Abstract
The serine/threonine kinase mammalian/mechanistic target of rapamycin (mTOR) integrates various environmental cues such as the presence of antigen, inflammation, and nutrients to regulate T cell growth, metabolism, and function. The tuberous sclerosis 1 (TSC1)/TSC2 complex negatively regulates the activity of an mTOR-containing multiprotein complex called mTOR complex 1. Recent studies have revealed an essential cell-intrinsic role for TSC1 in T cell survival, quiescence, and mitochondrial homeostasis. Given the emerging role of mTOR activity in the regulation of the quantity and quality of CD8 T cell responses, in this study, we examine the role of its suppressor, TSC1, in the regulation of antigen-specific primary and memory CD8 T cell responses to bacterial infection. Using an established model system of transgenic CD8 cell adoptive transfer and challenge with Listeria monocytogenes expressing a cognate antigen, we found that TSC1 deficiency impairs antigen-specific CD8 T cell responses, resulting in weak expansion, exaggerated contraction, and poor memory generation. Poor expansion of TSC1-deficient cells was associated with defects in survival and proliferation in vivo, while enhanced contraction was correlated with an increased ratio of short-lived effectors to memory precursors in the effector cell population. This perturbation of effector-memory differentiation was concomitant with decreased expression of eomesodermin among activated TSC1 knockout cells. Upon competitive adoptive transfer with wild-type counterparts and antigen rechallenge, TSC1-deficient memory cells showed moderate defects in expansion but not cytokine production. Taken together, these findings provide direct evidence of a CD8 T cell-intrinsic role for TSC1 in the regulation of antigen-specific primary and memory responses.
INTRODUCTION
CD8 T cells play a critical role in clearing several types of microbial infections by mounting robust cytotoxic T lymphocyte (CTL) responses that kill infected cells. A typical CTL response is characterized by the presence of an effector phase, a contraction phase, and a memory maintenance phase (1, 2). During the expansion phase, naive CD8 cells are activated by antigen-presenting cells (APCs) in secondary lymphoid organs and undergo rapid clonal expansion. At the peak of the response, the population of CD8 effectors is heterogeneous and consists of a majority of short-lived effector cells (SLECs) and a small pool of memory-precursor effector cells (MPECs) (3, 4). Following antigen clearance, 90 to 95% of the CD8 effectors undergo apoptosis during the contraction phase (5, 6). At this stage, MPECs are thought to preferentially contribute to the formation of a small but stable population of long-lived memory cells. These memory cells are capable of self-renewal in response to cytokines such as interleukin-15 (IL-15) and respond more rapidly than naive cells to antigen reexposure (7). A number of studies have correlated the kinetics of CD8 responses with external factors like the duration of antigen availability and the presence of costimulation (6, 8). More recently, a role for T cell receptor (TCR)-mediated signaling (9, 10) and cytokine-mediated signaling (11, 12) in shaping primary and memory CD8 responses has also come to the fore. However, the signaling mechanisms that integrate these diverse extracellular cues to modulate the magnitude and quality of CD8 immune responses remain to be fully understood.
The mammalian/mechanistic target of rapamycin (mTOR), an evolutionarily conserved serine/threonine kinase, plays a critical role in integrating environmental signals such as the presence of amino acids, nutrients, growth factors, and cytokines to determine cell growth and metabolic outcomes in eukaryotic cells (13, 14). Within the cell, mTOR exists as a part of two distinct multiprotein complexes called mTOR complex 1 (mTORc1) and mTORc2 (15). Recent studies have elegantly demonstrated that mTOR regulates a number of key aspects of T cell biology, including CD4 T helper cell differentiation and CD8 cell effector-memory differentiation (16–22), invariant NKT (iNKT) cell development and function (23), and regulatory T cell function (24). In particular, treatment with the mTORc1 inhibitor rapamycin increases the number of MPECs during the expansion phase and accelerates memory cell differentiation during the contraction phase in a viral infection model, suggesting that mTORc1 activity may negatively regulate these processes (17). Ex vivo, the temporal kinetics of mTORc1 activity during CD8 cell activation were found to play a critical role in determining effector versus memory fate by differentially regulating the expression of the T-box family transcription factors T-box expressed in T cells (T-bet) and eomesodermin (Eomes) (18). Other studies have also demonstrated a role for mTOR in the regulation of CD8 effector versus memory differentiation in vivo under conditions of homeostatic proliferation (25, 26).
The tuberous sclerosis (TSC) complex, a heterodimer of the tumor suppressor proteins TSC1 and TSC2, is an upstream negative regulator of mTORc1 activity (27). While TSC2 possesses GTPase-activating protein (GAP) activity, TSC1 is required to stabilize TSC2 and prevent its ubiquitin-mediated degradation (28, 29). Under resting conditions, the GAP activity of the TSC complex maintains the Ras family GTPase Rheb (Ras homolog enriched in brain) in an inactive, GDP-bound form. In the presence of nutrients, growth factors, or cytokines, receptor-mediated signals inhibit TSC activity and active GTP-bound Rheb promotes mTORc1 activity by stimulating mTOR phosphorylation at Ser2448 (30, 31). Several recent studies have demonstrated a vital role for TSC1 in T cell quiescence, survival, and mitochondrial homeostasis (32–35). Mice with a conditional deficiency of TSC1 in T cells showed a dramatic reduction of CD4 and CD8 cell numbers in the spleen, correlating with enhanced apoptosis via the intrinsic pathway. This was accompanied by hyperresponsiveness to TCR stimulation and a cell-autonomous loss of T cell quiescence. In addition, TSC1 has been shown to play an important role in terminal maturation and effector fate decision of the iNKT cells (36), iNKT cell anergy and anti-tumor immunity (37), regulatory T cell function (38), B cell development (39), innate immune responses and antigen presentation (40, 41), and mast cell survival and function (42). Given that mTORc1 activity plays a crucial role in effector/memory lineage decisions of CD8 cells, we examined the role of its regulator TSC1 in antigen-specific primary and memory CD8 responses. Preliminary results from a previous study suggest that TSC1flox/flox (TSC1f/f) CD4Cre mice contained fewer antigen-reactive CD8 cells and fewer gamma interferon (IFN-γ)-producing CD8 cells than their wild-type (WT) counterparts upon bacterial infection (33). However, since TSC1f/f CD4Cre mice have fewer mature T cells, a lower frequency of naive cells and a higher frequency of apoptotic T cells (than WT mice) prior to infection, these results have proven difficult to interpret.
Here we used a model of TCR-transgenic CD8 cell adoptive transfer, followed by infection with Listeria monocytogenes expressing a cognate antigen (43), to investigate a T cell-intrinsic role for TSC1 in the regulation of antigen-specific CD8 responses. The OT1 TCR contains Vα2 and Vβ5 variable segments and recognizes the SIINFEKL (OVA257-264) epitope of ovalbumin presented on H-2Kb. Using both individual and competitive adoptive transfers with WT cells, we showed that TSC1 deficiency impairs antigen-specific primary CD8 responses. Fewer TSC1-deficient CD8 cells than WT cells were present at the peak of the response, correlating with defects in in vivo proliferation and survival during the expansion phase. The TSC1 knockout (KO) population contained an increased ratio of SLECs to MPECs at the peak of the response, correlating with enhanced contraction. Upon competitive adoptive transfer of memory cells, fewer TSC1-deficient memory cells than WT memory cells were present at days 6 and 7 postchallenge, suggesting that TSC1 deficiency may also affect the quality of the memory cells formed. Taken together, our findings demonstrate a previously unknown role for TSC1 in the regulation of the kinetics of antigen-specific primary and memory CD8 responses by repressing cell death, promoting proliferation, and regulating effector-memory differentiation.
MATERIALS AND METHODS
Mice.
TSC1f/f mice and OT1 mice were obtained from The Jackson Laboratory, while CD4Cre mice were obtained from Taconic Farms. Mice were housed under specific-pathogen-free conditions and used in accordance with National Institutes of Health guidelines. The experiments described here were approved by the Institutional Animal Care and Use Committee of Duke University.
Flow cytometry.
Standard protocols were used to prepare single-cell suspensions from thymus, spleen, and lymph node samples from mice (in Iscove's modified Dulbecco medium containing 10% fetal bovine serum [FBS] and antibiotics). Red blood cells (RBCs) were lysed with ammonium-chloride-potassium (ACK) buffer. Samples were subsequently stained with antibodies in phosphate-buffered saline (PBS) containing 2% FBS, collected on a BD FACSCanto II cytometer, and analyzed with TreeStar FlowJo software. Fluorochrome-conjugated antibodies against CD8, Vα2, CD45.1, CD45.2, CD69, KLRG1, IL-7Rα, T-bet, Eomes, IFN-γ, and tumor necrosis factor alpha (TNF-α) were purchased from BioLegend. Bromodeoxyuridine (BrdU) incorporation assays were performed with a BD BioSciences kit as discussed below. 7-Aminoactinomycin D (7-AAD; Invitrogen) was added to the samples shortly before collection.
Adoptive transfer and infection with L. monocytogenes expressing ovalbumin.
Enrichment of Vα2+ cells from spleen and lymph node samples of congenically marked WT OT1 and TSC1f/f CD4Cre OT1 mice was performed with Miltenyi Biotec LS columns. Cells were incubated first with Vα2-phycoerythrin (PE) antibodies and then with anti-PE magnetic beads to isolate Vα2+ cells according to the manufacturer's protocol. Enriched samples were stained with appropriate antibodies and sorted on a MoFlo Astrios or FACSDiva sorter to obtain viable naive OT1 cells (7AAD− CD8+ Vα2+ CD44lo CD62Lhi).
For individual adoptive-transfer experiments, 104 such naive WT OT1 (CD45.2) or TSC1f/f CD4Cre OT1 cells (CD45.2) were transferred into WT CD45.1 CD45.2 recipients by intravenous injection. For competitive adoptive transfers, equal numbers of naive WT OT1 (CD45.1) and TSC1f/f CD4Cre OT1 (CD45.2) cells were mixed and 104 cells from this mixture were adoptively transferred into WT CD45.1 CD45.2 recipients by intravenous injection. In both cases, recipients were infected after 24 h with 104 CFU of L. monocytogenes expressing recombinant ovalbumin (Lm-Ova) (44, 45). Peripheral blood samples were collected in PBS containing 5 mM EDTA at 1, 2, 4, and 7 weeks postinfection. RBCs were lysed by ACK treatment, and samples were stained with fluorochrome-conjugated antibodies for analysis by flow cytometry. At certain time points, mice were sacrificed to monitor the CD8 response in the spleen.
The following gating strategy was applied. First, CD8+ Vα2+ cells were distinguished from among the total peripheral blood mononuclear cells (PBMCs) or splenocytes of the recipient. This CD8+ Vα2+ population contains adoptively transferred OT1 cells, as well as endogenous (belonging to the recipient mouse) CD8 cells that use Vα2. Next, the expression of congenic markers (CD45.1 and CD45.2) within this gated CD8+ Vα2+ population was analyzed in order to distinguish adoptively transferred cells from the endogenous population. Since recipient mice expressed both CD45.1 and CD45.2, the CD45.1+ CD45.2+ population represents the endogenous CD8+ Vα2+ cells. In the case of individual adoptive transfers, the adoptively transferred cells (either WT OT1 or TSC1f/f CD4Cre OT1) were CD45.1− CD45.2+. In the case of competitive adoptive transfers, the WT OT1 cells were CD45.1+ CD45.2− and the TSC1f/f CD4Cre OT1 cells were CD45.1− CD45.2+. Further calculations were performed as follows. For example, in competitive-transfer experiments, the percentage of WT OT1 cells among the total PBMCs was calculated as a product of the percentage of CD8+ Vα2+ cells among the total PBMCs and the percentage of CD45.1+ CD45.2− cells within the gated CD8+ Vα2+ population.
To investigate memory responses, WT OT1 (CD45.1) and TSC1f/f CD4Cre OT1 (CD45.2) memory cells (7AAD− CD8+ Vα2+ CD44hi) were sorted from recipients beyond 7 weeks postinfection. These cells were adoptively transferred into naive WT (CD45.1 CD45.2) recipients that were then challenged with 105 CFU of Lm-Ova. Expansion of memory cells in the spleen and peripheral blood was examined at days 6 and 7 postchallenge.
T cell activation and proliferation assays.
Splenocytes from TCRβ−/− δ−/− mice were loaded with 10 μM SIINFEKL (OVA257-264) peptide, incubated for 2 h at 37°C, and subsequently treated with 25 μg/ml mitomycin C to prevent cell division. Cells were washed repeatedly to remove excess mitomycin C before subsequent coculture. TCRβ−/− δ−/− splenocytes that were not loaded with SIINFEKL peptide but simply treated with mitomycin C were used as controls. For overnight activation, 0.2 × 106 naive OT1 cells were plated with 0.2 × 106 peptide-loaded or control APCs in 96-well plates and incubated for 16 to 18 h at 37°C. Surface expression of CD69 on viable CD8+ Vα2+ cells was analyzed by flow cytometry after staining with fluorochrome-conjugated antibodies. In some experiments, 5 × 106 splenocytes were incubated with different concentrations of SIINFEKL peptide in the present of GolgiPlug for 4 h, and frequencies of IFN-γ- and TNF-α-producing CD8+ Vα2+ T cells were analyzed by flow cytometry after intracellular staining with fluorochrome-conjugated antibodies. For proliferation assays, naive OT1 cells were first labeled with 10 μM carboxyfluorescein succinimidyl ester (CFSE) for 9 min at room temperature. About 0.4 × 106 CFSE-labeled OT1 cells were incubated with 0.8 × 106 peptide-loaded or control APCs in 48-well plates for 65 to 72 h at 37°C. After staining with fluorochrome-conjugated antibodies, the CFSE dilution among viable Vα2+ CD8 cells was analyzed by flow cytometry as a measure of cell division.
In vivo BrdU incorporation assay.
In vivo labeling of cells with BrdU was performed with the BD Biosciences BrdU Flow kit. Briefly, recipient mice received 1.5 mg of BrdU in PBS via intraperitoneal injection on day 5 postinfection. Peripheral blood samples were collected and mice were sacrificed at 16 h postinjection of BrdU. Single-cell suspensions of peripheral blood and spleen samples were stained for surface markers with fluorochrome-conjugated antibodies. Cells were subsequently fixed (BD Cytofix/Cytoperm buffer), permeabilized (BD Cytoperm Plus buffer), refixed (BD Cytofix/Cytoperm buffer), and treated with 300 μg/ml DNase for 1 h at 37°C according to the manufacturer's protocol. Following DNase treatment, intracellular staining was performed with an anti-BrdU antibody and samples were analyzed by flow cytometry.
Real-time quantitative PCR.
Viable naive OT1 cells (7AAD− CD8+ Vα2+ CD44lo CD62Lhi) were sorted from spleen and lymph node samples of WT OT1 and TSC1f/f CD4Cre OT1 mice, and viable effector cells (7AAD− CD8+ Vα2+ CD45.2+ CD44hi) were sorted at 7 days postinfection from recipients that received WT or TSC1f/f CD4Cre OT1 cells. Sorted cells were immediately lysed in TRIzol for RNA preparation. cDNA was made with the iScript Select cDNA synthesis kit (Bio-Rad) according to the manufacturer's protocol. Real-time quantitative PCR was conducted and analyzed as previously described (42). Expressed levels of target mRNAs were normalized to β-actin and calculated by the 2−ΔΔCT method. The primers used were as follows: T-bet forward, 5′-GGTGTCTGGGAAGCTGAGAG-3′; T-bet reverse, 5′-GAAGGACAGGAATGGGAACA-3′; Eomes forward, 5′-CCCTATGGCTCAAATTCCAC-3′; Eomes reverse, 5′-TGGGGTTGAGTCCGTTTATG-3′; prdm1 forward, 5′-TGGTATTGTCGGGACTTTGC-3′; prdm1 reverse, 5′-TGGGGACACTCTTTGGGTAG-3′; Bcl-2 forward, 5′-CCGGGAGAACAGGGTATGAT-3′; Bcl-2 reverse, 5′-GCACAGCGGGCATTGGGTTG-3′; Bcl-xL forward, 5′-GGTGAGTCGGATTGCAAGTT-3′; Bcl-xL reverse, 5′-TGTTCCCGTAGAGATCCACA-3′; Bad forward, 5′-GCACACGCCCTAGGCTTGAGG-3′; Bad reverse, 5′-GGAACATACTCTGGGCTGCTGGTC-3′; Bax forward, 5′-TGCTACAGGGTTTCATCCAGGATCG-3′; Bax reverse, 5′-TCATCTCCAATTCGCCGGAGACA-3′; β-actin forward, 5′-TGTCCACCTTCCAGCAGATGT-3′; β-actin reverse, 5′-AGCTCAGTAACAGTCCGCCTAGA-3′.
Statistical analysis.
Statistical significance was determined with the Student t test. P values are indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
RESULTS
TSC1 deficiency impairs antigen-specific CD8 responses in vivo.
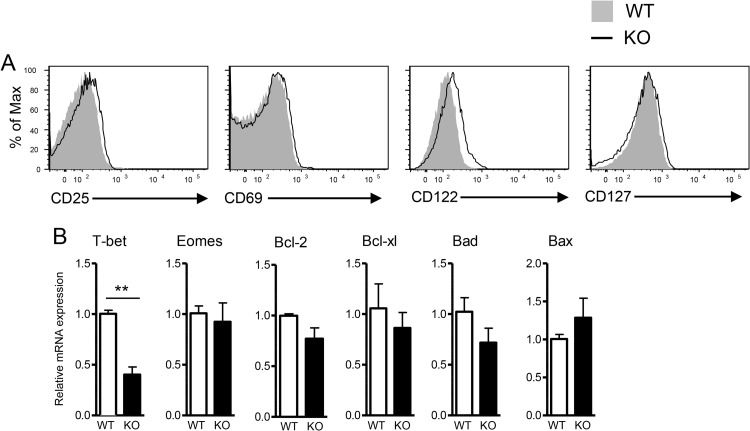
To better understand the role of TSC1 in the regulation of CD8 T cell-mediated immunity, we generated T cell-specific TSC1-deficient mice carrying the OT1 TCR (TSC1f/f CD4Cre OT1 mice). Naive OT1 cells (CD44lo CD62Lhi Vα2+ CD8+) from TSC1 KO OT1 mice expressed low levels of the activation makers CD25 and CD69 similar to those of their naive counterparts from WT OT1 controls. Expression of IL-7 receptor α (CD127) was also similar between naive WT and TSC1 KO OT1 T cells, while IL-15R β (CD122) expression was slightly increased in TSC1 KO OT1 T cells. In addition, expression of the prosurvival genes for Bcl-2 and Bcl-xL and the proapoptosis genes for Bad and Bax was not obviously different between naive WT and TSC1 KO OT1 T cells (Fig. 1B). T-bet and Eomes are two transcription factors that play important roles in effector/memory T cell differentiation. Although T-bet expression was about 60% lower in naive TSC1 KO OT1 T cells than in their naive WT OT1 counterparts, their Eomes mRNA levels were found to be similar.
FIG 1.
Phenotypic and gene expression analyses of TSC1-deficient naive OT1 T cells. (A) Expression of cell surface molecules in gated CD8+ Va2+ CD44lo CD62Lhi naive WT and TSC1 KO OT1 T cells. (B) mRNA levels of the molecules indicated in naive WT and TSC1 KO OT1 T cells. Total RNAs isolated from sorted CD8+ Va2+ CD44lo CD62Lhi WT and TSC1 KO OT1 T cells were subjected to reverse transcription and real-time quantitative PCR analysis. The mean ± the standard error of the mean was calculated for five mice per group. The data shown are representative of two independent experiments. *, P < 0.05; **, P < 0.01 (Student t test).
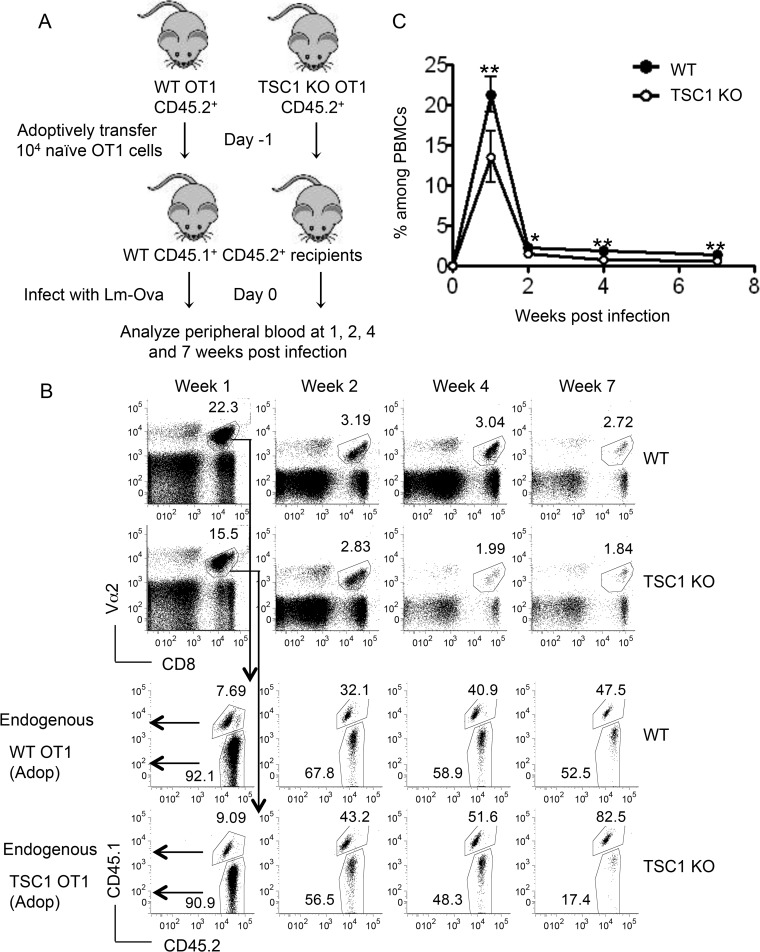
To examine how TSC1 may control CD8 T cell-mediated immune responses in vivo, we adoptively transferred 104 naive CD44lo CD62Lhi Vα2+ CD8 T cells from WT OT1 or TSC1f/f CD4Cre OT1 donors (CD45.2+) into congenically marked WT recipients (CD45.1+ CD45.2+). The recipient mice were subsequently infected with Lm-Ova (Fig. 2A). Adoptively transferred cells were tracked in the peripheral blood at 1, 2, 4, and 7 weeks postinfection by flow cytometry based on their congenic markers. In our analysis, we first gated on the Vα2+ CD8+ population, which contains both adoptively transferred OT1 cells and the recipient's endogenous CD8 cells that use Vα2 (Fig. 2B, top). When we examined the expression of congenic markers within this gated Vα2+ CD8+ population, adoptively transferred cells formed a distinct CD45.1− CD45.2+ population that was readily distinguishable from CD45.1+ CD45.2+ endogenous cells (Fig. 2B, bottom). As explained in Materials and Methods, the percentage of WT OT1 (or TSC1f/f CD4Cre OT1) cells among the total PBMCs was calculated as a product of the percentage of Vα2+ CD8+ cells among the total PBMCs and the percentage of adoptively transferred cells (CD45.1− CD45.2+) within the gated Vα2+ CD8+ population. Results from these flow cytometric analyses showed that adoptively transferred WT cells underwent robust clonal expansion, reaching peak numbers at week 1, and declined rapidly thereafter, leaving behind a stable pool of memory cells (Fig. 2B and C). The frequency of TSC1 KO OT1 cells, however, was significantly lower than that of their WT OT1 counterparts at all time points, suggesting that TSC1 deficiency may impair the ability of CD8 cells to mount a robust response to antigens. The reduced frequency of TSC1 KO cells at week 1 and later time points suggests that TSC1 may be required for optimal clonal expansion and memory formation/maintenance, respectively.
FIG 2.
TSC1 deficiency impairs antigen-specific CD8 responses. (A) Schematic representation of the experimental design showing individual adoptive transfers of naive WT OT1 or TSC1 KO OT1 cells (CD45.1− CD45.2+) into WT CD45.1+ CD45.2+ recipients. (B) Representative fluorescence-activated cell sorter analysis of peripheral blood samples showing percentages of Vα2+ CD8 cells among the total PBMCs (top). The analysis on the bottom was gated on this Vα2+ CD8+ population, and the adoptively transferred (CD45.1− CD45.2+) and endogenous (CD45.1+ CD45.2+) populations at the postinfection times indicated are shown. (C) Percentages of WT and TSC1 KO cells among the total PBMCs at the postinfection times indicated. The percentage of WT cells, for instance, was calculated as a product of the percentage of Vα2+ CD8+ cells among the total PBMCs and the percentage of WT cells (CD45.1− CD45.2+) within the Vα2+ CD8+ population. The mean ± the standard error of the mean was calculated for five mice per group. The data shown are representative of three independent experiments. *, P < 0.05; **, P < 0.01 (Student t test).
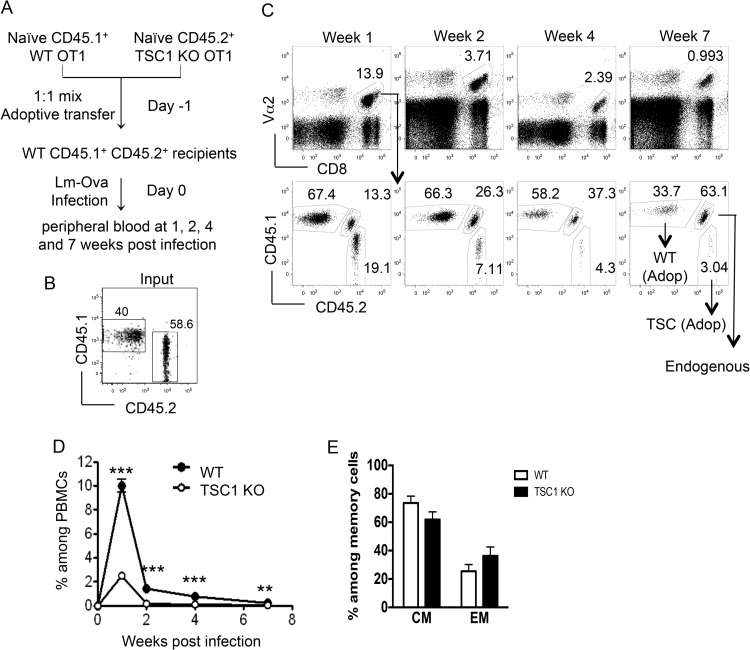
To account for possible differences in antigen clearance and to measure the responses of WT and TSC1 KO OT1 cells in the same host, we next performed competitive adoptive-transfer experiments. In these experiments, a mixture containing equal numbers of WT OT1 (CD45.1+) and TSC1f/f CD4Cre OT1 (CD45.2+) cells was injected into each WT (CD45.1+ CD45.2+) recipient (Fig. 3A and B). Impairment of the TSC1 KO CD8 response was more striking in the competitive model, with nearly four times as many WT cells as TSC1 KO cells being present among PBMCs at week 1 (Fig. 3C and D). This trend should perhaps not be surprising, given that TSC1 KO OT1 cells compete for antigen with an equal number of WT OT1 cells in the mixed-transfer system, as opposed to a rare population of endogenous T cells bearing Vα2+ Vβ5+ TCRs in the individual-transfer system. The TSC1 KO population also contracted dramatically, becoming less frequent than endogenous Vα2+ CD8 cells by week 2 (Fig. 3C). This was correlated with a marked paucity of TSC1 KO memory cells, as seen at weeks 4 and 7. At 7 weeks, the TSC1 KO memory cell population contained a slightly smaller central memory compartment and a slightly larger effector memory compartment than the WT memory cell population (Fig. 3E).
FIG 3.
TSC1-deficient CD8 cells are severely impaired in a competitive adoptive-transfer system. (A) Schematic representation of the experimental design showing competitive adoptive transfers of naive WT and TSC1 KO OT1 cells into WT CD45.1+ CD45.2+ recipients. (B) Donor naive OT1 T cell mixture before adoptive transfer showing the percentages of WT and TSC1 KO cells during adoptive transfer. (C) Representative fluorescence-activated cell sorter analysis of peripheral blood samples showing Vα2+ CD8 cells among the total PBMCs (top) and WT (CD45.1+ CD45.2−), TSC1 KO (CD45.1− CD45.2+), and endogenous (CD45.1+ CD45.2+) populations within the gated Vα2+ CD8 population (bottom) at the postinfection times indicated. Adop, adoptive transfer. (D) Percentages of WT and TSC1 KO cells among the total PBMCs at the postinfection times indicated. (E) Percentages of WT and TSC1 KO central memory (CM) and effector memory (EM) cells at 7 weeks after primary Lm-Ova infection. The mean ± the standard error of the mean was calculated for five mice per group. The data shown are representative of three independent experiments. **, P < 0.01; ***, P < 0.001 (Student t test).
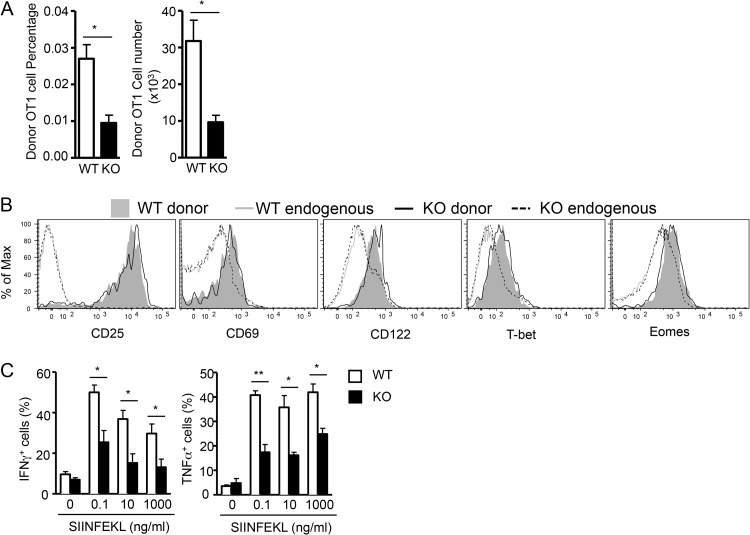
One scenario that could lead to lower TSC1 KO OT1 T cell numbers on day 7 after Lm-Ova infection is the following. If TSC1 KO OT1 cells underwent accelerated expansion, reaching peak responses well before day 7, early onset of contraction could result in lower TSC1 KO OT1 cell numbers on day 7. To investigate this possibility, we analyzed the WT and TSC1 KO OT1 populations at the early time point of day 4 postinfection. The percentages and absolute numbers of TSC1 KO OT1 T cells were about 60 to 70% lower than WT OT1 T cells on day 4 after Lm-Ova infection, arguing against the idea of an early peak response for TSC1 KO OT1 cells and suggesting that TSC1 KO OT1 T cells indeed show impaired antigen-specific expansion (Fig. 4A). At this time point, the TSC1 KO OT1 T cells expressed elevated CD25, CD69, and CD122 levels similar to those of WT OT1 T cells (Fig. 4B), indicating that the upregulation of these activation makers is not affected in OT1 T cells in the absence of TSC1. However, fewer TSC1 KO OT1 T cells than WT OT1 T cells expressed IFN-γ or TNF-α following ex vivo stimulation with SIINFEKL peptide for 5 h (Fig. 4C), suggesting a compromised effector function. These data underscore the finding that even at early time points, TSC1-deficient OT1 T cells fail to mount a robust response to Lm-Ova infection.
FIG 4.
TSC1 deficiency impairs antigen-specific early CD8 responses. Naive WT or TSC1 KO OT1 T cells were transferred into recipient mice that were then infected with Lm-Ova as shown in Fig. 2A. Mice were euthanized on day 4 after infection. (A) Percentages and total numbers of WT and TSC1 KO OT1 T cells in the spleen. The mean ± the standard error of the mean was calculated for five mice per group. (B) Cell surface expression of the molecules indicated. (C) Percentages of IFN-γ- or TNF-α-producing, donor-derived OT1 T cells. Splenocytes were stimulated with SIINFEKL peptide of the indicated concentrations in the presence of GolgiPlug for 5 h. Bar graphs show the mean percentages of IFN-γ- or TNF-α-producing donor cells ± the standard errors. The data shown are representative of three independent experiments. *, P < 0.05; **, P < 0.01 (Student t test).
Taken together, results from the individual and competitive adoptive-transfer systems suggest that TSC1 may play a critical cell-intrinsic role during both the expansion and contraction phases of antigen-specific CD8 responses.
Loss of TSC1 diminishes CD8 cell proliferation in vivo.
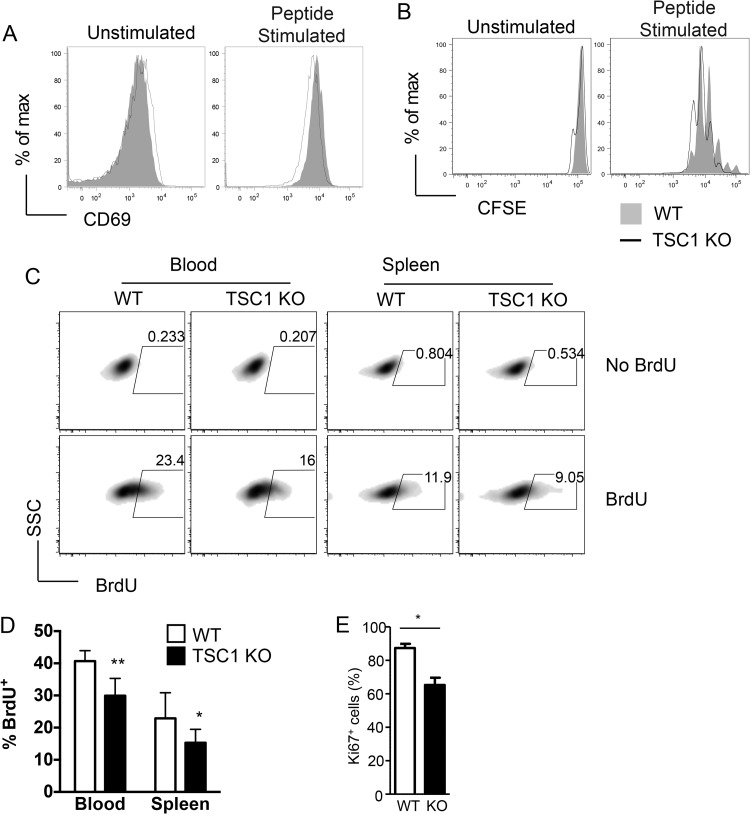
We sought to determine if the loss of TSC1 could affect the proliferative capacity of CD8 cells. To this end, we first cultured naive WT and TSC1 KO OT1 cells ex vivo with APCs that were either not loaded (unstimulated) or loaded with the SIINFEKL peptide (peptide stimulated). Upon overnight culture, WT and TSC1 KO cells showed comparable upregulation of CD69 (Fig. 5A). We also examined the ability of TSC1 KO OT1 cells to proliferate in response to peptide stimulation ex vivo by culturing CFSE-labeled naive WT and TSC1 KO OT1 cells with APCs that were not loaded or SIINFKL loaded for a period of 65 to 72 h. Analysis of CFSE dilution among OT1 cells by flow cytometry revealed that WT and TSC1 KO cells had undergone comparable rounds of cell division (Fig. 5B). Together, these results suggest that loss of TSC1 may not affect T cell activation or proliferation ex vivo.
FIG 5.
Defective antigen-driven proliferation of TSC1-deficient CD8 cells in vivo. (A) Representative histograms of CD69 expression on WT and TSC1 KO OT1 cells that were cultured overnight with APCs that were not loaded or loaded with SIINFEKL peptide. (B) Representative histograms showing the CFSE dilutions among WT and TSC1 KO OT1 cells that were cultured for 65 to 72 h with APCs that were not loaded or loaded with SIINFEKL peptide. (C) Representative density plots showing BrdU incorporation in WT and TSC1 KO OT1 cells in the peripheral blood and spleen. Following competitive adoptive transfers, mice were injected with BrdU on day 5 postinfection and tissues were harvested after 16 h for staining and flow cytometric analysis. SSC, side scatter. (D) Percentages of BrdU+ cells among WT OT1 and TSC1 KO OT1 populations in the peripheral blood and spleen. The mean ± the standard error of the mean was calculated for four mice per group. (E) Percentages of Ki67+ donor-derived OT1 T cells 4 days after Lm-Ova infection. The data shown are representative of two or three independent experiments. *, P < 0.05; **, P < 0.01 (Student t test).
To determine if WT and TSC1 KO OT1 cells proliferate comparably in vivo, we analyzed BrdU incorporation into these populations over a 16-h period during the expansion phase (on day 5 after Lm-Ova infection). Surprisingly, results from this experiment showed that a smaller pool of TSC1 KO cells than WT cells had proliferated during this period in both the peripheral blood and spleen (Fig. 5C and D). Consistent with these data, expression of Ki67, a marker of cell proliferation, was also reduced in TSC1 KO OT1 T cells on day 4 after Lm-Ova infection (Fig. 5E). Perturbations in proliferation in vivo, but not ex vivo, suggest the possibility that TSC1 deficiency may somehow limit the ability of CD8 cells to access or respond to antigen in vivo.
Enhanced CD8 cell death in the absence of TSC1.
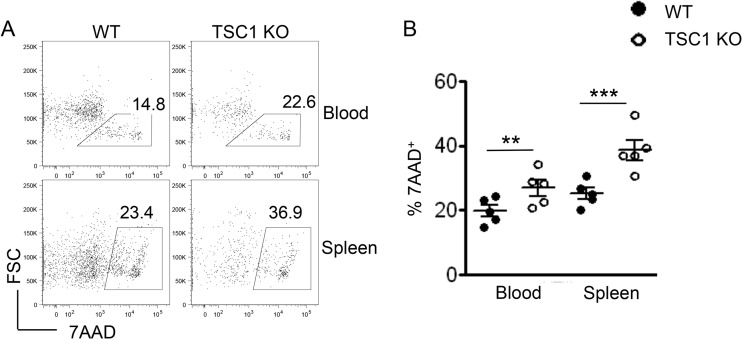
On the basis of results from previous studies that showed an increase in T cell apoptosis in the absence of TSC1, we hypothesized that enhanced cell death may also contribute to the poor expansion of TSC1 KO OT1 cells. To examine this possibility, we stained freshly isolated splenic and peripheral blood cells on day 6 postinfection with the exclusion dye 7AAD (Fig. 6A). Flow cytometric analysis revealed that a greater proportion of TSC1 KO OT1 cells than WT OT1 cells failed to exclude 7AAD (Fig. 6A and B), indicating that enhanced cell death in the TSC1 KO population may also play a role in curtailing expansion.
FIG 6.
Impaired survival of TSC1-deficient CD8 cells in vivo. (A) Representative fluorescence-activated cell sorter plots showing 7AAD staining within WT OT1 and TSC1 KO OT1 populations in the peripheral blood and spleen on day 6 postinfection (competitive adoptive transfers). FSC, forward scatter. (B) Percentages of 7AAD+ (nonviable) cells within WT OT1 and TSC1 KO OT1 populations in the peripheral blood and spleen on day 6 postinfection. The mean ± the standard error of the mean was calculated for five mice per group. The data shown are representative of two independent experiments. **, P < 0.01; ***, P < 0.001 (Student t test).
TSC1 deficiency alters effector-memory differentiation and promotes contraction.
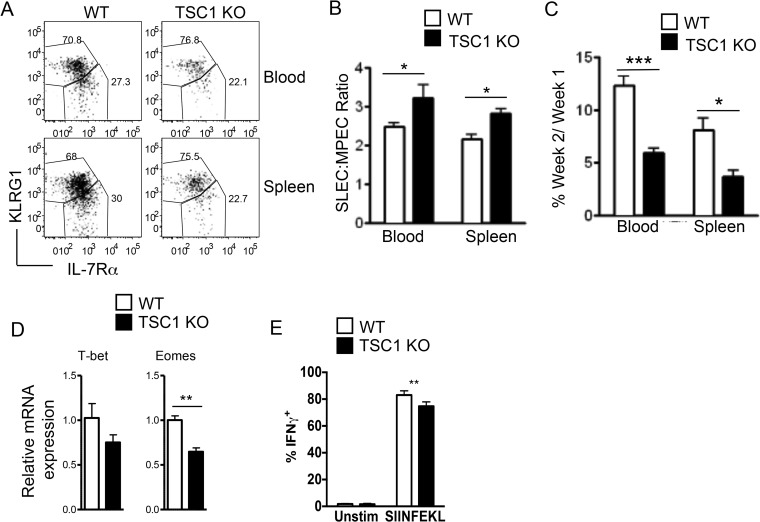
Given that impaired expansion of TSC1 KO OT1 cells is associated with defective proliferation and enhanced cell death, we next sought to determine if the absence of TSC1 could affect the dynamics of the CD8 response by altering effector-memory differentiation. To examine if effector-memory differentiation is affected by the loss of TSC1, we performed competitive adoptive transfers and examined the frequency of SLECs and MPECs among the WT OT1 and TSC1 KO OT1 populations at the peak of the response. Flow cytometric analysis revealed that the TSC1-deficient population contained a higher ratio of SLECs (KLRG1hi IL-7Rαlo) to MPECs (KLRG1lo IL-7Rαhi) than the TSC1 sufficient population (Fig. 7A and B) both in the peripheral blood and the spleen. These results are consistent with those from previous studies, which showed that sustained mTORc1 activity promotes T-bet expression and effector differentiation (18). We reasoned that a higher SLEC-to-MPEC ratio might be correlated with enhanced contraction. When we examined the frequency of cells surviving at week 2 postinfection as a percentage of the cells that were present at week 1, we found that only about 5% of the TSC1 KO cells survived the contraction phase, compared to more than 10% of the WT cells in peripheral blood (Fig. 7C). A similar trend was observed in the spleen, and these findings together suggest that loss of TSC1 may promote CD8 contraction.
FIG 7.
Loss of TSC1 alters CD8 cell effector-memory differentiation and promotes contraction. Mice received both naive WT and TSC1 KO OT1 T cells were infected with Lm-Ova in a fashion similar to that described in Fig. 3. (A) Representative fluorescence-activated cell sorter plots showing KLRG1 and IL-7Rα staining within WT OT1 and TSC1 KO OT1 populations in the peripheral blood and spleen on day 7 after infection. (B) Ratio of SLECs to MPECs within WT OT1 and TSC1 KO OT1 populations in the peripheral blood and spleen at week 1 postinfection. (C) Frequency of WT OT1 or TSC1 KO OT1 cells surviving in the peripheral blood and spleen at week 2 postinfection, as a percentage of the cells that were present at week 1. (D) T-bet and Eomes mRNA levels. Total RNA from sorted donor WT and TSC1 KO OT1 T cells 7 days after infection was subjected to reverse transcription and real-time quantitative PCR. (E) IFN-γ-producing cells within a donor OT1 T cell population. Seven days after infection, splenocytes were stimulated with SIINFEKL peptide in the presence of GolgiPlug for 5 h. The bar graph shows percentages of donor OT1 T cells that stained positive for IFN-γ. Unstim, unstimulated. All of the bar graphs represent the mean ± the standard error of the mean calculated for five mice per group. The data shown are representative of four (A to C), two (D), and three (E) independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Student t test).
To better understand the mechanisms by which TSC1 regulates effector-memory differentiation, we examined the expression of the transcription factors T-bet and Eomes in WT and TSC1 KO OT1 cells. A bevy of elegant studies have revealed a complex interplay between these closely related T-box transcription factors in CTL differentiation. While T-bet and Eomes act redundantly to induce CD8 effector functions, they have also been shown to act reciprocally to drive effector and memory cell differentiation, respectively (46). We found that Eomes mRNA levels were lower in TSC1 KO OT1 cells than those in WT controls at 7 days postinfection. Although T-bet mRNA also appeared to be lower in TSC1 KO OT1 cells, this difference was not statistically significant (Fig. 7D). It is possible that this decreased Eomes expression contributes to the higher SLEC-to-MPEC ratio that was observed at the peak of the response (Fig. 7A and B). Similar to but less severe than day 4 after Lm-Ova infection, fewer TSC1 KO OT1 cells than WT counterparts produced IFN-γ (Fig. 7E), which is also consistent with previous data reported by Yang et al. (33). In sum, these observations suggest that loss of TSC1 may perturb effector-memory differentiation and enhance CD8 contraction in the Lm-Ova model.
Moderate impairment of CD8 memory responses in the absence of TSC1.
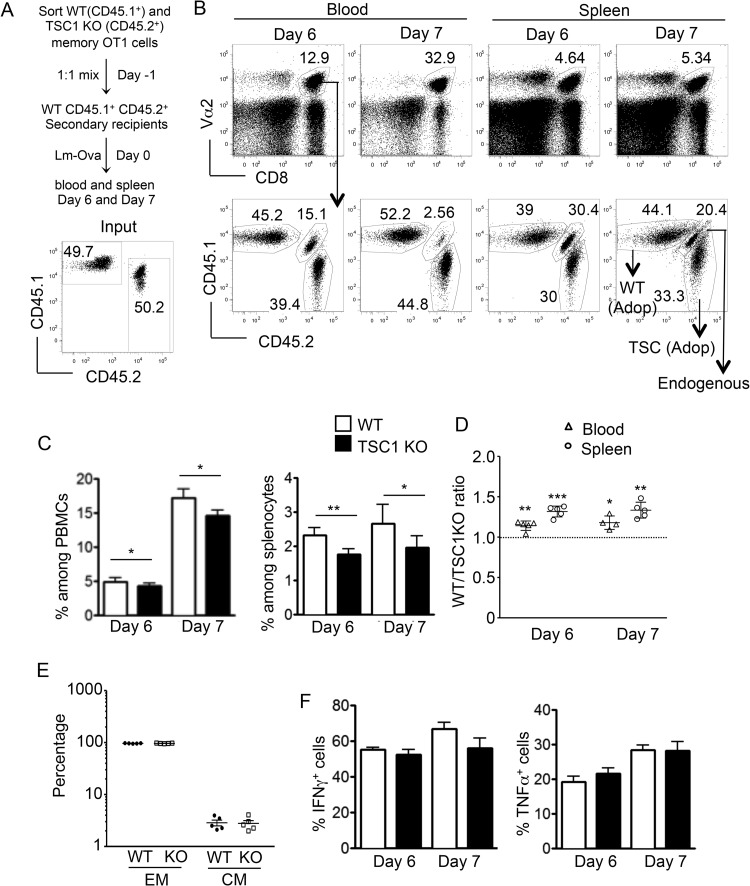
To assess the quality of TSC1-deficient memory cells in terms of their ability to respond to antigen reexposure, we adoptively transferred a mixture containing equal numbers of WT OT1 (CD45.1+) and TSC1 KO OT1 (CD45.2+) memory cells into congenically marked naive WT recipients (CD45.1+ CD45.2+). These recipients were subsequently challenged with a 10-fold higher dose (compared to the primary response) of Lm-Ova, and the memory response was monitored in the peripheral blood and spleen on days 6 and 7 postchallenge. While the ratio of adoptively transferred WT and TSC1 KO memory cells was close to 1:1 (Fig. 8A), flow cytometric analysis revealed a moderate reduction in TSC1 KO OT1 cell frequencies at days 6 and 7, compared to those of WT counterparts (Fig. 8B and C). These differences were more apparent in the spleen than in the peripheral blood, which is also supported by the increased ratios of WT to TSC1 KO OT1 cells in individual mice (Fig. 8D). Both adoptively transferred WT and TSC1 KO OT1 cells displayed similarly high percentages of CD44hi CD62Llo effector cells after Lm-Ova infection (Fig. 8E). Moreover, ex vivo SIINFEKL stimulation of splenocytes at days 6 and 7 postinfection revealed no significant differences in the production of effector cytokines such as IFN-γ and TNF-α (Fig. 8F). Though TSC1 deficiency severely limits the quantity of memory formation during primary responses, these results suggest that its effects on memory cell quality might be subtler.
FIG 8.
CD8 memory responses are moderately impaired in the absence of TSC1. (A) Schematic representation of the experimental design showing competitive adoptive transfers of WT OT1 and TSC1 KO OT1 memory cells into WT CD45.1+ CD45.2+ recipients. The percentages of WT and TSC1 KO cells during adoptive transfer are shown at the bottom. (B) Representative fluorescence-activated cell sorter analyses of peripheral blood and spleen samples showing the percentages of Vα2+ CD8 cells among the total PBMCs and splenocytes (top) and WT (CD45.1+ CD45.2−), TSC1 KO (CD45.1− CD45.2+), and endogenous (CD45.1+ CD45.2+) populations within the gated Vα2+ CD8 population (bottom) at the postchallenge times indicated. Adop, adoptive transfer. (C) Percentages of WT and TSC1 KO cells among the total PBMCs and splenocytes at the postchallenge times indicated. (D) WT-to-TSC1 KO CD8 cell ratios in individual mice. (E) Percentages of donor-derived WT and TSC1 KO effector memory (EM)- and central memory (CM)-like cells on day 7 after Lm-Ova infection. (F) Percentages of IFN-γ+ and TNF-α+ cells within the WT and TSC1 KO splenocyte populations at the postchallenge times indicated, as detected by intracellular staining and flow cytometry after 5 h of SIINFEKL stimulation. The mean ± the standard error of the mean was calculated for five mice per group. The data shown are representative of three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Student t test).
DISCUSSION
In this study, we demonstrated that loss of TSC1 in CD8 cells weakens antigen-specific primary CD8 responses. TSC1 KO CD8 cells showed poor expansion and an increased SLEC-to-MPEC ratio at the peak of the response, correlated with enhanced contraction and poor memory generation.
Our data suggest that impaired proliferation in vivo is a contributing factor in the decreased expansion of TSC1 KO CD8 cells in primary responses. Previous studies have examined a role for mTOR and TSC1 in the regulation of T cell proliferation. In ex vivo experiments, mTOR-deficient CD4 cells proliferated less than WT counterparts, and this defect was correlated with impaired upregulation of cyclin D3 expression (16). Results from our ex vivo assays, in which naive WT and TSC1 KO OT1 cells were stimulated with Ova peptide-loaded APCs, closely mirrored those from previous studies in which polyclonal TSC1-deficient CD4 and CD8 cells were stimulated with anti-CD3 antibodies (32). In both cases, TSC1-deficient cells did not show defects in proliferation and in fact appeared to proliferate slightly more than WT counterparts. Together, these results suggest that unlike a lack of mTOR activity, excessive mTOR activity may not be directly detrimental to T cell proliferation. Surprisingly, results from our in vivo BrdU incorporation and Ki67 staining experiments demonstrated a significant reduction in proliferating TSC1 KO OT1 cells during the expansion phase, suggesting that TSC1 may, in fact, regulate proliferation in vivo. One scenario that could reconcile these seemingly discrepant observations is that TSC1 deficiency might diminish the ability of CD8 cells to home to appropriate regions of secondary lymphoid organs and interact with APCs. This idea is supported by evidence that mTOR activity plays a role in downregulating the expression of CD62L and CCR7 (47), an adhesion molecule and a chemokine receptor, respectively, that are critical for lymph node and splenic white pulp homing of T cells (48–50). Further studies are required to test the hypothesis that chronic mTORc1 activity in TSC1-deficient CD8 cells can alter their trafficking patterns in a manner that limits interaction with APCs.
The propensity of TSC1 KO CD8 cells to undergo apoptosis may also contribute to impaired primary responses, accelerated contraction, and decreased memory formation of TSC1 KO CD8 cells following Lm-Ova infection. This observation is consistent with previous observations in different experimental settings (32, 33, 35). While TSC1 deficiency enhances mTORc1 activity, previous studies have shown that it is also associated with decreased mTORc2-Akt activity in T cells (32, 33). As an important prosurvival molecule, decreased Akt activity may contribute to impair CD8 cell responses. Additionally, TSC1-deficient T cells were shown to contain elevated reactive oxygen species and to exhibit decreased mitochondrial content and membrane potential, which may lead to the activation of the intrinsic death pathway. Though it stands to reason that increased mTORc1 activity might play a major role in perturbing antigen-specific CD8 responses in the absence of TSC1, further studies are required to dissect the contributions of the two mTOR complexes to the phenotype observed.
A complex interplay between the closely related transcription factors T-bet and Eomes underlies several aspects of CD8 cell function, including IFN-γ production and effector-versus-memory differentiation (51, 52). T-bet and Eomes show functional homology in several aspects of T cell function (53) but are inversely regulated and thought to act antagonistically during CD8 differentiation. Although previous work has shown that IL-12, a hallmark cytokine of cell-mediated immunity, promotes T-bet expression and represses Eomes expression in CD8 cells during L. monocytogenes infection via enhanced mTOR signaling (18, 54, 55), we have found that TSC1 deficiency and increased mTORc1 signaling cause decreased T-bet expression in CD8 cells. Consistently, TSC1 KO iNKT cells expressed reduced levels of T-bet and impaired IFN-γ-producing iNKT lineage differentiation (36). Studies have shown that both T-bet and Eomes are important for IFN-γ expression (56, 57), and the reduced frequency of IFN-γ production during primary responses may be associated with decreased T-bet in TSC1-deficient CD8 cells. Further studies are required to clearly define the molecular mechanisms by which TSC1 deficiency may modulate the complex transcriptional network to control CD8 effector-memory differentiation.
Although we sorted naive CD44− CD62Lhi OT1 T cells from TSC1f/f CD4Cre OT1 mice for the adoptive-transfer experiments, these cells might already display certain differences from WT naive OT1 cells. For example, TSC1 KO naive OT1 T cells expressed slightly higher levels of CD122/IL-15Rβ than WT controls. IL-15 plays important roles not only in memory CD8 T cell expansion and maintenance but also in CD8 T cell primary expansion (58, 59). Increased availability of IL-15 has been shown to delay contraction during primary CD8 T cell responses (60, 61). These observations suggest that elevated CD122 expression in TSC1 KO naive CD8 T cells might not cause impaired expansion of these cells during primary responses. Nevertheless, future studies using inducible TSC1-deficient OT1 T cells are needed to conclusively determine the contribution of upregulated expression of CD122 or abnormal expression of other molecules in TSC1 KO naive T cells to the impaired primary responses to bacterial infection.
While the quantity of memory cells was diminished in the absence of TSC1, we investigated the quality of the memory cells by using competitive adoptive transfers. In these experiments, memory TSC1 KO cells showed only moderate defects in expansion and cytokine production upon rechallenge, indicating a differential requirement of TSC1 for primary and memory CD8 cell responses to bacterial infection. Memory T cells differ from naive counterparts in both their migration patterns and their thresholds for activating stimuli (6). It is possible that these differences may mitigate the effects of TSC1 deficiency on memory cells, allowing them to expand more efficiently than TSC1-deficient naive cells. Additionally, TSC1 KO CD8 memory cells may adapt their signaling machinery to compensate for the loss of TSC1 activity. Further studies are required to examine these possibilities.
In conclusion, our study identifies a critical role for TSC1 in the regulation of both the quantitative and qualitative aspects of antigen-specific CD8 responses and suggests that impaired proliferation, enhanced cell death, and altered effector-memory differentiation contribute to impaired CD8 responses in the absence of TSC1.
ACKNOWLEDGMENTS
We thank Lynn Martinek, Nancy Martin, and Mike Cook at the Duke University Comprehensive Cancer Center flow cytometry facility for cell sorting and technical advice and Weiguo Zhang and You-Wen He for providing the Lm-Ova strains.
This work was supported by the National Institutes of Health (AI076357, AI079088, and AI101206) and the American Cancer Society (RSG-08-186-01-LIB).
S.K. designed and conducted experiments, analyzed data, and wrote the paper; J.Y., H.W., and Y.Q. conducted experiments and analyzed data. X.-P.Z. conceived and supervised the project, designed research, and edited the paper.
We have no competing financial interests to declare.
Footnotes
Published ahead of print 12 May 2014
REFERENCES
- 1.Williams MA, Bevan MJ. 2007. Effector and memory CTL differentiation. Annu. Rev. Immunol. 25:171–192. 10.1146/annurev.immunol.25.022106.141548 [DOI] [PubMed] [Google Scholar]
- 2.Zhang N, Bevan MJ. 2011. CD8+ T cells: foot soldiers of the immune system. Immunity 35:161–168. 10.1016/j.immuni.2011.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. 2007. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity 27:281–295. 10.1016/j.immuni.2007.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4:1191–1198. 10.1038/ni1009 [DOI] [PubMed] [Google Scholar]
- 5.Badovinac VP, Porter BB, Harty JT. 2002. Programmed contraction of CD8+ T cells after infection. Nat. Immunol. 3:619–626. 10.1038/nrm880 [DOI] [PubMed] [Google Scholar]
- 6.Porter BB, Harty JT. 2006. The onset of CD8+-T-cell contraction is influenced by the peak of Listeria monocytogenes infection and antigen display. Infect. Immun. 74:1528–1536. 10.1128/IAI.74.3.1528-1536.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox MA, Kahan SM, Zajac AJ. 2013. Anti-viral CD8 T cells and the cytokines that they love. Virology 435:157–169. 10.1016/j.virol.2012.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badovinac VP, Porter BB, Harty JT. 2004. CD8+ T cell contraction is controlled by early inflammation. Nat. Immunol. 5:809–817. 10.1038/ni1098 [DOI] [PubMed] [Google Scholar]
- 9.Teixeiro E, Daniels MA, Hamilton SE, Schrum AG, Bragado R, Jameson SC, Palmer E. 2009. Different T cell receptor signals determine CD8+ memory versus effector development. Science 323:502–505. 10.1126/science.1163612 [DOI] [PubMed] [Google Scholar]
- 10.Ou-Yang CW, Zhu M, Sullivan SA, Fuller DM, Zhang W. 2013. The requirement of linker for activation of T cells in the primary and memory responses of CD8 T cells. J. Immunol. 190:2938–2947. 10.4049/jimmunol.1203163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson LJ, Kolumam GA, Thomas S, Murali-Krishna K. 2006. Innate inflammatory signals induced by various pathogens differentially dictate the IFN-I dependence of CD8 T cells for clonal expansion and memory formation. J. Immunol. 177:1746–1754. 10.4049/jimmunol.177.3.1746 [DOI] [PubMed] [Google Scholar]
- 12.Keppler SJ, Rosenits K, Koegl T, Vucikuja S, Aichele P. 2012. Signal 3 cytokines as modulators of primary immune responses during infections: the interplay of type I IFN and IL-12 in CD8 T cell responses. PLoS One 7:e40865. 10.1371/journal.pone.0040865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zoncu R, Efeyan A, Sabatini DM. 2011. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 12:21–35. 10.1038/nrm3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sengupta S, Peterson TR, Sabatini DM. 2010. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol. Cell 40:310–322. 10.1016/j.molcel.2010.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guertin DA, Sabatini DM. 2007. Defining the role of mTOR in cancer. Cancer Cell 12:9–22. 10.1016/j.ccr.2007.05.008 [DOI] [PubMed] [Google Scholar]
- 16.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. 2009. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 30:832–844. 10.1016/j.immuni.2009.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. 2009. mTOR regulates memory CD8 T-cell differentiation. Nature 460:108–112. 10.1038/nature08155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao RR, Li Q, Odunsi K, Shrikant PA. 2010. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and eomesodermin. Immunity 32:67–78. 10.1016/j.immuni.2009.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, Worley PF, Powell JD. 2011. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat. Immunol. 12:295–303. 10.1038/ni.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Brien TF, Zhong XP. 2012. The role and regulation of mTOR in T-lymphocyte function. Arch. Immunol. Ther. Exp. 60:173–181. 10.1007/s00005-012-0171-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee K, Gudapati P, Dragovic S, Spencer C, Joyce S, Killeen N, Magnuson MA, Boothby M. 2010. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity 32:743–753. 10.1016/j.immuni.2010.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurebayashi Y, Nagai S, Ikejiri A, Ohtani M, Ichiyama K, Baba Y, Yamada T, Egami S, Hoshii T, Hirao A, Matsuda S, Koyasu S. 2012. PI3K-Akt-mTORC1-S6K1/2 axis controls Th17 differentiation by regulating Gfi1 expression and nuclear translocation of RORgamma. Cell Rep. 1:360–373. 10.1016/j.celrep.2012.02.007 [DOI] [PubMed] [Google Scholar]
- 23.Shin J, Wang S, Deng W, Wu J, Gao J, Zhong XP. 2014. Mechanistic target of rapamycin complex 1 is critical for invariant natural killer T-cell development and effector function. Proc. Natl. Acad. Sci. U. S. A. 111:E776–E783. 10.1073/pnas.1315435111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng H, Yang K, Cloer C, Neale G, Vogel P, Chi H. 2013. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature 499:485–490. 10.1038/nature12297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Q, Rao RR, Araki K, Pollizzi K, Odunsi K, Powell JD, Shrikant PA. 2011. A central role for mTOR kinase in homeostatic proliferation induced CD8+ T cell memory and tumor immunity. Immunity 34:541–553. 10.1016/j.immuni.2011.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Q, Rao R, Vazzana J, Goedegebuure P, Odunsi K, Gillanders W, Shrikant PA. 2012. Regulating mammalian target of rapamycin to tune vaccination-induced CD8+ T cell responses for tumor immunity. J. Immunol. 188:3080–3087. 10.4049/jimmunol.1103365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inoki K, Li Y, Zhu T, Wu J, Guan KL. 2002. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 4:648–657. 10.1038/ncb839 [DOI] [PubMed] [Google Scholar]
- 28.Benvenuto G, Li S, Brown SJ, Braverman R, Vass WC, Cheadle JP, Halley DJ, Sampson JR, Wienecke R, DeClue JE. 2000. The tuberous sclerosis-1 (TSC1) gene product hamartin suppresses cell growth and augments the expression of the TSC2 product tuberin by inhibiting its ubiquitination. Oncogene 19:6306–6316. 10.1038/sj.onc.1204009 [DOI] [PubMed] [Google Scholar]
- 29.Chong-Kopera H, Inoki K, Li Y, Zhu T, Garcia-Gonzalo FR, Rosa JL, Guan KL. 2006. TSC1 stabilizes TSC2 by inhibiting the interaction between TSC2 and the HERC1 ubiquitin ligase. J. Biol. Chem. 281:8313–8316. 10.1074/jbc.C500451200 [DOI] [PubMed] [Google Scholar]
- 30.Inoki K, Li Y, Xu T, Guan KL. 2003. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 17:1829–1834. 10.1101/gad.1110003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang J, Manning BD. 2008. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem. J. 412:179–190. 10.1042/BJ20080281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Brien TF, Gorentla BK, Xie D, Srivatsan S, McLeod IX, He YW, Zhong XP. 2011. Regulation of T-cell survival and mitochondrial homeostasis by TSC1. Eur. J. Immunol. 41:3361–3370. 10.1002/eji.201141411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang K, Neale G, Green DR, He W, Chi H. 2011. The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nat. Immunol. 12:888–897. 10.1038/ni.2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Q, Liu Y, Chen C, Ikenoue T, Qiao Y, Li CS, Li W, Guan KL, Zheng P. 2011. The tuberous sclerosis complex-mammalian target of rapamycin pathway maintains the quiescence and survival of naive T cells. J. Immunol. 187:1106–1112. 10.4049/jimmunol.1003968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang L, Zhang H, Li L, Xiao Y, Rao E, Miao Z, Chen H, Sun L, Li H, Liu G, Zhao Y. 2012. TSC1/2 signaling complex is essential for peripheral naive CD8+ T cell survival and homeostasis in mice. PLoS One 7:e30592. 10.1371/journal.pone.0030592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu J, Yang J, Yang K, Wang H, Gorentla B, Shin J, Qiu Y, Que LG, Foster WM, Xia Z, Chi H, Zhong XP. 2014. iNKT cells require TSC1 for terminal maturation and effector lineage fate decisions. J. Clin. Invest. 124:1685–1698. 10.1172/JCI69780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu J, Shin J, Xie D, Wang H, Gao J, Zhong XP. 2014. Tuberous sclerosis 1 promotes invariant NKT cell anergy and inhibits invariant NKT cell-mediated antitumor immunity. J. Immunol. 192:2643–2650. 10.4049/jimmunol.1302076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park Y, Jin HS, Lopez J, Elly C, Kim G, Murai M, Kronenberg M, Liu YC. 2013. TSC1 regulates the balance between effector and regulatory T cells. J. Clin. Invest. 123:5165–5178. 10.1172/JCI69751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benhamron S, Tirosh B. 2011. Direct activation of mTOR in B lymphocytes confers impairment in B-cell maturation and loss of marginal zone B cells. Eur. J. Immunol. 41:2390–2396. 10.1002/eji.201041336 [DOI] [PubMed] [Google Scholar]
- 40.Pan H, O'Brien TF, Wright G, Yang J, Shin J, Wright KL, Zhong XP. 2013. Critical role of the tumor suppressor tuberous sclerosis complex 1 in dendritic cell activation of CD4 T cells by promoting MHC class II expression via IRF4 and CIITA. J. Immunol. 191:699–707. 10.4049/jimmunol.1201443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan H, O'Brien TF, Zhang P, Zhong XP. 2012. The role of tuberous sclerosis complex 1 in regulating innate immunity. J. Immunol. 188:3658–3666. 10.4049/jimmunol.1102187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shin J, Pan H, Zhong XP. 2012. Regulation of mast cell survival and function by tuberous sclerosis complex 1. Blood 119:3306–3314. 10.1182/blood-2011-05-353342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Condotta SA, Richer MJ, Badovinac VP, Harty JT. 2012. Probing CD8 T cell responses with Listeria monocytogenes infection. Adv. Immunol. 113:51–80. 10.1016/B978-0-12-394590-7.00005-1 [DOI] [PubMed] [Google Scholar]
- 44.Shen H, Slifka MK, Matloubian M, Jensen ER, Ahmed R, Miller JF. 1995. Recombinant Listeria monocytogenes as a live vaccine vehicle for the induction of protective anti-viral cell-mediated immunity. Proc. Natl. Acad. Sci. U. S. A. 92:3987–3991. 10.1073/pnas.92.9.3987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pope C, Kim SK, Marzo A, Masopust D, Williams K, Jiang J, Shen H, Lefrancois L. 2001. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J. Immunol. 166:3402–3409. 10.4049/jimmunol.166.5.3402 [DOI] [PubMed] [Google Scholar]
- 46.Kallies A. 2008. Distinct regulation of effector and memory T-cell differentiation. Immunol. Cell Biol. 86:325–332. 10.1038/icb.2008.16 [DOI] [PubMed] [Google Scholar]
- 47.Sinclair LV, Finlay D, Feijoo C, Cornish GH, Gray A, Ager A, Okkenhaug K, Hagenbeek TJ, Spits H, Cantrell DA. 2008. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat. Immunol. 9:513–521. 10.1038/ni.1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weninger W, Manjunath N, von Andrian UH. 2002. Migration and differentiation of CD8+ T cells. Immunol. Rev. 186:221–233. 10.1034/j.1600-065X.2002.18618.x [DOI] [PubMed] [Google Scholar]
- 49.Ebert LM, Schaerli P, Moser B. 2005. Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues. Mol. Immunol. 42:799–809. 10.1016/j.molimm.2004.06.040 [DOI] [PubMed] [Google Scholar]
- 50.Nolz JC, Starbeck-Miller GR, Harty JT. 2011. Naive, effector and memory CD8 T-cell trafficking: parallels and distinctions. Immunotherapy 3:1223–1233. 10.2217/imt.11.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, Shen H, Cereb N, Yang SY, Lindsten T, Rossant J, Hunter CA, Reiner SL. 2003. Control of effector CD8+ T cell function by the transcription factor eomesodermin. Science 302:1041–1043. 10.1126/science.1090148 [DOI] [PubMed] [Google Scholar]
- 52.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. 2002. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science 295:338–342. 10.1126/science.1065543 [DOI] [PubMed] [Google Scholar]
- 53.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. 2005. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 6:1236–1244. 10.1038/ni1268 [DOI] [PubMed] [Google Scholar]
- 54.Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. 2006. Cutting edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J. Immunol. 177:7515–7519. 10.4049/jimmunol.177.11.7515 [DOI] [PubMed] [Google Scholar]
- 55.Rao RR, Li Q, Gubbels Bupp MR, Shrikant PA. 2012. Transcription factor Foxo1 represses T-bet-mediated effector functions and promotes memory CD8+ T cell differentiation. Immunity 36:374–387. 10.1016/j.immuni.2012.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cruz-Guilloty F, Pipkin ME, Djuretic IM, Levanon D, Lotem J, Lichtenheld MG, Groner Y, Rao A. 2009. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J. Exp. Med. 206:51–59. 10.1084/jem.20081242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu Y, Ju S, Chen E, Dai S, Li C, Morel P, Liu L, Zhang X, Lu B. 2010. T-bet and eomesodermin are required for T cell-mediated antitumor immune responses. J. Immunol. 185:3174–3183. 10.4049/jimmunol.1000749 [DOI] [PubMed] [Google Scholar]
- 58.Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R. 2002. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 195:1541–1548. 10.1084/jem.20020369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schluns KS, Williams K, Ma A, Zheng XX, Lefrancois L. 2002. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J. Immunol. 168:4827–4831. 10.4049/jimmunol.168.10.4827 [DOI] [PubMed] [Google Scholar]
- 60.Yajima T, Yoshihara K, Nakazato K, Kumabe S, Koyasu S, Sad S, Shen H, Kuwano H, Yoshikai Y. 2006. IL-15 regulates CD8+ T cell contraction during primary infection. J. Immunol. 176:507–515. 10.4049/jimmunol.176.1.507 [DOI] [PubMed] [Google Scholar]
- 61.Rubinstein MP, Lind NA, Purton JF, Filippou P, Best JA, McGhee PA, Surh CD, Goldrath AW. 2008. IL-7 and IL-15 differentially regulate CD8+ T-cell subsets during contraction of the immune response. Blood 112:3704–3712. 10.1182/blood-2008-06-160945 [DOI] [PMC free article] [PubMed] [Google Scholar]