Abstract
Acinetobacter baumannii is a leading cause of ventilator-associated pneumonia in intensive care units, and the increasing rates of antibiotic resistance make treating these infections challenging. Consequently, there is an urgent need to develop new antimicrobials to treat A. baumannii infections. One potential therapeutic option is to target bacterial systems involved in maintaining appropriate metal homeostasis, processes that are critical for the growth of pathogens within the host. The A. baumannii inner membrane zinc transporter ZnuABC is required for growth under low-zinc conditions and for A. baumannii pathogenesis. The expression of znuABC is regulated by the transcriptional repressor Zur. To investigate the role of Zur during the A. baumannii response to zinc limitation, a zur deletion mutant was generated, and transcriptional changes were analyzed using RNA sequencing. A number of Zur-regulated genes were identified that exhibit increased expression both when zur is absent and under low-zinc conditions, and Zur binds to predicted Zur box sequences of several genes affected by zinc levels or the zur mutation. Furthermore, the zur mutant is impaired for growth in the presence of both high and low zinc levels compared to wild-type A. baumannii. Finally, the zur mutant exhibits a defect in dissemination in a mouse model of A. baumannii pneumonia, establishing zinc sensing as a critical process during A. baumannii infection. These results define Zur-regulated genes within A. baumannii and demonstrate a requirement for Zur in the A. baumannii response to the various zinc levels experienced within the vertebrate host.
INTRODUCTION
Acinetobacter baumannii is a Gram-negative, opportunistic pathogen that is a significant source of hospital-acquired infections, predominately in intensive care units (1, 2). The most common type of infection caused by A. baumannii is ventilator-associated pneumonia; however, A. baumannii can cause a range of infections, including wound and burn infections, sepsis, urinary tract infections, meningitis, and osteomyelitis (1, 3, 4). Additionally, A. baumannii infections have been frequently identified in American military personnel returning from the Middle East, and community-acquired infections have been reported in southeast Asia and Australia (5–8). Exacerbating morbidity and mortality caused by A. baumannii is the high rate of acquired antibiotic resistance, and multidrug- and pan-drug-resistant strains of A. baumannii are increasingly being identified (9–11). These facts underscore the need for new therapeutic strategies to treat A. baumannii infection.
All organisms, including bacteria, require nutrient metals for growth due to a requirement for these metals as cofactors for enzymes required for numerous physiological processes. In turn, some vertebrate hosts sequester these essential metals from invading pathogens as a defense mechanism termed nutritional immunity (12, 13). Calprotectin (CP) is a vertebrate protein involved in nutritional immunity that is found at sites of inflammation, such as infected tissues (14). CP is a heterodimer of S100A8 and S100A9 with one metal binding site that coordinates zinc (Zn) and a second site capable of coordinating Zn or manganese (Mn) (15, 16). Furthermore, CP is antimicrobial against several pathogens, including A. baumannii, in a manner dependent on its metal-chelating properties (15, 17–22). Following A. baumannii pulmonary infection, CP is observed in the lungs of infected mice, and its abundance and distribution reflect the inflammatory response to infection (23). Importantly, CP is required for controlling A. baumannii pneumonia, as S100A9−/− mice exhibit higher bacterial organ burdens than wild-type mice (18).
In a transposon mutagenesis screen to uncover factors involved in the A. baumannii response to CP, an inner membrane Zn transporter, ZnuABC, was identified (18). Deletion of the gene encoding the permease subunit of this system, znuB, results in a mutant that is defective for growth in Zn-limited conditions. Moreover, in a mouse model of A. baumannii pneumonia, the znuB deletion mutant is defective for growth in lungs and for dissemination to the liver, and this defect is dependent on the presence of CP. Finally, znuA, znuB, and znuC are upregulated in the presence of the Zn chelator TPEN (18). These results demonstrate the importance of CP-mediated Zn sequestration to the host during infection and that Zn acquisition is required for A. baumannii virulence. Additionally, these data suggest that A. baumannii responds to Zn starvation through modification of specialized metal acquisition systems.
Within the A. baumannii genome, znuA is located upstream of znuB and znuC but in the opposite orientation (18). Immediately upstream of A. baumannii znuBC, and in a predicted operon with these genes, is a gene encoding a candidate Fur family transcriptional repressor termed Zur (zinc uptake regulator). In Zn-replete conditions, Zur proteins bind Zn and recognize a conserved Zur box sequence, thereby repressing target genes (24). In Zn-depleted conditions, Zur is no longer bound to Zn; therefore, it is released from the Zur box sequence, alleviating target gene repression. A. baumannii Zur has been demonstrated to bind Zn and the Zur box located upstream of zur znuCB in a metal-dependent fashion (18).
Using a consensus Zur box sequence, other putative Zur boxes have been identified within the A. baumannii genome, resulting in a list of putative Zur-regulated genes (18). This list includes genes encoding proteins involved in Zn acquisition, such as ZnuABC, two putative outer membrane Zn transporters, ZnuD1 and ZnuD2, and a TonB/ExbB/ExbD (designated tonBZn) system that likely provides the energy for Zn transport through the outer membrane. The observed upregulation of znuA, znuB, znuC, znuD1, znuD2, and tonBZn under low-Zn conditions supports Zur regulation of these genes and their role in Zn acquisition (18). Here, we define the broader Zur regulon through the creation and analysis of a zur deletion mutant (Δzur::Km). We also show that Zur binds to several predicted Zur box sequences and that Zur-regulated genes are differentially regulated in the presence of various Zn levels, including in CP-mediated Zn-limiting conditions. Furthermore, we demonstrate that Zur is required for A. baumannii adaptation to both high and low levels of Zn. Finally, using a mouse model of A. baumannii pneumonia, Zur is shown to be required for A. baumannii dissemination in a manner dependent on the presence of CP, highlighting the importance of Zur during A. baumannii infection.
MATERIALS AND METHODS
Bacterial strains and reagents.
All experiments were performed using Acinetobacter baumannii ATCC 17978 or a mutant derivative. Cloning was performed in Escherichia coli DH5α. Strains were cultured in Luria broth (LB) at 37°C unless otherwise noted. Kanamycin was used at a 40 μg/ml concentration (Km40) when required for selection. Selection on ampicillin was at 100 μg/ml (Amp100) for E. coli and 500 μg/ml (Amp500) for A. baumannii. All antibiotics were purchased from Sigma. Recombinant human calprotectin was expressed and purified as previously described (17).
Generation of a zur mutant.
For generation of a strain inactivated for zur, approximately 1,000 bp of DNA in both the 5′ and 3′ flanking regions surrounding zur were amplified from A. baumannii genomic DNA, and the kanamycin resistance gene aph was amplified from the vector pUCK1 (25). The three PCR products were stitched together using overlap extension PCR, and the construct was then cloned into pCR2.1 (Invitrogen) and its sequence verified. The deletion construct replaces the zur gene with the kanamycin cassette and retains 12 bp (corresponding to 4 amino acids) of the zur open reading frame (ORF) on the 5′ and 3′ ends. The deletion construct was digested out of pCR2.1 with BamHI and XbaI and ligated into pFLP2 (26). This vector was then transformed into A. baumannii by electroporation, and bacterial integrants were selected for on LB Km40 agar with overnight growth at 37°C. Transformants were patched onto LB Km40 or LB with 5% sucrose, and merodiploids were Kmr and sucrose sensitive. To resolve the integrated plasmid, merodiploid strains were grown overnight in 3 ml LB and incubated overnight at 37°C with shaking. Cultures were serially diluted, plated on LB agar with 5% sucrose, and incubated overnight at 37°C. Transformants were patched on LB Km40 and LB 5% sucrose agar and incubated at 37°C overnight. The Kmr and sucrose-resistant strains were screened for the loss of zur and replacement with aph by multiple PCRs and Southern blotting using both zur-specific and aph-specific probes. All relevant primers are listed in Table S2 in the supplemental material.
Construction of the zur complementation vector.
The promoter from the 16S gene r01 was amplified from A. baumannii genomic DNA incorporating EcoRI sites at the 5′ and 3′ ends. Following EcoRI digestion, this promoter was ligated into pWH1266. The zur gene was amplified from A. baumannii genomic DNA incorporating a C-terminal cMyc tag (zurMyc) and a BamHI site at the 5′ and 3′ ends. zurMyc then was digested with BamHI and ligated downstream of the r01 promoter. The integrity of the r01 promoter and zurMyc sequences was confirmed by sequencing. The empty vector, pr01pro, and the complementation vector, pzurMyc, each were transformed into the Δzur::Km strain by electroporation. In addition, the empty vector was also transformed into wild-type A. baumannii to serve as an additional control, accounting for any potential growth differences associated with the vector. All relevant primers are listed in Table S2 in the supplemental material.
Bacterial growth assay in TPEN and in high Zn.
Overnight bacterial cultures of wild-type A. baumannii or the Δzur::Km strain were subcultured 1:50 in fresh LB for 1 h. For experiments involving complementation, all strains harbored plasmids and were grown in LB Amp500 (ampicillin at 500 μg/ml). Following overnight growth, strains were back diluted 1:50 into LB with selection for 1 h. Back-diluted cultures were then reseeded 1:100 into LB containing various concentrations of tetrakis-(2-pyridylmethyl)ethylenediamine (TPEN) from 0 to 80 μM, with or without addition of 25 μM ZnCl2, MnCl2, CaCl2, or CuCl2 or 10 μM FeSO4. For experiments assessing growth at high Zn levels, back-diluted cultures were reseeded 1:100 into LB containing a range of ZnCl2 concentrations from 0 to 500 μM. For all assays, bacteria were grown at 37°C with shaking. For graphs depicting percent growth, the data from the 8-h time point are presented. Data were averaged from at least three replicates, and statistical significance was determined by Student's t test or two-way analysis of variance (ANOVA).
RNA sequencing (RNA-seq).
Wild-type A. baumannii and the Δzur::Km mutant were grown overnight in 3 ml LB. Cultures were reseeded 1:50 in LB and grown for 1 h at 37°C with shaking. These cultures were reseeded 1:100 in LB and grown for 7 h at 37°C with shaking. Cultures were pelleted at 4°C with 2,500 × g for 8 min and then air dried on ice. Each pellet was suspended in 1 ml TRIzol and transferred to tubes containing lysing matrix B (MP Biomedicals). Bacteria were lysed using a FastPrep-24 (MP Biomedicals) bead beater for 45 s at 6 m/s. Two hundred μl chloroform then was added to each tube. After brief vortexing, the tubes were centrifuged for 15 min at 4°C and the upper layer was transferred to a new tube. RNA was purified using the RNeasy preparation kit (Qiagen) according to the RNeasy lipid tissue directions. DNA contamination was removed by adding 8 μl RQ1 (Promega), 12 μl 10× RQ1 buffer, and 2 μl RNase inhibitor (Promega) to each sample and incubating at 37°C for 2 h. Samples were then cleaned up using the RNeasy miniprep RNA cleanup protocol (Qiagen). To ensure purity of the RNA from DNA contamination, an aliquot of RNA was removed for reverse transcription (RT), including no-RT controls, and assessed by PCR. Prior to sequencing, RNA was quantified on the Synergy 2 with Gen5 2.00 software (BioTek).
Vanderbilt Technologies for Advanced Genomics Core Facility (VANTAGE) prepared RNA-seq libraries from 1.5 μg of A. baumannii total RNA using the following protocol. First, the integrity of the total RNA was evaluated using the Agilent Bioanalyzer Nano RNA chip. The Ribo-Zero rRNA removal kit for Gram-negative bacteria (Epicentre) was used to remove rRNA by following the manufacturer's protocol. The rRNA-reduced RNA was used as an input to the TruSeq stranded mRNA sample preparation kit (Illumina), skipping the mRNA selection step and going directly into the RNA fragmentation and random hexamer priming step. The RNA was converted into double-stranded cDNA, adapter ligated, and enriched with PCR, replacing the enzyme from the kit with KAPA HiFi DNA polymerase to create the final cDNA sequencing library. The cDNA library underwent quality control (QC) by running on an Agilent Bioanalyzer HS DNA assay to confirm the final library size and on an Agilent Mx3005P quantitative PCR (qPCR) machine using the KAPA Illumina library quantification kit to determine concentration. A 2 nM stock was created, and samples were pooled by molarity for multiplexing. From the pool, a 10.5 pM concentration was loaded into each well for the flow cell on the Illumina cBot for cluster generation. The flow cell was then loaded onto an Illumina HiSeq 2500, utilizing v3 chemistry and HTA 1.8 for a paired-end 50-bp run. The raw sequencing reads in BCL format were processed through CASAVA-1.8.2 for FASTQ conversion and demultiplexing. The RTA chastity filter was used, and only the PF (pass filter) reads were retained for further analysis.
Transcriptome analysis.
The Illumina HiSeq 2500-generated FASTQ reads were first processed by using the Bayesian adapter trimmer Scythe (version 0.992; http://github.com/vsbuffalo/scythe) to trim 3′ adaptor sequence contaminants from the reads. EDGE-pro v1.3 (Estimated Degree of Gene Expression in Prokaryotes) software (27) then was used to align the reads with Bowtie2 v2.1.0 (28) and estimate gene expression directly from the alignment output. The FASTA of the reference genome sequence (.fna), protein translation table with coordinates of protein coding genes (.ptt), and a table containing coordinates of tRNA and rRNA genes were downloaded from NCBI (ftp://ftp.ncbi.nih.gov/GenBank/genomes/Bacteria/Acinetobacter_ baumannii_ATCC_17978_uid17477). The .fna, .ptt, and .rnt files were concatenated from the main genome and two native plasmids into single input files, and they were used as inputs into EDGE-pro. EDGE-pro was run with default parameters, except for defining the read length as 50 bp (−l 50) and using 16 threads (−t 16) on a 64-core Linux server. On average, more than 96% of the reads were uniquely aligned. Alignment statistics and other QC metrics were calculated from the aligned BAM file using the RSeQC program suite (29). The output of EDGE-pro was an RPKM value table for the genome and plasmids. A script provided with EDGE-pro called edgeToDeseq.perl was used to concatenate the output of the various samples into a single count table for input into DESeq. The expression level of each gene was determined using DESeq (30) in the statistical programming package R-3.0.0 (http://www.r-project.org). Differences in expression comparing wild-type and Δzur::Km strains were considered significant based on a P value less than or equal to 0.01.
Quantitative RT-PCR.
Bacteria were grown as done for RNA-seq but under various Zn levels. For TPEN experiments, bacteria were grown in LB with or without 25 μM TPEN. For CP experiments, bacteria were grown in 60% CP buffer (16) and 40% LB with or without 250 μg/ml CP. For high-Zn experiments, bacteria were grown in LB with or without 100 μM ZnCl2. RNA was prepared as described for RNA-seq. Reverse transcription was performed using 2 μg of RNA and the Moloney murine leukemia virus reverse transcriptase (Promega) by following the manufacturer's instructions. Quantitative PCR was performed using iQ SYBR green Supermix (Bio-Rad) and the cDNA described above, including a no-RT negative control and no-DNA control for each primer pair. PCR was completed on a CFX96 qPCR cycler (Bio-Rad). Fold changes were calculated from threshold cycle (CT) values from at least three biological replicates after normalizing to 16S rRNA (18). The quantitative reverse transcription-PCR (qRT-PCR) primer sequences used were previously published for znuA, znuB, znuC, znuD1, znuD2, and tonBZn (18), and the rest are listed in Table S2 in the supplemental material.
Zur purification.
Zur was purified as previously described (18). Briefly, E. coli BL21(DE3) containing the Zur expression vector was grown at 37°C with shaking to an optical density at 600 nm (OD600) of 0.6. Protein expression was induced with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) and grown an additional 6 h at 37°C. Cells were harvested by centrifugation at 6,000 × g and suspended in lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazole, 1 mg/ml lysozyme). Cells were lysed by five passes through an EmulsiFlex homogenizer (Aventin, Inc., Ottawa, ON, Canada) at 20,000 lb/in2. Lysates were centrifuged at 8,000 × g to remove unlysed cells and debris, and then insoluble material was removed by ultracentrifugation at 100,000 × g for 1 h at 4°C. The supernatants were applied to a nickel-nitrilotriacetic acid column preequilibrated with lysis buffer. The column was washed with 20 bed volumes of wash buffer (50 mM NaH2PO4, 300 mM NaCl, 25 mM imidazole), followed by two washes with wash buffer containing 150 mM imidazole. Zur was eluted in two bed volumes of wash buffer containing 250 mM imidazole. CaCl2 was added to the eluted sample to a final concentration of 2.5 mM. The His tag was removed by thrombin cleavage. Two units of restriction-grade thrombin (Novagen) was added, and the sample was cleaved and dialyzed overnight at 4°C into 20 mM Tris-HCl, pH 8.0, 150 mM NaCl, and 2.5 mM CaCl2. To isolate cleaved Zur, the purification steps described above were repeated, and final Zur eluate was dialyzed into 20 mM Tris-HCl, pH 8.0, 150 mM NaCl, and 5% glycerol.
Electrophoretic mobility shift assay.
Predicted Zur box sequences were previously identified upstream of several A. baumannii genes (18). Oligonucleotides were designed for several putative Zur box-containing genes that covered the predicted Zur box sequence plus 3 to 5 additional base pairs. These oligonucleotide sequences, along with their respective negative controls, are listed in Table S2 in the supplemental material. Negative-control oligonucleotides were designed for regions immediately adjacent to the putative Zur box sequence. One of each oligonucleotide pair was radiolabeled with [γ-32P]ATP (PerkinElmer) using the T4 polynucleotide kinase (New England BioLabs), and excess radiolabel was removed with ProbeQuant G50 microcolumns (GE Healthcare). Labeled oligonucleotide was annealed to 5-fold unlabeled complementary oligonucleotide in duplex buffer (30 mM HEPES, pH 8.0, 100 mM potassium acetate) at 95°C for 5 min and then slowly cooled to room temperature. Unlabeled oligonucleotide probes were generated by incubating complementary oligonucleotides at a 1:1 ratio in duplex buffer at 95°C for 5 min and then slowly cooled to room temperature. Purified Zur was mixed with labeled oligonucleotide in the presence of binding buffer [10 mM Tris, pH 8.0, 1 mM EDTA, 1 mM dithiothreitol (DTT), 60 mM potassium glutamate, 150 μg/ml bovine serum albumin (BSA), 10% glycerol, 150 mM KCl, 50 ng/μl poly(dI · dC)] containing 100 μM ZnCl2 and with or without unlabeled probe competitor. Binding reaction mixtures were incubated at room temperature for 10 min and then loaded directly onto 15% polyacrylamide gel that was prerun in 1× Tris-borate-EDTA (TBE) running buffer at 10 mA for 30 min. Samples were run at 20 mA at room temperature for 15 min. The gel was dried using a vacuum dryer, exposed to a phosphor screen for at least 5 h, and imaged on an FLA7000IP Typhoon (GE Healthcare). All oligonucleotide sequences are listed in Table S2 in the supplemental material.
Mouse model of pneumonia.
All of the animal infection experiments were approved by the Vanderbilt University Institutional Animal Care and Use Committee. Wild-type C57BL/6 mice were obtained from Jackson Laboratories. S100A9−/− mice were a gift from Wolfgang Nacken (Institute of Experimental Dermatology, University of Münster, Münster, Germany). Nine-week-old mice were inoculated with a 1:1 mixture of wild-type A. baumannii and the Δzur::Km mutant totaling 5 × 108 CFU of bacteria in 30 μl phosphate-buffered saline (PBS). At 36 h postinfection, mice were euthanized and CFU were enumerated in the lungs and livers following tissue homogenization and dilution plating on LB and LB Km40. Data presented represent a combination of three separate experiments. The limit of detection of the assay is 200 CFU/g. Significance was determined by a Mann-Whitney nonparametric test.
RESULTS
Zur regulates numerous genes in A. baumannii.
In order to investigate the role of Zur in the response of A. baumannii to altered Zn levels, a zur deletion mutant was generated in A. baumannii ATCC 17978 using allelic exchange. This mutant was utilized to identify genes whose expression is affected by Zur. RNA-seq was employed to evaluate gene expression changes in the Δzur::Km mutant compared to the wild type following growth in Zn-replete conditions. There were 76 genes that had statistically significant (P < 0.1) increases in expression in the Δzur::Km mutant compared to that in the wild type (Table 1). Furthermore, nine of the original 16 predicted Zur-regulated genes had increased expression at least 2-fold, including znuA, tonBZn, exbB, exbD, znuD1, znuD2, rpmE2, A1S_3411, and A1S_3412 (18). In addition to these candidate Zur-regulated genes, other genes predicted to be involved in metal homeostasis exhibited significantly increased transcript abundance, including a putative ferric siderophore receptor (A1S_0092). A Co/Zn/Cd efflux system (A1S_1044 to A1S_1045) exhibited a >2-fold increase in gene expression; however, this change was not statistically significant. Interestingly, an operon involved in histidine catabolism, which is analogous to the histidine utilization (Hut) system widely conserved among bacteria, has increased expression in the Δzur::Km mutant (31). The expression changes for this Hut system were not statistically significant; however, the expression of the entire system increased more than 2-fold in the Δzur::Km mutant. There were numerous genes with higher expression in the Δzur::Km mutant that are not predicted to have a Zur box, suggesting that these genes are indirectly regulated by Zur. The other transcriptional regulators with increased transcript levels in the Δzur::Km mutant could account for these expression changes and suggest that A. baumannii encodes additional regulators that respond to Zn levels. Finally, the transcripts of several genes encoding secreted proteins and virulence factors were more abundant in the Δzur::Km mutant, suggesting a link between Zur and pathogenesis.
TABLE 1.
Genes with increased expression in the Δzur::Km mutant straina
| Locus and category | Fold change | Annotationb |
|---|---|---|
| Metal homeostasis | ||
| A1S_3411 | 214.8 | G3E family GTPase |
| A1S_0391 | 144.9 | rpmE2 50S ribosomal protein L31 |
| A1S_2892 | 41.0 | TonB-dependent receptor protein (ZnuD1) |
| A1S_0092 | 33.6 | Ferric siderophore receptor protein |
| A1S_3475 | 11.7 | TonB-dependent receptor protein (ZnuD2) |
| A1S_0146 | 6.8 | High-affinity Zn transport protein (ZnuA) |
| A1S_0452 | 5.7 | Hypothetical protein (TonB) |
| A1S_0453 | 3.7 | Biopolymer transport protein (ExbB) |
| A1S_0454 | 2.6 | Biopolymer transport protein (ExbD) |
| Transcriptional regulators | ||
| A1S_0094 | 7.6 | lrp regulon transcriptional regulator (AsnC family) |
| A1S_1539 | 2.6 | ArsR family transcriptional regulator |
| A1S_0739 | 2.4 | Transcriptional regulator (TetR family) |
| A1S_0253 | 2.2 | Transcriptional regulator (AcrR family) |
| A1S_0560 | 2.1 | Transcriptional regulator (PBP2_LTTR_aromatics_like) |
| A1S_1007 | 2.1 | Transcriptional regulator LysR |
| A1S_0399 | 1.8 | LysR family transcriptional regulator |
| Transporters | ||
| A1S_1530 | 7.1 | SSS family major sodium/proline symporter |
| A1S_1402 | 2.3 | Amino acid efflux transmembrane protein |
| A1S_1670 | 1.7 | Secretion protein HlyD |
| Secreted proteins and virulence factors | ||
| A1S_3476 | 6.3 | Secretory lipase |
| A1S_1586 | 5.1 | EsvK1 |
| A1S_1587 | 3.3 | EsvK2 |
| A1S_3329 | 3.3 | EsvJ-NlpD |
| A1S_0390 | 3.2 | HopJ; HopJ type III effector protein |
| A1S_1600 | 2.1 | Lysozyme |
| Phage proteins | ||
| A1S_1591 | 11.9 | A1S_1591 phage major capsid protein HK97 |
| A1S_1590 | 7.1 | A1S_1590 peptidase U35 phage prohead HK97 |
| A1S_1588 | 5.1 | Phage terminase-like protein large subunit |
| A1S_1748 | 2.6 | Replication protein; phage_X; phage X family |
| A1S_1145 | 1.7 | Cro protein |
| Signal peptides | ||
| A1S_2889 | 2.3 | Signal peptide |
| A1S_0032 | 2.2 | Signal peptide |
| A1S_2885 | 1.9 | Signal peptide |
| A1S_0847 | 1.8 | Signal peptide |
| tRNA | ||
| A1S_3216 | 3.0 | trnF |
| A1S_0332 | 1.8 | trnF |
| Other | ||
| A1S_0971 | 8.1 | MetH B12-dependent methionine synthase |
| A1S_3410 | 2.2 | Acyltransferase (COG1835) |
| A1S_0389 | 1.7 | Camphor resistance protein CrcB |
| Hypothetical/uncharacterized proteins | ||
| A1S_3412 | 18.8 | Hypothetical protein |
| A1S_0093 | 51.1 | Hypothetical protein |
| A1S_3693 | 11.4 | Uncharacterized protein |
| A1S_3694 | 11.0 | Hypothetical protein |
| A1S_1593 | 5.5 | Hypothetical protein |
| A1S_3704 | 5.4 | Uncharacterized protein; UniProt |
| A1S_1592 | 5.0 | Hypothetical protein |
| A1S_3699 | 4.9 | Uncharacterized protein; UniProt |
| A1S_2988 | 4.7 | Hypothetical protein |
| A1S_3696 | 4.4 | Uncharacterized protein; UniProt |
| A1S_1595 | 4.0 | Hypothetical protein |
| A1S_3695 | 3.8 | Uncharacterized protein; UniProt |
| A1S_3700 | 3.5 | Uncharacterized protein; UniProt |
| A1S_3705 | 3.3 | Uncharacterized protein; UniProt |
| A1S_1596 | 3.2 | Hypothetical protein |
| A1S_1589 | 3.0 | Hypothetical protein |
| A1S_1594 | 3.0 | Hypothetical protein |
| A1S_0648 | 2.9 | Hypothetical protein |
| A1S_0561 | 2.8 | Hypothetical protein |
| A1S_3551 | 2.6 | Uncharacterized protein; UniProt |
| A1S_0183 | 2.4 | Hypothetical protein |
| A1S_3697 | 2.3 | Uncharacterized protein; UniProt |
| A1S_3698 | 2.2 | Uncharacterized protein; UniProt |
| A1S_3607 | 2.1 | Uncharacterized protein; UniProt |
| A1S_2347 | 1.9 | Hypothetical protein |
| A1S_3791 | 1.9 | Uncharacterized protein; UniProt |
| A1S_1157 | 1.9 | Hypothetical protein |
| A1S_0565 | 1.9 | Hypothetical protein |
| A1S_2893 | 1.9 | Hypothetical protein |
| A1S_3444 | 1.8 | Hypothetical protein |
| A1S_3703 | 1.8 | Uncharacterized protein; UniProt |
| A1S_0795 | 1.8 | Hypothetical protein |
| A1S_3702 | 1.7 | Uncharacterized protein; UniProt |
| A1S_3800 | 1.7 | Uncharacterized protein; UniProt |
| A1S_1161 | 1.7 | Hypothetical protein |
| A1S_3240 | 1.7 | Hypothetical protein |
Statistically significant changes were determined at P ≤ 0.01 and are listed in Table S1 in the supplemental material.
Annotations are listed as designated in the NCBI or UniProt database.
An additional 68 genes exhibited statistically significant decreases in transcript abundance in the Δzur::Km mutant (Table 2). This list largely includes genes encoding proteins involved in metabolic processes and hypothetical proteins. Of interest, a set of genes involved in fatty acid synthesis (A1S_0103 to A1S_0109) were all at least 2-fold decreased in the Δzur::Km mutant. Additionally, at least seven genes involved in benzoate metabolism were decreased in expression in the Δzur::Km strain at least 2-fold or more. The complete list of annotated genes with the associated raw reads, fold changes, and significance values is presented in Table S1 in the supplemental material.
TABLE 2.
Genes with decreased expression in the Δzur::Km mutant straina
| Locus and category | Fold change | Annotationb |
|---|---|---|
| Metal homeostasis | ||
| A1S_0145 | 391.9 | Zn transport system transcriptional repressor (Zur) |
| Amino acid metabolism | ||
| A1S_0737 | 7.6 | 5-Methyltetrahydropteroyltriglutamate/homocysteine S-methyltransferase |
| A1S_0926 | 3.3 | Choline dehydrogenase |
| A1S_0925 | 3.0 | Choline dehydrogenase |
| A1S_0927 | 2.9 | Betaine aldehyde dehydrogenase |
| A1S_0924 | 2.7 | Choline dehydrogenase |
| A1S_1726 | 2.6 | Aspartate ammonia-lyase |
| Transporters | ||
| A1S_3272 | 5.4 | MFS family transporter |
| A1S_2449 | 3.4 | Aromatic amino acid APC transporter |
| A1S_3220 | 2.6 | Cation efflux system protein |
| A1S_1418 | 2.3 | MFS permease |
| A1S_2212 | 2.0 | Proline/glycine betaine transporter |
| Sucrose metabolism | ||
| A1S_0804 | 4.1 | Trehalose-6-phosphate phosphatase |
| Regulators | ||
| A1S_2042 | 2.9 | TetR family transcriptional regulator |
| A1S_0928 | 2.8 | Transcriptional regulator BetI |
| A1S_0548 | 1.9 | TetR family transcriptional regulator |
| A1S_1320 | 1.8 | Transcriptional regulator SoxR |
| Signal peptides | ||
| A1S_3253 | 4.8 | Signal peptide |
| A1S_1292 | 1.9 | Signal peptide |
| tRNA | ||
| A1S_0333 | 2.3 | trnM |
| Other | ||
| A1S_0058 | 3.8 | Glycosyltransferase |
| A1S_0738 | 3.6 | Flavoprotein oxidoreductase |
| A1S_1386 | 3.5 | Catalase |
| A1S_1639 | 2.9 | PPIase; rotamase |
| A1S_1699 | 2.3 | Acetoin:26-dichlorophenolindophenol oxidoreductase subunit alpha |
| A1S_0820 | 2.2 | Peptidoglycan-binding LysM |
| A1S_1387 | 2.0 | Oxidoreductase |
| A1S_2363 | 1.9 | Xanthine dehydrogenase large subunit |
| A1S_2054 | 1.7 | Thioesterase |
| Hypothetical/uncharacterized proteins | ||
| A1S_3512 | 117.9 | Uncharacterized protein; UniProt |
| A1S_3661 | 10.2 | Uncharacterized protein; UniProt |
| A1S_1319 | 9.1 | Hypothetical protein |
| A1S_0736 | 9.0 | Hypothetical protein |
| A1S_2091 | 4.4 | Hypothetical protein |
| A1S_2294 | 4.1 | Hypothetical protein |
| A1S_0627 | 4.1 | Hypothetical protein |
| A1S_2041 | 4.0 | Hypothetical protein |
| A1S_3481 | 3.8 | Uncharacterized protein; UniProt |
| A1S_1385 | 3.4 | Hypothetical protein |
| A1S_0779 | 3.2 | Hypothetical protein |
| A1S_3786 | 3.1 | Uncharacterized protein; UniProt |
| A1S_3636 | 2.7 | Uncharacterized protein; UniProt |
| A1S_1233 | 2.7 | Hypothetical protein |
| A1S_3155 | 2.7 | Hypothetical protein |
| A1S_2228 | 2.6 | Hypothetical protein |
| A1S_3580 | 2.4 | Uncharacterized protein; UniProt |
| A1S_3303 | 2.3 | Hypothetical protein |
| A1S_3792 | 2.3 | Uncharacterized protein; UniProt |
| A1S_2696 | 2.2 | Hypothetical protein |
| A1S_2074 | 2.2 | Hypothetical protein |
| A1S_2843 | 2.1 | Hypothetical protein |
| A1S_3717 | 2.1 | Uncharacterized protein; UniProt |
| A1S_3673 | 2.1 | Uncharacterized protein; UniProt |
| A1S_3865 | 2.1 | Uncharacterized protein; UniProt |
| A1S_1435 | 2.0 | Hypothetical protein |
| A1S_1952 | 2.0 | Hypothetical protein |
| A1S_3637 | 2.0 | Uncharacterized protein; UniProt |
| A1S_3715 | 2.0 | Uncharacterized protein; UniProt |
| A1S_3675 | 2.0 | Uncharacterized protein; UniProt |
| A1S_3725 | 2.0 | Uncharacterized protein; UniProt |
| A1S_1293 | 1.9 | Hypothetical protein |
| A1S_1760 | 1.9 | Hypothetical protein |
| A1S_3750 | 1.9 | Uncharacterized protein; UniProt |
| A1S_0549 | 1.9 | Hypothetical protein |
| A1S_1450 | 1.8 | Hypothetical protein |
| A1S_1294 | 1.8 | Hypothetical protein |
| A1S_3659 | 1.8 | Uncharacterized protein; UniProt |
| A1S_2179 | 1.8 | Hypothetical protein |
| A1S_3644 | 1.8 | Uncharacterized protein; UniProt |
| A1S_3362 | 1.7 | Hypothetical protein |
Statistically significant changes were determined at P ≤ 0.01 and are listed in Table S1 in the supplemental material.
Annotations are listed as designated in the NCBI or UniProt database.
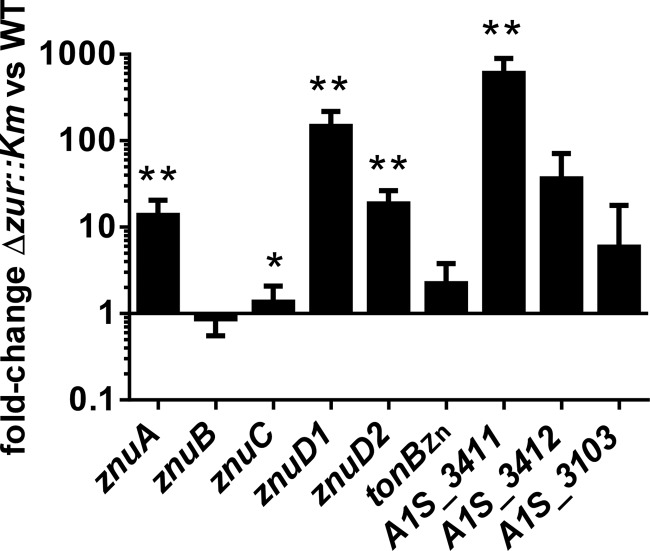
To validate the observed expression changes in the RNA-seq experiments, we performed qRT-PCR comparing the expression of several genes in the Δzur::Km mutant to that of the wild type (Fig. 1). These experiments confirmed the expression changes observed by RNA-seq; however, in most cases qRT-PCR demonstrated a greater effect of zur inactivation. Taken together, these results reveal the impact of zur inactivation on gene expression in A. baumannii.
FIG 1.
A. baumannii has altered gene expression in the absence of Zur. In order to validate the expression changes identified by RNA-seq, wild-type and Δzur::Km strains were grown under conditions used for RNA-seq. RNA was isolated and cDNA generated. Expression changes for selected genes from the RNA-seq experiment were assessed by qPCR on cDNA prepared as described in the text. The graph depicts fold changes in the Δzur::Km strain relative to the wild type from at least three separate experiments. Statistical significance was determined by Student's t test using a reference value of 1.0. *, P ≤ 0.05; **, P ≤ 0.0005.
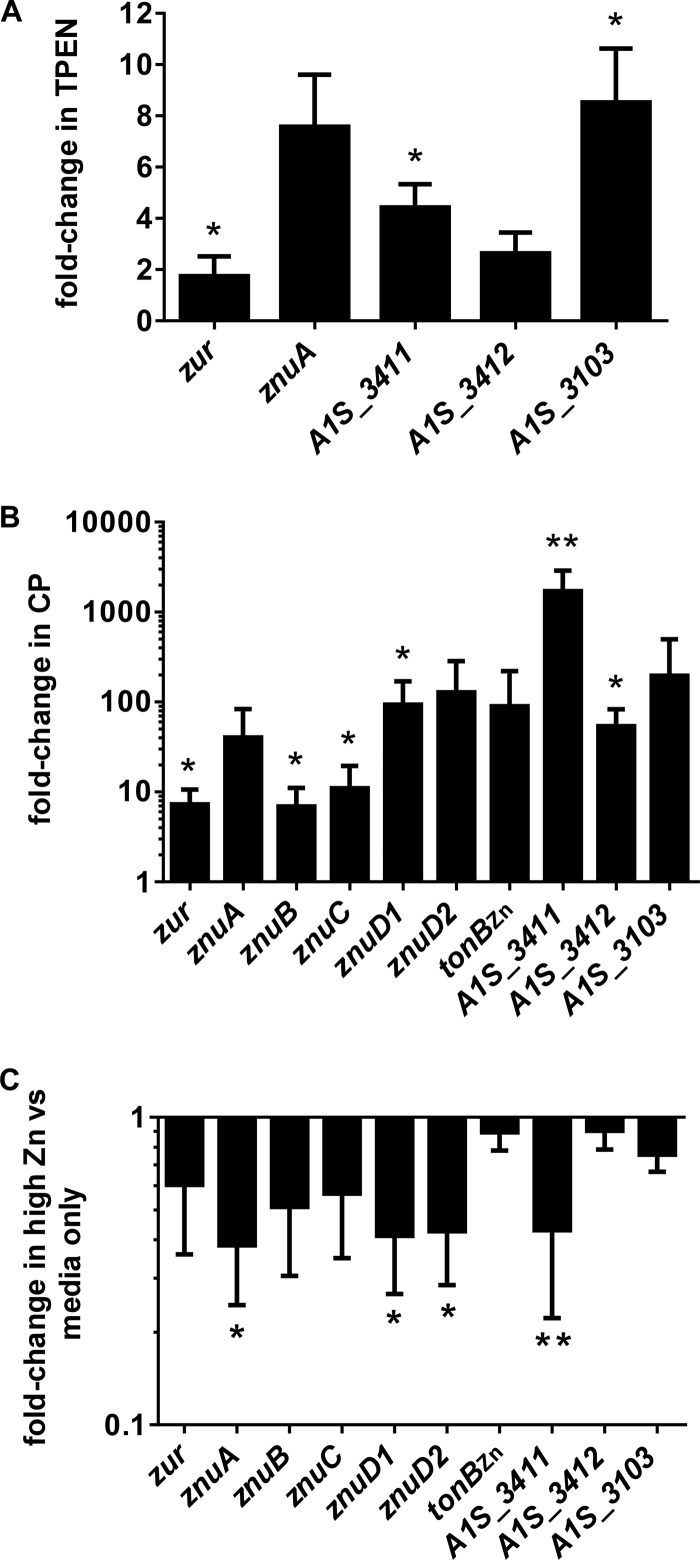
Alterations in Zn levels affect the expression of candidate Zur-regulated genes.
The canonical model for Zur-mediated gene regulation suggests that Zur derepresses expression of target genes in low-Zn conditions (32). Consistent with this, several predicted Zur-regulated genes have been shown to be upregulated in the presence of the Zn chelator TPEN, including znuA, znuB, znuC, znuD1, znuD2, and tonBZn (18). qRT-PCR analyses demonstrate that zur, A1S_3103, A1S_3411, and A1S_3412 are also upregulated in A. baumannii grown in the presence of 25 μM TPEN (Fig. 2A). Furthermore, all of the above-described genes are upregulated to an even greater magnitude in the presence of the Zn-chelating protein CP (Fig. 2B). This same set of genes all are downregulated in bacteria grown under conditions of excess Zn, supporting a model whereby Zur represses gene expression when it binds Zn (Fig. 2C). Together, these data support the conclusion that alterations in Zn levels affect the expression of candidate Zur-regulated genes.
FIG 2.
Expression of putative Zur-regulated gene changes in response to various Zn levels. (A) Expression of selected genes in bacteria grown in the presence of 25 μM Zn chelator TPEN. (B) Expression of selected genes in bacteria grown in the presence of 250 μg/ml vertebrate protein CP. (C) Expression of selected genes in Zn-replete conditions (100 μM ZnCl2). Gene expression was determined by qRT-PCR. The graph depicts fold changes under treated conditions relative to untreated conditions (LB only). Data represent at least three separate experiments. Statistical significance was determined by Student's t test using a reference value of 1.0. *, P ≤ 0.05; **, P ≤ 0.005.
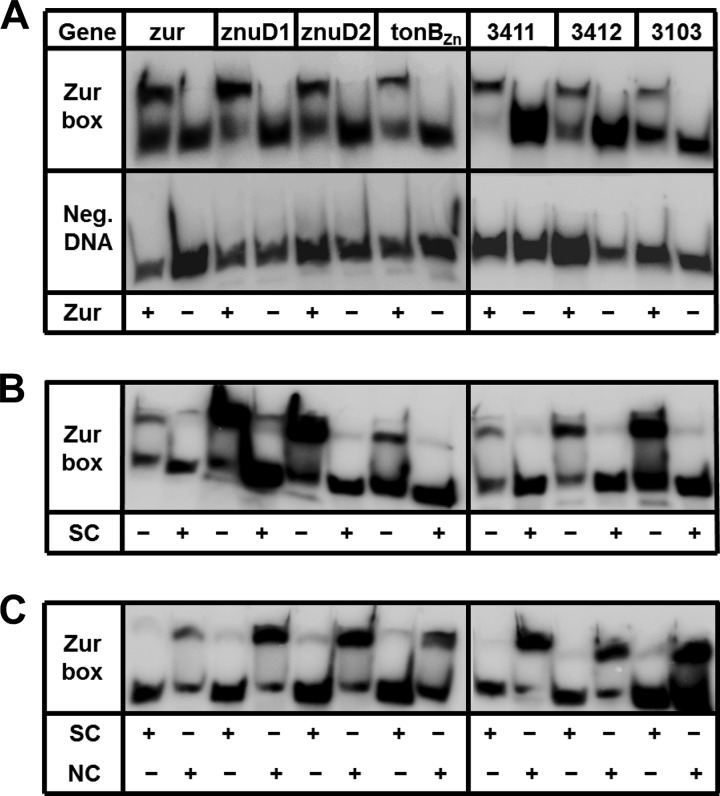
Zur binds to predicted Zur box sequences.
The impact of zur inactivation on gene expression can be due to either direct or indirect regulation by Zur. To determine if Zur recognizes and binds to the predicted Zur boxes of putative target genes, we performed electrophoretic mobility shift assays in Zn-replete conditions. Purified Zur was incubated with radiolabeled target DNA corresponding to either the predicted Zur box sequence or a negative-control sequence of DNA immediately adjacent to the predicted Zur box. We assessed Zur binding to the Zur boxes upstream of several Zur-regulated genes, including znuD1, znuD2, tonBZn, A1S_3103, A1S_3411, and A1S_3412. Zur binds to the Zur boxes of each tested gene but not the negative-control sequences (Fig. 3A). Incubation of purified Zur with labeled Zur box sequences in the presence of an unlabeled specific competitor DNA competes off Zur from the labeled Zur box sequence (Fig. 3B). Finally, purified Zur incubated with Zur box sequence in the presence of the unlabeled nonspecific competitor DNA does not prevent Zur binding to the labeled Zur box sequence (Fig. 3C). Together, these results demonstrate that Zur specifically binds to the predicted Zur box sequences upstream of znuD1, znuD2, tonBZn, A1S_3103, A1S_3411, and A1S_3412 and implicates these genes as being directly regulated by Zur.
FIG 3.
Zur recognizes Zur box sequences of target genes. To assess direct binding of Zur to predicted Zur boxes upstream of putative Zur-regulated genes, electrophoretic mobility shift assays were performed using purified Zur incubated with radiolabeled target DNA. Zur boxes from select Zur-regulated genes were assessed. For gene labels, 3411 is A1S_3411, 3412 is A1S_3412, and 3103 is A1S_3103. (A) Purified Zur incubated with either the Zur box sequence or negative-control sequence (Neg. DNA), which is DNA immediately adjacent to the Zur box sequence of the respective gene. Each DNA sequence was incubated in the presence or absence of particular Zur proteins, as designated in the bottom row by a plus or minus sign. (B) Purified Zur incubated with Zur box sequence in the presence or absence of an unlabeled specific competitor DNA (SC; Zur box sequence from panel A) as designated in the bottom row by a plus or minus sign. (C) Purified Zur incubated with Zur box sequence in the presence of the unlabeled specific competitor DNA (SC) or a nonspecific competitor DNA (NS; negative-control sequence used in panel A) as designated in the bottom row by a plus or minus sign.
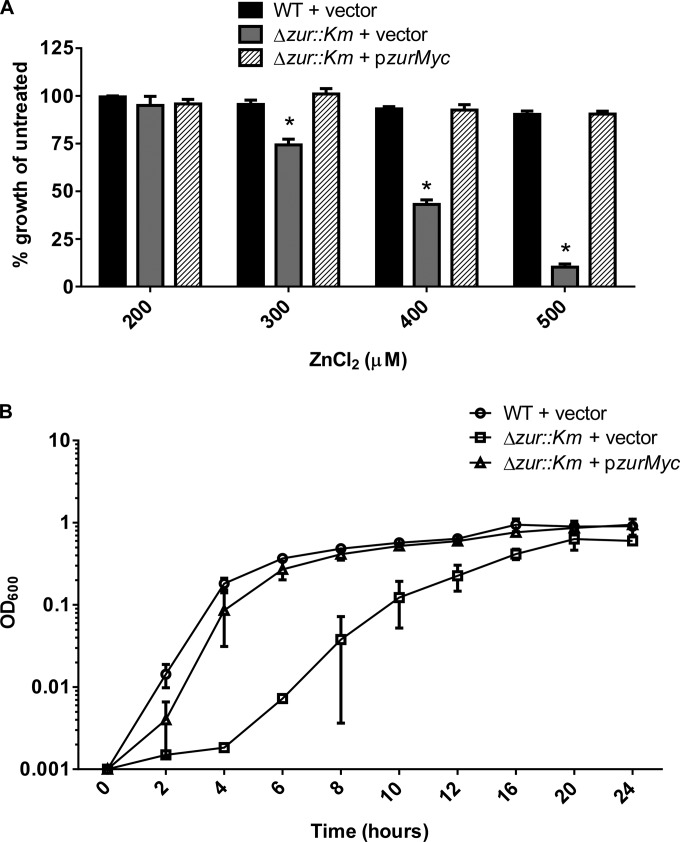
Zur is required for A. baumannii growth in high Zn.
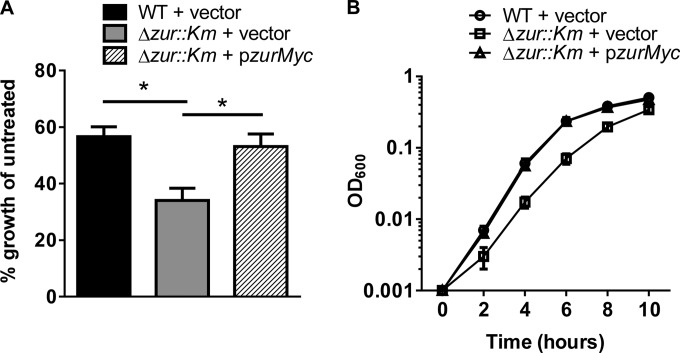
Zn and other transition metals are toxic at high levels, necessitating the strict regulation of metal import. Toxicity is prevented in part through the repression of acquisition systems by metalloregulators such as Zur. In the absence of Zur, A. baumannii has increased expression of Zn uptake systems, which may result in increased Zn uptake and higher susceptibility to Zn toxicity. To test this hypothesis, we grew wild-type A. baumannii and the Δzur::Km mutant in the presence of increasing concentrations of ZnCl2 (Fig. 4A). In these experiments, wild-type growth was unaffected by increased Zn, whereas the Δzur::Km strain displayed a dose-dependent growth defect. Expression of zur in trans restored the growth of the Δzur::Km strain to wild-type levels (Fig. 4B). These data demonstrate a role for Zur in avoiding Zn toxicity through repression of Zn uptake systems when Zn levels are high.
FIG 4.
Zur is required for growth at high levels of Zn. (A) Wild-type (WT) and Δzur::Km bacteria were grown in the presence of concentrations of ZnCl2 increasing from 0 to 500 μM. The graph depicts percent growth of WT plus vector, the strain with Δzur::Km plus vector, and the strain with Δzur::Km plus pzurMyc relative to untreated (LB only) cells from an 8-h time point. (B) The WT plus vector, the Δzur::Km strain plus vector, and the Δzur::Km strain plus pzurMyc were grown in the presence of 400 μM ZnCl2. The graph shows OD600 over time up to 24 h and depicts data from three separate experiments. Statistical significance was determined by two-way ANOVA. *, P ≤ 0.0005.
Zur is required for A. baumannii growth in low Zn.
The large number of genes altered in expression in the Δzur::Km mutant led us to investigate whether Zur also is required for A. baumannii growth under low-Zn conditions. Wild-type and Δzur::Km strains were grown in 20 μM the Zn chelator TPEN, a concentration previously demonstrated to inhibit growth of A. baumannii (18), and the growth of TPEN-treated wild-type and Δzur::Km strains was compared to the growth of untreated strains for 10 h. These experiments revealed that the Δzur::Km strain exhibits a decrease in fitness compared to that of wild-type A. baumannii under Zn-limiting conditions (Fig. 5A and B). The growth defect of the Δzur::Km mutant can be complemented by the expression of zur in trans, demonstrating that this phenotype can be attributed to the loss of zur. Taken together, these results reveal that A. baumannii requires Zur for adaptation to high and low levels of Zn (Fig. 4 and 5).
FIG 5.
Zur is required for growth in Zn-limiting conditions. (A) WT plus vector, the Δzur::Km strain plus vector, and the Δzur::Km strain plus pzurMyc grown in the presence of 20 μM TPEN. The graph depicts percent growth of TPEN-treated WT plus vector, Δzur::Km plus vector, and Δzur::Km plus pzurMyc cells over untreated (LB only) cells from an 8-h time point. (B) WT plus vector, the Δzur::Km strain plus vector, and the Δzur::Km strain plus pzurMyc grown in the presence of 20 μM TPEN. The graph shows the OD600 of each strain over time up to 10 h. Data presented are consolidated from three separate experiments. Statistical significance was determined by Student's t test. *, P ≤ 0.005.
Zur contributes to A. baumannii dissemination to the liver in a mouse model of pneumonia.
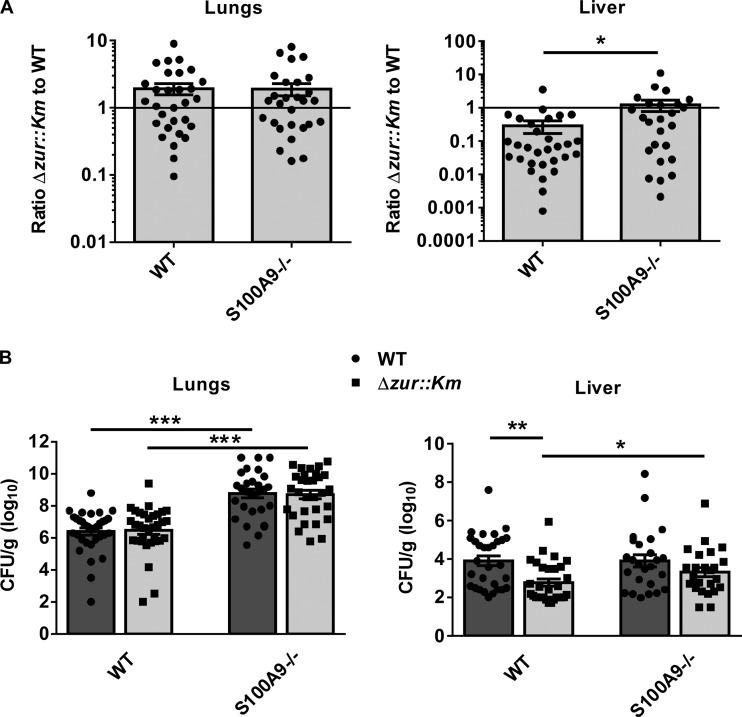
The importance of Zur to growth in the presence of various Zn concentrations suggests that Zur enables A. baumannii to respond to alterations in Zn concentrations encountered within the vertebrate host. In order to test this hypothesis, we performed a coinfection experiment using wild-type and Δzur::Km strains in a murine model of A. baumannii pneumonia. Wild-type mice were inoculated intranasally with a 1:1 mixture of wild-type and Δzur::Km strains (5 × 108 CFU total). After 36 h, animals were sacrificed, bacterial burdens in lungs and livers were determined following tissue homogenization, and a competitive index was calculated (Fig. 6A). Equivalent numbers of wild-type and Δzur::Km bacteria were recovered from the lungs. However, the wild type outcompetes the Δzur::Km strain in measures of dissemination to the liver in this model. Figure 6B shows the CFU/g (log10) for each organ. These results reveal a contribution of Zur to A. baumannii dissemination to the liver following pulmonary infection.
FIG 6.
Zur contributes to dissemination to the liver during A. baumannii pneumonia. WT or S100A9−/− mice were coinfected intranasally with WT and Δzur::Km strains. At 36 h postinfection, bacterial organ burdens were determined in the lungs and livers. (A) Graphs depict the competitive index of the Δzur::Km to the WT strain and are consolidated from three separate experiments. (B) Graphs depict CFU/g (log10) of the Δzur::Km (light gray bars, squares) or WT (dark gray bars, circles) strain and are consolidated from three separate experiments. Statistical significance was determined by the Mann-Whitney nonparametric test. *, P ≤ 0.05; **, P ≤ 0.0005; ***, P ≤ 0.0001.
CP-dependent metal chelation is antimicrobial toward A. baumannii, and zur is upregulated in the presence of CP (18). In keeping with this, we assessed the contribution of CP to replication and dissemination of the Δzur::Km strain following pulmonary challenge in the coinfection model described above using CP-deficient mice (S100A9−/−). Consistent with prior published observations (18), S100A9−/− mice exhibit increased susceptibility to A. baumannii infection, as evidenced by increased organ burdens in the lungs and livers (Fig. 6A and B). The loss of CP also restores the ability of the Δzur::Km strain to compete with the wild type in the liver, implicating CP as a primary contributor to the Zn-starved environment experienced by A. baumannii during infection. Together, these results suggest that the reduced bacterial burdens of the Δzur::Km strain compared to those of the wild type is the result of an inability of this mutant strain to compete with CP and obtain Zn during infection.
DISCUSSION
Zur proteins are metalloregulators that are important for regulation of genes required to adapt to Zn limitation and to avoid Zn toxicity. When Zn is readily available, Zn-bound Zur recognizes a conserved Zur box sequence upstream of target genes, thereby repressing transcription. In situations where Zn is scarce, Zur is no longer bound to the Zur box, and target genes are derepressed. Zur responds to Zn at femtomolar levels via high-affinity Zn binding sites within the homodimer in order to regulate target genes (33–35). The extreme sensitivity of Zur to Zn levels reflects the importance of proper metal homeostasis within the bacterial cell. In the present study, we have assessed the contribution of Zur to the A. baumannii response to various Zn levels, both in vitro and in a murine model of pneumonia.
Assessment of global transcriptional changes in A. baumannii Δzur::Km revealed a large number of genes that are regulated by Zur, either directly or indirectly. Some of the genes found to be Zur regulated in A. baumannii have homologs shown to be Zur or Zn regulated in other bacteria, largely those genes involved in metal homeostasis (36–51). These include znuA, znuB, znuC, znuD1, znuD2, rpmE2, czcD, and a gene encoding a putative siderophore receptor, A1S_0092. Interestingly, the most highly upregulated gene in the Δzur::Km strain was A1S_3411. This gene encodes a protein predicted to belong to the conserved COG0523 family, and genes of this family have also been detected in other Zur regulons or upregulated in Zn-limiting conditions (41–43, 45, 51). The function of this highly conserved family of proteins is still not known; however, they are projected to be metal-binding P-loop GTPases involved in metallocenter formation (52). Also consistent with Zur regulators in other bacterial species, A. baumannii Zur regulates TonB-dependent receptors (42–44). In this regard, we identified a TonB system that is also Zur regulated. This could reflect a specialized system in A. baumannii, an organism that encodes several predicted TonB systems (53, 54). Furthermore, other regulators, transporters, virulence factors, and secreted proteins have been identified as being Zur or Zn regulated in various bacterial species (39–45, 48, 50). One unique group of Zur-regulated genes identified in this analysis are those belonging to the Hut system. The Hut system is responsible for the catabolism of histidine to the end products glutamate, ammonia, and formate/formamide (31). A functional link between histidine catabolism and adaptation to Zn-limiting conditions has yet to be defined.
The lack of significant upregulation of znuB and znuC in the Δzur::Km mutant for both RNA-seq and qRT-PCR could be the result of an unidentified regulatory element being disrupted following deletion of the upstream zur gene or the loss of transcript stability in the absence of zur. Alternatively, the basal levels of ZnuB and ZnuC may be higher; therefore, a dramatic increase in expression under low-Zn conditions is not required. Finally, it is possible that zur and znuBC are cotranscribed and their regulation simply reflects that of zur, which is not highly upregulated. In other words, due to the toxicity that could occur following the extensive upregulation of a regulator, such as Zur, zur levels may be controlled to prevent overexpression. The downstream znuB and znuC expression levels consequently are not as high. Notably, modest upregulation of znuB and/or znuC in the Δzur::Km mutant or under Zn-limiting conditions has been reported previously for other organisms (39, 41, 42, 44, 48).
We observed a substantial number of genes upregulated in the Δzur::Km strain that are not predicted to contain Zur box sequences. These genes likely are regulated by Zur indirectly through derepression of other Zur targets, perhaps one of the six regulators with changed expression in the zur mutant. Alternatively, the increased expression of genes without an identifiable Zur box in the Δzur::Km strain may be due to Zur directly regulating a subset of genes through a motif distinct from that of the Zur box. There is precedence for noncanonical DNA-binding consensus sequences for the closely related Fur regulator in other organisms (55–57). In this vein, our electrophoretic mobility shift assays show that Zur binds to the predicted Zur box of several genes not previously shown to be Zur regulated. Although the functions of these genes have yet to be empirically determined, direct Zur regulation links these genes to Zn homeostasis.
As predicted, A. baumannii Zur-regulated genes decrease expression in Zn-replete conditions, and derepression of Zur target genes was observed when Zn was limiting, including in the presence of the host protein CP. Notably, the Zur-regulated genes were found at dramatically increased levels in the presence of CP compared to A. baumannii grown in the presence of TPEN or to the Δzur::Km strain. Overall, these data support the Zn dependency of the Zur-mediated regulation. Furthermore, these data suggest that in low-Zn conditions, there is a synergistic effect of host metal chelation and Zur binding. First, limited Zn prevents bacteria from populating Zn-dependent proteins involved in numerous processes, such as metabolism, transcription, and translation, thereby slowing bacterial growth. Second, Zn chelation adequately sequesters Zn from Zur; therefore, repression of Zur target genes is relieved, allowing bacteria to adapt to these conditions.
On the other hand, the loss of zur increases A. baumannii sensitivity to Zn toxicity, which is consistent with other reports of a Zur homolog in the cyanobacterium Anabaena sp. strain PCC 7120 and Xanthomonas campestris pv. campestris (43, 58). The increased toxicity to high levels of Zn likely reflects the derepression of Zur-regulated Zn acquisition systems and a concomitant increase in intracellular Zn. The mechanisms of Zn toxicity are not clear; however, they may involve competition with other metal ions in metalloproteins and enzyme inhibition (59, 60). Many bacteria express Zn efflux systems to keep intracellular Zn at subtoxic levels, such as ZntA and ZitA, two well-characterized transporters in E. coli; however, Zn efflux systems have yet to be described in A. baumannii (61, 62). RNA-seq data identified a putative Zn efflux system (A1S_1044-A1S_1045) that exhibits a >2-fold increase in expression in the Δzur::Km mutant. A1S_1044 and A1S_1045 share identity with genes of a Co/Zn/Cd efflux system (CzcD). Perhaps expression of an efflux system when the level of Zn is low aids in the maintenance of Zn homeostasis and prevention of Zn toxicity that may be encountered due to upregulation of Zn uptake systems. Notably, A. baumannii lacking zur is defective for growth in Zn-starved conditions. The improper regulation of proteins involved in Zn uptake or in modification of the metalloproteome or A. baumannii metabolism could result in a growth defect of the Δzur::Km strain under these conditions. Supplementation with Zn, Mn, or Cu but not Fe or Ca complements growth of the zur mutant under low-Zn conditions, suggesting that the growth defect is metal dependent but not necessarily Zn specific (see Fig. S1 in the supplemental material). One possible explanation for these data is that an excess of divalent cations with affinity for TPEN can outcompete Zn for the TPEN and liberate Zn for A. baumannii to access. An alternative possibility is that A. baumannii can adapt to low Zn by modifying its metalloproteome through the distribution of non-Zn metals in order to maintain at least minimal processes required for growth. We are currently investigating these and other possibilities.
Using a mouse model of A. baumannii pneumonia, we demonstrated that Zur is necessary for dissemination to the liver. The organ-specific phenotype could have several biological explanations. First, there may be different metal levels in infected lungs than in infected livers. Since the loss of CP restored growth of the Δzur::Km mutant to wild-type levels, this suggests that the Δzur::Km phenotype is the result of metal limitation that would be alleviated when CP is absent. CP is expressed in the lungs following A. baumannii infection; however, CP levels in the A. baumannii-infected liver have not been evaluated. Alternatively, since CP is also a proinflammatory mediator, it is possible that the growth defects in the liver, for both the zur and znuB deletion mutants (18), are the result of a modified immune response that is more detrimental to these A. baumannii mutants. Finally, the decreased burdens in the liver may specifically represent a dissemination defect as opposed to a growth defect, such that the Δzur::Km strain is less able to survive in the blood and, consequently, fewer bacteria reach the secondary site. Further studies are required to investigate the role of Zur and other Zn homeostasis mutants during A. baumannii infection.
Our data describe the Zn regulon in A. baumannii, including genes involved in metal homeostasis, metabolism, and virulence. Moreover, we have demonstrated a role for Zur in the ability of A. baumannii to grow in the presence of various levels of Zn and within the host during infection. In general, the ability of bacterial pathogens to respond to the various metal levels in the host is crucial to establishing infection because of the requirement for metals in a variety of physiological processes. In view of the increasing rates of antibiotic resistance in A. baumannii and other bacteria, identification of new targets for antimicrobials is urgently needed. Systems involved in bacterial metal homeostasis are attractive possibilities due to their importance in bacterial physiology and growth as well as the conservation of these systems among bacterial species.
Supplementary Material
ACKNOWLEDGMENTS
We thank the members of the Skaar laboratory for their editorial comments on the manuscript.
Work presented in the paper was funded by grants R01 AI1091771 and R01 AI101171 from the National Institutes of Health (NIH). E.P.S. is a Burroughs Wellcome Fund Investigator in the Pathogenesis of Infectious Diseases. B.L.M. is supported by grant F32-AI108192 from the National Institute of Allergy and Infectious Disease (NIAID) and also during the course of this work through the Childhood Infection Research Program (ChIRP; T32-AI095202).
The content of this article does not necessarily represent the views of the NIH or NIAID and is solely the responsibility of the authors.
Footnotes
Published ahead of print 9 May 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.01650-14.
REFERENCES
- 1.Doyle JS, Buising KL, Thursky KA, Worth LJ, Richards MJ. 2011. Epidemiology of infections acquired in intensive care units. Semin. Respir. Crit. Care Med. 32:115–138. 10.1055/s-0031-1275525 [DOI] [PubMed] [Google Scholar]
- 2.Gaynes R, Edwards JR. 2005. Overview of nosocomial infections caused by gram-negative bacilli. Clin. Infect. Dis. 41:848–854. 10.1086/432803 [DOI] [PubMed] [Google Scholar]
- 3.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. CMR. 21:538–582. 10.1128/CMR.00058-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis KA, Moran KA, McAllister CK, Gray PJ. 2005. Multidrug-resistant Acinetobacter extremity infections in soldiers. Emerg. Infect. Dis. 11:1218–1224. 10.3201/1108.050103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calhoun JH, Murray CK, Manring MM. 2008. Multidrug-resistant organisms in military wounds from Iraq and Afghanistan. Clin. Orthop. Relat. Res. 466:1356–1362. 10.1007/s11999-008-0212-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sebeny PJ, Riddle MS, Petersen K. 2008. Acinetobacter baumannii skin and soft-tissue infection associated with war trauma. Clin. Infect. Dis. 47:444–449. 10.1086/590568 [DOI] [PubMed] [Google Scholar]
- 7.Whitman TJ. 2007. Infection control challenges related to war wound infections in the ICU setting. J. Trauma 62:S53. 10.1097/TA.0b013e318065aa71 [DOI] [PubMed] [Google Scholar]
- 8.Falagas ME, Karveli EA, Kelesidis I, Kelesidis T. 2007. Community-acquired Acinetobacter infections. Eur. J. Clin. Microbiol. Infect. Dis. 26:857–868. 10.1007/s10096-007-0365-6 [DOI] [PubMed] [Google Scholar]
- 9.Wang SH, Sheng WH, Chang YY, Wang LH, Lin HC, Chen ML, Pan HJ, Ko WJ, Chang SC, Lin FY. 2003. Healthcare-associated outbreak due to pan-drug resistant Acinetobacter baumannii in a surgical intensive care unit. J. Hosp. Infect. 53:97–102. 10.1053/jhin.2002.1348 [DOI] [PubMed] [Google Scholar]
- 10.Hasan B, Perveen K, Olsen B, Zahra R. 2014. Emergence of carbapenem-resistant Acinetobacter baumannii in hospitals in Pakistan. J. Med. Microbiol. 63:50–55. 10.1099/jmm.0.063925-0 [DOI] [PubMed] [Google Scholar]
- 11.Durante-Mangoni E, Zarrilli R. 2011. Global spread of drug-resistant Acinetobacter baumannii: molecular epidemiology and management of antimicrobial resistance. Future Microbiol. 6:407–422. 10.2217/fmb.11.23 [DOI] [PubMed] [Google Scholar]
- 12.Weinberg ED. 1975. Nutritional immunity. Host's attempt to withhold iron from microbial invaders. JAMA 231:39–41 [DOI] [PubMed] [Google Scholar]
- 13.Hood MI, Skaar EP. 2012. Nutritional immunity: transition metals at the pathogen-host interface. Nat. Rev. Microbiol. 10:525–537. 10.1038/nrmicro2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gebhardt C, Nemeth J, Angel P, Hess J. 2006. S100A8 and S100A9 in inflammation and cancer. Biochem. Pharmacol. 72:1622–1631. 10.1016/j.bcp.2006.05.017 [DOI] [PubMed] [Google Scholar]
- 15.Damo SM, Kehl-Fie TE, Sugitani N, Holt ME, Rathi S, Murphy WJ, Zhang Y, Betz C, Hench L, Fritz G, Skaar EP, Chazin WJ. 2013. Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. Proc. Natl. Acad. Sci. U. S. A. 110:3841–3846. 10.1073/pnas.1220341110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kehl-Fie TE, Chitayat S, Hood MI, Damo S, Restrepo N, Garcia C, Munro KA, Chazin WJ, Skaar EP. 2011. Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus. Cell Host Microbe 10:158–164. 10.1016/j.chom.2011.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, Torres VJ, Anderson KL, Dattilo BM, Dunman PM, Gerads R, Caprioli RM, Nacken W, Chazin WJ, Skaar EP. 2008. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319:962–965. 10.1126/science.1152449 [DOI] [PubMed] [Google Scholar]
- 18.Hood MI, Mortensen BL, Moore JL, Zhang Y, Kehl-Fie TE, Sugitani N, Chazin WJ, Caprioli RM, Skaar EP. 2012. Identification of an Acinetobacter baumannii zinc acquisition system that facilitates resistance to calprotectin-mediated zinc sequestration. PLoS Pathog. 8:e1003068. 10.1371/journal.ppat.1003068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. 2009. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 5:e1000639. 10.1371/journal.ppat.1000639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCormick A, Heesemann L, Wagener J, Marcos V, Hartl D, Loeffler J, Heesemann J, Ebel F. 2010. NETs formed by human neutrophils inhibit growth of the pathogenic mold Aspergillus fumigatus. Microbes Infect. 12:928–936. 10.1016/j.micinf.2010.06.009 [DOI] [PubMed] [Google Scholar]
- 21.Bianchi M, Niemiec MJ, Siler U, Urban CF, Reichenbach J. 2011. Restoration of anti-Aspergillus defense by neutrophil extracellular traps in human chronic granulomatous disease after gene therapy is calprotectin-dependent. J. Allergy Clin. Immunol. 127:1243–1252. 10.1016/j.jaci.2011.01.021 [DOI] [PubMed] [Google Scholar]
- 22.Liu JZ, Jellbauer S, Poe AJ, Ton V, Pesciaroli M, Kehl-Fie TE, Restrepo NA, Hosking MP, Edwards RA, Battistoni A, Pasquali P, Lane TE, Chazin WJ, Vogl T, Roth J, Skaar EP, Raffatellu M. 2012. Zinc sequestration by the neutrophil protein calprotectin enhances Salmonella growth in the inflamed gut. Cell Host Microbe 11:227–239. 10.1016/j.chom.2012.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore JL, Becker KW, Nicklay JJ, Boyd KL, Skaar EP, Caprioli RM. 2013. Imaging mass spectrometry for assessing temporal proteomics: analysis of calprotectin in Acinetobacter baumannii pulmonary infection. Proteomics 10.1002/pmic.201300046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaballa A, Helmann JD. 1998. Identification of a zinc-specific metalloregulatory protein, Zur, controlling zinc transport operons in Bacillus subtilis. J. Bacteriol. 180:5815–5821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menard R, Sansonetti PJ, Parsot C. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 175:5899–5906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77–86. 10.1016/S0378-1119(98)00130-9 [DOI] [PubMed] [Google Scholar]
- 27.Magoc T, Wood D, Salzberg SL. 2013. EDGE-pro: estimated degree of gene expression in prokaryotic genomes. Evol. Bioinform. Online 9:127–136. 10.4137/EBO.S11250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9:357–359. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L, Wang S, Li W. 2012. RSeQC: quality control of RNA-seq experiments. Bioinformatics 28:2184–2185. 10.1093/bioinformatics/bts356 [DOI] [PubMed] [Google Scholar]
- 30.Anders S, Huber W. 2010. Differential expression analysis for sequence count data. Genome Biol. 11:R106. 10.1186/gb-2010-11-10-r106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bender RA. 2012. Regulation of the histidine utilization (hut) system in bacteria. Microbiol. Mol. Biol. Rev. 76:565–584. 10.1128/MMBR.00014-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hantke K. 2001. Bacterial zinc transporters and regulators. Biometals 14:239–249. 10.1023/A:1012984713391 [DOI] [PubMed] [Google Scholar]
- 33.Outten CE, O'Halloran TV. 2001. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 292:2488–2492. 10.1126/science.1060331 [DOI] [PubMed] [Google Scholar]
- 34.Outten CE, Tobin DA, Penner-Hahn JE, O'Halloran TV. 2001. Characterization of the metal receptor sites in Escherichia coli Zur, an ultrasensitive zinc(II) metalloregulatory protein. Biochemistry 40:10417–10423. 10.1021/bi0155448 [DOI] [PubMed] [Google Scholar]
- 35.Shin JH, Jung HJ, An YJ, Cho YB, Cha SS, Roe JH. 2011. Graded expression of zinc-responsive genes through two regulatory zinc-binding sites in Zur. Proc. Natl. Acad. Sci. U. S. A. 108:5045–5050. 10.1073/pnas.1017744108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin JH, Oh SY, Kim SJ, Roe JH. 2007. The zinc-responsive regulator Zur controls a zinc uptake system and some ribosomal proteins in Streptomyces coelicolor A3(2). J. Bacteriol. 189:4070–4077. 10.1128/JB.01851-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panina EM, Mironov AA, Gelfand MS. 2003. Comparative genomics of bacterial zinc regulons: enhanced ion transport, pathogenesis, and rearrangement of ribosomal proteins. Proc. Natl. Acad. Sci. U. S. A. 100:9912–9917. 10.1073/pnas.1733691100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patzer SI, Hantke K. 2000. The zinc-responsive regulator Zur and its control of the znu gene cluster encoding the ZnuABC zinc uptake system in Escherichia coli. J. Biol. Chem. 275:24321–24332. 10.1074/jbc.M001775200 [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Qiu Y, Gao H, Guo Z, Han Y, Song Y, Du Z, Wang X, Zhou D, Yang R. 2009. Characterization of Zur-dependent genes and direct Zur targets in Yersinia pestis. BMC Microbiol. 9:128. 10.1186/1471-2180-9-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaballa A, Wang T, Ye RW, Helmann JD. 2002. Functional analysis of the Bacillus subtilis Zur regulon. J. Bacteriol. 184:6508–6514. 10.1128/JB.184.23.6508-6514.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schroder J, Jochmann N, Rodionov DA, Tauch A. 2010. The Zur regulon of Corynebacterium glutamicum ATCC 13032. BMC Genomics 11:12. 10.1186/1471-2164-11-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim CK, Hassan KA, Penesyan A, Loper JE, Paulsen IT. 2013. The effect of zinc limitation on the transcriptome of Pseudomonas protegens Pf-5. Environ. Microbiol. 15:702–715. 10.1111/j.1462-2920.2012.02849.x [DOI] [PubMed] [Google Scholar]
- 43.Napolitano M, Rubio MA, Santamaria-Gomez J, Olmedo-Verd E, Robinson NJ, Luque I. 2012. Characterization of the response to zinc deficiency in the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 194:2426–2436. 10.1128/JB.00090-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pawlik MC, Hubert K, Joseph B, Claus H, Schoen C, Vogel U. 2012. The zinc-responsive regulon of Neisseria meningitidis comprises 17 genes under control of a Zur element. J. Bacteriol. 194:6594–6603. 10.1128/JB.01091-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maciag A, Dainese E, Rodriguez GM, Milano A, Provvedi R, Pasca MR, Smith I, Palu G, Riccardi G, Manganelli R. 2007. Global analysis of the Mycobacterium tuberculosis Zur (FurB) regulon. J. Bacteriol. 189:730–740. 10.1128/JB.01190-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis LM, Kakuda T, DiRita VJ. 2009. A Campylobacter jejuni znuA orthologue is essential for growth in low-zinc environments and chick colonization. J. Bacteriol. 191:1631–1640. 10.1128/JB.01394-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nielubowicz GR, Smith SN, Mobley HL. 2010. Zinc uptake contributes to motility and provides a competitive advantage to Proteus mirabilis during experimental urinary tract infection. Infect. Immun. 78:2823–2833. 10.1128/IAI.01220-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graham AI, Hunt S, Stokes SL, Bramall N, Bunch J, Cox AG, McLeod CW, Poole RK. 2009. Severe zinc depletion of Escherichia coli: roles for high affinity zinc binding by ZinT, zinc transport and zinc-independent proteins. J. Biol. Chem. 284:18377–18389. 10.1074/jbc.M109.001503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lindsay JA, Foster SJ. 2001. zur: a Zn(2+)-responsive regulatory element of Staphylococcus aureus. Microbiology 147:1259–1266 [DOI] [PubMed] [Google Scholar]
- 50.Sigdel TK, Easton JA, Crowder MW. 2006. Transcriptional response of Escherichia coli to TPEN. J. Bacteriol. 188:6709–6713. 10.1128/JB.00680-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gabriel SE, Miyagi F, Gaballa A, Helmann JD. 2008. Regulation of the Bacillus subtilis yciC gene and insights into the DNA-binding specificity of the zinc-sensing metalloregulator Zur. J. Bacteriol. 190:3482–3488. 10.1128/JB.01978-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haas CE, Rodionov DA, Kropat J, Malasarn D, Merchant SS, de Crecy-Lagard V. 2009. A subset of the diverse COG0523 family of putative metal chaperones is linked to zinc homeostasis in all kingdoms of life. BMC Genomics 10:470. 10.1186/1471-2164-10-470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zimbler DL, Arivett BA, Beckett AC, Menke SM, Actis LA. 2013. Functional features of TonB energy transduction systems of Acinetobacter baumannii. Infect. Immun. 81:3382–3394. 10.1128/IAI.00540-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith MG, Gianoulis TA, Pukatzki S, Mekalanos JJ, Ornston LN, Gerstein M, Snyder M. 2007. New insights into Acinetobacter baumannii pathogenesis revealed by high-density pyrosequencing and transposon mutagenesis. Genes Dev. 21:601–614. 10.1101/gad.1510307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Friedman YE, O'Brian MR. 2003. A novel DNA-binding site for the ferric uptake regulator (Fur) protein from Bradyrhizobium japonicum. J. Biol. Chem. 278:38395–38401. 10.1074/jbc.M306710200 [DOI] [PubMed] [Google Scholar]
- 56.Baichoo N, Helmann JD. 2002. Recognition of DNA by Fur: a reinterpretation of the Fur box consensus sequence. J. Bacteriol. 184:5826–5832. 10.1128/JB.184.21.5826-5832.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao H, Zhou D, Li Y, Guo Z, Han Y, Song Y, Zhai J, Du Z, Wang X, Lu J, Yang R. 2008. The iron-responsive Fur regulon in Yersinia pestis. J. Bacteriol. 190:3063–3075. 10.1128/JB.01910-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang DJ, Li XJ, He YQ, Feng JX, Chen B, Tang JL. 2005. The zinc uptake regulator Zur is essential for the full virulence of Xanthomonas campestris pv. campestris. Mol. Plant Microbe Interact. 18:652–658. 10.1094/MPMI-18-0652 [DOI] [PubMed] [Google Scholar]
- 59.Vallee BL, Falchuk KH. 1993. The biochemical basis of zinc physiology. Physiol. Rev. 73:79–118 [DOI] [PubMed] [Google Scholar]
- 60.Dineley KE, Votyakova TV, Reynolds IJ. 2003. Zinc inhibition of cellular energy production: implications for mitochondria and neurodegeneration. J. Neurochem. 85:563–570. 10.1046/j.1471-4159.2003.01678.x [DOI] [PubMed] [Google Scholar]
- 61.Grass G, Fan B, Rosen BP, Franke S, Nies DH, Rensing C. 2001. ZitB (YbgR), a member of the cation diffusion facilitator family, is an additional zinc transporter in Escherichia coli. J. Bacteriol. 183:4664–4667. 10.1128/JB.183.15.4664-4667.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rensing C, Mitra B, Rosen BP. 1997. The zntA gene of Escherichia coli encodes a Zn(II)-translocating P-type ATPase. Proc. Natl. Acad. Sci. U. S. A. 94:14326–14331. 10.1073/pnas.94.26.14326 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.