Abstract
Porphyromonas gingivalis is a Gram-negative obligate anaerobic bacterium and is considered a keystone pathogen in the initiation of periodontitis, one of the most widespread infectious diseases. Bacterial bis-(3′-5′) cyclic GMP (cyclic di-GMP [c-di-GMP]) serves as a second messenger and is involved in modulating virulence factors in numerous bacteria. However, the role of this second messenger has not been investigated in P. gingivalis, mainly due to a lack of an annotation regarding diguanylate cyclases (DGCs) in this bacterium. Using bioinformatics tools, we found a protein, PGN_1932, containing a GGDEF domain. A deletion mutation in the pgn_1932 gene had a significant effect on the intracellular c-di-GMP level in P. gingivalis. Genetic analysis showed that expression of the fimA and rgpA genes, encoding the major protein subunit of fimbriae and an arginine-specific proteinase, respectively, was downregulated in the pgn_1932 mutant. Correspondingly, FimA protein production and the fimbrial display on the mutant were significantly reduced. Mutations in the pgn_1932 gene also had a significant impact on the adhesive and invasive capabilities of P. gingivalis, which are required for its pathogenicity. These findings provide evidence that the PGN_1932 protein is both responsible for synthesizing c-di-GMP and involved in biofilm formation and host cell invasion by P. gingivalis by controlling the expression and biosynthesis of FimA.
INTRODUCTION
Bis-(3′-5′) cyclic GMP (cyclic di-GMP [c-di-GMP]) was discovered in Gluconacetobacter xylinus as a bacterial cellular synthase by Moshe Benziman and colleagues more than 2 decades ago and is now considered a common bacterial second messenger (1). To date, most studies have focused on diguanylate cyclases (DGCs), c-di-GMP-specific phosphodiesterases (PDEs), and c-di-GMP receptors/effectors, such as PilZ domain proteins, responsible for downstream biological processes of c-di-GMP signaling (2). DGC cyclizes two GTP molecules into the bis-cyclic diguanylic acid (c-di-GMP) (3). The GGDEF domains found in typical DGCs appear to be sufficient for diguanylate cyclase activity (4–6). In contrast, PDE enzymes are responsible for c-di-GMP hydrolysis, which converts a c-di-GMP into a linear di-GMP, and an EAL or HD-GYP domain is required for this PDE activity (7, 8). DGC and PDE enzymes are found in about 85% of bacteria, including many bacteria considered to be human pathogens, as determined by genome sequence analysis (9). The active domains of these enzymes are highly conserved between/among bacterial species. A good example is the DGC domain of hmsT from Yersinia pestis, which complements a mutation in adrA of Salmonella enterica (10). It is also striking that many bacteria possess a large array of DGC and PDE enzymes. For example, 62 genes of Vibrio cholerae and 29 genes of Escherichia coli K-12 were found to encode proteins with DGC and PDE domains, suggesting that a complex system governing c-di-GMP levels exists in these bacteria (9, 11). Thus, intracellular levels of c-di-GMP were found to be modulated in V. cholerae in response to cell density and to different growth phases (11).
The association between c-di-GMP levels and bacterial virulence varies by bacterial species. For instance, an increase in intracellular c-di-GMP concentrations in a vieA mutant with a deficiency in PDE activity led to an attenuation of disease in an infant mouse model of cholera (5). Similarly, deletion of all DGC genes increased bacterial virulence in a mouse model of Brucella melitensis infection, while deletion of all PDE genes reduced the virulence, indicating a negative relationship between c-di-GMP levels and bacterial virulence in vivo (12). As a second messenger, c-di-GMP plays a key role in many cellular functions, such as regulation of the cell cycle, motility, and biofilm formation, as well as in dispersion. Mechanisms of c-di-GMP signaling affecting virulence may therefore be associated with the role of c-di-GMP in a bacterium's ability to adhere to and/or invade host cells, its cytotoxicity, its resistance to oxidative stress, and the modulation of immune responses (5, 13, 14). Numerous studies have led to a consistent finding that c-di-GMP promotes biofilm formation. Biosynthesis of cellulose, fimbriae, exopolysaccharides, and alginate, all of which are well-known biofilm-related molecules, requires c-di-GMP synthesis in many bacterial species, such as Escherichia coli, Pseudomonas aeruginosa, and Salmonella enterica (15–18).
Despite the fact that c-di-GMP acts as a second messenger in bacteria and has been the focus of molecular microbiology for the past 25 years, studies pursuing the role of c-di-GMP in oral bacteria are very limited. However, through genome analyses, Treponema denticola was reported to possess proteins with GGDEF, EAL, and PilZ domains, suggesting that c-di-GMP may also serve as an important regulatory molecule in T. denticola (19). In fact, a recent study showed that a PilZ-like c-di-GMP binding protein plays important roles in the motility, biofilm formation, and virulence of T. denticola (20). Interestingly, an in vitro study demonstrated that extracellular c-di-GMP inhibited the adherence of Streptococcus mutans to tooth surfaces and reduced biofilm formation by >50% compared to that for untreated controls, although the mechanism was unknown (21).
A number of potential virulence factors have been identified in Porphyromonas gingivalis, including cysteine proteinases (gingipains), lipopolysaccharide (LPS), a capsule, and fimbriae. The majority of P. gingivalis clinical isolates, especially those isolated at the bottoms of periodontal pockets, are fimbriated (22–24). Major fimbriae (long fimbriae) composed of FimA represent a well-studied virulence factor that contributes to colonization, biofilm formation, cell invasion, bone resorption, and evasion of host defense mechanisms (25, 26). Two genes (pgn_0239 and pgn_0282) have been annotated as a putative type I phosphodiesterase-nucleotide pyrophosphatase and a 2′,3′-cyclic-nucleotide 2′-phosphodiesterase precursor, respectively, by analysis of the genome sequence of strain ATCC 33277 (27). However, the potential role of c-di-GMP has not yet been investigated in P. gingivalis, mainly due to a lack of annotation of DGC enzymes.
In this study, we identified, using bioinformatics tools, a GGDEF domain in PGN_1932, which was initially annotated as a hypothetical protein. Mutation in this putative dgc (pgn_1932) gene led to significant alterations in the virulence properties of P. gingivalis, such as decreased adherence and invasive abilities. We further demonstrate that the expression and production of FimA are significantly reduced in the pgn_1932 mutant compared to those in the wild-type strain ATCC 33277.
MATERIALS AND METHODS
Bacterial and eukaryotic cell culture conditions.
The bacterial strains and host cell lines that have been used in this work are listed in Table 1. P. gingivalis strains were grown in Trypticase soy broth (TSB; Becton, Dickinson and Company) or on TSB blood agar plates supplemented with yeast extract (1 mg/ml), hemin (5 μg/ml), and menadione (1 μg/ml) in an anaerobic chamber (85% N2, 10% H2, 5% CO2) at 37°C. The following antibiotics were used under the appropriate conditions: gentamicin (50 μg/ml) and erythromycin (10 μg/ml). HeLa cells and human periodontal ligament fibroblasts (HPDLFs) were used as the host for bacterial attachment and invasion studies. HeLa cells were cultured at 37°C under a 5% CO2 atmosphere in Dulbecco's modified Eagle medium (DMEM; Life Technologies) supplemented with 10% fetal bovine serum (FBS; Invitrogen). HPDLFs were cultured in fibroblast medium supplemented with FBS and fibroblast growth supplement (ScienCell Research Laboratories) in a poly-l-lysine-coated flask at 37°C in 5% CO2 according to the manufacturer's protocol.
TABLE 1.
Bacterial strains and host cell lines used in this study
| Strain or cell line | Relevant characteristic(s) | Source or reference |
|---|---|---|
| P. gingivalis strains | ||
| ATCC 33277 | Type strain from ATCC | Lab collection |
| 1932E | P. gingivalis mutant with the pgn_932 gene inactivated by insertion of an ermF-ermAM cassette, Emra | This study |
| FAE | P. gingivalis mutant with the fimA gene inactivated by insertion of an ermF-ermAM cassette, Emr | 46 |
| Host cells | ||
| HeLa | Human epithelial cell line | ATCC |
| HPDLF | Human periodontal ligament fibroblast | ScienCell Research Laboratories |
Emr, resistance to erythromycin.
Bioinformatics.
To identify open reading frames that contain a GGDEF domain, a BLAST search (28) was carried out. Short query sequences that characterize a GGDEF domain (including GGDEF, GGDDF, GGEEF, and GGEDF) were used to search against sequences in the nonredundant protein sequence database of P. gingivalis ATCC 33277. All the hits from the BLAST search were then subjected to InterProScan (29), Motif Scan (30), and ScanProsite (31) searches for further confirmation.
RNA isolation and quantitative PCR (qPCR).
P. gingivalis strains were grown anaerobically in 5 ml of TSB. Bacteria were harvested by centrifugation at 10,000 × g and homogenized in TRIzol reagent (Invitrogen). The RNA in the supernatant was then purified using an RNeasy mini-spin column (Qiagen). RNA samples were digested on the column with RNase-free DNase. The total RNA was tested using an Agilent 2100 bioanalyzer to ensure the quality of the samples. Real-time reverse transcription-PCR (RT-PCR) analysis was performed by using a QuantiTect SYBR green RT-PCR kit (Qiagen) on an iCycler MyiQ real-time PCR detection system (Bio-Rad Laboratories, Inc., Hercules, CA) according to the manufacturer's instructions. Primers are listed in Table 2. The expression levels of the investigated genes for the test samples were determined relative to the expression level of the untreated calibrator sample by using the comparative cycle threshold (ΔCT) method. ΔCT values were calculated by subtracting the average CT value of the test sample from the average CT value of the calibrator sample and were then used to calculate the ratio between the two by assuming 100% amplification efficiency. By loading the same amount of total RNA for any comparable samples, ΔCT represents the difference in gene expression between the samples.
TABLE 2.
Primers used in this study
| Primer name | Primer sequencea | Application |
|---|---|---|
| Pgn_1932F1 | GGTGGTTTGCTGTGGAAAGT | Creation of the pgn_1932 mutant |
| Pgn1932R1erm | gatgttgcaaataccgatgagcGTTGAGGTGGCCAATGAGTT | |
| Pgn1932F2erm | cctctagagtcgacctgcagTCTTCCGCTTTTGGTCACTT | |
| Pgn_1932R2 | ATCCTTGCGCATAGAACCAG | |
| 1932expF | CAACAACATATGTGGACGGCGAGCTACCTCTTC | Creation of recombinant PGN_1932 |
| 1932expR | CAACAAGGATCCTTATTTCTCTTTTTTAGCCGTTACAAATTG | |
| rgpAF160 | GTCACCGTCTGCATCGATCG | qPCR for rgp gene |
| rgpAR160 | AGTCGGCCAGAAAGTAACGC | |
| fimAF88 | CGGAACGAATAACCCAGAGA | qPCR for fimA gene |
| fimAR88 | CTGACCAACGAGAACCCACT | |
| fimR124F | TAACCACGGGTAGCCATTTC | qPCR for fimR gene |
| fimR124R | GAGTCGTTCTGCTGCTGTTG | |
| sod235F | AATTCCACCACGGTAAGCAC | qPCR for sod gene |
| sod235R | GAGCCGAATTGTTTGTCGAT | |
| Pgn0178-113F | CCACTTCTTCGGGCTTCAC | qPCR for pgn_0178 gene |
| Pgn0178-113R | CAATGGCGTATCCCTCGTAT | |
| Pgn0179-104F | TACACAAGCGAAGCTCTCCAA | qPCR for pgn_0179 gene |
| Pgn0179-104R | TGAAATTTCCAATCGTATTC | |
| Pgn0181-128F | GACGATCACATGCTGGAAGA | qPCR for pgn_0181 gene |
| Pgn0181-128F | ATTGGGCAGGTCAGTACACC | |
| Pgn0183-131F | CGGCTTCCTTGTACCCTATG | qPCR for pgn_0183 gene |
| Pgn0183-131R | CTTTCCAACGGCTACCATCT | |
| uvrAII-112F | GAAGGAACGGTGGAGGAAC | qPCR for uvrAII gene |
| uvrAII-112R | GGCATGCCCCGATAGGATTG | |
| PG16S-F | TGTAGATGACTGATGGTGAAA | qPCR for 16S rRNA gene |
| PG16S-R | ACTGTTAGCAACTACCGATGT |
The sequences corresponding to those of the erythromycin resistance (Emr) gene are underlined and in lowercase.
Construction of pgn_1932 mutants.
An insertional trx (pgn_1932) mutant was generated by using ligation-independent cloning of products obtained by PCR-mediated mutagenesis (LIC-PCR) (32–34). A 2.1-kb ermF-ermAM cassette was introduced into the pgn_1932 gene by three steps of PCR to yield a pgn_1932–erm–pgn_1932 DNA fragment including an insertion mutation and a deletion mutation, as described previously (33). The specific primers used are listed in Table 2. The final PCR products were then introduced into P. gingivalis ATCC 33277 by electroporation. The pgn_1932-deficient mutant was generated via a double-crossover event that replaced the pgn_1932 gene with the pgn_1932–erm–pgn_1932 DNA fragment in the ATCC 33277 chromosome. The mutants were selected on TSB plates containing erythromycin (5 μg/ml). The insertion mutation was confirmed by PCR analysis, and the mutant was designated P. gingivalis 1932E.
Bacterial attachment and invasion assays.
Attachment of P. gingivalis to saliva-coated surfaces was quantified by the method of O'Toole and Kolter (35). Saliva collection was approved by the Institutional Review Board of Meharry Medical College, and saliva was collected from periodontally healthy donors. After centrifugation and filter sterilization (pore size, 0.22 μm) to remove cell debris, saliva was incubated in a 96-well polystyrene plate (Corning Incorporated) for 1 h. The plate was then washed with phosphate-buffered saline (PBS; 100 mM NaH2PO4, 150 mM NaCl). P. gingivalis strains were grown in TSB to mid-log phase (optical density at 600 nm [OD600] = 1.0) and collected by centrifugation. The bacterial cells were resuspended in prereduced (1/4) Tris-buffered saline TSB (1 volume of TSB and 3 volumes of PBS). The cells (5 × 107) were added to each well of a 96-well plate that had been precoated with human whole saliva and incubated at 37°C in an anaerobic chamber for 24 h. The bacterial biofilms were stained with 1% crystal violet for 15 min. After washing with PBS three times, the biofilms were destained with 200 μl of 95% ethanol for 30 s; 125 μl of absolute ethanol from each well was transferred to a fresh well for reading at 570 nm in a Benchmark Plus microplate spectrophotometer (Bio-Rad).
To determine the attachment of P. gingivalis to host cells, HeLa cells or HPDLFs (5 × 105) were seeded in the well of a six-well plate for 16 h to reach an ∼80% confluence. Cells were infected with P. gingivalis strains ATCC 33277, 1932E, and FAE (the fimA mutant) at a multiplicity of infection (MOI) of 100 for 1 h. To inhibit bacterial invasion, cytochalasin D (2.5 μg/ml) was preincubated with the cells for 30 min prior to addition of the bacteria and remained present throughout the invasion assay (36). The unbound bacteria were removed by washing thrice in PBS. One milliliter of sterile distilled water was added to each well, and the plate was incubated for 15 min at room temperature to lyse eukaryotic cells. The surface-bound and internalized bacterial cells were collected by centrifugation and resuspended in 100 μl Tris-EDTA (TE) buffer (Invitrogen). To release the bacterial DNA, the bacterial suspensions were boiled at 95°C for 20 min. The number of bacterial cells was measured by qPCR using primers specific for P. gingivalis 16S rRNA.
The invasive ability of P. gingivalis and its mutants was determined using an antibiotic protection assay (37). Host cells (5 × 105) were seeded in a six-well plate, and after they were infected with P. gingivalis strains ATCC 33277, 1932E, and FAE for 1 h, the cells were washed with PBS to remove the unbound bacteria and cultured for another 4 h in the presence of the antibiotics gentamicin (300 μg/ml) and metronidazole (200 μg/ml) to eliminate extracellular bacteria. The wells were then washed thrice with PBS and lysed with sterile distilled H2O. The internalized bacteria were collected by centrifugation and plated on TSB blood agar plates. The plates were incubated anaerobically at 37°C for 7 days, and the CFU of P. gingivalis were then enumerated.
ELISA.
Enzyme-linked immunosorbent assay (ELISA) was performed according to our previous description, but with some modifications (35). Briefly, Nunc immune modules (Nalgene) were coated with proteins (50 μg) that had been extracted by sonication of P. gingivalis strains grown in TSB for 16 h at 4°C. The modules were washed twice with PBS containing 0.1% Tween 20, pH 7.4. The modules were then blocked with 3% bovine serum albumin in PBS-Tween 20 for 2 h at 37°C. A series of dilutions of anti-FimA polyclonal antibodies (32) was then applied to the modules, and the modules were incubated for 3 h at room temperature. The modules were washed and incubated with horseradish peroxidase-conjugated antibodies against rabbit IgG (1:3,000; Amersham Biosciences) for 1 h at room temperature. After the modules were washed five times with PBS, peroxide substrate (Sigma) was added to each module. The reaction was stopped by the addition of 100 μl of 1 N H2SO4. The results were read at 450 nm on a Benchmark Plus microplate reader (Bio-Rad). All samples were assayed in triplicate for each sample.
Transmission electron microscopy of P. gingivalis.
Transmission electron microscopy of P. gingivalis was conducted as previously described (38). P. gingivalis strains ATCC 33277 and 1932E were grown anaerobically for 16 h in TSB. Bacterial cells were collected by centrifugation and resuspended in PBS. Twenty microliters of each bacterial culture was applied to a Formvar-coated copper grid (200 mesh; Electron Microscopy Sciences) and air dried. The bacterial cells were then negatively stained with 0.5% ammonium molybdate for 60 s and observed under an FEI Tecnai T12 transmission electron microscope (Philips) operated at 100 kV.
Measurement of the intracellular c-di-GMP level in P. gingivalis.
P. gingivalis strains ATCC 33277 and 1932E were grown anaerobically in TSB at 37°C to an OD600 of ∼1.0. The cells were harvested by centrifugation and resuspended in 1 ml PBS. To remove surface molecules, the cell suspension was sonicated by a sonicator (Fisher Scientific) for three pulses for 20 s at the output setting of 1 as described previously (39). The intact cells were pelleted by centrifugation for 30 s at maximum speed in a benchtop centrifuge and then resuspended in 150 μl of 40% (vol/vol) acetonitrile–40% (vol/vol) methanol–0.1 N formic acid (FA) for 30 min at −20°C. The supernatant was collected for analysis by liquid chromatography (LC) combined with tandem mass spectrometry (MS/MS). Samples were first dried on a SpeedVac apparatus and resolved in 20 μl buffer A (0.1% FA in water); 2 μl of each sample was injected into a nano-LC system (NanoLC-1DPlus; Eksigent) connected to a nanocolumn (75 μm by 10 cm packed with 5-μm-particle-size C18 resin; Michrom Bioresources) in line with an LTQ linear ion trap mass spectrometer (Thermo Fisher) and run with a 5 to 35% buffer B gradient (0.1% FA in acetonitrile) over 30 min. MS spectra were acquired in positive-ion mode in three consequent scans: (i) a full MS scan from a 200 to 1,000 m/z interval, (ii) single ion monitoring (SIM) from a 688.0 to 690.0 m/z interval, and (iii) a data-dependent MS/MS scan, retaining and then fragmenting the most abundant peak from the SIM parent ion scan. Chemically synthesized c-di-GMP (KeraFAST) was used as a standard. The general mass spectrometric settings were as follows: spray voltage, 2.1 kV; no sheath and auxiliary gas flow; ion transfer tube temperature, 200°C; collision-induced dissociation fragmentation (for MS/MS), 35% normalized collision energy; activation q, 0.25; activation time, 30 ms. The minimal threshold for the dependent scans was set to 500 counts, and a dynamic exclusion list was used with the following settings: repeat count of 1, repeat duration of 2 s, exclusion list size of 200, exclusion duration of 90 s, and exclusion mass width of 0.2% relative to the reference mass.
Statistical analyses.
Student's t test was used to determine the statistical significance of the differences in gene expression profiles, the attachment and invasion abilities, and the growth rates of the P. gingivalis strains. A P value of <0.05 was considered significant. Values are shown as means ± standard deviations (SDs).
RESULTS
Exploration of putative DGCs in P. gingivalis.
Initial analysis of the annotated genome of P. gingivalis ATCC 33277 detected two genes, pgn_0239 and pgn_0282, as a putative type I phosphodiesterase-nucleotide pyrophosphatase and a 2′,3′-cyclic-nucleotide 2′-phosphodiesterase precursor, respectively. However, a diguanylate cyclase (DGC) activity was not annotated. In search for putative DGCs using bioinformatics tools, including BLAST, InterProScan, Motif Scan, and ScanProsite searches, we identified one protein with a GGDEF domain encoded by pgn_1932 with a high degree of certainty. PGN_1932 with 526 amino acid residues was initially annotated as a conserved hypothetical protein (27). The structural features of PGN_1932, however, suggest that this protein likely functions as a DGC involved in the synthesis of c-di-GMP in P. gingivalis.
Role of PGN_1932 in the adhesive and invasive abilities of P. gingivalis.
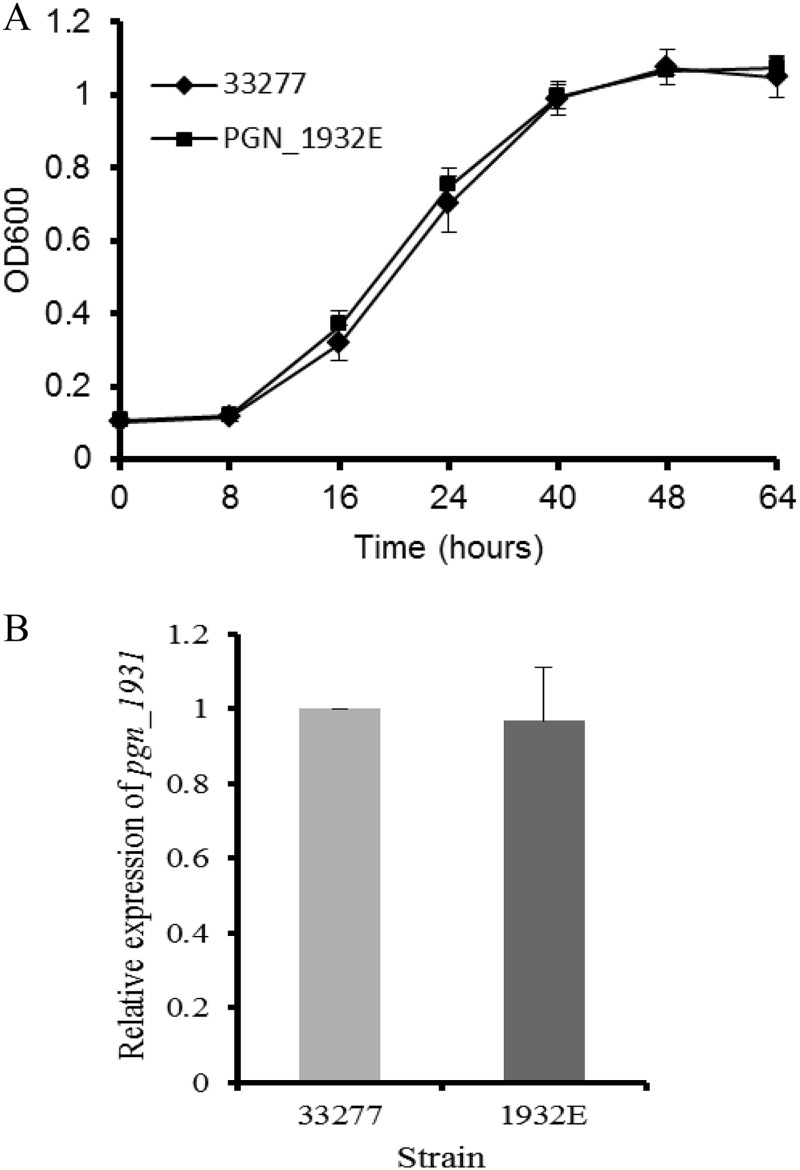
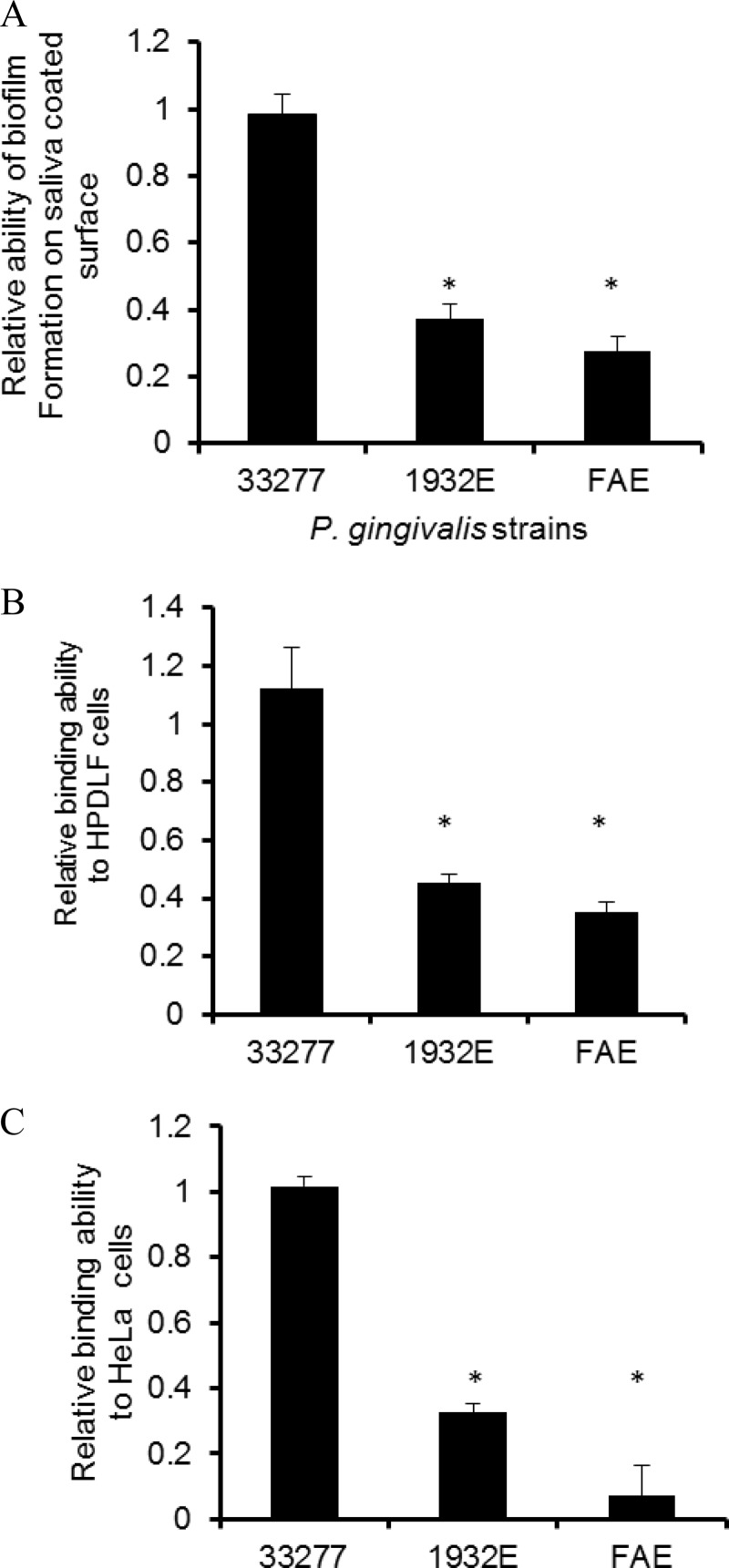
To explore the possible role of the PGN_1932 protein, we generated insertion and deletion mutations in the pgn_1932 gene, which carries an ermF-ermAM cassette and has lost the GGDEF domain. As shown in Fig. 1A, the mutation did not have a central effect on the pgn_1932 mutant, as there was no alteration in its growth rate compared to that of P. gingivalis ATCC 33277. Additionally, expression of the pgn_1931 gene, which is located immediately downstream of the pgn_1932 gene, in the pgn_1932 mutant was examined and compared to that of the parent strain. Expression of the downstream gene was not significantly altered in the mutant (Fig. 1B), suggesting that the mutation in the pgn_1932 gene has no polar effect on the downstream genes. Since the level of c-di-GMP has been shown to be linked in some bacteria to their pathogenic virulence properties (such as biofilm formation), we examined the adherence and invasive capabilities of mutant 1932E. P. gingivalis strains were first tested for their ability to bind to the saliva-coated surface that mimics a tooth's surface. Similar to the fimA mutant (FAE), strain 1932E showed a significantly reduced ability to stay on saliva-coated surfaces compared to that found for the parent strain, ATCC 33277 (Fig. 2A). It is well-known that P. gingivalis can colonize both tooth surfaces and soft tissue surfaces of the human oral cavity (25). Therefore, we also examined the ability of these P. gingivalis strains to adhere to epithelial (HeLa) cells and human periodontal ligament fibroblasts (HPDLFs). Consistent with the observations from previous studies, P. gingivalis strain ATCC 33277 bound to both HPDLFs and HeLa cells (40, 41) (Fig. 2B and C). Further, the mutations in the fimA gene or the pgn_1932 gene had a substantial impact on the abilities of these P. gingivalis mutants to adhere to epithelial cells or fibroblasts, suggesting that the pgn_1932 gene is involved in the control of biofilm formation on the surfaces of teeth and periodontal tissues.
FIG 1.
Comparison of the growth curves and gene expression of P. gingivalis ATCC 33277 and its mutant 1932E. (A) P. gingivalis cells were grown in standard TSB for 64 h. Bacterial growth is indicated by the means of the optical densities (OD600s) of the bacterial cultures. Error bars represent SDs (n = 4 experiments). (B) The expression levels of the gene (pgn_1931) located immediately downstream of pgn_1932 were measured by real-time RT-PCR. Each bar represents relative gene expression in the pgn_1932 mutant compared to that in P. gingivalis ATCC 33277 (which was given a value of 1). Error bars represent standard deviations (n = 3).
FIG 2.
Comparison of attachment ability of P. gingivalis ATCC 33277, the dgc mutant (1932E), and the fimA mutant (FAE). P. gingivalis strains were exposed to a saliva-coated surface (A), HPDLFs (B), and (C) HeLa cells for 1 h. The numbers of bacterial cells remaining on the surfaces were determined, and the relative binding abilities of the mutants were compared to the binding ability of wild-type strain ATCC 33277, which was given a value of 1. Error bar represents SDs (n = 4 experiments). *, a statistically significant difference between the wild type and the mutants (P < 0.05, t test,).
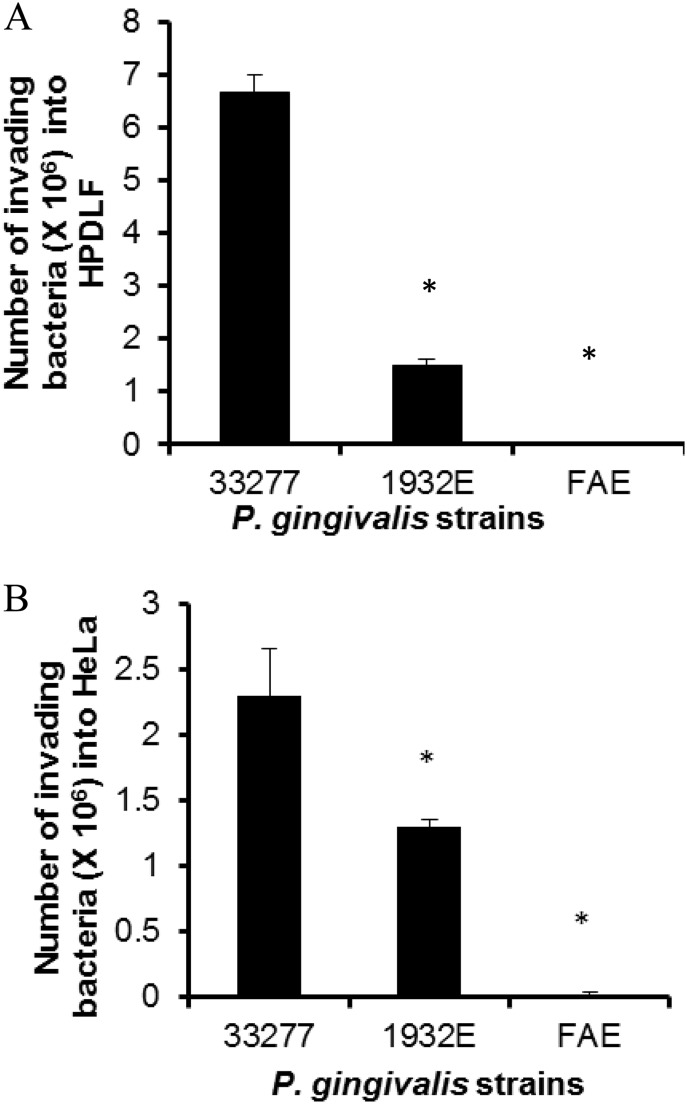
In addition, the abilities of these strains to invade HeLa cells and HPDLFs was also examined using an antibiotic protection assay (36). Consistent with their adherence capabilities, both the fimA and the pgn_1932 mutants showed a decreased invasive ability (Fig. 3A and B), which likely results from a reduced interaction between the mutants and the host cells. However, we cannot rule out the possibility that the mutation in the pgn_1932 gene has an impact on the internalization of P. gingivalis. Interestingly, we also observed that HPDLFs were about 3 times more susceptible than HeLa cells to invasion by P. gingivalis ATCC 33277. However, these differential susceptibilities between HeLa cells and HPDLFs were not detected when these two kinds of cells were exposed to the fimA or pgn_1932 mutant. These results imply an involvement, directly or indirectly, of both the fimA and the pgn_1932 genes in recognition of specific receptors on the surfaces of these epithelial cell and fibroblast lines.
FIG 3.
Comparison of invasive ability of P. gingivalis ATCC 33277 and the dgc (1932E) and fimA (FAE) mutants. HPDLFs (A) and HeLa cells (B) were infected with P. gingivalis strains at an MOI of 100 for 1 h, and the extracellular bacterial cells were eliminated by the use of antibiotics. The intracellular bacterial cells were plated on TSB blood agar plates. Each bar represents the average number of CFU of four independent experiments. *, a statistically significant difference between the wild type and the mutants (P < 0.05, t test).
Fimbria expression and production in the pgn_1932 mutant.
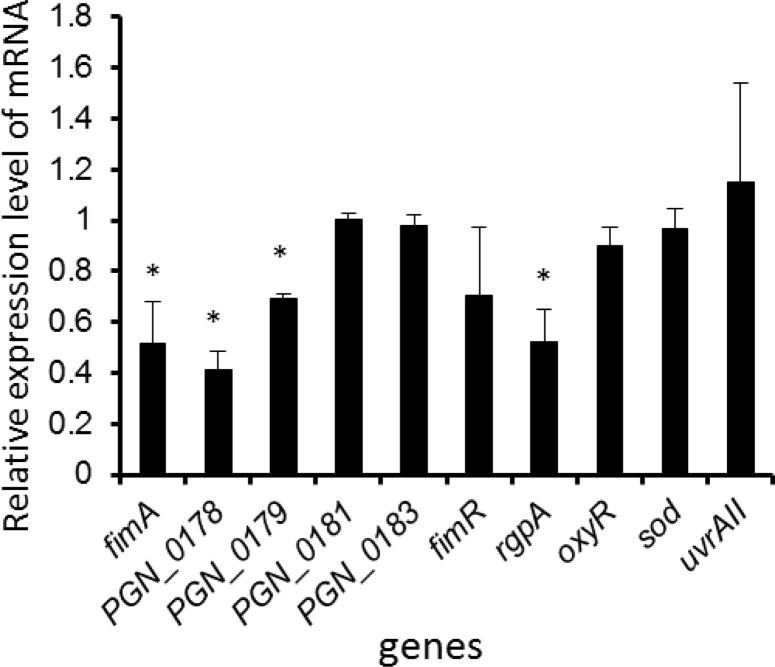
To assess how the pgn_1932 gene affects P. gingivalis adherence and invasion, we isolated and purified total RNA samples from the pgn_1932 mutant and from its parent strain, ATCC 33277. The mRNA levels of several genes potentially involved in the attachment of P. gingivalis were determined using quantitative RT-PCR and compared for the wild-type and mutant strains. The expression of four genes was downregulated at least 2-fold: fimA, pgn_0178 plus pgn_0179 (two genes located immediately upstream of fimA), and rgpA (Fig. 4).
FIG 4.
Differential expression of genes in P. gingivalis ATCC 33277 and the dgc mutant (1932E). Total RNAs were extracted from the P. gingivalis strains. The expression levels of the genes were measured by real-time RT-PCR. Each bar represents the relative gene expression in the dgc mutant compared to that in P. gingivalis ATCC 33277 (which was given a value of 1). Error bars represent standard deviations (n = 3). *, a significant difference between the gene expression level in the dgc mutant and that in P. gingivalis ATCC 33277 (P < 0.05, t test).
However, the levels of expression of pgn_0181 and pgn_0182 (two genes downstream of fimA), along with those of rgp (which encodes an arginine-specific gingipain), fimR (the transcriptional activator of fimA), oxyR (the transcriptional repressor of fimA), and sod (which encodes superoxide dismutase), were not altered. The results suggest that the PGN_1932 protein is required for the expression of fimA and some other genes in its locus.
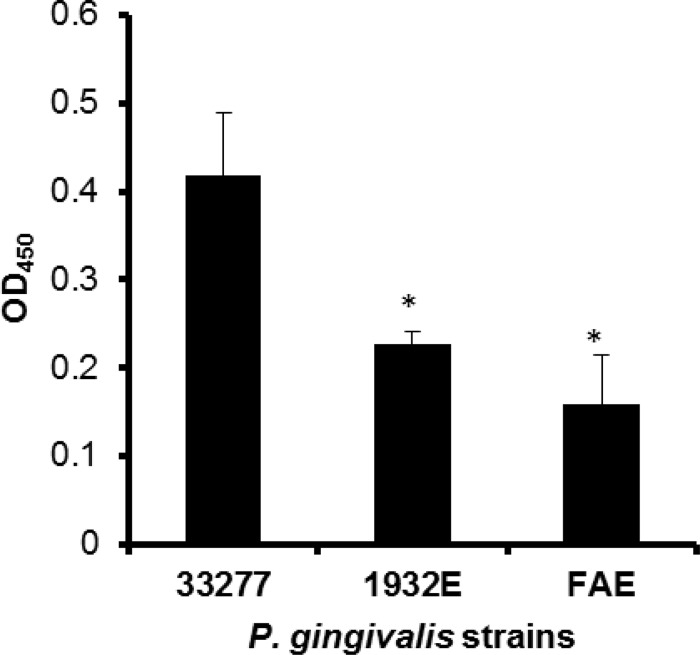
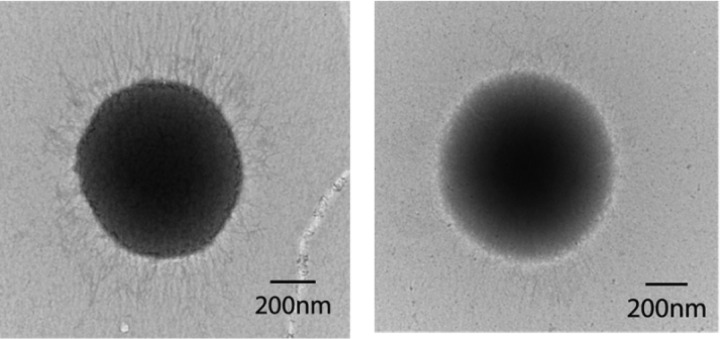
The analysis of production of the FimA protein in the pgn_1932 mutant by ELISA showed that the FimA levels on cell surfaces were significantly decreased in the fimA and the pgn_1932 mutants compared to those found on wild-type strain ATCC 33277 (Fig. 5). Not surprisingly, the biosynthesis of P. gingivalis long fimbriae was also greatly reduced in the pgn_1932 mutant compared to wild-type strain ATCC 33277, determined using a transmission electron microscopy (Fig. 6).
FIG 5.
Differential expression of FimA in P. gingivalis ATCC 33277, the dgc mutant (1932E), and the fimA mutant (FAE). Nunc immune modules were coated with equal amounts of the P. gingivalis surface protein from each strain. FimA levels were measured using ELISA with anti-FimA antibodies and horseradish peroxidase-tagged secondary antibodies. The absorbance was measured at 450 nm. The results represent the averages of three independent experiments. Statistical significance was measured by Student's t test. *, P < 0.05.
FIG 6.
Transmission electron microscopic analysis of P. gingivalis fimbriae. P. gingivalis strains ATCC 33277 (left) and 1932E (right) were prepared by negative staining with ammonium molybdate. Fimbrial structures were visualized using transmission electron microscopy.
Involvement of PGN_1932 in controlling c-di-GMP levels in P. gingivalis.
To determine if PGN_1932 acts as a diguanylate cyclase, we measured and compared the intracellular c-di-GMP concentrations in wild-type strain ATCC 33277 and its dgc mutant, 1932E, using liquid chromatography combined with tandem mass spectrometry (LC-MS/MS). Parent ion peaks of c-di-GMP (691 m/z) were detected in both strains. They both had similar retention times and exhibited the characteristic two MS/MS signature fragment peaks (248 and 540 m/z) seen in the c-di-GMP standard. The ion intensity peak of c-di-GMP was reduced 2.3-fold in the dgc mutant (1932E) compared to that in wild-type strain ATCC 33277, as shown in Table 3 (P = 0.005). This result suggests that the PNG_1932 protein is likely a diguanylate cyclase responsible for the synthesis of c-di-GMP in P. gingivalis.
TABLE 3.
c-di-GMP levels detected in P. gingivalis strains
| P. gingivalis strain | Retention time (min) | Parent ion intensity of c-di-GMP | Change relative to ATCC 33277 |
|---|---|---|---|
| ATCC 33277 | 15.95 | 83.1 ± 2.5 | 1.00 |
| 1932E | 15.92 | 33.8 ± 2.3a | 0.40 |
P = 0.005, t test.
DISCUSSION
Cyclic di-GMP is considered a truly universal bacterial second messenger that is presumably represented in all major bacterial phyla, on the basis of the distribution of enzymes responsible for c-di-GMP synthesis and degradation, such as DGCs and PDEs (42). Another striking feature of this signaling system is that c-di-GMP-metabolizing enzymes are abundant in some bacterial species. For example, among a total of 21,587 proteins in Thermotogae, 1.07% contain DGC- and PDE-specific domains (43). Unlike bacteria with numerous c-di-GMP enzymes, only two genes were previously annotated as phosphodiesterases in the genome of P. gingivalis ATCC 33277 (27). In the present study, we identified a DGC encoded by pgn_1932, annotated as a conserved hypothetical protein, using bioinformatics tools. The results of LC-MS/MS analyses revealed the presence of c-di-GMP in P. gingivalis, and we also demonstrated a significant decrease in intracellular c-di-GMP levels in the pgn_1932 mutant compared to those in parent train ATCC 33277. This indicates the involvement of PGN_1932 in c-di-GMP synthesis. Therefore, three c-di-GMP-metabolizing enzymes have now been identified in P. gingivalis ATCC 33277; collectively, they represent only 0.0014% of a total of 2,090 proteins. This number is much lower than that found in most bacteria with a c-di-GMP signaling system. However, it is highly possible that there may be more P. gingivalis proteins functioning as DGCs or PDEs, since deletion of the GGDEF domain from PGN_1932 did not completely eliminate c-di-GMP synthesis, although it reduced the c-di-GMP level more than 2-fold. This observation suggests that another protein(s) may also be involved in c-di-GMP synthesis. It should be noted that a P. gingivalis 83 protein (PG_1987) having 89% identity with PGN_1932 is annotated as a CRISPR-associated Csm1 family protein. A recent report suggests that CRISPRs may be involved in the regulation of insertion sequence transposition in P. gingivalis and in the development of bacterial diversity (44). It will be interesting to determine in the future if PGN_1932 has a similar function.
The important question is if c-di-GMP signaling is involved in the regulation of the pathogenicity of P. gingivalis. It has been reported that reducing or abolishing c-di-GMP in bacteria, in general, leads to much lower level of biofilm formation. Previous studies reported that attachment and invasive capabilities are characteristic features of P. gingivalis, and both of these are essential for bacterial infection (25). Consistent with the results of studies with other Gram-negative bacteria, such as P. aeruginosa (for which a reduction of cyclic di-GMP levels helped eliminate a P. aeruginosa biofilm from the spleens of infected mice [45]), we observed a significantly lower level of intracellular c-di-GMP in the dgc mutant (1932E); further, this mutant produced lower levels of biofilm on the surfaces of saliva-coated wells, epithelial cells, and human periodontal ligament fibroblasts. The observations provide support for the hypothesis that the intracellular level of c-di-GMP mediated by the PGN_1932 protein at least in part controls the lifestyle of P. gingivalis. While a higher intracellular level of c-di-GMP serves as a signal that promotes P. gingivalis cell attachment to surfaces, these cells may stay in a planktonic lifestyle when the intracellular levels of c-di-GMP decrease. Therefore, the association of the intracellular c-di-GMP level with the attachment ability of P. gingivalis may provide an effective strategy for the control of biofilm formation. Furthermore, as expected, we also observed that the dgc mutant, like the fimA mutant, showed a reduced ability to invade epithelial cells and fibroblasts. It is speculated that the reduced expression and/or production of FimA is the mechanism by which both the adhesive and invasive abilities were inhibited in the dgc mutant. This hypothesis is based on a previous observation that FimA is necessary for the adhesive and invasive abilities of P. gingivalis (37, 46). In this study, we provide strong evidence demonstrating that the expression of fimA was downregulated at the transcriptional level and that the production of FimA and the cell surface display of long fimbriae were also decreased in the dgc mutant. Therefore, we conclude that c-di-GMP levels are associated with a pathway that can control the adherence and invasion properties of P. gingivalis by regulating FimA expression and production.
Besides examination of fimA expression at the transcriptional level, we also analyzed the mRNA levels of other genes involved in fimA expression and the production of FimA in the dgc mutant. It has been reported that the fimA locus is composed of five genes, including two genes (pgn_0178 and pgn_0179) immediately upstream of fimA and two genes (pgn_0181 and pgn_0183) immediately downstream of fimA (47). A distal promoter region upstream of pgn_0178 and a proximal promoter region upstream of fimA were also identified (48). Moreover, a polycistronic transcript with mRNAs of pgn_0178, pgn_0179, and fimA has been observed. In this study, we found that only the three upstream genes of the fimA locus were downregulated in the dgc mutant. This observation could be explained by an involvement of c-di-GMP signaling in fimA expression via the distal promoter region of fimA. An interesting finding from the analysis of differential gene expression in the dgc mutant was a decreased level of mRNA of genes encoding arginine-specific gingipain (rgpA and rgpB), using primers corresponding to the conserved region of rgpA and rgpB. Since Rgp is considered to be involved in the maturation of FimA and the synthesis of long fimbriae (49, 50), the downregulation of rgp would presumably contribute to reduced fimbrial production and expression, which would lead to a decreased adhesive ability of P. gingivalis. However, we could not exclude the possibility of a direct role of RgpA in biofilm formation on saliva-coated surfaces and host cells, since previous studies have shown that the C-terminal adhesin domains of RgpA mediate the binding of P. gingivalis to epithelial cells and to human gingival fibroblasts (51).
It is also noteworthy that two transcriptional factors (FimR and OxyR) of fimA did not appear to be involved in c-di-GMP signaling. FimR acts as a transcriptional activator of the fimA gene (47), while OxyR is a transcriptional repressor of the fimA gene (32). Expression of the mRNAs of both transcriptional factors was not affected by the mutation in the pgn_1932 gene compared to the expression seen in wild-type strain ATCC 33277 and the pgn_1932 mutant. We also showed that the expression of superoxide dismutase (sod), which is mainly dependent on OxyR activity, was not altered in the pgn_1932 mutant, suggesting that c-di-GMP signaling is not involved in the activation of OxyR. Based on these observations, we note that the regulation of fimA expression by c-di-GMP could follow a unique pathway and not require the two known transcriptional factors (FimR and OxyR) of fimA. Therefore, the results presented here provide inspiration for further investigation of c-di-GMP signaling in P. gingivalis.
In conclusion, despite the fact that c-di-GMP signaling has been detected in a wide range of bacterial species, no information regarding the existence of this signal in P. gingivalis has been available to date. We show here that c-di-GMP is likely generated in P. gingivalis by a newly identified diguanylate cyclase, PGN_1932. Analyzing the role of c-di-GMP in bacterial pathogenicity, we found that c-di-GMP is involved in regulating the adhesive and invasive abilities of P. gingivalis. Therefore, the knowledge that the intracellular c-di-GMP level is associated with the attachment ability of P. gingivalis may lead to an effective strategy for bacterial biofilm control.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants DE020915 and DE022428 (to H.X.) from NIDCR and MD007593 and MD007586 from NIMHD.
Footnotes
Published ahead of print 14 April 2014
REFERENCES
- 1.Ross P, Weinhouse H, Aloni Y, Michaeli D, Weinberger-Ohana P, Mayer R, Braun S, de Vroom E, van der Marel GA, van Boom JH, Benziman M. 1987. Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature 325:279–281. 10.1038/325279a0 [DOI] [PubMed] [Google Scholar]
- 2.Sondermann H, Shikuma NJ, Yildiz FH. 2012. You've come a long way: c-di-GMP signaling. Curr. Opin. Microbiol. 15:140–146. 10.1016/j.mib.2011.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tal R, Wong HC, Calhoon R, Gelfand D, Fear AL, Volman G, Mayer R, Ross P, Amikam D, Weinhouse H, Cohen A, Sapir S, Ohana P, Benziman M. 1998. Three cdg operons control cellular turnover of cyclic di-GMP in Acetobacter xylinum: genetic organization and occurrence of conserved domains in isoenzymes. J. Bacteriol. 180:4416–4425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ausmees N, Mayer R, Weinhouse H, Volman G, Amikam D, Benziman M, Lindberg M. 2001. Genetic data indicate that proteins containing the GGDEF domain possess diguanylate cyclase activity. FEMS Microbiol. Lett. 204:163–167. 10.1111/j.1574-6968.2001.tb10880.x [DOI] [PubMed] [Google Scholar]
- 5.Tischler AD, Camilli A. 2005. Cyclic diguanylate regulates Vibrio cholerae virulence gene expression. Infect. Immun. 73:5873–5882. 10.1128/IAI.73.9.5873-5882.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aldridge P, Paul R, Goymer P, Rainey P, Jenal U. 2003. Role of the GGDEF regulator PleD in polar development of Caulobacter crescentus. Mol. Microbiol. 47:1695–1708. 10.1046/j.1365-2958.2003.03401.x [DOI] [PubMed] [Google Scholar]
- 7.Schmidt AJ, Ryjenkov DA, Gomelsky M. 2005. The ubiquitous protein domain EAL is a cyclic diguanylate-specific phosphodiesterase: enzymatically active and inactive EAL domains. J. Bacteriol. 187:4774–4781. 10.1128/JB.187.14.4774-4781.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galperin MY, Natale DA, Aravind L, Koonin EV. 1999. A specialized version of the HD hydrolase domain implicated in signal transduction. J. Mol. Microbiol. Biotechnol. 1:303–305 [PMC free article] [PubMed] [Google Scholar]
- 9.Galperin MY. 2004. Bacterial signal transduction network in a genomic perspective. Environ. Microbiol. 6:552–567. 10.1111/j.1462-2920.2004.00633.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simm R, Fetherston JD, Kader A, Romling U, Perry RD. 2005. Phenotypic convergence mediated by GGDEF-domain-containing proteins. J. Bacteriol. 187:6816–6823. 10.1128/JB.187.19.6816-6823.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beyhan S, Odell LS, Yildiz FH. 2008. Identification and characterization of cyclic diguanylate signaling systems controlling rugosity in Vibrio cholerae. J. Bacteriol. 190:7392–7405. 10.1128/JB.00564-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petersen E, Chaudhuri P, Gourley C, Harms J, Splitter G. 2011. Brucella melitensis cyclic di-GMP phosphodiesterase BpdA controls expression of flagellar genes. J. Bacteriol. 193:5683–5691. 10.1128/JB.00428-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alm RA, Bodero AJ, Free PD, Mattick JS. 1996. Identification of a novel gene, pilZ, essential for type 4 fimbrial biogenesis in Pseudomonas aeruginosa. J. Bacteriol. 178:46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulasakara H, Lee V, Brencic A, Liberati N, Urbach J, Miyata S, Lee DG, Neely AN, Hyodo M, Hayakawa Y, Ausubel FM, Lory S. 2006. Analysis of Pseudomonas aeruginosa diguanylate cyclases and phosphodiesterases reveals a role for bis-(3′-5′)-cyclic-GMP in virulence. Proc. Natl. Acad. Sci. U. S. A. 103:2839–2844. 10.1073/pnas.0511090103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romling U, Rohde M, Olsen A, Normark S, Reinkoster J. 2000. AgfD, the checkpoint of multicellular and aggregative behaviour in Salmonella typhimurium regulates at least two independent pathways. Mol. Microbiol. 36:10–23. 10.1046/j.1365-2958.2000.01822.x [DOI] [PubMed] [Google Scholar]
- 16.Merighi M, Lee VT, Hyodo M, Hayakawa Y, Lory S. 2007. The second messenger bis-(3′-5′)-cyclic-GMP and its PilZ domain-containing receptor Alg44 are required for alginate biosynthesis in Pseudomonas aeruginosa. Mol. Microbiol. 65:876–895. 10.1111/j.1365-2958.2007.05817.x [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Preston JF, III, Romeo T. 2004. The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J. Bacteriol. 186:2724–2734. 10.1128/JB.186.9.2724-2734.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Da Re S, Ghigo JM. 2006. A CsgD-independent pathway for cellulose production and biofilm formation in Escherichia coli. J. Bacteriol. 188:3073–3087. 10.1128/JB.188.8.3073-3087.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frederick JR, Sarkar J, McDowell JV, Marconi RT. 2011. Molecular signaling mechanisms of the periopathogen, Treponema denticola. J. Dent. Res. 90:1155–1163. 10.1177/0022034511402994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bian J, Liu X, Cheng YQ, Li C. 2013. Inactivation of cyclic di-GMP binding protein TDE0214 affects the motility, biofilm formation, and virulence of Treponema denticola. J. Bacteriol. 195:3897–3905. 10.1128/JB.00610-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan W, Qu T, Zhao H, Su L, Yu Q, Gao J, Wu B. 2010. The effect of c-di-GMP (3′-5′-cyclic diguanylic acid) on the biofilm formation and adherence of Streptococcus mutans. Microbiol. Res. 165:87–96. 10.1016/j.micres.2008.10.001 [DOI] [PubMed] [Google Scholar]
- 22.Suzuki Y, Yoshimura F, Takahashi K, Tani H, Suzuki T. 1988. Detection of fimbriae and fimbrial antigens on the oral anaerobe Bacteroides gingivalis by negative staining and serological methods. J. Gen. Microbiol. 134:2713–2720 [DOI] [PubMed] [Google Scholar]
- 23.Suzuki Y, Yoshimura F, Tani H, Suzuki T. 1988. Fimbriae from the oral anaerobe Bacteroides gingivalis: a screening of clinical isolates from various places. Adv. Dent. Res. 2:301–303 [DOI] [PubMed] [Google Scholar]
- 24.Noiri Y, Li L, Yoshimura F, Ebisu S. 2004. Localization of Porphyromonas gingivalis-carrying fimbriae in situ in human periodontal pockets. J. Dent. Res. 83:941–945. 10.1177/154405910408301210 [DOI] [PubMed] [Google Scholar]
- 25.Lamont RJ, Jenkinson HF. 1998. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol. Mol. Biol. Rev. 62:1244–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M, Shakhatreh MA, James D, Liang S, Nishiyama S, Yoshimura F, Demuth DR, Hajishengallis G. 2007. Fimbrial proteins of porphyromonas gingivalis mediate in vivo virulence and exploit TLR2 and complement receptor 3 to persist in macrophages. J. Immunol. 179:2349–2358. 10.4049/jimmunol.179.4.2349 [DOI] [PubMed] [Google Scholar]
- 27.Naito M, Hirakawa H, Yamashita A, Ohara N, Shoji M, Yukitake H, Nakayama K, Toh H, Yoshimura F, Kuhara S, Hattori M, Hayashi T, Nakayama K. 2008. Determination of the genome sequence of Porphyromonas gingivalis strain ATCC 33277 and genomic comparison with strain W83 revealed extensive genome rearrangements in P. gingivalis. DNA Res. 15:215–225. 10.1093/dnares/dsn013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410. 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- 29.Zdobnov EM, Apweiler R. 2001. InterProScan—an integration platform for the signature-recognition methods in InterPro. Bioinformatics 17:847–848. 10.1093/bioinformatics/17.9.847 [DOI] [PubMed] [Google Scholar]
- 30.Pagni M, Ioannidis V, Cerutti L, Zahn-Zabal M, Jongeneel CV, Hau J, Martin O, Kuznetsov D, Falquet L. 2007. MyHits: improvements to an interactive resource for analyzing protein sequences. Nucleic Acids Res. 35:W433–W437. 10.1093/nar/gkm352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Castro E, Sigrist CJ, Gattiker A, Bulliard V, Langendijk-Genevaux PS, Gasteiger E, Bairoch A, Hulo N. 2006. ScanProsite: detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res. 34:W362–W365. 10.1093/nar/gkl124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu J, Lin X, Xie H. 2008. OxyR is involved in coordinate regulation of expression of fimA and sod genes in Porphyromonas gingivalis. FEMS Microbiol. Lett. 282:188–195. 10.1111/j.1574-6968.2008.01116.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu J, Lin X, Xie H. 2009. Regulation of hemin binding proteins by a novel transcriptional activator in Porphyromonas gingivalis. J. Bacteriol. 191:115–122. 10.1128/JB.00841-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aslanidis C, de Jong PJ. 1990. Ligation-independent cloning of PCR products (LIC-PCR). Nucleic Acids Res. 18:6069–6074. 10.1093/nar/18.20.6069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Toole GA, Kolter R. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 28:449–461. 10.1046/j.1365-2958.1998.00797.x [DOI] [PubMed] [Google Scholar]
- 36.Lamont RJ, Chan A, Belton CM, Izutsu KT, Vasel D, Weinberg A. 1995. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect. Immun. 63:3878–3885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie H, Cai S, Lamont RJ. 1997. Environmental regulation of fimbrial gene expression in Porphyromonas gingivalis. Infect. Immun. 65:2265–2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng C, Wu J, Xie H. 2011. Differential expression and adherence of Porphyromonas gingivalis FimA genotypes. Mol. Oral Microbiol. 26:388–395. 10.1111/j.2041-1014.2011.00626.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Massie JP, Reynolds EL, Koestler BJ, Cong JP, Agostoni M, Waters CM. 2012. Quantification of high-specificity cyclic diguanylate signaling. Proc. Natl. Acad. Sci. U. S. A. 109:12746–12751. 10.1073/pnas.1115663109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Metzger Z, Blasbalg J, Dotan M, Weiss EI. 2009. Enhanced attachment of Porphyromonas gingivalis to human fibroblasts mediated by Fusobacterium nucleatum. J. Endod. 35:82–85. 10.1016/j.joen.2008.10.011 [DOI] [PubMed] [Google Scholar]
- 41.Weiss EI, Shaniztki B, Dotan M, Ganeshkumar N, Kolenbrander PE, Metzger Z. 2000. Attachment of Fusobacterium nucleatum PK1594 to mammalian cells and its coaggregation with periodontopathogenic bacteria are mediated by the same galactose-binding adhesin. Oral Microbiol. Immunol. 15:371–377. 10.1034/j.1399-302x.2000.150606.x [DOI] [PubMed] [Google Scholar]
- 42.Romling U, Galperin MY, Gomelsky M. 2013. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol. Mol. Biol. Rev. 77:1–52. 10.1128/MMBR.00043-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romling U, Simm R. 2009. Prevailing concepts of c-di-GMP signaling. Contrib. Microbiol. 16:161–181. 10.1159/000219379 [DOI] [PubMed] [Google Scholar]
- 44.Watanabe T, Nozawa T, Aikawa C, Amano A, Maruyama F, Nakagawa I. 2013. CRISPR regulation of intraspecies diversification by limiting IS transposition and intercellular recombination. Genome Biol. Evol. 5:1099–1114. 10.1093/gbe/evt075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christensen LD, van Gennip M, Rybtke MT, Wu H, Chiang WC, Alhede M, Hoiby N, Nielsen TE, Givskov M, Tolker-Nielsen T. 2013. Clearance of Pseudomonas aeruginosa foreign-body biofilm infections through reduction of the cyclic di-GMP level in the bacteria. Infect. Immun. 81:2705–2713. 10.1128/IAI.00332-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin X, Wu J, Xie H. 2006. Porphyromonas gingivalis minor fimbriae are required for cell-cell interactions. Infect. Immun. 74:6011–6015. 10.1128/IAI.00797-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishikawa K, Yoshimura F, Duncan MJ. 2004. A regulation cascade controls expression of Porphyromonas gingivalis fimbriae via the FimR response regulator. Mol. Microbiol. 54:546–560. 10.1111/j.1365-2958.2004.04291.x [DOI] [PubMed] [Google Scholar]
- 48.Park Y, Xie H, Lamont RJ. 2007. Transcriptional organization of the Porphyromonas gingivalis fimA locus. FEMS Microbiol. Lett. 273:103–108. 10.1111/j.1574-6968.2007.00782.x [DOI] [PubMed] [Google Scholar]
- 49.Nakayama K, Yoshimura F, Kadowaki T, Yamamoto K. 1996. Involvement of arginine-specific cysteine proteinase (Arg-gingipain) in fimbriation of Porphyromonas gingivalis. J. Bacteriol. 178:2818–2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuboniwa M, Amano A, Hashino E, Yamamoto Y, Inaba H, Hamada N, Nakayama K, Tribble GD, Lamont RJ, Shizukuishi S. 2009. Distinct roles of long/short fimbriae and gingipains in homotypic biofilm development by Porphyromonas gingivalis. BMC Microbiol. 9:105. 10.1186/1471-2180-9-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen T, Duncan MJ. 2004. Gingipain adhesin domains mediate Porphyromonas gingivalis adherence to epithelial cells. Microb. Pathog. 36:205–209. 10.1016/j.micpath.2003.12.001 [DOI] [PubMed] [Google Scholar]