Abstract
Previously, we reported that in a rat model of sporadic Alzheimer’s disease (AD) generated by exogenous administration of Aβ1–42 (250 pmol/d for 2 wk) via mini-osmotic pump, the animals exhibited learning and memory impairment, which could be attributed to the deleterious alterations in the levels of cognition-related signalling molecules. We showed that 4 wk of treadmill exercise totally prevented these impairments. Here, we evaluated the effect of exercise on non-cognitive function and basal synaptic transmission in the Cornu Ammonis 1 (CA1) area using the same AD model. Our results indicated that the anxiety behaviour of Aβ-treated rats was prevented by 4 wk of treadmill exercise. Exercised/Aβ-infused rats spent a longer time in the centre area of the open field (OF), elevated plus maze (EPM) paradigms and the light area of the light-dark (LD) box, which were similar to those of control and exercise rats. Furthermore, under basal conditions the aberrant up-regulation of calcineurin (PP2B) and reduction of phosphorylated Ca2+/calmodulin dependent protein kinase II (p-CaMKII) levels induced by AD-like pathology were normalised by the exercise regimen. We conclude that regular exercise may exert beneficial effects on both cognitive and non-cognitive functions in this AD model.
Keywords: Alzheimer’s disease, anxiety, basal synaptic transmission, calcineurin (PP2B), calcium-calmodulin dependent protein kinase II (CaMKII), exercise
Introduction
Alzheimer’s disease (AD) affects millions worldwide and is characterised by irreversible and progressive neurodegeneration. Due to the uncertain aetiology and complexity of the disease pathology, an effective cure for AD remains elusive. Current FDA-approved pharmacological treatments of AD only temporarily ameliorate cognitive deficits but correction of behavioural abnormalities requires additional medication. In addition to progressive memory loss, AD patients frequently exhibit non-cognitive symptoms such as depression, anxiety and aggressiveness, which worsen as the disease progresses. Thus, there is an urgent need for a novel therapeutic approach that can improve cognitive deficits as well as alleviate non-cognitive disturbances.
Current evidence suggests a beneficial effect of regular exercise on both cognitive and non-cognitive functions in humans and experimental animals. Exercised animals show an enhanced performance in spatial learning and memory tasks such as the Morris water maze and radial arm water maze (Khabour et al., 2013; Suijo et al., 2013). Exercise can also modify non-spatial learning and memory as shown in passive avoidance and object recognition (Bechara and Kelly, 2013; Hosseini et al., 2013; Radahmadi et al., 2013; Souza et al., 2013). In addition, epidemiological studies demonstrate that cognitive impairments resulting from normal aging, brain insults or pathology can also be rescued by regular exercise (Erickson et al., 2012). In addition to exerting a beneficial effect on cognitive function, exercise also has been shown to be as effective as other pharmacological treatments or psychotherapies for anxiety and depressive disorders (Broman-Fulks et al., 2004; Broman-Fulks and Storey, 2008; Bartley et al., 2013). In this study, we investigated whether 4 wk of treadmill exercise could prevent anxiety-like behaviours, impaired basal synaptic transmission in CA1 area and aberrant alterations in molecular pathways (i.e. CaMKII, calcineurin, BDNF) implicated in AD pathology. Our data consistently reveal a neuroprotective effect of regular treadmill exercise at the behavioural, cellular and molecular levels in our AD model.
Materials and method
Animals
Adult male Wistar rats weighing 176–200 g at the beginning of the experiments were purchased from Charles River Laboratories, USA. Upon arrival, rats were separated in groups of six and housed in Plexiglas cages in a climate-controlled room (25 °C) on a 12 h/12 h light/ dark cycle and provided a regular chow diet and water ad libitum. Rats were allowed a week for acclimatization and randomly assigned into 4 groups: control, exercise, amyloid-infused (Aβ) and exercise/amyloid-infused (Ex/Aβ). Animal experiments followed the instructions from National Research Council’s Guide of The Care and Use of Laboratory Animals and with the approval of Institutional Animal Care and Use Committee at the University of Houston.
Exercise protocol
Rats ran on a motorized rodent treadmill (Columbus Instruments) between 9:00 am and 4:00 pm, 5 d/wk for 4 wk as described (Vollert et al., 2011; Zagaar et al., 2012; Dao et al., 2013). Before exercise training, rats were familiarised with the treadmill environment, and then ran in sessions (15 min each) with a 5- min break in between sessions to avoid muscle fatigue. During the first 2 wk, rats ran 2 daily sessions at a speed of 10 m/min while during the 3rd and 4th wk, rats ran 3 and 4 sessions, respectively, at a speed of 15 m/min. In order to encourage the rats to run continuously, the metal bar grid at the beginning of the running lanes constantly delivered a mild foot shock (intensity=0.5 mA).
Alzheimer’s disease model
The i.c.v. infusion of amyloid peptide is considered to be an established model of AD as other studies, using various Aβ peptide species, or a mixture thereof, employing the same model have reported learning and memory impairment in addition to reduced cholinergic activity, cholinergic dysfunction and deleterious biochemical changes in levels of AD-related molecules (Nitta et al., 1994; Itoh et al., 1996; Srivareerat et al., 2009). After 2 wk of exercise, the Aβ and Ex/Aβ groups were implanted with osmotic mini-pumps (Alzet, Cupertino, USA) containing Aβ1–42 peptides (AnaSpec Inc., San Jose, USA). Previous studies in our lab showed infusion of inactive reversed (Aβ42-1) peptides or saline did not affect the test parameters (Srivareerat et al., 2009); thus, the control and exercise groups underwent a sham operation. The peptides were dissolved in a solution containing 64.9% distilled water, 35% acetonitrile, and 0.1% trifluoroacetate (TFA) to prevent peptide aggregation in the pump. The pumps were assembled, filled with 100 μl of Aβ1–42 solution (250 pmol/day), and primed in isotonic (0.9%) saline at 37 °C overnight. The infusion rate was 100 μl. Rats were anaesthetised with an i.p. injection of a mixture of ketamine (75 mg/kg) and xylazine (2.5 mg/kg) (Webster Veterinary, Devens, MA). The infusion cannula was implanted stereotaxically into the cerebral lateral right ventricle (AP: −0.3, L: 1.2, V: 4) and fixed with dental cement. The pump was placed in a sub dermal pocket in the back of the rat. The surgery site was closed with wound clips and kept aseptic with tincture of iodine, 60×diluted chlorohexidine and triple antibiotic ointment.
Behavioural tests
In order to investigate the effect of regular treadmill exercise on non-cognitive performance in Aβ rats, a battery of non-cognitive behavioural tests was conducted 4 wk after the start of the exercise in the following order:
Open field (OF)
The OF task is a common test used to assess anxiety level, exploratory behaviour and locomotor activity in rodents (Gould et al., 2009). The OF apparatus was constructed from an open area (40×60 cm) surrounded by Plexiglas walls (50 cm high). The rat was placed in the centre of the chamber and allowed to explore the environment. The activity was detected by infrared light sensors and quantified by Opto-Varimex Micro Activity Meter v2.00 software (Optomax, Columbus Instruments, USA). Sensors (6 sensors/cage) were positioned to reveal a two-dimensional cage and monitor rearing. Every movement was detected by beam breaks, which were recorded by computer for further analysis. Each experiment was conducted with one rat per chamber and recorded in 3-min test intervals for a total of 30 min. A 25×25 cm area in the middle of the chamber was defined as the centre area. Total activity, total distance travelled and percentage of time spent in the centre area were analysed. The OF apparatus was cleaned with 70% ethyl alcohol and aired after each measurement.
Light-dark box (LD)
The LD paradigm is a sensitive test of disorders involving generalized anxiety (Araujo et al., 2012). The LD box consists of a light compartment (27×27×27 cm) and a dark compartment (black walls and floor, 27×18×27 cm) separated by a single partition with an opening (7×7 cm), which allowed passage between the two compartments (Salim, et al., 2010). The apparatus was placed in an enclosed area of a behavioural room under standard lighting conditions with only one observer in the room. The experiment lasted 5 min. Anxiety behaviour was quantified by the length of time spent in the light compartment.
Elevated plus maze (EPM)
Another sensitive test is the EPM, which consists of two open and two closed arms (10 cm×50 cm length) that intersect at the middle creating a ‘+’ shape. The EPM was elevated 50 cm above the ground and placed in an enclosed area of the behavioural room. The procedure was carried out as previously described (Xiang et al., 2011). Briefly, the rat was placed at the intersection area (10×10 cm) of the four arms and allowed to explore the apparatus for 5 min. The rat’s movements during the testing period were recorded by a digital camera that was connected to a computer for data collection. Time spent in the closed arms, centre area and total distance travelled were analysed.
Basal synaptic transmission recordings
Seventeen days after the beginning of Aβ infusion, rats were anaesthetised by i.p. injection of urethane (1.2 g/kg) and prepared for recording as described (Aleisa et al., 2006). Briefly, the head was fixed in the stereotaxic frame with the nose bar positioned at 0.0. The skull was exposed after shaving and cutting a mid-line starting slightly behind the eyes. Two holes were drilled for placing the stimulating and recording electrodes to record from area CA1 of the hippocampus. On the left side of the brain, the stimulating electrode was placed at a 5° angle toward the midline (AP: −3, L: 3.5, V: 2.8) to stimulate the Schaffer collateral/commissural pathway. A glass recording electrode filled with 1% fast green dye in 2 M NaCl was inserted in the pre-drilled hole to record from the pyramidal cell layer of area CA1 (AP: −3, L: 2, V: 2). Once a robust p-spike was evoked, there was no simulation for 30-min to allow stabilisation. The input/output (I/O) curve was constructed by plotting various stimulus intensities (input) against the evoked field excitatory post-synaptic response (fEPSP) slope (output), a measure of synaptic strength. The voltages at which the response was minimal, 30% of the maximum, and maximal were also recorded.
Western blotting
Hippocampus dissection
At the end of all treatments, rats were euthanized by a lethal urethane injection into the heart. The brain was immediately removed and placed on a filter paper soaked with 0.2 M sucrose on top of a covered Petri dish filled with dry ice. The CA1 area of the right hippocampus was grossly dissected out and stored at −80 °C for later use.
Tissue homogenisation and protein estimation
Hippocampal tissue was homogenised with 200 μl lysis buffer as described (Zagaar et al., 2012). Then, the samples were sonicated 3 times, 5 s/time (Vibra cell, Sonics and Materials Inc., USA). A microBCA assay kit was used to estimate the amount of protein in each sample (Pierce Chemical Rockford, USA).
Immunoblotting and detection
Blotting procedures were carried out as reported (Zagaar et al., 2012, 2013; Dao et al., 2013). Briefly, the samples (approximately 15 μg of total protein per sample) were processed using a high throughput E-PAGE 48 system (Invitrogen Corp. Carlsbad, USA). The proteins were transferred onto a PVDF membrane via a dry blot system (Invitrogen Corp. Carlsbad, USA) and detected with specific primary antibodies and subsequent conjugation with secondary horseradish peroxidase antibodies. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control. Antibody dilution was as follows: mouse monoclonal anti-p-CaMKII (1:500); rabbit polyclonal anti- t-CaMKII (1:1000); rabbit polyclonal anti-BDNF (1:500); rabbit polyclonal anti-PP2B (1:1000); rabbit polyclonal anti-GAPDH (1:1000); secondary anti-mouse/rabbit antibodies (1:5000). All antibodies were purchased from Santa Cruz Technology except GAPDH antibody, which was purchased from Cell Signalling Inc., Boston, USA. The protein bands were visualised using commercial chemiluminescence reagents (Santa Cruz Biotechnology) and AlphaEase software and quantified by densitometry.
Statistical analysis
Unpaired t-test was used to compare two groups while one-way analysis of variance (ANOVA) followed by Tukey post-hoc test was used to compare all groups. All statistical analyses were done with GraphPad Prism software. p<0.05 indicates statistical significance. Data were expressed as mean±S.E.M.
Results
Non-cognitive disturbances caused by AD pathology were totally prevented by regular treadmill exercise
The open field test
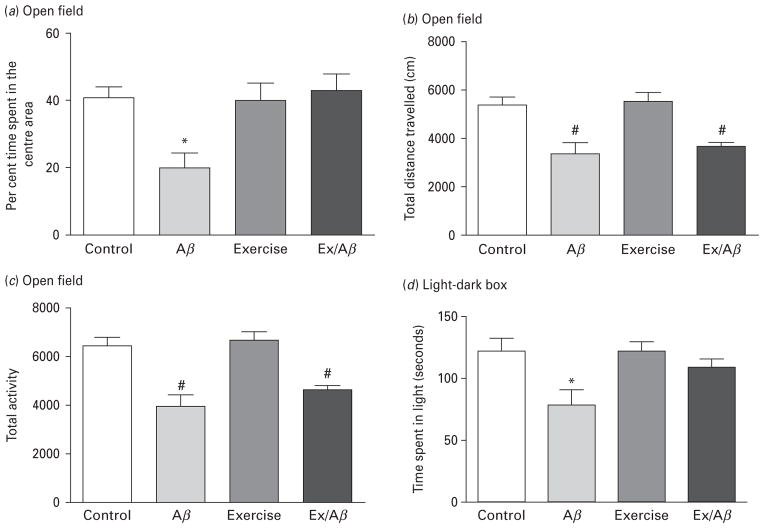
In the OF apparatus, the time a rat spent in the centre area is a measure of anxiety levels. Time spent in the centre area indicates high exploratory behaviours and low anxiety levels. In this study, the Aβ rats spent an average of 20.19±4.43 per cent time in the centre area, which was significantly shorter than the control rats (40.87±3.38%), exercise (40.07±5.41%), Ex/Aβ (43.40± 4.61%) (p=0.05–0.01) (Fig. 1a). Thus, it seems that regular exercise exerts an anxiolytic effect in our AD model. Also, in the OF apparatus the rat’s locomotor activity is determined by total distance travelled and total activity. The Aβ rats travelled an average distance of 3371.97±426.33 cm, which was similar to that of Ex/Aβ rats (3658.55± 155.28 cm), but statistically different from the control and exercise rats (control: 5386.46±309.94 cm, exercise: 5526.84±348.04 cm, p=0.01–0.001) (Fig. 1b). A similar trend was observed with total activity in the OF, where rats from Aβ and Ex/Aβ groups exhibited the same level of locomotor activity, but significantly different from those of control and exercise groups (p=0.01–0.001) (Fig. 1c). These data indicate that locomotor activity is impaired in AD model and this impairment was not prevented by our regimen of regular exercise.
Fig. 1.
Alzheimer’s disease (AD) pathology produced increased anxiety, which was prevented by moderate treadmill exercise. Rats were subjected to non-cognitive tests including open field (OF) apparatus (a–c) and light-dark (LD) box (d). Aβ rats spent significantly less time in the centre area in the OF test (a) and the light area of the LD box (d) compared to control, exercise and Ex/Aβ rats. The reduction in the locomotor activity was not prevented with exercise as Aβ and Ex/Aβ rats showed similar reduced total distance travelled (b) and total activity (c) compared to control and exercise rats. (*) denotes significant difference from all groups, (#) indicates significant difference from control and exercise groups (p<0.05, 8–10 rats/group).
The light-dark compartment test
We have also utilised the light-dark box apparatus to test anxiety-like behaviours. Because rodents are nocturnal, they have a tendency to stay in dark areas. Therefore, more time spent in the light area indicates less anxiety (Salim et al., 2010; Vollert et al., 2011). In our study, Aβ rats spent a significant amount of the entire testing time in the dark box compared to all other groups. Control, exercise and Ex/Aβ groups spent a similar length of time in the light compartment exploring, while Aβ rats spent significantly less time in the light area (control: 121.74± 10.50 s, Aβ: 69.80± 10.67 s, exercise: 123.70±8.18 s, Ex/Aβ: 108.00±6.39 s, p=0.05–0.01) (Fig. 1d). These results indicate that anxiety in AD model was alleviated by regular exercise.
The elevated plus maze (EPM) test
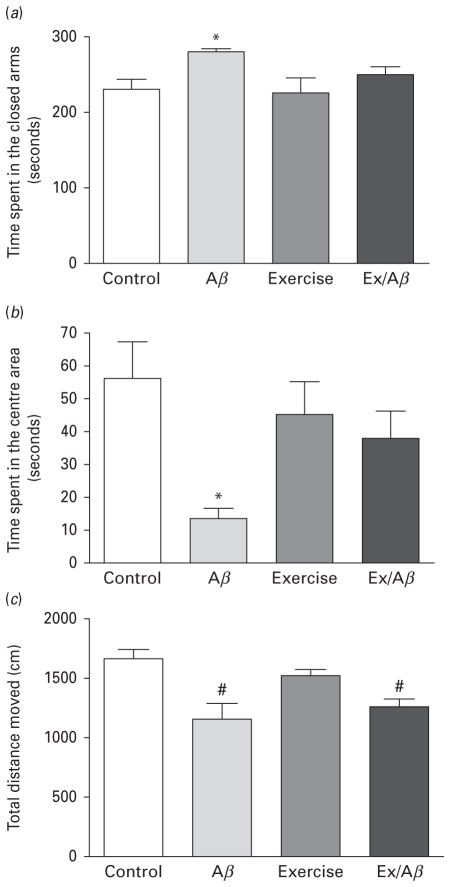
The EPM is used as a simple assay for assessing anxiety based on the natural aversion of rodents to elevated open areas (Xiang et al., 2011). Duration of time spent in the closed arms and centre area measures anxiety levels. Consistent with our findings in the OF paradigm, AD rats experienced increased anxiety as they spent significantly more time in the closed arms (control: 230.22± 13.62 s, Aβ: 279.78±4.33 s, exercise: 225.37±19.49 s, Ex/Aβ: 250.36±10.18 s, p=0.01–0.02) (Fig. 2a) and much less time in the centre area (control: 56.25±10.69 s, Aβ: 13.42±3.12 s, exercise: 45.07±9.97 s, Ex/Aβ: 37.90±8.22, p=0.05–0.002) (Fig. 2b) compared to all other groups. Additionally, the EPM data showed that the locomotor activity of Aβ-infused rats was impaired and our exercise regimen did not prevent this impairment as the Aβ and Ex/Aβ rats travelled similar distances during the whole experiment (Aβ: 1152.23±134.25 cm, Ex/Aβ: 1260.22± 71.28 cm), and was significantly different than the control and exercise rats (control: 1662.17±84.19 cm, exercise: 1528.57±48.67 cm, p =0.01–0.03) (Fig. 2c).
Fig. 2.
Elevated plus maze (EPM) exploration in Aβ rats with and without exercise training. (a): Time spent in the closed arms, (b): Time spent in the centre area, (c): Total distance travelled. Aβ rats showed increased anxiety-like behaviours as indicated by significantly longer time spent in the closed arms and shorter time in the centre area compared to control, exercise, and Ex/Aβ rats. The impaired locomotor activity of Aβ rats (shorter distance travelled) was not prevented by treadmill exercise. (*) p<0.05 compared to all groups, (#) p<0.05 compared to control and exercise groups, 8–10 rats/group.
Regular treadmill exercise prevented AD-induced impaired basal synaptic transmission
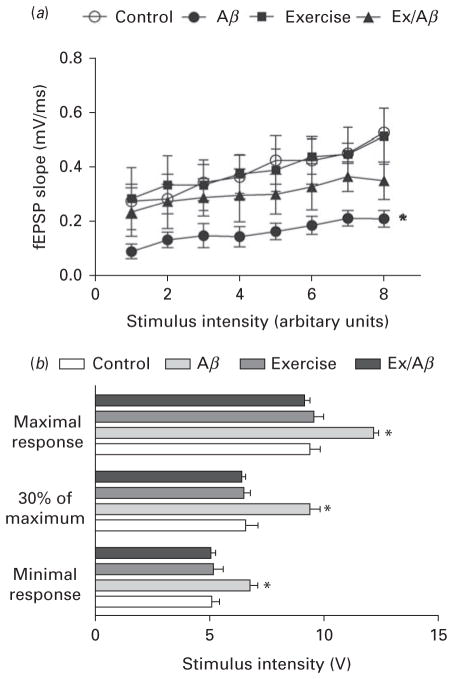
We evaluated the effect of AD pathology and/or exercise on basal synaptic transmission in CA1 area by constructing input-output (I/O) curves using extracellular recording. The I/O curve changes when synaptic strength is altered (Alzoubi et al., 2010, 2011). Our data indicated that Aβ rats exhibited impaired basal synaptic transmissions in area CA1. In all groups, as the stimulus intensity increased, the fEPSP slope increased. However, the I/O curve of Aβ rats was shifted to the right with a significantly lower fEPSP slope at all stimulus intensities compared to all other groups (p=0.01) (Fig. 3a). For instance, at minimal intensity, the fEPSP slope of Aβ rats was 0.09±0.01 mV/ms, which was markedly lower compared to other groups (control: 0.28±0.03 mV/ms, Ex: 0.29±0.05 mV/ms, Ex/Aβ: 0.24± 0.04 mV/ms).
Fig. 3.
Basal synaptic transmission is impaired in CA1 area of Aβ rats and this impairment is prevented by moderate treadmill exercise. (a): the input-output (I/O) curves are evoked by gradual increases in stimulus intensity. The right side shift of the I/O curve of Aβ rats indicates impaired basal synaptic transmission (b): Stimulus intensity required to produce minimal, 30% of maximum, and maximal response. Aβ rats required a significantly stronger voltage to elicit the same response compared to control, exercise, and Ex/Aβ rats. (*) indicates significant difference compared to other groups at all stimulus intensities. Values are mean±S.E.M., n=4–6 rats/ group.
The basal synaptic transmission in area CA1 in various groups was further assessed by measuring the magnitude of the voltage required for eliciting the minimal (30% of maximal) and maximal responses. As illustrated in Fig. 3b, Aβ rats required a significantly higher voltage to produce the same response in CA1 area with control, exercise and Ex/Aβ rats at minimal and maximal intensities (p=0.001–0.01). A mean voltage of 6.77±0.30 mV was required to evoke minimal response in Aβ rats while Ex/Aβ rats required 5.06±0.17 mV to produce the same response, which was similar to the control (5.08±0.34 mV) and exercise (5.16±0.44 mV) rats (Fig. 3b).
Regular treadmill exercise prevents reduction in basal levels of p-CaMKII in AD rat model
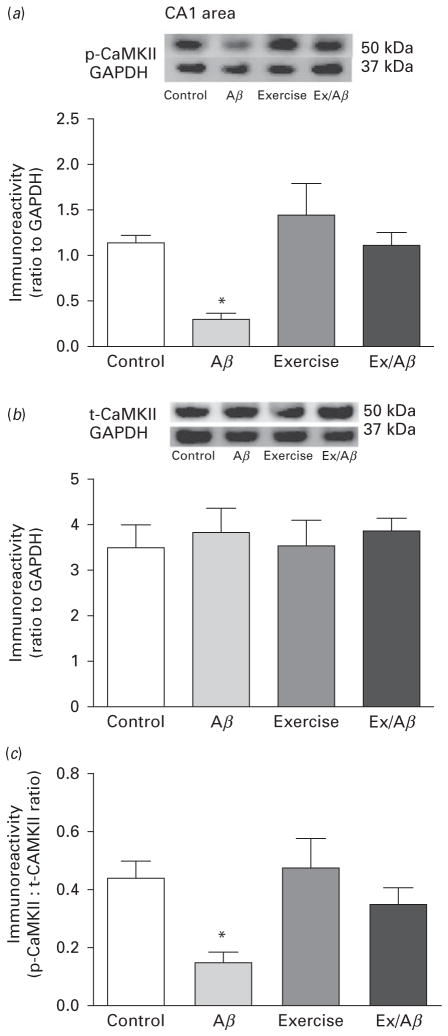
Calcium/calmodulin dependent protein kinase II (CaMKII) has been extensively studied as a key signalling molecule in synaptic plasticity and cognition. (Cammarota et al., 2002); however, the role of CaMKII in modulating non-cognitive functions is not well defined. One recent study showed that connexin36 deficient mice displayed enhanced anxiety accompanied by a reduction of CaMKII protein levels in the striatum (Zlomuzica et al., 2012). Our data revealed that the basal levels of phosphorylated (p-) CaMKII, an active form of CaMKII, in CA1 area were significantly reduced compared to other groups (control: 1.14±0.08, Aβ: 0.29± 0.07, exercise: 1.44±0.35, Ex/Aβ: 1.11±0.14, p=0.05–0.01) (Fig. 4a). The basal levels of total CaMKII (t-CaMKII) remained unchanged across all groups (control: 3.52± 0.483, Aβ: 3.859±0.53, exercise: 3.576±0.529, Ex/Aβ: 3.887±0.297) (Fig. 4b). As a result, the ratio of p-CaMKII to t-CaMKII of Aβ rats was significantly lower than those of control, exercise and Ex/Aβ rats (control: 0.44± 0.06, Aβ: 0.15±0.04, exercise: 0.48±0.10, Ex/Aβ: 0.35± 0.06, p=0.05) (Fig. 4c).
Fig. 4.
Basal levels of p-CaMKII (a), t-CaMKII (b), and p-CaMKII/t-CaMKII ratio (c) in CA1 area. The basal levels of p-CaMKII in Aβ rats were significantly reduced compared to other groups while the levels of t-CaMKII were similar across all groups. Thus, the p-CaMKII/t-CaMKII ratio of Aβ rats was significantly smaller than those of control, exercise, and Ex/Aβ rats, which indicated an impaired phosphorylation process. (*) indicates significant difference from control, exercise, and Ex/Aβ, p=0.05–0.01. Values are mean±S.E.M., n=4–6 rats/group. Insets are representative blots.
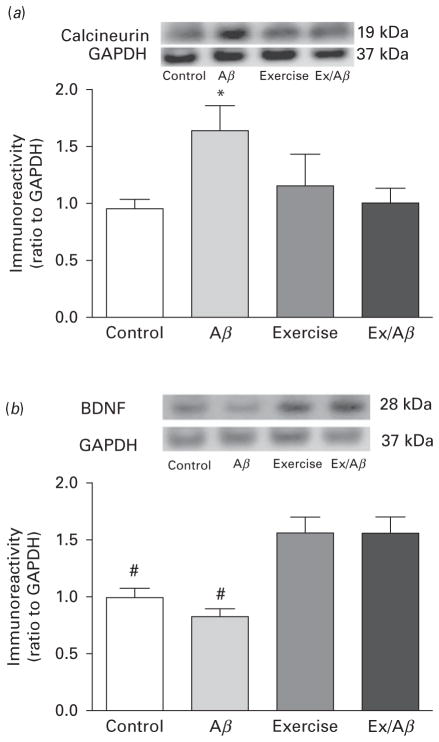
The Aβ-induced aberrant increase in basal levels of calcineurin (PP2B) was normalised in Aβ/Ex rats
Calcineurin (PP2B) is a phosphatase that inactivates CaMKII, thus returning the constitutive activity of CaMKII back to normal. In our Aβ AD model, the basal levels of PP2B were markedly up-regulated (1.65±0.216) in area CA1 (Fig. 5a). Nevertheless, 4 wk of treadmill exercise prevented the abnormal increase of PP2B in Aβ rats, as the basal levels of Ex/Aβ rats (1.011±0.12) were similar to those of the control and exercise rats (control: 0.96± 0.08, exercise: 0.99±0.07) (Fig. 5a).
Fig. 5.
Basal levels of calcineurin (a) and BDNF (b) in CA1 area. Exogenous administration of Aβ1–42 peptides increased the basal levels of calcineurin protein but did not affect BDNF protein levels. Moderate treadmill exercise prevented the aberrant up-regulation of calcineurin induced in Aβ rats as its basal levels in Ex/Aβ rats are similar to those of control and exercise rats. The basal levels of BDNF in exercised rats including Ex/Aβ rats were significantly increased compared to those of Aβ and control rats. (*) indicates significant difference compared to all groups (p<0.05). (#) indicates significant difference compared to control and exercise groups (p<0.05–0.01). Values are mean±S.E.M., n=4–6 rats/group. Insets are representative blots.
Basal levels of BDNF were highly up-regulated in exercised rats
Brain-derived neurotrophic factor (BDNF) can exert a pleiotropic effect in the central nervous system as it can affect neuronal growth, synaptic transmission and plasticity and pathogenesis of psychiatric disorders (Hong et al., 2011). While the basal levels of BDNF protein in Aβ rats were not significantly different than control rats, those of both exercise and Ex/Aβ rats were significantly increased compared to that of control rats (control: 1.00±0.05, Aβ: 0.83±0.07, exercise: 1.57±0.14, Ex/Aβ: 1.57±0.14, p=0.01) (Fig. 5b). These data indicate that exercise-induced elevated levels of BDNF might be beneficial to prevent behavioural disturbances caused by AD pathology.
Discussion
Even though AD is considered to be mainly a learning and memory disorder, non-cognitive functions are also negatively affected at all stages of the disease. In addition to the progressive memory loss, the clinical population of AD patients may also show various symptoms ranging from olfactory impairment, gait and balance dysfunction to psychiatric problems such as depression, anxiety, aggression, disinhibition, hallucinations and delusions (Raudino, 2013). Thus, reproducing an AD model in animals is a challenging task. Nevertheless, most studies have successfully recapitulated the cognitive impairment but only sparingly addressed the non-cognitive disturbances of the disease in animal models. In this study, we report neuropsychiatric symptoms (i.e. anxiety) in a rat model of sporadic AD achieved by exogenous administration of amyloid peptides. In agreement with our findings, other non-transgenic and transgenic studies of 3×Tg-AD mice have also reported depression-like behaviour, increased anxiety and apathy (Filali et al., 2012; Chen et al., 2013).
Our data revealed that AD pathology produced non-cognitive disturbances and these detrimental effects were prevented by prior regular exercise. Ample evidence suggests regular exercise is beneficial for learning and memory loss caused by sleep deprivation, maternal deprivation stress, addiction and toxic chemical insults (Vollert et al., 2011; Zagaar et al., 2012, 2013; Dimatelis et al., 2013; Lynch et al., 2013). A recent study showed that in 3×Tg-AD mouse model of AD, voluntary exercise prevented anxiety, lack of exploration and emotionality (Garcia-Mesa et al., 2012). However, the mechanism by which exercise exerts a neuroprotective effect on AD brains remains unclear. To this extent, it has been postulated that by reducing the AD-induced oxidative stress burden (i.e. decreasing abnormal elevated lipid peroxidation), exercise ameliorates non-cognitive symptoms associated with the disease (Cakir et al., 2010; Garcia-Mesa et al., 2012).
The present findings revealed that regular treadmill exercise produced a positive effect on our AD model in several tests of anxiety as shown in the open field apparatus, light-dark box and elevated plus maze paradigm. The exercise-alleviated behavioural and psychiatric symptoms seen in our AD model are accompanied by a normalised basal synaptic transmission and prevention of deleterious alterations in the levels of important signalling molecules such as phosphorylated CaMKII, calcineurin and BDNF. These experimental data correspond well with the anxiolytic effects of exercise in AD patients reported in the clinical literature (Teri et al., 2003; Williams and Tappen, 2007, 2008).
In addition to the non-cognitive disturbances caused by amyloid infusion, electrophysiological recordings in CA1 pyramidal cells reveal that 2 wk infusion of Aβ1–42 shifts the input/out (I/O) curves of Aβ rats to the right side indicating impaired basal synaptic transmissions. Also, these Schaffer collaterals synapses require much higher voltage to elicit the same response in controls. In agreement with our findings, studies in transgenic AD mice (Tg2576, AβPPPS1-21 and Tau22) show learning and memory deficits and impairment of the prefrontal and perirhinal cortex synaptic plasticity (Tamagnini et al., 2012, Lo et al., 2013). Furthermore, clinical studies in AD patients demonstrate an impaired long-term potentiation (LTP)-like response in cortical areas (Koch et al., 2012) and this synaptic dysfunction was also observed in other brain regions (e.g. hippocampus) in various animal models of AD (Nalbantoglu et al., 1997; Chapman et al., 1999; Stephan et al., 2001, Srivareerat et al., 2009).
It is noteworthy that our exercise regimen does not seem to alter basal synaptic transmission in cognitively normal rats as neither the I/O curve nor fEPSP slopes measured in CA1 area was affected by exercise training. However, even though it is not significant, there is a trend showing that exercise seems to lower the voltage in normal rats to produce minimal and maximal responses. Nevertheless, our data indicate that 4 wk of moderate treadmill exercise protects the Schaffer collaterals synapses against the deleterious effect of AD pathology. For example, the right side shift of the I/O curve observed in the Aβ rats is not seen in animals with exercise training inasmuch as the I/O curve of Ex/Aβ rats is similar to that of control rats. Furthermore, the voltages required to elicit minimal and maximal response in Ex/Aβ and control rats are not different. Perhaps by increasing synaptic excitability and fostering synaptic connections between neurons, exercise prepares the hippocampal synapses to withstand amyloid toxicity.
What possible mechanisms might account for the protective effects of exercise against brain function impairment caused by amyloid peptides? One of the potential candidates is probably preservation of molecules that exert effects on both the peripheral and central nervous system; cardinal among these molecules is CaMKII, particularly the α-subtype. Even though α-CaMKII is the most abundant protein found in the brain, it is also present in skeletal muscle with a seemingly non-functional kinase role (Chin, 2004). Additionally, it is well documented that α-CaMKII plays a key role in learning and memory processes as well as long-term potentiation (LTP) induction and persistency (Sanhueza and Lisman, 2013). Genetic knockouts of the α-CaMKII gene result in cognitive deficits and LTP blockage (Silva et al., 1992; Stevens et al., 1994; Matsuo et al., 2009). Mice that are heterozygous for a null mutation of α-CaMKII exhibited both memory impairment and behaviour indicating mood change (Yamasaki et al., 2008). In our study, we reported a significant reduction in levels of CaMKII in the CA1 area of Aβ rats. This alteration was abolished in Ex/Aβ rats. In agreement with our finding, other studies have also indicated that regular exercise increased expression of hippocampal CaMKII mRNA in rats (Egan et al., 2010) and CaMKII expression and activity in human skeletal muscles (Rose et al., 2007). These exercise-induced molecular effects seem to translate into an ability of exercise to prevent anxiety caused by AD. Interestingly, over-expression of α-CaMKII in the mouse forebrain produced increased anxiety coupled with offensive aggression (Hasegawa et al., 2009).
BDNF, a member of the nerve growth factor family, is a critical molecule responsible for neuronal development and synaptic plasticity (Leal et al., 2013). The Aβ rats did not show a reduction in the basal levels of BDNF indicating a possible compensatory mechanism for the initial effect of toxic amyloid peptides. However, in our study the exercised groups, including exercise alone and Ex/Aβ rats, showed a significant elevation in the basal levels of BDNF in CA1 area compared to those of the sedentary control. The pleiotropic effects of exercise-induced BDNF are well documented, thus BDNF might be a potential mechanism behind the neuroprotective effects of exercise in AD. BDNF is produced in peripheral tissues (Lommatzsch et al., 1999) and crosses the blood-brain barrier (Poduslo and Curran, 1996) where it probably acts synergistically with available BDNF in the central nervous system to produce a beneficial effect on brain function.
Even though the exact mechanisms by which exercise exerts a beneficial effect on brain function are still under investigation, findings from our study provide valuable insights into molecular pathways that could be involved in both AD and regular exercise. In short, it can be concluded that regular treadmill exercise prevented both cognitive and non-cognitive deficits associated with AD. Thus, it is possible that exercise can be used as a therapeutic approach against diseases that are associated with memory problems and/or anxiety symptoms.
Acknowledgments
This study was funded by a number of SGP grants from the University of Houston (KA) and the National Institutes of Health (1R15AG039008; JLE) and (NIH R15 G103327, SS).
Footnotes
Statement of Interest
The authors disclose no conflicts of biomedical or financial interest.
References
- Aleisa AM, Alzoubi KH, Alkadhi KA. Chronic but not acute nicotine treatment reverses stress-induced impairment of LTP in anesthetized rats. Brain Res. 2006;1097:78–84. doi: 10.1016/j.brainres.2006.04.070. [DOI] [PubMed] [Google Scholar]
- Alzoubi KH, Aleisa AM, Alkadhi KA. In vivo expression of ganglionic long-term potentiation in superior cervical ganglia from hypertensive aged rats. Neurobiol Aging. 2010;31:805–812. doi: 10.1016/j.neurobiolaging.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Alzoubi KH, Alhaider IA, Tran TT, Mosely A, Alkadhi KK. Impaired neural transmission and synaptic plasticity in superior cervical ganglia from beta-amyloid rat model of Alzheimer’s disease. Curr Alzheimer Res. 2011;8:377–384. doi: 10.2174/156720511795745311. [DOI] [PubMed] [Google Scholar]
- Araujo J, Maximino C, de Brito TM, da Silva AWB, Oliveira KRM, de Jesus Oliveira Batista E, Morato S, Herculano AM, Gouveia A., Jr Behavioural and pharmacological aspects of anxiety in the light/dark preference test. Neuromethods. 2012;66:191–202. [Google Scholar]
- Bartley CA, Hay M, Bloch MH. Meta-analysis: aerobic exercise for the treatment of anxiety disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45C:34–39. doi: 10.1016/j.pnpbp.2013.04.016. [DOI] [PubMed] [Google Scholar]
- Bechara RG, Kelly AM. Exercise improves object recognition memory and induces BDNF expression and cell proliferation in cognitively enriched rats. Behav Brain Res. 2013;245:96–100. doi: 10.1016/j.bbr.2013.02.018. [DOI] [PubMed] [Google Scholar]
- Broman-Fulks JJ, Storey KM. Evaluation of a brief aerobic exercise intervention for high anxiety sensitivity. Anxiety Stress Coping. 2008;21:117–128. doi: 10.1080/10615800701762675. [DOI] [PubMed] [Google Scholar]
- Broman-Fulks JJ, Berman ME, Rabian BA, Webster MJ. Effects of aerobic exercise on anxiety sensitivity. Behav Res Ther. 2004;42:125–136. doi: 10.1016/S0005-7967(03)00103-7. [DOI] [PubMed] [Google Scholar]
- Cakir B, Kasimay O, Kolgazi M, Ersoy Y, Ercan F, Yegen BC. Stress-induced multiple organ damage in rats is ameliorated by the antioxidant and anxiolytic effects of regular exercise. Cell Biochem Funct. 2010;28:469–479. doi: 10.1002/cbf.1679. [DOI] [PubMed] [Google Scholar]
- Cammarota M, Bevilaqua LR, Viola H, Kerr DS, Reichmann B, Teixeira V, Bulla M, Izquierdo I, Medina JH. Participation of CaMKII in neuronal plasticity and memory formation. Cell Mol Neurobiol. 2002;22:259–267. doi: 10.1023/A:1020763716886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, Younkin SG, Hsiao KK. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- Chen Y, Liang Z, Blanchard J, Dai CL, Sun S, Lee MH, Grundke-Iqbal I, Iqbal K, Liu F, Gong CX. A non-transgenic mouse model (icv-STZ mouse) of Alzheimer’s disease: similarities to and differences from the transgenic model (3×Tg-AD mouse) Mol Neurobiol. 2013;47:711–725. doi: 10.1007/s12035-012-8375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin ER. The role of calcium and calcium/calmodulin-dependent kinases in skeletal muscle plasticity and mitochondrial biogenesis. Proc Nutr Soc. 2004;63:279–286. doi: 10.1079/PNS2004335. [DOI] [PubMed] [Google Scholar]
- Dao AT, Zagaar MA, Levine AT, Salim S, Eriksen JL, Alkadhi KA. Treadmill exercise prevents learning and memory impairment in Alzheimer’s disease-like pathology. Curr Alzheimer Res. 2013;10:507–515. doi: 10.2174/1567205011310050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimatelis JJ, Hendricks S, Hsieh J, Vlok NM, Bugarith K, Daniels WM, Russell VA. Exercise partly reverses the effect of maternal separation on hippocampal proteins in 6-hydroxydopamine-lesioned rat brain. Exp Physiol. 2013;98:233–244. doi: 10.1113/expphysiol.2012.066720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan B, Carson BP, Garcia-Roves PM, Chibalin AV, Sarsfield FM, Barron N, McCaffrey N, Moyna NM, Zierath JR, O’Gorman DJ. Exercise intensity-dependent regulation of peroxisome proliferator-activated receptor coactivator-1 mRNA abundance is associated with differential activation of upstream signalling kinases in human skeletal muscle. J Physiol. 2010;588:1779–1790. doi: 10.1113/jphysiol.2010.188011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Weinstein AM, Lopez OL. Physical activity, brain plasticity, and Alzheimer’s disease. Arch Med Res. 2012;43:615–621. doi: 10.1016/j.arcmed.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filali M, Lalonde R, Theriault P, Julien C, Calon F, Planel E. Cognitive and non-cognitive behaviors in the triple transgenic mouse model of Alzheimer’s disease expressing mutated APP, PS1, and Mapt (3×Tg-AD) Behav Brain Res. 2012;234:334–342. doi: 10.1016/j.bbr.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Garcia-Mesa Y, Gimenez-Llort L, Lopez LC, Venegas C, Cristofol R, Escames G, Acuna-Castroviejo D, Sanfeliu C. Melatonin plus physical exercise are highly neuroprotective in the 3×Tg-AD mouse. Neurobiol Aging. 2012;33:1124. e1113, 1129. doi: 10.1016/j.neurobiolaging.2011.11.016. [DOI] [PubMed] [Google Scholar]
- Gould T, Dao DT, Kovacsics CE. The open field test. Neuromethods. 2009;42:1–20. [Google Scholar]
- Hasegawa S, Furuichi T, Yoshida T, Endoh K, Kato K, Sado M, Maeda R, Kitamoto A, Miyao T, Suzuki R, Homma S, Masushige S, Kajii Y, Kida S. Transgenic up-regulation of alpha-CaMKII in forebrain leads to increased anxiety-like behaviors and aggression. Mol Brain. 2009;2:6. doi: 10.1186/1756-6606-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong CJ, Liou YJ, Tsai SJ. Effects of BDNF polymorphisms on brain function and behavior in health and disease. Brain Res Bull. 2011;86:287–297. doi: 10.1016/j.brainresbull.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Hosseini N, Alaei H, Reisi P, Radahmadi M. The effect of treadmill running on passive avoidance learning in animal model of Alzheimer disease. Int J Prev Med. 2013;4:187–192. [PMC free article] [PubMed] [Google Scholar]
- Itoh A, Nitta A, Nadai M, Nishimura K, Hirose M, Hasegawa T, Nabeshima T. Dysfunction of cholinergic and dopaminergic neuronal systems in beta-amyloid protein–infused rats. J Neurochem. 1996;66:1113–1117. doi: 10.1046/j.1471-4159.1996.66031113.x. [DOI] [PubMed] [Google Scholar]
- Khabour OF, Alzoubi KH, Alomari MA, Alzubi MA. Changes in spatial memory and BDNF expression to simultaneous dietary restriction and forced exercise. Brain Res Bull. 2013;90:19–24. doi: 10.1016/j.brainresbull.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Koch G, Di Lorenzo F, Bonni S, Ponzo V, Caltagirone C, Martorana A. Impaired LTP- but not LTD-like cortical plasticity in Alzheimer’s disease patients. Journal of Alzheimer’s disease JAD. 2012;31:593–599. doi: 10.3233/JAD-2012-120532. [DOI] [PubMed] [Google Scholar]
- Leal G, Comprido D, Duarte CB. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.04.005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Lo AC, Iscru E, Blum D, Tesseur I, Callaerts-Vegh Z, Buee L, De Strooper B, Balschun D, D’Hooge R. Amyloid and Tau Neuropathology Differentially Affect Prefrontal Synaptic Plasticity and Cognitive Performance in Mouse Models of Alzheimer’s Disease. Journal of Alzheimer’s disease JAD. 2013;37(1):109–25. doi: 10.3233/JAD-122296. [DOI] [PubMed] [Google Scholar]
- Lommatzsch M, Braun A, Mannsfeldt A, Botchkarev VA, Botchkareva NV, Paus R, Fischer A, Lewin GR, Renz H. Abundant production of brain-derived neurotrophic factor by adult visceral epithelia. Implications for paracrine and target-derived neurotrophic functions. Am J Pathol. 1999;155:1183–1193. doi: 10.1016/S0002-9440(10)65221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA. Exercise as a novel treatment for drug addiction: a neurobiological and stage-dependent hypothesis. Neurosci Biobehav Rev. 2013;37(8):1622–44. doi: 10.1016/j.neubiorev.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo N, Yamasaki N, Ohira K, Takao K, Toyama K, Eguchi M, Yamaguchi S, Miyakawa T. Neural activity changes underlying the working memory deficit in alpha-CaMKII heterozygous knockout mice. Front Behav Neurosci. 2009;3:20. doi: 10.3389/neuro.08.020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbantoglu J, Tirado-Santiago G, Lahsaini A, Poirier J, Goncalves O, Verge G, Momoli F, Welner SA, Massicotte G, Julien JP, Shapiro ML. Impaired learning and LTP in mice expressing the carboxy terminus of the Alzheimer amyloid precursor protein. Nature. 1997;387:500–505. doi: 10.1038/387500a0. [DOI] [PubMed] [Google Scholar]
- Nitta A, Itoh A, Hasegawa T, Nabeshima T. beta-Amyloid protein-induced Alzheimer’s disease animal model. Neurosci Lett. 1994;170:63–66. doi: 10.1016/0304-3940(94)90239-9. [DOI] [PubMed] [Google Scholar]
- Poduslo JF, Curran GL. Permeability at the blood-brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Brain Res Mol Brain Res. 1996;36:280–286. doi: 10.1016/0169-328x(95)00250-v. [DOI] [PubMed] [Google Scholar]
- Radahmadi M, Alaei H, Sharifi MR, Hosseini N. The effect of synchronized running activity with chronic stress on passive avoidance learning and body weight in rats. Int J Prev Med. 2013;4:430–437. [PMC free article] [PubMed] [Google Scholar]
- Raudino F. Non-cognitive symptoms and related conditions in the Alzheimer’s disease: a literature review. Neurological sciences: official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2013;38(8):1275–82. doi: 10.1007/s10072-013-1424-7. [DOI] [PubMed] [Google Scholar]
- Rose AJ, Frosig C, Kiens B, Wojtaszewski JF, Richter EA. Effect of endurance exercise training on Ca2+ calmodulin-dependent protein kinase II expression and signalling in skeletal muscle of humans. J Physiol. 2007;583:785–795. doi: 10.1113/jphysiol.2007.138529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim S, Asghar M, Chugh G, Taneja M, Xia Z, Saha K. Oxidative stress: a potential recipe for anxiety, hypertension and insulin resistance. Brain Res. 2010;1359:178–185. doi: 10.1016/j.brainres.2010.08.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanhueza M, Lisman J. The CaMKII/NMDAR complex as a molecular memory. Mol Brain. 2013;6:10. doi: 10.1186/1756-6606-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Paylor R, Wehner JM, Tonegawa S. Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- Souza LC, Filho CB, Goes AT, Fabbro LD, de Gomes MG, Savegnago L, Oliveira MS, Jesse CR. Neuroprotective effect of physical exercise in a mouse model of Alzheimer’s disease induced by beta-Amyloid1-40 peptide. Neurotox Res. 2013;24:148–163. doi: 10.1007/s12640-012-9373-0. [DOI] [PubMed] [Google Scholar]
- Srivareerat M, Tran TT, Alzoubi KH, Alkadhi KA. Chronic psychosocial stress exacerbates impairment of cognition and long-term potentiation in beta-amyloid rat model of Alzheimer’s disease. Biol Psychiatry. 2009;65:918–926. doi: 10.1016/j.biopsych.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Stephan A, Laroche S, Davis S. Generation of aggregated beta-amyloid in the rat hippocampus impairs synaptic transmission and plasticity and causes memory deficits. The Journal of neuroscience. 2001;21:5703–5714. doi: 10.1523/JNEUROSCI.21-15-05703.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CF, Tonegawa S, Wang Y. The role of calcium-calmodulin kinase II in three forms of synaptic plasticity. Curr Biol. 1994;4:687–693. doi: 10.1016/s0960-9822(00)00153-6. [DOI] [PubMed] [Google Scholar]
- Suijo K, Inoue S, Ohya Y, Odagiri Y, Takamiya T, Ishibashi H, Itoh M, Fujieda Y, Shimomitsu T. Resistance exercise enhances cognitive function in mouse. Int J Sports Med. 2013;34:368–375. doi: 10.1055/s-0032-1323747. [DOI] [PubMed] [Google Scholar]
- Tamagnini F, Burattini C, Casoli T, Balietti M, Fattoretti P, Aicardi G. Early impairment of long-term depression in the perirhinal cortex of a mouse model of Alzheimer’s disease. Rejuvenation research. 2012;15:231–234. doi: 10.1089/rej.2011.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teri L, Gibbons LE, McCurry SM, Logsdon RG, Buchner DM, Barlow WE, Kukull WA, LaCroix AZ, McCormick W, Larson EB. Exercise plus behavioral management in patients with Alzheimer disease: a randomized controlled trial. JAMA. 2003;290:2015–2022. doi: 10.1001/jama.290.15.2015. [DOI] [PubMed] [Google Scholar]
- Vollert C, Zagaar M, Hovatta I, Taneja M, Vu A, Dao A, Levine A, Alkadhi K, Salim S. Exercise prevents sleep deprivation-associated anxiety-like behavior in rats: potential role of oxidative stress mechanisms. Behav Brain Res. 2011;224:233–240. doi: 10.1016/j.bbr.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Williams CL, Tappen RM. Effect of exercise on mood in nursing home residents with Alzheimer’s disease. Am J Alzheimer’s Dis Other Dement. 2007;22:389–397. doi: 10.1177/1533317507305588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, Tappen RM. Exercise training for depressed older adults with Alzheimer’s disease. Aging Mental Health. 2008;12:72–80. doi: 10.1080/13607860701529932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang X, Huang W, Haile CN, Kosten TA. Hippocampal GluR1 associates with behavior in the elevated plus maze and shows sex differences. Behav Brain Res. 2011;222:326–331. doi: 10.1016/j.bbr.2011.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki N, et al. Alpha-CaMKII deficiency causes immature dentate gyrus, a novel candidate endophenotype of psychiatric disorders. Mol Brain. 2008;1:6. doi: 10.1186/1756-6606-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagaar M, Alhaider I, Dao A, Levine A, Alkarawi A, Alzubaidy M, Alkadhi K. The beneficial effects of regular exercise on cognition in REM sleep deprivation: behavioral, electrophysiological and molecular evidence. Neurobiol Dis. 2012;45:1153–1162. doi: 10.1016/j.nbd.2011.12.039. [DOI] [PubMed] [Google Scholar]
- Zagaar M, Dao A, Levine A, Alhaider I, Alkadhi K. Regular exercise prevents sleep deprivation associated impairment of long-term memory and synaptic plasticity in the CA1 area of the hippocampus. Sleep. 2013;36:751–761. doi: 10.5665/sleep.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlomuzica A, Viggiano D, Degen J, Binder S, Ruocco LA, Sadile AG, Willecke K, Huston JP, Dere E. Behavioral alterations and changes in Ca/calmodulin kinase II levels in the striatum of connexin36 deficient mice. Behav Brain Res. 2012;226:293–300. doi: 10.1016/j.bbr.2011.08.028. [DOI] [PubMed] [Google Scholar]