal level of RpoE2 activity was found to be higher than that in wild-type cells. In addition, RpoE2 could still be activated by stress, a persistent activity partly dependent on SMc00322, another (orphan) HW-type histidine kinase. Nevertheless, even in the double rsiC SMc00322 mutant, RpoE2 was still activated at a level that was low but sufficient enough to make the cells resistant to osmotic stress. Therefore, we assume that additional systems, either histidine kinases or small phosphodonors (like acetyl phosphate) (38), are able to phosphorylate RsiB1/B2. In this respect, S. meliloti is very different from C. crescentus, in which the absence of the PhyK histidine kinase leads to complete inactivity of SigT (24, 35). Another kinase, LovK, could complement the absence of PhyK only when it was overexpressed and even better when its cognate regulator, LovR, was absent (35). However, we believe that RsiC is normally the main or unique contributor to RsiB1/B2 phosphorylation in a wild-type S. meliloti background, since RpoE2 activation was not significantly affected in SMc00322 mutants under stress conditions (Fig. 2). Therefore, we assume that RsiC, through its phosphatase activity, prevents undesired, nonspecific phosphorylation of RsiB1/B2 (cross talk), as previously proposed for other bifunctional kinases (36, 39). Interestingly, in Erythrobacter litoralis, two HW-type kinases (EL368 and EL346) were recently shown to phosphorylate PhyR in vitro (40), but their contribution to EcfG activity in vivo has not been tested so far, particularly in the presence of the cis-encoded HW-type kinase (ELI10220). In Mesorhizobium loti, yeast two-hybrid screening revealed that the putative RsiB/PhyR ortholog mlr3700 was able to interact with the HW-type kinase mlr9680 (41), but the phosphorylation of mlr3700 was not investigated. These observations suggest the existence of cross talk in other Alphaproteobacteria.
In addition to its protective role against physiologically irrelevant cross talk, the negative regulatory action of RsiC may serve a negative feedback regulatory role to reset the response once the stress is gone. In Sphingomonas sp. strain FR1, such a function was proposed to be carried out by the putative PhyP phosphatase, as the phyP deletion was found to be lethal, supposedly because of overactivation of σEcfG (18). In C. crescentus, it has been suggested that LovK can act as a phosphatase toward PhyR when its cognate response regulator LovR is overexpressed, which led the authors to propose that LovK plays a negative regulatory role similar to that of PhyP in Sphingomonas (35). However, we succeeded in deleting rsiC in S. meliloti, while constitutive activation of RpoE2 is known to be lethal (5). This indicates that additional systems, including the anti-sigma factors RsiA1/A2 (5, 23), and possibly additional phosphatase activities or protease activities, as recently shown in B. abortus (15), are acting to limit RpoE2 overactivation. In addition, if RsiC were acting as a negative feedback regulator, we would expect rsiC expression to be under RpoE2 control, since in Sphingomonas sp. strain FR1 phyP expression is under σEcfG control (18), while in C. crescentus, lovK (and not phyK) expression is under SigT control (35). However, rsiC transcription is RpoE2 independent (5, 9, 10, and unpublished data) and was recently found to be controlled by the heat shock sigma factor RpoH1 (42). Nevertheless, the RpoE2 response was found not to be affected in an rpoH1 mutant at either high temperature (42) or acidic pH (43), which is consistent with the data obtained in the present paper with rsiC mutants.
Apart from histidine kinases/phosphatases, which play a direct role in regulating the phosphorylation status of RsiB1/RsiB2, other actors may indirectly contribute to this regulation. Thus, in C. crescentus and M. extorquens, mutation of a single-domain response regulator unrelated to the EcfG system (LovR and Mext_0407, respectively) resulted in up- or downregulation of the EcfG response, respectively (35, 44). Even though the biological relevance of these findings is not always clear, we cannot rule out that such additional levels of regulation also exist in S. meliloti.
Although several histidine kinases and/or phosphatases now have been proposed to be part of the EcfG sigma factor activation cascade in Alphaproteobacteria, the nature of inducing signals and how they would be perceived by these proteins are still unknown. Strikingly, although EcfG sigma factors generally are activated by numerous stress conditions, only a limited number of proteins appear to control each transduction cascade in wild-type situations (Fig. 1A). Even more surprisingly, these proteins carry variable motifs in their putative N-terminal sensor domains and do or do not contain putative transmembrane domains, which suggests they could be located in different cell compartments, i.e., the membrane (PhyK, SMc00322, and PhyP) or the cytoplasm (RsiC and LovK) (28). Although they remain to be experimentally tested, these observations suggest that these proteins could each perceive a different signal, possibly of different origins. Therefore, these proteins probably are not the primary sensors of environmental cues, and additional actors likely are involved in stress perception and control of the kinase/phosphatase activities. A challenge for the future will be to identify these actors in order to understand how the systems are able to integrate multiple environmental changes.
Supplementary Material
ACKNOWLEDGMENTS
We thank Anke Becker for providing Tn5 mutant strains, Bénédicte Bastiat, Marie-Françoise Jardinaud, Nikita Malkov, and Jean-Jacques Bono for discussions and advice, Ludivine Castan for technical help with some experiments, and Frans de Bruijn, Pauline Blanquet, and Pierre Dupuy for critical reading of the manuscript.
This work was supported, in part, by the Département Santé des Plantes et Environnement of INRA and by the French Laboratory of Excellence project “TULIP” (ANR-10-LABX-41 and ANR-11-IDEX-0002-02).
Footnotes
Published ahead of print 2 May 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.01623-14.
REFERENCES
- 1.Price CW. 2011. General stress response in Bacillus subtilis and related gram positive bacteria, p 301–318 In Storz G, Hengge R. (ed), Bacterial stress responses, 2nd ed. ASM Press, Washington, DC [Google Scholar]
- 2.Hecker M, Pane-Farre J, Volker U. 2007. SigB-dependent general stress response in Bacillus subtilis and related gram-positive bacteria. Annu. Rev. Microbiol. 61:215–236. 10.1146/annurev.micro.61.080706.093445 [DOI] [PubMed] [Google Scholar]
- 3.Battesti A, Majdalani N, Gottesman S. 2011. The RpoS-mediated general stress response in Escherichia coli. Annu. Rev. Microbiol. 65:189–213. 10.1146/annurev-micro-090110-102946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hengge R. 2011. The general stress response in Gram-negative bacteria, p 251–289 In Storz G, Hengge R. (ed), Bacterial stress responses, 2nd ed. ASM Press, Washington, DC [Google Scholar]
- 5.Sauviac L, Philippe H, Phok K, Bruand C. 2007. An extracytoplasmic function sigma factor acts as a general stress response regulator in Sinorhizobium meliloti. J. Bacteriol. 189:4204–4216. 10.1128/JB.00175-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fléchard M, Fontenelle C, Trautwetter A, Ermel G, Blanco C. 2009. Sinorhizobium meliloti rpoE2 is necessary for H2O2 stress resistance during the stationary growth phase. FEMS Microbiol. Lett. 290:25–31. 10.1111/j.1574-6968.2008.01401.x [DOI] [PubMed] [Google Scholar]
- 7.Fléchard M, Fontenelle C, Blanco C, Goude R, Ermel G, Trautwetter A. 2010. RpoE2 of Sinorhizobium meliloti is necessary for trehalose synthesis and growth in hyperosmotic media. Microbiology 156:1708–1718. 10.1099/mic.0.034850-0 [DOI] [PubMed] [Google Scholar]
- 8.Barra-Bily L, Fontenelle C, Jan G, Fléchard M, Trautwetter A, Pandey SP, Walker GC, Blanco C. 2010. Proteomic alterations explain phenotypic changes in Sinorhizobium meliloti lacking the RNA chaperone Hfq. J. Bacteriol. 192:1719–1729. 10.1128/JB.01429-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sallet E, Roux B, Sauviac L, Jardinaud MF, Carrère S, Faraut T, de Carvalho-Niebel F, Gouzy J, Gamas P, Capela D, Bruand C, Schiex T. 2013. Next-generation annotation of prokaryotic genomes with EuGene-P: application to Sinorhizobium meliloti 2011. DNA Res. 20:339–354. 10.1093/dnares/dst014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlüter JP, Reinkensmeier J, Barnett MJ, Lang C, Krol E, Giegerich R, Long SR, Becker A. 2013. Global mapping of transcription start sites and promoter motifs in the symbiotic α-proteobacterium Sinorhizobium meliloti 1021. BMC Genomics 14:156. 10.1186/1471-2164-14-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Humann JL, Ziemkiewicz HT, Yurgel SN, Kahn ML. 2009. Regulatory and DNA repair genes contribute to the desiccation resistance of Sinorhizobium meliloti Rm1021. Appl. Environ. Microbiol. 75:446–453. 10.1128/AEM.02207-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staron A, Sofia HJ, Dietrich S, Ulrich LE, Liesegang H, Mascher T. 2009. The third pillar of bacterial signal transduction: classification of the extracytoplasmic function (ECF) sigma factor protein family. Mol. Microbiol. 74:557–581. 10.1111/j.1365-2958.2009.06870.x [DOI] [PubMed] [Google Scholar]
- 13.Alvarez-Martinez CE, Lourenco RF, Baldini RL, Laub MT, Gomes SL. 2007. The ECF sigma factor sigma(T) is involved in osmotic and oxidative stress responses in Caulobacter crescentus. Mol. Microbiol. 66:1240–1255. 10.1111/j.1365-2958.2007.06005.x [DOI] [PubMed] [Google Scholar]
- 14.Delory M, Hallez R, Letesson JJ, De Bolle X. 2006. An RpoH-like heat shock sigma factor is involved in stress response and virulence in Brucella melitensis 16M. J. Bacteriol. 188:7707–7710. 10.1128/JB.00644-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HS, Caswell CC, Foreman R, Roop RM, Crosson S. 2013. The Brucella abortus general stress response system regulates chronic mammalian infection and is controlled by phosphorylation and proteolysis. J. Biol. Chem. 288:13906–13916. 10.1074/jbc.M113.459305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francez-Charlot A, Frunzke J, Reichen C, Ebneter JZ, Gourion B, Vorholt JA. 2009. Sigma factor mimicry involved in regulation of general stress response. Proc. Natl. Acad. Sci. U. S. A. 106:3467–3472. 10.1073/pnas.0810291106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gourion B, Francez-Charlot A, Vorholt JA. 2008. PhyR is involved in the general stress response of Methylobacterium extorquens AM1. J. Bacteriol. 190:1027–1035. 10.1128/JB.01483-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaczmarczyk A, Campagne S, Danza F, Metzger LC, Vorholt JA, Francez-Charlot A. 2011. Role of Sphingomonas sp. strain Fr1 PhyR-NepR-σEcfG cascade in general stress response and identification of a negative regulator of PhyR. J. Bacteriol. 193:6629–6638. 10.1128/JB.06006-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gourion B, Sulser S, Frunzke J, Francez-Charlot A, Stiefel P, Pessi G, Vorholt JA, Fischer HM. 2009. The PhyR-sigma(EcfG) signalling cascade is involved in stress response and symbiotic efficiency in Bradyrhizobium japonicum. Mol. Microbiol. 73:291–305. 10.1111/j.1365-2958.2009.06769.x [DOI] [PubMed] [Google Scholar]
- 20.Martínez-Salazar JM, Salazar E, Encarnación S, Ramirez-Romero MA, Rivera J. 2009. Role of the extracytoplasmic function sigma factor RpoE4 in oxidative and osmotic stress responses in Rhizobium etli. J. Bacteriol. 191:4122–4132. 10.1128/JB.01626-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abromaitis S, Koehler JE. 2013. The Bartonella quintana extracytoplasmic function sigma factor RpoE has a role in bacterial adaptation to the arthropod vector environment. J. Bacteriol. 195:2662–2674. 10.1128/JB.01972-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vercruysse M, Fauvart M, Jans A, Beullens S, Braeken K, Cloots L, Engelen K, Marchal K, Michiels J. 2011. Stress response regulators identified through genome-wide transcriptome analysis of the (p)ppGpp-dependent response in Rhizobium etli. Genome Biol. 12:R17. 10.1186/gb-2011-12-2-r17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bastiat B, Sauviac L, Bruand C. 2010. Dual control of Sinorhizobium meliloti RpoE2 sigma factor activity by two PhyR-type two-component response regulators. J. Bacteriol. 192:2255–2265. 10.1128/JB.01666-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lourenco RF, Kohler C, Gomes SL. 2011. A two-component system, an anti-sigma factor and two paralogous ECF sigma factors are involved in the control of general stress response in Caulobacter crescentus. Mol. Microbiol. 80:1598–1612. 10.1111/j.1365-2958.2011.07668.x [DOI] [PubMed] [Google Scholar]
- 25.Herrou J, Foreman R, Fiebig A, Crosson S. 2010. A structural model of anti-anti-σ inhibition by a two-component receiver domain: the PhyR stress response regulator. Mol. Microbiol. 78:290–304. 10.1111/j.1365-2958.2010.07323.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herrou J, Rotskoff G, Luo Y, Roux B, Crosson S. 2012. Structural basis of a protein partner switch that regulates the general stress response of α-proteobacteria. Proc. Natl. Acad. Sci. U. S. A. 109:E1415–E1423. 10.1073/pnas.1116887109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campagne S, Damberger FF, Kaczmarczyk A, Francez-Charlot A, Allain FH, Vorholt JA. 2012. Structural basis for sigma factor mimicry in the general stress response of Alphaproteobacteria. Proc. Natl. Acad. Sci. U. S. A. 109:E1405–E1414. 10.1073/pnas.1117003109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staron A, Mascher T. 2010. General stress response in alpha-proteobacteria: PhyR and beyond. Mol. Microbiol. 78:271–277. 10.1111/j.1365-2958.2010.07336.x [DOI] [PubMed] [Google Scholar]
- 29.Glazebrook J, Walker GC. 1991. Genetic techniques in Rhizobium meliloti. Methods Enzymol. 204:398–418. 10.1016/0076-6879(91)04021-F [DOI] [PubMed] [Google Scholar]
- 30.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 31.Antoine R, Locht C. 1992. Isolation and molecular characterization of a novel broad-host-range plasmid from Bordetella bronchiseptica with sequence similarities to plasmids from gram-positive organisms. Mol. Microbiol. 6:1785–1799. 10.1111/j.1365-2958.1992.tb01351.x [DOI] [PubMed] [Google Scholar]
- 32.Barbieri CM, Stock AM. 2008. Universally applicable methods for monitoring response regulator aspartate phosphorylation both in vitro and in vivo using Phos-tag-based reagents. Anal. Biochem. 376:73–82. 10.1016/j.ab.2008.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karniol B, Vierstra RD. 2004. The HWE histidine kinases, a new family of bacterial two-component sensor kinases with potentially diverse roles in environmental signaling. J. Bacteriol. 186:445–453. 10.1128/JB.186.2.445-453.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ulrich LE, Zhulin IB. 2010. The MiST2 database: a comprehensive genomics resource on microbial signal transduction. Nucleic Acids Res. 38:D401–D407. 10.1093/nar/gkp940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foreman R, Fiebig A, Crosson S. 2012. The LovK-LovR two-component system is a regulator of the general stress pathway in Caulobacter crescentus. J. Bacteriol. 194:3038–3049. 10.1128/JB.00182-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ardissone S, Viollier PH. 2012. Stressed by a Lov triangle. J. Bacteriol. 194:3035–3037. 10.1128/JB.00423-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casino P, Rubio V, Marina A. 2010. The mechanism of signal transduction by two-component systems. Curr. Opin. Struct. Biol. 20:763–771. 10.1016/j.sbi.2010.09.010 [DOI] [PubMed] [Google Scholar]
- 38.Wolfe AJ. 2010. Physiologically relevant small phosphodonors link metabolism to signal transduction. Curr. Opin. Microbiol. 13:204–209. 10.1016/j.mib.2010.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siryaporn A, Goulian M. 2010. Characterizing cross-talk in vivo avoiding pitfalls and overinterpretation. Methods Enzymol. 471:1–16. 10.1016/S0076-6879(10)71001-6 [DOI] [PubMed] [Google Scholar]
- 40.Correa F, Ko WH, Ocasio V, Bogomolni RA, Gardner KH. 2013. Blue light regulated two-component systems: enzymatic and functional analyses of light-oxygen-voltage (LOV)-histidine kinases and downstream response regulators. Biochemistry 52:4656–4666. 10.1021/bi400617y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimoda Y, Shinpo S, Kohara M, Nakamura Y, Tabata S, Sato S. 2008. A large scale analysis of protein-protein interactions in the nitrogen-fixing bacterium Mesorhizobium loti. DNA Res. 15:13–23. 10.1093/dnares/dsm028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barnett MJ, Bittner AN, Toman CJ, Oke V, Long SR. 2012. Dual RpoH sigma factors and transcriptional plasticity in a symbiotic bacterium. J. Bacteriol. 194:4983–4994. 10.1128/JB.00449-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Lucena DK, Puhler A, Weidner S. 2010. The role of sigma factor RpoH1 in the pH stress response of Sinorhizobium meliloti. BMC Microbiol. 10:265. 10.1186/1471-2180-10-265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Metzger LC, Francez-Charlot A, Vorholt JA. 2013. Single-domain response regulator involved in the general stress response of Methylobacterium extorquens. Microbiology 159:1067–1076. 10.1099/mic.0.066068-0 [DOI] [PubMed] [Google Scholar]
- 45.Wayne KJ, Li S, Kazmierczak KM, Tsui HC, Winkler ME. 2012. Involvement of WalK (VicK) phosphatase activity in setting WalR (VicR) response regulator phosphorylation level and limiting cross-talk in Streptococcus pneumoniae D39 cells. Mol. Microbiol. 86:645–660. 10.1111/mmi.12006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meade HM, Long SR, Ruvkun GB, Brown SE, Ausubel FM. 1982. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J. Bacteriol. 149:114–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pobigaylo N, Szymczak S, Nattkemper TW, Becker A. 2008. Identification of genes relevant to symbiosis and competitiveness in Sinorhizobium meliloti using signature-tagged mutants. Mol. Plant Microbe Interact. 21:219–231. 10.1094/MPMI-21-2-0219 [DOI] [PubMed] [Google Scholar]
- 48.Quandt J, Hynes MF. 1993. Versatile suicide vectors which allow direct selection for gene replacement in gram-negative bacteria. Gene 127:15–21. 10.1016/0378-1119(93)90611-6 [DOI] [PubMed] [Google Scholar]
- 49.Figurski DH, Helinski DR. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. U. S. A. 76:1648–1652. 10.1073/pnas.76.4.1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lefebre MD, Valvano MA. 2002. Construction and evaluation of plasmid vectors optimized for constitutive and regulated gene expression in Burkholderia cepacia complex isolates. Appl. Environ. Microbiol. 68:5956–5964. 10.1128/AEM.68.12.5956-5964.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.