Abstract
Objective
To use multimodality imaging to explore the relationship of biomarkers of inflammation, T-cell activation and monocyte activation with coronary calcification and subclinical vascular disease in a population of HIV-infected patients on antiretroviral therapy (ART).
Design
Cross-sectional.
Methods
A panel of soluble and cellular biomarkers of inflammation and immune activation was measured in 147 HIV-infected adults on ART with HIV RNA less than 1000 copies/ml and low-density lipoprotein cholesterol (LDL-C) 130 mg/dl or less. We examined the relationship of biomarkers to coronary calcium (CAC) score and multiple ultrasound measures of subclinical vascular disease.
Results
Overall, median (interquartile range, IQR) age was 46 (40–53) years; three-quarters of participants were male and two-thirds African-American. Median 10-year Framingham risk score was 6%. Participants with CAC more than 0 were older, less likely to be African-American and had higher current and lower nadir CD4+ T-cell counts. Most biomarkers were similar between those with and without CAC; however, soluble CD14 was independently associated with CAC after adjustment for traditional risk factors. Among those with a CAC score of zero, T-cell activation and systemic inflammation correlated with carotid intima–media thickness and brachial hyperemic velocity, respectively. Compared with normal participants and those with CAC only, participants with increasing degrees of subclinical vascular disease had higher levels of sCD14, hs-CRP and fibrinogen (all P<0.05).
Conclusion
Soluble CD14 is independently associated with coronary artery calcification, and, among those with detectable calcium, predicts the extent of subclinical disease in other vascular beds. Future studies should investigate the utility of multimodality imaging to characterize vascular disease phenotypes in this population.
Keywords: carotid intima–media thickness, coronary artery calcium, endothelial function, HIV, inflammation, microbial translocation, soluble CD14
Introduction
There is increasing evidence that chronic HIV infection is associated with an elevated risk of coronary heart disease [1,2]. The risk persists after adjustment for traditional risk factors and may be partially attributed to inflammation and residual immune dysregulation despite effective antiretroviral therapy (ART) [3–5]. The mechanisms of immune dysregulation in treated HIV infection are complex and include activation of the innate and adaptive immune system resulting from low-level viral replication [6], adipose tissue dysfunction [7], coinfections such as cytomegalovirus [8] and translocation of microbial products from a damaged gut [9]. Recent studies suggest that T-cell activation [10–12] and monocyte activation [13–16] are associated with vascular disease in HIV-infected patients.
Coronary artery calcification (CAC) detected by computed tomography (CT) is an accepted surrogate marker of coronary atherosclerosis that provides superior cardiovascular disease (CVD) risk discrimination than other surrogate markers in the general population [17]. A meta-analysis of older studies found no association of HIV infection with presence of CAC (pooled odds ratio 0.95) [18], though recent studies suggest that treated HIV-infected patients have higher calcium scores than matched controls [19,20]. Interestingly, HIV infection also appears to be associated with more noncalcified atherosclerotic plaque by CT angiography [16], suggesting that CAC alone may not detect the true burden of disease in this population. This is supported by another study in which over one-third of HIV-infected patients without detectable CAC had carotid atherosclerosis by ultrasound [20].
Biomarkers of more generalized inflammation such as high-sensitivity C-reactive protein (hs-CRP) or interleukin- 6 (IL-6) are strongly predictive of CVD events but are only weakly associated with CAC in the general population [21,22], suggesting that generalized inflammation may influence CVD risk primarily through alternative mechanisms (i.e. plaque rupture and thrombosis). In the HIV-infected population, biomarkers of generalized inflammation are strongly predictive of cardiovascular events [5], but these biomarkers have not been related to coronary calcification in published studies [16,19].
In this study, we therefore aimed, for the first time in HIV, to comprehensively examine the relationship of biomarkers of inflammation, T-cell activation and monocyte activation with CAC in a population of HIV-infected patients on modern ART. We further hypothesized that among patients with and without coronary artery calcium, these biomarkers would be related to the extent of subclinical disease in other vascular beds.
Materials and methods
Study design
This study is a cross-sectional analysis of 147 HIV-infected adults on ART who underwent comprehensive cardiometabolic risk assessment and immunophenotyping at the time of enrolment into the Stopping Atherosclerosis and Treating Unhealthy bone with RosuvastatiN in HIV (SATURN-HIV) trial. SATURN-HIV is a randomized, double-blind, placebo-controlled trial designed to measure the effect of rosuvastatin 10 mg daily on the progression of subclinical vascular disease. All individuals were at least 18 years of age, on stable ART for at least 12 weeks with HIV-1 RNA less than 1000 copies/ml, without known coronary disease or diabetes, no statin intake for at least the last 6 months and with a fasting low-density lipoprotein cholesterol (LDL-C) of 130 mg/dl or less. Additional entry criteria included evidence of heightened T-cell activation (CD8+CD38+HLA-DR+ ≥19%) and/or increased inflammation (hs-CRP ≥2 mg/l). The study was approved by the Institutional Review Board of University Hospitals Case Medical Center (Cleveland, Ohio, USA) and written informed consent was obtained from each study participant.
Study evaluations
Self-reported demographics and medical history were obtained along with a targeted physical examination, including height, weight, waist and hip measurements. Blood was drawn after at least a 12-h fast for glucose, insulin and lipoproteins. HOMA-IR was calculated from the fasting glucose and insulin measurements [23]. Ten-year Framingham Risk Score and Reynold’s Risk Score were calculated using published risk calculators [24–26]. HIV-1 RNA level and CD4+ cell count were obtained as part of routine clinical care. Trunk and limb fat were measured by whole-body dual-energy absorptiometry (DEXA) [27]. Epicardial and peri-aortic fat were measured by CT [10].
Coronary artery calcium score
All individuals had a baseline CT scan of the chest to quantify CAC burden. A 64-slice multidetector CT scanner (Somatom Sensation 64; Siemens Healthcare, Malvern, Pennsylvania, USA) was used with 30 × 0.6 mm collimation, 330 ms rotation time and 120 kV tube voltage. Three-millimetre slices were obtained from the carina to the diaphragm with prospective ECG gating at 60% of the R-R interval. Calcified coronary lesions were defined as areas of at least 6 pixels with density more than 130 Hounsfield units (HU). A single reader (R.G.) quantified total coronary calcium score using the Agatston method.
Immune activation and inflammation markers
Soluble plasma biomarkers of monocyte activation (soluble CD14 and CD163), systemic inflammation [hs-CRP, IL-6, tumour necrosis factor-α receptor I (sTNFR-I)], endothelial activation [soluble vascular cell adhesion molecule-1 (sVCAM-1)], coagulation (D-dimer and fibrinogen) and calcium homeostasis [osteoprotegerin (OPG) and receptor activator of nuclear factor-κB ligand (RANKL)] were measured. Soluble CD14 and soluble CD163 were measured by ELISA (R&D Systems, Minneapolis, Minnesota, USA). Inter-assay variability ranged from 0.4 to 8.6% for soluble CD14 and from 0.7 to 18.3% for sCD163. IL-6, sTNFR-I, sVCAM-1 and OPG were determined by quantitative sandwich ELISAs (R&D Systems and Biomedica, Vienna, Austria). Inter-assay variability ranged from 2.02 to 15.36%, from 3.66 to 5.77%, from 4.76 to 8.77%, and from 3.79 to 11.03, respectively. Hs-CRP and fibrinogen were determined by particle-enhanced immunonepholometric assays on a BNII nephelometer (Siemens, Munich, Germany). Inter-assay variability ranged from 3.01 to 6.46% and from 3.42 to 7.59%, respectively. D-dimer was determined by immuno-turbidometric assay on a STA-R Coagulation Analyser (DiagnosticaStago, Parsippany-Troy Hills, New Jersey, USA). Inter-assay variability ranged from 1.54 to 9.03%. RANKL was determined by singleplex immunoassay (Millipore, Billerica, Massachusetts, USA). Inter-assay variability ranged from 6.63 to 15.81%.
Monocyte and T-cells were phenotyped by flow cytometry as previously described [10,28]. CD4+ and CD8+ T-cell activation was defined as coexpression of CD38 and HLA-DR. Monocyte phenotype was determined by the relative expression of CD14, CD16 and surface tissue factor (TF).
Vascular ultrasound
A high-resolution B-mode ultrasound scan of the carotid arteries was performed using a Philips iU22 ultrasound system with an L9–3 MHz linear array transducer according to the consensus protocol of the American Society of Echocardiography [29]. Mean–mean and mean–max common carotid artery intima-media thickness (CCA-IMT) were measured as described previously [10]. Complete scans of the bilateral common carotid arteries (CCAs), internal carotid arteries (ICAs) and external carotid arteries (ECAs) were used to identify plaque, defined as IMT greater than 1.5 mm or more than 50% thicker than the adjacent vessel. Carotid atherosclerosis was defined as the presence of plaque or a mean–max CCA-IMT greater than 1.0 mm.
Brachial artery reactivity testing was performed using the same L9–3 MHz transducer and 5-min occlusion time according to a previously described AIDS Clinical Trials Group protocol [30] with omission of nitroglycerin-mediated dilation testing. Flow-mediated dilation (FMD) of the brachial artery was calculated as the percentage change in brachial artery diameter from baseline to 60 s postreactive hyperemia. The average velocity-time integral (VTI) of the third, fourth and fifth beats of reactive hyperemic flow after cuff release was used as a measure of microvascular function. Abnormal endothelial function was defined as having below median values of both FMD (<4%) and hyperemic VTI (<77 cm).
All vascular ultrasound measurements were performed offline by a single reader (C.T.L.) using semi-automated edge detection software (Medical Imaging Applications LLC, Coralville, Iowa, USA).
Statistical analysis
Baseline characteristics of participants were described using medians and interquartile ranges for continuous variables and frequencies and percentages for categorical ones. These characteristics were compared between the CAC present and absent groups using Wilcoxon signed rank tests or chi-square tests, as appropriate. The Wilcoxon test was also utilized to compare median values of biomarkers of inflammation and immune activation in the two CAC groups. Differences among median values of sCD14 in three CAC groups were assessed using the Kruskal–Wallis test. Correlations between biomarkers with measures of vascular structure and function in patients with and without detectable CAC levels were assessed using Spearman’s rho. The probability of CAC greater than 0 was modelled as a function of sCD14 and other covariates using binary multivariable logistic regression.
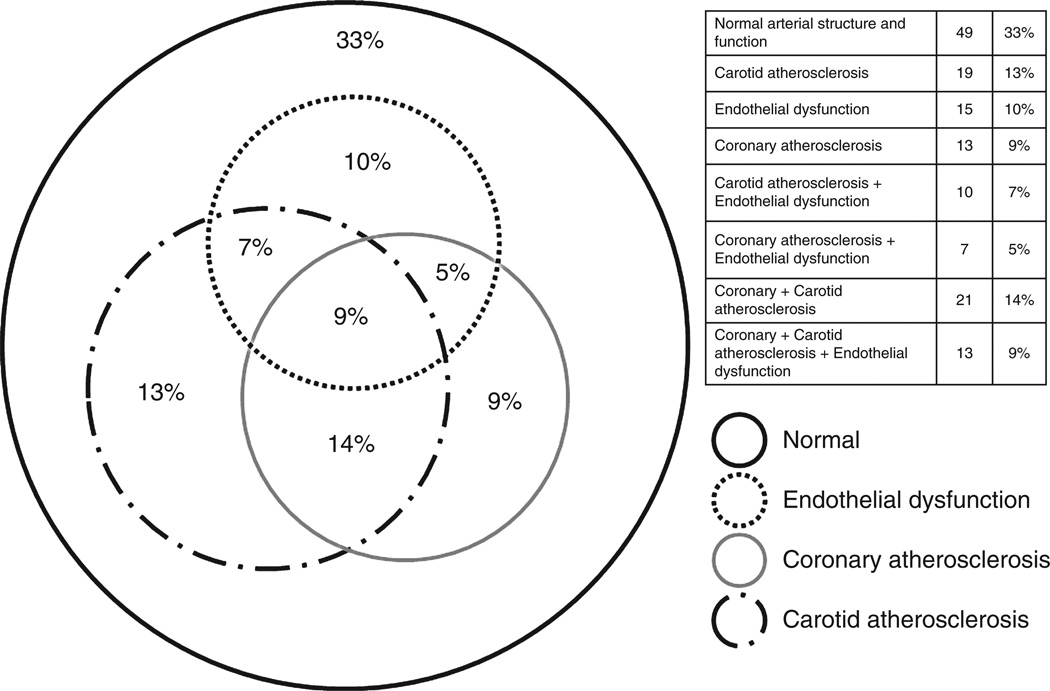
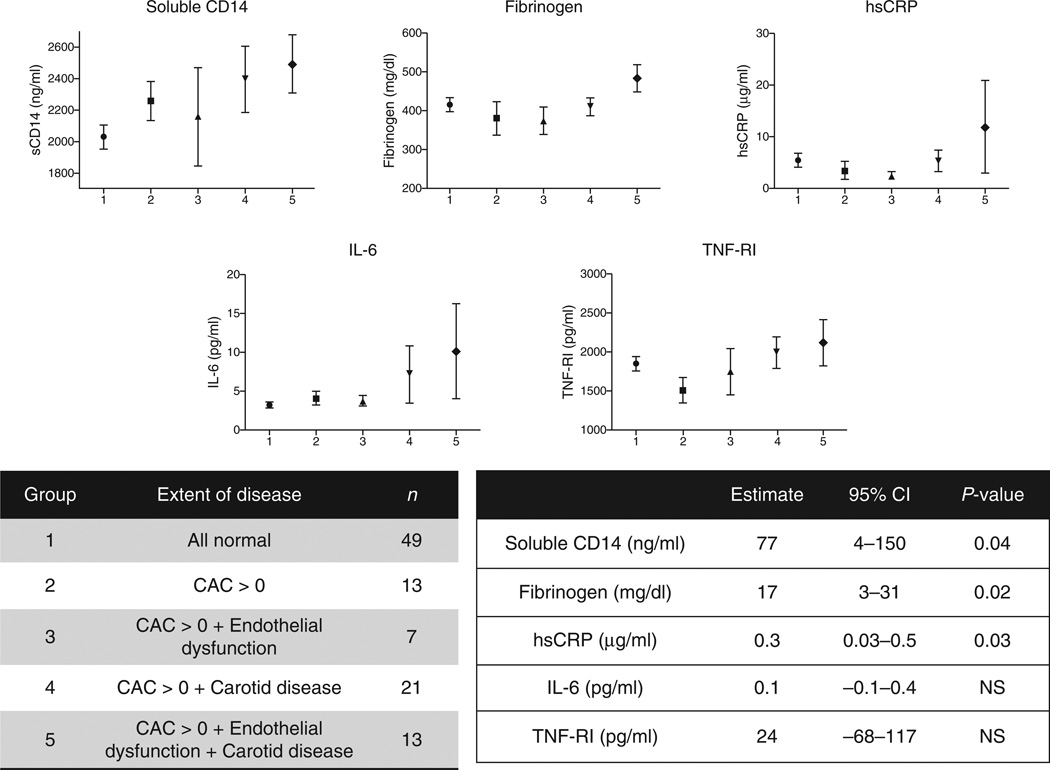
The overlap of coronary atherosclerosis (CAC >0), carotid atherosclerosis (plaque in any carotid vessel or mean-max CCA-IMT >1 mm) and endothelial dysfunction (FMD and hyperemic VTI both <75th percentile) was visually described using a Venn diagram. Participants with detectable coronary calcium were further compared with normal individuals by categorizing the participants into five groups (i.e. vascular disease phenotypes). These five ordered groups represent an increasing burden of vascular disease from normal (Group 1) to extensive subclinical disease involving multiple vascular beds (Group 5). Linear regression was used to analyse the trend of five biomarkers of interest across the five ordered groups.
All statistical tests were two-sided with a 0.05 significance level. Nominal P values are presented throughout. Analyses were performed with SAS version 9.2 (SAS Institute, Cary, North Carolina, USA).
Results
The baseline characteristics of study participants are displayed in Table 1. Overall, median age was 46 years. Three-quarters of participants were men, and two-thirds were African-American. Median time since HIV diagnosis was 12 years, and median current CD4+ T-cell count was 613 cells/µl; 50% were currently on protease-inhibitor based ART, but only 5% were currently taking a thymidine analogue. Median 10-year Framingham Risk Score was 3.0% and Reynold’s Risk Score was 2.1%. Compared with those without detectable coronary calcium, participants with CAC greater than 0 were older, less commonly African-American and had higher current CD4+ T-cell counts and lower nadir CD4+ cell counts. They had higher concentration of LDL-C and global risk scores but had similar indices of insulin sensitivity and prevalence of metabolic syndrome [31]. Ectopic fat volumes were higher among those with CAC greater than 0 and there was a trend towards lower limb adipose tissue volumes (P<0.1).
Table 1.
Baseline characteristics of study participants.
| Demographics | Overall (n = 147) | CAC=0 (n = 93) | CAC >0 (N = 54) | P |
|---|---|---|---|---|
| Age (years) | 46 (40–53) | 44 (35–49) | 50 (44–56) | <0.0001 |
| Male | 115 (78%) | 71 (76%) | 44 (81%) | >0.1 |
| African-American | 101 (69%) | 71 (76%) | 30 (56%) | 0.04 |
| HIV parameters | ||||
| Current CD4+ cell count (cells/µl) | 613 (425–853) | 647 (438–878) | 513 (420–754) | 0.04 |
| Nadir CD4+ cell count (cells/µl) | 179 (86–298) | 197 (105–327) | 133 (40–274) | 0.04 |
| HIV duration (years) | 12 (6.2–18) | 11 (6.3–17) | 13(5.8–19) | >0.1 |
| ART duration (years) | 5.3 (3.2–9.8) | 5.3 (3.3–9.3) | 5.8 (3.2–11) | >0.1 |
| PI duration (years) | 3.5 (0.7–7.1) | 3.3 (0.8–6.6) | 4.3 (0.6–7.4) | >0.1 |
| Undetectable viral load (<48 copies/ml) | 104 (70%) | 68 (73%) | 36 (67%) | >0.1 |
| Traditional cardiovascular risk factors | ||||
| SBP (mmHg) | 121 (112–132) | 121 (110–132) | 122 (114–132) | >0.1 |
| HDL cholesterol (mg/dl) | 46 (37–57) | 47 (38–57) | 44 (37–57) | >0.1 |
| LDL cholesterol (mg/dl) | 97 (77–113) | 92 (74–107) | 106 (87–121) | 0.02 |
| Non-HDL cholesterol (mg/dl) | 123 (102–144) | 119 (99–144) | 129 (107–148) | >0.1 |
| Creatinine clearance (ml/min) | 112 (86–140) | 116 (88–148) | 106 (84–128) | >0.1 |
| HOMA-IR | 1.8 (1.1–3.3) | 1.9 (1.1–3.2) | 1.6 (1.0–3.4) | >0.1 |
| Metabolic syndrome | 114 (22%) | 74 (20%) | 40 (26%) | >0.1 |
| Current smoker | 93 (63%) | 59 (63%) | 34 (63%) | >0.1 |
| Family history of MI | 46 (31%) | 26 (28%) | 20 (37%) | >0.1 |
| 10-year Framingham risk score (%) | 3.0 (1.0–7.0) | 2.0 (1.0–6.0) | 6.0 (3.0–10) | <0.0001 |
| Reynold’s risk score | 2.1 (0.9–5.1) | 1.6 (0.6–3.1) | 4.2 (1.6–6.5) | <0.0001 |
| Body fat composition | ||||
| BMI (kg/m2) | 27 (23–30) | 27 (24–30) | 26 (23–30) | >0.1 |
| Total limb adipose tissue (kg) | 8.8 (4.9–14) | 9.8 (5.5–15) | 7.6 (4.3–12) | 0.08 |
| Total trunk adipose tissue (kg) | 13 (7.7–18) | 13 (8.2–18) | 13 (6.7–18) | >0.1 |
| Epicardial adipose tissue (cm3) | 68 (47–92) | 62 (45–82) | 76 (52–122) | 0.02 |
| Thoracic peri-aortic adipose tissue (cm3) | 9.2 (6.3–14) | 8.8 (6.3–11) | 10 (6.5–19) | 0.04 |
| Current medication use | ||||
| Antihypertensive medication use | 36 (24%) | 18 (19%) | 18 (33%) | 0.06 |
| Fibrate use | 4 (3%) | 1 (1%) | 3 (6%) | >0.1 |
| Fish oil use | 12 (8%) | 8 (9%) | 4 (7%) | >0.1 |
| Current PI use | 72 (49%) | 46 (49%) | 26 (48%) | >0.1 |
| Current ZDV or D4T use | 8 (5%) | 4 (4%) | 4 (7%) | >0.1 |
| Current abacavir use | 7 (5%) | 4 (4%) | 3 (6%) | >0.1 |
Data presented as median (interquartile range) for continuous variables or frequency (percentage) for categorical variables. ART, antiviral therapy; CAC, coronary artery calcium; D4T, stavudine; HDL, high-density lipoprotein; HOMA-IR, Homeostasis Model Assessment–Insulin Resistance; LDL, low-density lipoprotein; MI, myocardial infarction; PI, protease inhibitor; ZDV, zidovudine.
Biomarkers of inflammation and immune activation were generally similar between participants with and without detectable CAC, with two exceptions (Table 2). Soluble CD14 concentration was higher and TF expression on inflammatory (CD14+CD16+) monocytes was lower among those with CAC greater than 0. When participants were further stratified into three categories, there was a graded increase in sCD14 [median (IQR) 2057 (1677–2413), 2212 (1914–2469) and 2504 (1956–2918) ng/ml for CAC 0, CAC 1–99 and CAC >100, respectively; P=0.017]. This statistically significant relationship between sCD14 and CAC >0 persisted (P=0.028) in a multivariable logistic regression model that adjusted for age, sex, race, nadir CD4+ cell count, LDL-C and limb fat.
Table 2.
Biomarkers of inflammation and immune activation by coronary artery calcium category.
| CAC=0 (n = 93) | CAC >0 (n = 54) | P | |
|---|---|---|---|
| CD8+CD38+HLA-DR+ T cells (%) | 13 (9–18) | 11 (7.4–17) | >0.1 |
| CD4+CD38+HLA-DR+ T cells (%) | 5.1 (3.8–6.9) | 4.9 (3.4–6.2) | >0.1 |
| CD14+CD16+ monocytes (%) | 22 (17–32) | 26 (19–38) | >0.1 |
| CD14dimCD16+ monocytes (%) | 12 (7.8–15) | 9.9 (7.8–14) | >0.1 |
| CD14+CD16+TF+ monocytes (%) | 13 (7.9–18) | 10 (6.9–15) | 0.03 |
| CD14dimCD16+ TF+ monocytes (%) | 21 (14–29) | 18 (14–24) | >0.1 |
| Soluble CD14 (ng/ml) | 2057 (1677–2413) | 2246 (1927–2781) | 0.03 |
| Soluble CD163 (ng/ml) | 651 (487–829) | 630 (476–904) | >0.1 |
| Interleukin-6 (pg/ml) | 2.5 (1.9–4.4) | 3.0 (2.2–5.7) | 0.08 |
| TNF-α receptor I (pg/ml) | 1581 (1297–2330) | 1581 (1191–2208) | >0.1 |
| High sensitivity CRP (µg/ml) | 1.7 (0.8–4.8) | 2.0 (0.6–5.3) | >0.1 |
| Soluble VCAM (ng/ml) | 638 (530–795) | 687 (575–839) | >0.1 |
| D-dimer (µg/ml) | 0.17 (0.11–0.32) | 0.16 (0.13)-0.29 | >0.1 |
| Fibrinogen (mg/dl) | 394 (334–513) | 367 (339–477) | >0.1 |
| OPG (pmol/l) | 3.7 (3.1–4.8) | 4.1 (3.0–5.1) | >0.1 |
| RANKL (pg/ml) | 10 (3.0–29) | 8.2 (2.0–29) | >0.1 |
Data presented as median (interquartile range). CRP, C-reactive protein; OPG, osteoprotegerin; RANKL, receptor activator of nuclear factor kappa-B ligand; TNF, tumour necrosis factor; VCAM, vascular cell adhesion molecule.
The associations of biomarkers of inflammation and immune activation with ultrasound-derived measures of vascular disease differed according to whether CAC was present or absent (Table 3). For example, CD4+ and CD8+ T-cell activation was associated with CCA-IMT only among those without coronary calcium. Despite the lack of a relationship with FMD, higher levels of inflammation were consistently associated with worse microvascular function as measured by hyperemic VTI among those with and without CAC.
Table 3.
Correlations of biomarkers with measures of vascular structure and function stratified by coronary artery calcium status.
| CCA-IMT |
Plaque |
FMD |
Hyperemic VTI |
|||||
|---|---|---|---|---|---|---|---|---|
| CAC=0 | CAC >0 | CAC=0 | CAC >0 | CAC=0 | CAC >0 | CAC=0 | CAC >0 | |
| CD8+CD38+HLA-DR+ T cells (%) | 0.249 | * | * | * | * | * | * | * |
| CD4+ CD38+HLA-DR+ T cells (%) | 0.314 | * | * | 0.279 | * | * | * | * |
| CD14+CD16+ monocytes (%) | * | * | −0.259 | * | * | * | * | * |
| CD14dimCD16+ monocytes (%) | * | * | * | * | * | * | * | * |
| CD14+CD16+TF+ monocytes (%) | * | * | * | * | * | * | * | * |
| CD14dimCD16 +TF+ monocytes (%) | * | * | * | * | * | * | * | * |
| Soluble CD14 (ng/ml) | * | * | * | * | * | * | * | * |
| Soluble CD163 (ng/ml) | * | * | * | * | * | * | * | * |
| Interleukin-6 (pg/ml) | * | * | −0.221 | * | * | * | −0.287 | −0.523 |
| TNF-α receptor I (pg/ml) | * | * | −0.291 | * | 0.210 | * | * | −0.353 |
| High-sensitivity CRP (µg/ml) | * | * | * | * | * | * | −0.295 | −0.353 |
| sVCAM-1 (ng/ml) | 0.283 | * | 0.215 | * | * | * | * | * |
| D-dimer (µg/ml) | * | * | * | * | * | * | * | * |
| Fibrinogen (mg/dl) | * | 0.418 | * | * | * | * | −0.328 | −0.328 |
| OPG (pmol/l) | * | 0.270 | 0.234 | * | * | * | * | * |
| RANKL (pg/ml) | * | * | * | * | * | * | 0.228 | * |
Spearman correlation coefficient is shown for all statistically significant relationships. CAC, coronary artery calcium; CCA-IMT, common carotid artery intima-media thickness; CRP, C-reactive protein; FMD, flow-mediated dilation of the brachial artery; OPG, osteoprotegerin; RANKL, receptor activator of nuclear factor kappa-B ligand; sVCAM-1, soluble vascular cell adhesion molecule-1; TNF, tumour necrosis factor; VTI, velocity-time integral.
P > 0.05.
Over two-thirds of our cohort had some degree of subclinical atherosclerosis or endothelial dysfunction and nearly one in 10 had evidence of extensive vascular disease in all three vascular territories (Fig. 1). Compared with those with normal vascular structure and function or coronary calcification alone, patients with increasing extent of subclinical disease in addition to CAC had higher levels of sCD14 and other biomarkers of inflammation (Fig. 2). The 13 participants with the most extensive vascular disease consistently had the highest levels of inflammation and immune activation.
Figure 1. Distribution of vascular phenotypes representing the extent of vascular disease in the study cohort.
Figure 2. Relationship of soluble CD14 and biomarkers of inflammation with the extent of vascular disease among participants with detectable coronary artery calcium.
The five ordered groups represent an increasing burden of vascular disease from normal (Group 1) to extensive subclinical disease involving multiple vascular beds (Group 5). Point estimates are the mean plasma concentration of the biomarker and error bars represent standard error of the mean. P value is for trend across all five ordered categories. IL-6, interleukin-6; NS, nonsignificant; TNF-RI, tumour necrosis factor-α receptor I.
Discussion
We have demonstrated in this cross-sectional study of a comprehensively phenotyped cohort of HIV-infected patients treated with modern ART that inflammation and immune activation correlates to the extent of vascular disease in multiple vascular beds. Soluble CD14, a surrogate of monocyte activation, is a particularly strong marker of the extent of subclinical vascular disease. Our finding that vascular disease is common but heterogeneous in this population is an important guiding principle for the design and interpretation of future studies of CVD risk in HIV.
CD14 is a coreceptor on innate immune cells that, together with Toll-like receptor-4 and lipopolysaccharide (LPS)-binding protein, allows these cells to scavenge circulating bacterial LPS and releases nuclear factor-κB dependent inflammatory cytokines [32]. The soluble form of CD14 (sCD14) is shed by activated monocytes and has been proposed as a surrogate marker of LPS-induced activation [9], although the liver also produces sCD14 as an acute phase protein in response to IL-6 [33]. Plasma sCD14 and LPS are elevated in chronic HIV infection and are associated with poor immunologic response to ART [34], progression of HIV-1 [35] and HIV-2 [36] disease, and mortality [37,38].
In the general population, plasma sCD14 levels have recently been independently associated with myocardial infarction, coronary heart disease and all-cause mortality among older (>65 years) men and women in the Cardiovascular Health Study (CHS) [39]. Several genome-wide single-nucleotide polymorphisms (SNPs) explain about one-third of the variance of sCD14 in this population; however, none of these SNPs was associated with mortality or cardiovascular disease after adjustment for multiple testing. Genetic polymorphisms that increase sCD14 have been variably associated with coronary heart disease in several other cohorts [32,40,41]. Similar to our findings, soluble CD14 was also associated with subclinical vascular disease as measured by carotid IMT or ankle-brachial index in CHS [39]. In the European PRIME study, sCD14 was not associated with CVD events despite correlations with other biomarkers of inflammation, endothelial activation and coagulation [42].
This is the first published study to demonstrate a relationship between sCD14 and CAC in HIV-infected patients that is independent of traditional risk factors. This is in agreement with two other studies that have associated sCD14 levels with both pathologic carotid IMT thickening [43] and progression of IMT over time [13]. A large number of other studies, however, have failed to demonstrate an association between sCD14 and carotid disease [10,44], endothelial function [45] and coronary atherosclerosis in men [15,16]. In a study of HIV-infected women, sCD14 levels were significantly elevated compared with controls, but did not appear to be associated with number or types of coronary plaque [46]. Interestingly, these same studies have variably shown associations of vascular disease with LPS [45] or other markers of monocyte activation such as soluble CD163 [15,16,46].
Although this study lacked a HIV-negative control group, the magnitude of plasma sCD14 levels observed among study participants with increasing extent of subclinical vascular disease can be placed in the context of HIV-uninfected controls from our laboratory and the general population using the same assay. Mean concentrations of three HIV-uninfected control populations from our laboratory ranged from 1340 to 1740 ng/ml [47–49]. In the CHS, mean (SD) concentration was 1641 (348) ng/ml [39]. In the present study, mean sCD14 of patients in Group 5 (CAC>0 and endothelial dysfunction and carotid disease; mean sCD14 2495 ng/ml) was elevated but equal to the approximate upper bound of normal in the CHS (1.5× the interquartile range above the 75th percentile) [39].
Together with this previous literature, our data suggest that even modest amounts of chronic monocyte activation may contribute to the development of subclinical atherosclerosis in treated HIV infection. In addition, monocyte activation may trigger acute vascular events through alternative pathways (i.e. thrombosis) [28,50], though this study was not designed to evaluate that hypothesis. In light of early data from SATURN-HIV suggesting that rosuvastatin effectively reduces sCD14 [51], we are particularly optimistic about the potential for statins to reduce CVD risk in patients with residual immune activation despite ART.
Hsue et al. [20] have previously described the extent of carotid disease in patients with and without coronary artery calcium. Our study confirms the finding that 30–50% of HIV-infected patients without detectable coronary calcium have subclinical carotid disease. We agree that this may represent an earlier stage of atherosclerosis that is mediated by different pathways. For example, although T-cell activation is known to be associated with carotid disease [10–12], our data suggest that this relationship is strongest among those who have not yet developed detectable coronary calcium (i.e. earlier stageatherosclerosis). Our data also suggest that microvascular function measured by hyperemic VTI – in contrast to FMD – is correlated with systemic inflammation markers such as interleukin-6 and may add significantly to CVD risk prediction in patients with CAC scores of zero. In the general population, hyperemic VTI, but not FMD, is predictive of CVD events in an otherwise low-risk population [52].
Our study also highlights the utility of multimodality imaging for the evaluation of CVD risk. To our knowledge, ours is the first study to combine endothelial function testing together with carotid ultrasound and CAC scoring as a measure of the extent of vascular disease in the HIV-infected population. This method elucidates relationships that may otherwise go undetected. For example, although sCD14 was not associated with carotid disease in an initial analysis of this cohort [10] and was not correlated with ultrasound measures of vascular disease in this current study (Table 3), a combined measure of the extent of vascular disease showed a clear relationship with sCD14 (Fig. 2). This could be replicated in larger HIV-infected cohort studies that have measured CAC, carotid IMT and brachial endothelial function in the same participants, although long-term follow-up will be required to determine the prognosis of the various subclinical vascular disease phenotypes. In addition, a combined vascular endpoint may be useful in trials of interventions that aim to reduce CVD risk by reducing inflammation in this population.
Limitations
The overall study size was robust considering the extensive characterization of the study participants; however, we may have lacked power to detect minor relationships within the subgroup analyses. Our study also shares the limitations of all cross-sectional analyses, including the possibility of residual confounding and inability to determine causality. Because this study investigated a specific HIV-infected population with normal LDL-C, the generalizability to the HIV-infected population with high lipid levels should be determined in future studies. Finally, the large number of statistical comparisons raises the risk of type-I error.
Conclusion
Soluble CD14 is independently associated with CAC, and, among those with detectable calcium, predicts the extent of subclinical disease in other vascular beds. Similarly, other biomarkers of inflammation and immune activation may be associated with ultrasound measures of vascular disease in patients without detectable CAC. A combined multimodality measure of vascular disease phenotypes may help elucidate the pathophysiology of atherosclerosis in HIV-infected patients on ART and could serve as a useful endpoint in trials that aim to reduce CVD risk in this population.
Acknowledgements
C.T.L. and G.A.M. did the data collection, study design, data analysis, drafting manuscript. Y.J. and S.D. did the data analysis. C.E.O. and R.C.G did the study design, drafting manuscript. N.T.F. did the data collection, study design, drafting manuscript. M.M.L. did the study design, drafting manuscript. N.S. and D.E.L. did the data collection, administrative support.
This project was supported by an award from the National Institutes of Health (NR012642 to G.M.). Technical assistance was provided by the Center for AIDS Research, Case Western Reserve University (P30 AI36219). The study is registered with clinicaltrials.gov, number NCT01218802.
Footnotes
Conflicts of interest
C.T.L. has received grants from Bristol-Myers Squibb and the Medtronic Foundation. S.D. currently serves on a DSMB of a Johnson and Johnson study. G.A.M. has served as a scientific advisor or speaker for Bristol-Myers Squibb, GlaxoSmithKline, Tibotec and Gilead Sciences, has received research grants from Bristol-Myers Squibb, GlaxoSmithKline and Gilead Sciences, and is currently serving as the DSMB Chair for a Pfizer-sponsored study. Y.J., C.O., R.G., S.D., N.T.F., M.M.L., N.S. and D.E.L. have no disclosures.
References
- 1.Boccara F, Lang S, Meuleman C, Ederhy S, Mary-Krause M, Costagliola D, et al. HIV and coronary heart disease: time for a better understanding. J Am Coll Cardiol. 2013;61:511–523. doi: 10.1016/j.jacc.2012.06.063. [DOI] [PubMed] [Google Scholar]
- 2.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:1–9. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV-infected adults. J Infect Dis. 2012;205(Suppl 3):S375–S382. doi: 10.1093/infdis/jis200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Lelyveld SF, Gras L, Kesselring A, Zhang S, De Wolf F, Wensing AM, et al. Long-term complications in patients with poor immunological recovery despite virological successful HAART in Dutch ATHENA cohort. AIDS. 2012;26:465–474. doi: 10.1097/QAD.0b013e32834f32f8. [DOI] [PubMed] [Google Scholar]
- 5.Duprez DA, Neuhaus J, Kuller LH, Tracy R, Belloso W, De Wit S, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One. 2012;7:e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunt PW. HIV and inflammation: mechanisms and consequences. Current HIV/AIDS Rep. 2012;9:139–147. doi: 10.1007/s11904-012-0118-8. [DOI] [PubMed] [Google Scholar]
- 7.Giralt M, Domingo P, Villarroya F. Adipose tissue biology and HIV-infection. Best practice & research. Clin Endocrinol Metab. 2011;25:487–499. doi: 10.1016/j.beem.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Hunt PW, Martin JN, Sinclair E, Epling L, Teague J, Jacobson MA, et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis. 2011;203:1474–1483. doi: 10.1093/infdis/jir060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandler NG, Douek DC. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nat Rev Microbiol. 2012;10:655–666. doi: 10.1038/nrmicro2848. [DOI] [PubMed] [Google Scholar]
- 10.Longenecker C, Funderburg N, Jiang Y, Debanne S, Storer N, Labbato D, et al. Markers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV Med. 2013;14:385–390. doi: 10.1111/hiv.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis. 2011;203:452–463. doi: 10.1093/infdis/jiq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. T cell activation predicts carotid artery stiffness among HIV-infected women. Atherosclerosis. 2011;217:207–213. doi: 10.1016/j.atherosclerosis.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis. 2012;206:1558–1567. doi: 10.1093/infdis/jis545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, et al. Arterial inflammation in patients with HIV. JAMA. 2012;308:379–386. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zanni MV, Abbara S, Lo J, Wai B, Hark D, Marmarelis E, et al. Increased coronary atherosclerotic plaque vulnerability by coronary computed tomography angiography in HIV-infected men. AIDS. 2013;27:1263–1272. doi: 10.1097/QAD.0b013e32835eca9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burdo TH, Lo J, Abbara S, Wei J, Delelys ME, Preffer F, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204:1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O’Leary D, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788–795. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hulten E, Mitchell J, Scally J, Gibbs B, Villines TC. HIV positivity, protease inhibitor exposure and subclinical atherosclerosis: a systematic review and meta-analysis of observational studies. Heart. 2009;95:1826–1835. doi: 10.1136/hrt.2009.177774. [DOI] [PubMed] [Google Scholar]
- 19.Lo J, Abbara S, Shturman L, Soni A, Wei J, Rocha-Filho JA, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010;24:243–253. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsue PY, Ordovas K, Lee T, Reddy G, Gotway M, Schnell A, et al. Carotid intima-media thickness among human immunodeficiency virus-infected patients without coronary calcium. Am J Cardiol. 2012;109:742–747. doi: 10.1016/j.amjcard.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenny NS, Brown ER, Detrano R, Folsom AR, Saad MF, Shea S, et al. Associations of inflammatory markers with coronary artery calcification: results from the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2010;209:226–229. doi: 10.1016/j.atherosclerosis.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamirani YS, Pandey S, Rivera JJ, Ndumele C, Budoff MJ, Blumenthal RS, et al. Markers of inflammation and coronary artery calcification: a systematic review. Atherosclerosis. 2008;201:1–7. doi: 10.1016/j.atherosclerosis.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 24.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611–619. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 26.Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR. C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation. 2008;118 doi: 10.1161/CIRCULATIONAHA.108.814251. 2243-22512244p following 2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McComsey GA, Kitch D, Sax PE, Tebas P, Tierney C, Jahed NC, et al. Peripheral and central fat changes in subjects randomized to abacavir-lamivudine or tenofovir-emtricitabine with atazanavir-ritonavir or efavirenz: ACTG Study A5224s. Clin Infect Dis. 2011;53:185–196. doi: 10.1093/cid/cir324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Funderburg NT, Zidar DA, Shive C, Lioi A, Mudd J, Musselwhite LW, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndromes. Blood. 2012;120:4599–4608. doi: 10.1182/blood-2012-05-433946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Torriani FJ, Komarow L, Parker RA, Cotter BR, Currier JS, Dube MP, et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: the ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol. 2008;52:569–576. doi: 10.1016/j.jacc.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 32.Arroyo-Espliguero R, Avanzas P, Jeffery S, Kaski JC. CD14 and toll-like receptor 4: a link between infection and acute coronary events? Heart. 2004;90:983–988. doi: 10.1136/hrt.2002.001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bas S, Gauthier BR, Spenato U, Stingelin S, Gabay C. CD14 is an acute-phase protein. J Immunol. 2004;172:4470–4479. doi: 10.4049/jimmunol.172.7.4470. [DOI] [PubMed] [Google Scholar]
- 34.Lederman MM, Calabrese L, Funderburg NT, Clagett B, Medvik K, Bonilla H, et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis. 2011;204:1217–1226. doi: 10.1093/infdis/jir507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marchetti G, Cozzi-Lepri A, Merlini E, Bellistri GM, Castagna A, Galli M, et al. Microbial translocation predicts disease progression of HIV-infected antiretroviral-naive patients with high CD4R cell count. AIDS. 2011;25:1385–1394. doi: 10.1097/QAD.0b013e3283471d10. [DOI] [PubMed] [Google Scholar]
- 36.Thiebaut R, Charpentier C, Damond F, Taieb A, Antoine R, Capeau J, et al. Association of soluble CD14 and inflammatory biomarkers with HIV-2 disease progression. Clin Infect Dis. 2012;55:1417–1425. doi: 10.1093/cid/cis708. [DOI] [PubMed] [Google Scholar]
- 37.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Justice AC, Freiberg MS, Tracy R, Kuller L, Tate JP, Goetz MB, et al. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin Infect Dis. 2012;54:984–994. doi: 10.1093/cid/cir989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reiner AP, Lange EM, Jenny NS, Chaves PH, Ellis J, Li J, et al. Soluble CD14: genomewide association analysis and relationship to cardiovascular risk and mortality in older adults. Arterioscler Thromb Vasc Biol. 2013;33:158–164. doi: 10.1161/ATVBAHA.112.300421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koenig W, Khuseyinova N, Hoffmann MM, Marz W, Frohlich M, Hoffmeister A, et al. CD14 C(-260)–>T polymorphism, plasma levels of the soluble endotoxin receptor CD14, their association with chronic infections and risk of stable coronary artery disease. J Am Coll Cardiol. 2002;40:34–42. doi: 10.1016/s0735-1097(02)01937-x. [DOI] [PubMed] [Google Scholar]
- 41.Morange PE, Saut N, Alessi MC, Frere C, Hawe E, Yudkin JS, et al. Interaction between the C-260T polymorphism of the CD14 gene and the plasma IL-6 concentration on the risk of myocardial infarction: the HIFMECH study. Atherosclerosis. 2005;179:317–323. doi: 10.1016/j.atherosclerosis.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 42.Morange PE, Tiret L, Saut N, Luc G, Arveiler D, Ferrieres J, et al. TLR4/Asp299Gly, CD14/C-260T, plasma levels of the soluble receptor CD14 and the risk of coronary heart disease: the PRIME Study. Eur J Hum Genet. 2004;12:1041–1049. doi: 10.1038/sj.ejhg.5201277. [DOI] [PubMed] [Google Scholar]
- 43.Merlini E, Luzi K, Suardi E, Barassi A, Cerrone M, Martinez JS, et al. T-cell phenotypes, apoptosis and inflammation in HIV+ patients on virologically effective cART with early atherosclerosis. PLoS One. 2012;7:e46073. doi: 10.1371/journal.pone.0046073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaplan RC, Landay AL, HodisHN, Gange SJ, Norris PJ, Young M, et al. Potential cardiovascular disease risk markers among HIV-infected women initiating antiretroviral treatment. J Acquir Immune Defic Syndr. 2012;60:359–368. doi: 10.1097/QAI.0b013e31825b03be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blodget E, Shen C, Aldrovandi G, Rollie A, Gupta SK, Stein JH, et al. Relationship between microbial translocation and endothelial function in HIV infected patients. PLoS One. 2012;7:e42624. doi: 10.1371/journal.pone.0042624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fitch KV, Srinivasa S, Abbara S, Burdo TH, Williams KC, Eneh P, et al. Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. J Infect Dis. 2013;208:1737–1746. doi: 10.1093/infdis/jit508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayne E, Funderburg NT, Sieg SF, Asaad R, Kalinowska M, Rodriguez B, et al. Increased platelet and microparticle activation in HIV infection: upregulation of P-selectin and tissue factor expression. J Acquir Immune Defic Syndr. 2012;59:340–346. doi: 10.1097/QAI.0b013e3182439355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lederman MM, Calabrese L, Funderburg NT, Clagett B, Medvik K, Bonilla H, et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis. 2011;204:1217–1226. doi: 10.1093/infdis/jir507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Funderburg NT, Zidar DA, Shive C, Lioi A, Mudd J, Musselwhite LW, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood. 2012;120:4599–4608. doi: 10.1182/blood-2012-05-433946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Funderburg NT, Mayne E, Sieg SF, Asaad R, Jiang W, Kalinowska M, et al. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood. 2010;115:161–167. doi: 10.1182/blood-2009-03-210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Funderburg NT, Jiang Y, Debanne SM, Storer N, Labbato D, Clagett B, et al. Rosuvastatin treatment reduces markers of monocyte activation in HIV infected subjects on antiretroviral therapy. Clin Infect Dis. 2013 doi: 10.1093/cid/cit748. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anderson TJ, Charbonneau F, Title LM, Buithieu J, Rose MS, Conradson H, et al. Microvascular function predicts cardiovascular events in primary prevention: long-term results from the Firefighters and Their Endothelium (FATE) study. Circulation. 2011;123:163–169. doi: 10.1161/CIRCULATIONAHA.110.953653. [DOI] [PubMed] [Google Scholar]