Abstract
We previously showed that Brucella abortus rough mutant strain 2308 ΔATP (called the ΔrfbE mutant in this study) exhibits reduced intracellular survival in RAW264.7 cells and attenuated persistence in BALB/c mice. In this study, we performed microarray analysis to detect genes with differential expression between the ΔrfbE mutant and wild-type strain S2308. Interestingly, acid shock protein 24 gene (asp24) expression was significantly upregulated in the ΔrfbE mutant compared to S2308, as confirmed by quantitative reverse transcription-PCR (qRT-PCR) and Western blotting. Further studies using additional strains indicated that the upregulation of asp24 occurred only in rough mutants with disrupted O-antigen export system components, including the ATP-binding protein gene rfbE (bab1_0542) and the permease gene rfbD (bab1_0543), while the ΔwboA rough mutant (which lacks an O-antigen synthesis-related glycosyltransferase) and the RB51 strain (a vaccine strain with the rough phenotype) showed no significant changes in asp24 expression compared to S2308. In addition, abolishing the intracellular O-antigen synthesis of the ΔrfbE mutant by deleting the wboA gene (thereby creating the ΔrfbE ΔwboA double-knockout strain) recovered asp24 expression. These results indicated that asp24 upregulation is associated with intracellular O-antigen synthesis and accumulation but not with the bacterial rough phenotype. Further studies indicated that asp24 upregulation in the ΔrfbE mutant was associated neither with bacterial adherence and invasion nor with cellular necrosis on RAW264.7 macrophages. However, proper expression of the asp24 gene favors intracellular survival of Brucella in RAW264.7 cells and HeLa cells during an infection. This study reveals a novel mechanism for asp24 upregulation in B. abortus mutants.
INTRODUCTION
Brucella spp. are facultative intracellular bacteria that infect both animals and humans (1–3). Brucellosis is one of the most widespread zoonotic diseases in the world, especially in developing countries (2, 4). The Brucella genus is currently divided into 10 species according to preference for specific animal hosts, including the six classical species (Brucella abortus, B. suis, B. melitensis, B. neotomae, B. canis, and B. ovis) and newly recognized species (B. ceti, B. microti, B. pinnipedialis, and B. inopinata) (5, 6). Brucella has no classical virulence factors, such as exotoxins, cytolysins, capsules, fimbriae, plasmids, lysogenic phages, drug-resistant forms, antigenic variations, or endotoxic lipopolysaccharide (LPS) molecules (7); its virulence relies on the ability to invade and multiply intracellularly in both phagocytic cells and nonphagocytic cells (8).
Brucella LPS is recognized as a main virulence factor for resisting phagocytosis and enhancing survival in macrophages (9–11). The LPS consists of three key components, namely, lipid A, core sugar, and O-antigen (12), among which the O-antigen is critical for the virulence of classical Brucella species (B. melitensis, B. abortus, and B. suis) (13, 14). B. abortus lipid A possesses a diaminoglucose backbone, and the acyl groups are longer (C18-C19 and C28) and are linked to the core only by amide bonds (12). The O-antigen of Brucella is a homopolymer of 4,6-dideoxy-4-formamido-α-d-mannopyranosyl residues joined by an α-1,2 linkage in A-epitope-dominant strains, but every fifth residue is joined by an α-1,3 linkage in M-epitope-dominant strains (15, 16). The Brucella wboA gene is capable of encoding a glycosyltransferase that has been demonstrated to be essential for the biosynthesis of the Brucella O-antigen (17). Disruption of the wboA gene in B. abortus 2308, B. melitensis 16M, and B. suis resulted in rough mutants that were unable to synthesize the O-antigen (18). The main genes involved in LPS biosynthesis in Brucella spp. include those for GDP-mannose dehydratase (Gmd), perosamine synthetase (Per), phosphoglucomutase (Pgm), phosphomannomutase (Pmm), mannose isomerase (ManB), mannose guanylyltransferase (ManC), O-antigen export permease (Wzm), ATP-binding protein (Wzt), WbkB, methionyl tRNA formyltransferase (WbkC), and N-formyl-perosaminyltransferase (WbkA). Among these genes, wzm and wzt (called rfbD and rfbE in this study) encode putative integral membrane components of ATP-binding cassette (ABC) transporters (12, 19) that flip the O-antigen from the cytoplasmic face to the periplasmic face of the inner membrane (20).
Our previous study showed that B. abortus rough mutant strain 2308 ΔATP (called the ΔrfbE mutant in this study) exhibits reduced intracellular survival in RAW264.7 cells and attenuated persistence in BALB/c mice. In this study, we performed microarray analysis to detect genes with differential expression between the ΔrfbE mutant and wild-type (WT) strain S2308. Interestingly, acid shock protein 24 gene (asp24) expression was significantly upregulated in the ΔrfbE mutant compared to S2308, as confirmed by quantitative reverse transcription-PCR (qRT-PCR) and Western blotting. Acid shock protein 24 (Asp24) is a protein previously reported to be induced in acidic environments; in Brucella, Asp24 expression is optimal at pH values below 4.0 and within the first 3 h of acid exposure (21). The exact role of Asp24 in pathogenesis remains unknown. In this study, we demonstrated upregulated asp24/Asp24 expression in B. abortus O-antigen transporter mutants. Further study revealed that the upregulation is associated with intracellular O-antigen synthesis and accumulation. Therefore, we discovered a novel mechanism for asp24 upregulation and showed that its altered expression affects intracellular survival of Brucella in host cells.
MATERIALS AND METHODS
Strains, plasmids, and culture conditions.
All strains and plasmids used in the study are listed in Table 1. B. abortus S2308 and its derivatives were cultured on tryptic soy agar (TSA) (Difco) or in tryptic soy broth (TSB) at 37°C with 5% CO2. Escherichia coli strains were cultured at 37°C in Luria Broth (LB). When appropriate, 100 μg/ml ampicillin or 20 μg/ml chloramphenicol (Sigma) was added.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Characteristics | Source or reference |
|---|---|---|
| Strains | ||
| B. abortus strains | ||
| S2308 | WT; parental strain; smooth phenotype | ATCC |
| RB51 | Vaccine strain; rough phenotype | Q. Wu |
| ΔrfbE | Deletion strain for rfbE gene; rough phenotype | 23 |
| ΔrfbE(pBBR-rfbE) | Cmr; ΔrfbE strain carrying the complementary plasmid pBBR-rfbE; smooth phenotype | This study |
| ΔrfbD | Deletion strain for rfbD gene; rough phenotype | This study |
| ΔwboA | Deletion strain for wboA gene; rough phenotype | This study |
| ΔrfbE ΔwboA | Deletion strain for rfbE and wboA genes; rough phenotype | This study |
| ΔrfbE Δasp24 | Deletion strain for rfbE and asp24 genes; rough phenotype | This study |
| ΔrfbE Δasp24(pBBR-asp24) | Cmr; ΔrfbE Δasp24 strain carrying the complementary plasmid pBBR-asp24; rough phenotype | This study |
| S2308(pBBR-asp24) | Cmr; WT carrying the plasmid pBBR-asp24; smooth phenotype | This study |
| ΔrfbE(pBBR-asp24) | Cmr; ΔrfbE strain carrying the plasmid pBBR-asp24; rough phenotype | This study |
| E. coli strains | ||
| DH5α | F− ϕ80lacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK+) phoA supE44 thi-1 gyrA96 relA1 λ− | Invitrogen |
| BL21(DE3) | F− ompT hsdSB(rB− mB−) gal dcm(DE3) | Invitrogen |
| Plasmids | ||
| pBBR1MCS1 | Cmr; Broad-host-range cloning vector | 25 |
| pET28a(+) | Kanr, prokaryotic expression vector | Novagen |
| pSC | Ampr; pUC19 plasmid containing sacB gene | 23 |
| pSCΔwboA | Ampr; pSC plasmid containing the ΔwboA fragment; used to construct deletion strain | This study |
| pSCΔasp24 | Ampr; pSC plasmid containing the Δasp24 fragment; used to construct deletion strain | This study |
| pBBR-rfbE | Cmr; pBBR1MCS1 containing the rfbE gene flanked by its upstream and downstream regions | This study |
| pBBR-asp24 | Cmr; pBBR1MCS1 containing the asp24 gene flanked by its upstream and downstream regions | This study |
DNA microarray analysis.
Total RNAs were extracted from S2308 and the ΔrfbE mutant by use of TRIzol RNA isolation reagents (Invitrogen). Genomic DNA contamination was removed through treatment with a Turbo DNA-free kit (Ambion). The B. abortus bv. 1 strain 9-941 gene expression array was manufactured by Agilent (Agilent Technologies) and consisted of 3,334 genes. Sample preparation and microarray hybridization were performed based on the manufacturer's standard protocols. Briefly, 1 μg of total RNA from each sample was amplified and transcribed into fluorescent cRNA according to the Agilent Quick Amp labeling protocol (version 5.7; Agilent Technologies). Labeled cRNAs were hybridized onto a whole-genome oligonucleotide array (8 × 15K; Agilent Technologies). After slide washes, the arrays were scanned using an Agilent G2505B scanner. Agilent Feature Extraction software (version 10.7.3.1) was used to analyze acquired array images. Quantile normalization and subsequent data processing were performed using the GeneSpring GX v11.5.1 31 software package (Agilent Technologies). Differentially expressed genes were identified through volcano plot filtering.
Real-time PCR assay.
Validation of the microarray data was performed using qRT-PCR. Genes which showed expression differences of >5-fold in the microarray assay were selected for validation. Primers used are listed in Table 2. Total RNA was extracted as described above. RNA (1 μg) was reverse transcribed into cDNA by using a PrimeScript RT-PCR kit (TaKaRa) according to the manufacturer's instructions. One microliter of cDNA was used in a 20-μl RT-PCR mixture containing 10 μl 2× GoTaq qPCR master mix (Promega), 0.5 μl (each) forward and reverse primers (10 μM [each]), and 8 μl double-distilled water (ddH2O). The mixture was incubated at 95°C for 2 min, and then 40 cycles of 95°C for 15 s and 60°C for 1 min were carried out with a Mastercycler ep Realplex system (Eppendorf). For each gene, PCRs were performed in triplicate, and relative transcription levels were determined by the 2−ΔΔCT method, using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control for data normalization.
TABLE 2.
Primers used in this study
| Primer | Oligonucleotide sequence (5′ to 3′)a | Target geneb | Product size (bp) |
|---|---|---|---|
| CrfbE-F | CGCGTCGACCACAGGATCTTACCCTGTTG | rfbE containing the promoter region | 1,712 |
| CrfbE-R | CGCGGATCCTCATGCTATAGCTCCCATTC | rfbE containing the promoter region | 1,712 |
| rfbD-UF | TGCACTGCAGTGCACGAAATAGGTCCAGGTCTCG | Upstream fragment of rfbD | 1,250 |
| rfbD-UR | TACGCCACTTCGGGATCCCGTGAGCCTCCTCCTAACCTG | Upstream fragment of rfbD | 1,250 |
| rfbD-DF | AGGAGGCTCACGGGATCCCGAAGTGGCGTACCTTCCAG | Downstream fragment of rfbD | 1,140 |
| rfbD-DR | TCCCCCGGGGGAGTGAACGAATGCCTTGATAC | Downstream fragment of rfbD | 1,140 |
| wboA-UF | GCTCTAGAGCAATTCCGGTGGGTTGATT | Upstream fragment of wboA | 1,013 |
| wboA-UR | AACCTACATCTATTGGCGTCTTCCGCCTCGGTACTTAA | Upstream fragment of wboA | 1,013 |
| wboA-DF | TTAAGTACCGAGGCGGAAGACGCCAATAGATGTAGGTT | Downstream fragment of whoA | 1,091 |
| wboA-DR | GCTCTAGAGCTTGATAAAGGCATGTAATGTG | Downstream fragment of whoA | 1,091 |
| asp24-UF | GCTCTAGAGCAGCCACCAGCCAAGAAGATAGG | Upstream fragment of asp24 | 1,153 |
| asp24-UR | CGGGATCCCGTACTCTTTTACAGGTGCGCCGC | Upstream fragment of asp24 | 1,153 |
| asp24-DF | CGGGATCCCGCCCAGAAGCCGCAGGAATAAG | Downstream fragment of asp24 | 1,089 |
| asp24-DR | GCTCTAGAGCGGGTGCGATTGTGAATGTCTCCT | Downstream fragment of asp24 | 1,089 |
| Asp24-F | CGCGGATCCAATACGGATAAACCAGCAGC | Coding region of asp24 | 474 |
| Asp24-R | CCGCTCGAGTTATTCCTGCGGCTTCTGG | Coding region of asp24 | 474 |
| Casp24-F | GGGGTACCCCAAGACGAGCGGAAACGAC | asp24 containing the promoter region | 1,467 |
| Casp24-R | GCTCTAGAGCCATTGGACGACTTTGTTGAC | asp24 containing the promoter region | 1,467 |
| RT-0004F | CGTCCTATCGCGATCTTTTC | BruAb1_0004 | 189 |
| RT-0004R | GGACATTCAGCGCATTGTAG | BruAb1_0004 | 189 |
| RT-1070F | CCTCGCAGTTACAGGCTTTC | BruAb2_1070 | 216 |
| RT-1070R | TGCCGGTAATCATCAACTCA | BruAb2_1070 | 216 |
| RT-1334F | GCGGTCCTATCGATCTTGAG | BruAb1_1334 | 178 |
| RT-1334R | GTTACCTTGCCGTCACCATT | BruAb1_1334 | 178 |
| RT-1735F | ATGCCTGTTTCAATCCGAAC | BruAb1_1735 | 156 |
| RT-1735R | ATGCCTGTTTCAATCCGAAC | BruAb1_1735 | 156 |
| RT-0035F | AGCTTCGGCTGATGAATGAC | BruAb1_0035 | 115 |
| RT-0035R | TCGAAAGTCAGCATGGTCTG | BruAb1_0035 | 115 |
| RT-1556F | AAGCTTTGGCATGATTACCG | BruAb1_1556 | 87 |
| RT-1556R | TCCAGAACGCAAACATCAAC | BruAb1_1556 | 87 |
| RT-1726F | CACGCTCGAAAAGAAGCAC | BruAb1_1726 | 96 |
| RT-1726R | CGCTTGAGAGATGCGATATG | BruAb1_1726 | 96 |
| RT-1735F | ATGCCTGTTTCAATCCGAAC | BruAb1_1735 | 156 |
| RT-1735R | AATTCACTTGTCCGCAAACC | BruAb1_1735 | 156 |
| RT-0400F | AGAGCAAGCAGCTCTCGAAC | BruAb2_0400 | 185 |
| RT-0400R | CGGGGATTCTTAAACCCAAT | BruAb2_0400 | 185 |
| RT-0811F | GTTCGATTGCGAGGTGTCTT | BruAb2_0811 | 156 |
| RT-0811R | TTCGGAAAACAGGAACATGA | BruAb2_0811 | 156 |
| RT-1055F | ATCGCAAAATCCTCAAATCG | BruAb2_1055 | 189 |
| RT-1055R | CGGGATTATAGAAGCCGTCA | BruAb2_1055 | 189 |
| RT-1241F | CGTATATCCAGCTCGGTGGT | BruAb1_1241 | 231 |
| RT-1241R | CGATGTGGTAGTTGGTCGTG | BruAb1_1241 | 231 |
| RT-0657F | CGTATATCCAGCTCGGTGGT | BruAb1_0657 | 231 |
| RT-0657R | CGATGTGGTAGTTGGTCGTG | BruAb1_0657 | 231 |
| RT-0822F | ATACGCCGATGGTCAAGAAG | BruAb1_0822 | 169 |
| RT-0822R | TCAACAGCACGCTTGTTTTC | BruAb1_0822 | 169 |
| RT-1878F | GGCAAGAACAAGGACCACAT | BruAb1_1878 | 184 |
| RT-1878R | CCCAATAGTTGGTCGGAATG | BruAb1_1878 | 184 |
| RT-1239F | CGTTTGATCATCGGATCCTT | BruAb1_1239 | 192 |
| RT-1239R | TATCGAGAAGACGCTTGTGC | BruAb1_1239 | 192 |
| RT-1255F | ACGACGAAGACCACGGTTAC | BruAb1_1255 | 228 |
| RT-1255R | GTTGGTGAAGAACGGCGTAT | BruAb1_1255 | 228 |
| RT-1238F | GTCATAACTTCGGCGGTCAT | BruAb1_1238 | 106 |
| RT-1238R | TTGCCCTTGAACACCTTACC | BruAb1_1238 | 106 |
| RT-1242F | AGACGCCATAAGGCAGAGAA | BruAb1_1242 | 99 |
| RT-1242R | CTTGCCATGAAGCATGACTG | BruAb1_1242 | 99 |
| RT-1243F | TGCCTACCGTAAACCAGCTT | BruAb1_1243 | 168 |
| RT-1243R | GACGAACCTTGGCAACCTTA | BruAb1_1243 | 168 |
| RT-0505F | ACCTCGTTTTCGTCGAATTG | BruAb2_0505 | 140 |
| RT-0505R | GACAGCATCGTTGACTTCCA | BruAb2_0505 | 140 |
| RT-GAPDH-F | GACATTCAGGTCGTCGCCATCA | GAPDH | 188 |
| RT-GAPDH-R | TCTTCCTTCCACGGCAGTTCGG | GAPDH | 188 |
Underlining indicates restriction endonuclease recognition sequences.
B. abortus locus tags listed are for genes in B. abortus strain 9-941.
Plasmid construction.
All primers used in the study are listed in Table 2. Suicide plasmids were constructed using an overlap PCR assay as previously reported (22). Briefly, the upstream and downstream fragments of the target gene were amplified by independent PCRs and purified by gel extraction before being used as templates for a second round of PCR. The resultant product, containing joined flanking sequences, was gel purified, digested with appropriate enzymes, and cloned into the pSC plasmid (pUC19-sacB plasmid) (22, 23). The recombinant suicide plasmids were transformed into DH5α cells (Invitrogen) for propagation and then extracted to construct the mutants.
The complementation plasmids pBBR-rfbE and pBBR-asp24 were constructed using conventional methods (23, 24). The rfbE and asp24 fragments, containing respective promoter and terminator regions, were amplified by PCR. The joined flanking sequences for rfbE and asp24 were digested by use of BamHI/KpnI and BamHI/SalI, respectively, and then inserted into the same digested sites of the pBBR1MCS1 plasmid (25). The pBBR-rfbE or pBBR-asp24 plasmid was propagated in E. coli DH5α cells.
Mutants and complemented strain construction.
The B. abortus mutants were constructed by allelic replacement, using a two-step strategy as previously reported (26). Briefly, B. abortus strain 2308 competent cells were prepared through two washes with ice-cold sterile water. A suicide plasmid (0.5 to 1.0 μg) was transformed into competent cells by electroporation. The singly exchanged recombinants were selected by plating on TSA containing ampicillin, and then colonies were inoculated into TSB without antibiotics. The second exchanged recombinants were selected by plating on TSA containing 5% sucrose. All colonies were selected and verified by PCR or Western blotting.
The complemented strains were also constructed by electroporation. The recombinants were then selected by plating on TSA containing chloramphenicol. The colonies were verified through PCR or Western blotting.
Asp24 protein expression and rabbit antiserum preparation.
The coding region of the asp24 gene was amplified from the B. abortus S2308 genome by PCR using the primers Asp24-F and Asp24-R (Table 2) and then cloned into pET28a(+) (Novagen). The plasmid was introduced into E. coli BL21(DE3) for IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible expression of recombinant proteins and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Coomassie blue staining. The recombinant proteins were purified using HisTrap chelating high-performance columns (Amersham) and were assayed quantitatively using a bicinchoninic acid (BCA) protein assay kit (Beyotime). The rabbit antiserum was prepared as reported previously (27). Samples with enzyme-linked immunosorbent assay (ELISA) titers over 1:10,000 qualified for Western blotting.
Extraction and Western blotting of Brucella total protein and LPS.
B. abortus S2308 and its derivatives were cultured in TSB medium, and the bacterial whole cells were obtained by centrifugation. Bacterial pellets were then diluted to an optical density at 600 nm (OD600) of 10 and inactivated at 80°C for 1 h. Total bacterial proteins were extracted by boiling with SDS sample loading buffer (Beyotime). LPS was isolated by SDS-proteinase K extraction as described previously (28). Samples were loaded onto a 12% polyacrylamide gel for SDS-PAGE analysis. The gels were then transferred onto a nitrocellulose membrane (Whatman) for Western blotting.
The membranes were blocked for 1 h in phosphate-buffered saline (PBS) with 5% skimmed milk at room temperature. After being washed four times in PBS-0.1% Tween 20 (PBST) for 5 min, the membranes were incubated overnight with primary antibody (Brucella O-antigen monoclonal antibody A76 12G12 F12, rabbit anti-Asp24 polyclonal antibody, or mouse anti-D15 monoclonal antibody) diluted in PBST, washed four times in PBST for 5 min, incubated for 1 h with secondary antibody (IRDye 800CW-conjugated donkey anti-rabbit polyclonal antibody or IRDye 680RD-conjugated donkey anti-mouse polyclonal antibody) (Li-Cor) in PBST-0.02% SDS, and finally washed four times in PBST for 5 min. The blots were visualized with an Odyssey two-color infrared imaging system (Li-Cor). Quantitative analysis of the Asp24 protein was performed by gray scanning, using the Brucella surface antigen D15 as the internal control.
Bacterial adherence, invasion, and intracellular survival assays.
RAW264.7 cells were used to determine the effects of the Brucella asp24 gene on the bacterial adherence, invasion, and intracellular survival capacities. The cells were seeded at about 2 × 105 cells per well in 24-well plates and grown in Dulbecco modified Eagle medium (DMEM) (Biowest) with 10% fetal bovine serum (FBS) (Biowest) at 37°C with 5% CO2 for 24 h. For the adherence assay, the cell monolayer was washed twice with DMEM and infected with B. abortus S2308 or its derivatives at a multiplicity of infection (MOI) of 200, as previously described (29). Bacteria were centrifuged onto the cells at 400 × g for 5 min, and cells were then incubated at 37°C for 1 h. Nonadherent bacteria were removed by rinsing the wells twice with PBS (HyClone). The cells were released from the plate by adding 100 μl of 0.2% Triton X-100 in sterile water, and subsequently 900 μl of DMEM was added. This cell suspension was 10-fold serially diluted with PBS and spread onto TSA plates to determine the number of viable bacteria.
For the invasion assay, cell culture, bacterial infection, and bacterial counting were performed as described above for the bacterial adherence assay, except that the extracellular bacteria were killed by incubation of the monolayers with DMEM containing gentamicin (100 μg/ml) for 1 h after incubation with bacteria and three washes with PBS.
For the bacterial intracellular survival assay, cell culture and bacterial counting were performed as described above for the bacterial adherence assay. RAW264.7 cells were infected with B. abortus S2308 or its derivatives at an MOI of 100 and incubated in DMEM with 3% FBS and 50 μg/ml gentamicin. The cells were then washed and lysed at 1, 12, 24, 36, 48, and 60 h postinfection (hpi) to determine the amount of bacterial recovery. HeLa cells were also used to investigate the effect of asp24 on bacterial intracellular survival. The cells were infected with S2308, the ΔrfbE mutant, or S2308(pBBR-asp24) at a high infection dose (MOI of 500) to increase the number of invasive bacteria, with the bacterial CFU determined at 2, 10, 24, and 48 hpi, or infected with the ΔrfbE, ΔrfbE Δasp24, or ΔrfbE(pBBR-asp24) strain, with the bacterial CFU determined at 1, 12, 24, and 48 hpi.
All assays were performed in triplicate wells, and the results are averages for at least three independent experiments.
Immunofluorescence assay.
RAW264.7 cells cultured on glass coverslips with a diameter of 15 mm (Thermo Fisher Scientific) were infected with B. abortus S2308 or the ΔrfbE mutant at a low infection dose (MOI of 30) to conveniently observe intracellular survival of individual bacteria. The infected cells were fixed with 3.7% (wt/vol) paraformaldehyde at 0, 6, 12, 24, 36, 48, 60, and 72 hpi. Indirect immunofluorescence assay was performed as previously reported (29), using rabbit anti-B. abortus serum (diluted 1:500) as the primary antibody and Alexa Fluor 488-conjugated goat anti-rabbit IgG (diluted 1:1,000) (Invitrogen) as the secondary antibody.
Determination of Brucella-induced macrophage death.
RAW264.7 cells in 24-well plates were infected with B. abortus S2308 or derivatives at an MOI of 200. Culture supernatants were collected at 8 and 24 hpi, and levels of lactate dehydrogenase (LDH) were determined by CytoTox 96 nonradioactive cytotoxicity assay (Promega) according to the manufacturer's instructions. Brucella-induced macrophage death was further analyzed using a propidium iodide (PI)-Hoechst 33342 staining kit (Beyotime) and a fluorescein isothiocyanate (FITC)-annexin-PI staining kit (Vazyme) at 4, 8, or 15 hpi.
Acid induction and tolerance assay.
B. abortus S2308 or its derivatives were cultured to mid-logarithmic phase (OD600 = 1.0), and then a bacterial suspension (107 CFU/ml) was prepared in TSB with a pH of 7.3, 5.5, 4.5, or 3.8. After 2 h of incubation at 37°C, cells were serially diluted and plated on TSA to determine the bacterial CFU. The bacterial survival percentages were calculated with respect to numbers of CFU obtained from bacteria incubated in TSB at pH 7.3 (100% survival).
Statistical analysis.
Statistical analysis was performed using GraphPad Prism software. All results are presented as means ± standard deviations (SD) for at least three independent experiments. Statistical significance was determined by either an unpaired, two-tailed Student t test or, in the case of groups, one-way analysis of variance (ANOVA) followed by Tukey's test.
RESULTS
asp24 expression is significantly upregulated in the ΔrfbE mutant.
Genes with differential expression between B. abortus S2308 and the ΔrfbE mutant were identified using a B. abortus gene expression array containing 3,334 genes. A total of 283 genes that exhibited altered expression (>2-fold difference) were identified, among which 114 genes (114/3,334 genes [3.42%]) were upregulated and 169 genes (169/3,334 genes [5.07%]) were downregulated in the ΔrfbE mutant relative to B. abortus S2308 (see Table S1 in the supplemental material). Among these, 21 genes with at least 5-fold increases in expression in the microarray analysis were further validated using the qRT-PCR assay. The results revealed that only asp24 gene expression was significantly changed in the ΔrfbE mutant. Compared to the case in B. abortus S2308, the asp24 gene showed 24-fold more expression in the ΔrfbE mutant. Other selected genes showed changes of <3-fold by qRT-PCR analysis (Table 3).
TABLE 3.
Transcriptional levels of Brucella genes examined using qRT-PCR
| B. abortus 9-941 ORF | Predicted protein function | 2−ΔΔCT valuea |
|---|---|---|
| BruAb1_0004 | Molybdopterin biosynthesis protein MoeB | 1.08 |
| BruAb2_1070 | Flagellar biosynthesis protein FliQ | 1.17 |
| BruAb1_1334 | Acid shock protein | 24.41 |
| BruAb1_1735 | Hypothetical protein | 1.46 |
| BruAb1_0035 | Hypothetical protein | 1.02 |
| BruAb1_1556 | Hypothetical protein | 0.82 |
| BruAb1_1726 | Hypothetical protein | 1.63 |
| BruAb1_1735 | Hypothetical protein | 1.49 |
| BruAb2_0400 | Hypothetical protein | 1.63 |
| BruAb2_0811 | Hypothetical protein | 0.34 |
| BruAb2_1055 | IS3 family transposase OrfB | 2.14 |
| BruAb1_1241 | Elongation factor G | 0.93 |
| BruAb1_0657 | Omp2b, porin | 1.26 |
| BruAb1_0822 | NADH dehydrogenase subunit G | 1.10 |
| BruAb1_1878 | Succinate dehydrogenase flavoprotein subunit | 1.21 |
| BruAb1_1239 | 30S ribosomal protein S10 | 1.25 |
| BruAb1_1255 | Elongation factor Tu | 1.00 |
| BruAb1_1238 | 50S ribosomal protein L3 | 1.28 |
| BruAb1_1242 | 30S ribosomal protein S7 | 2.04 |
| BruAb1_1243 | 30S ribosomal protein S12 | 0.90 |
| BruAb2_0505 | Glycine cleavage system protein H | 1.44 |
A value of 1 indicates that the gene is similarly expressed under both conditions (ΔrfbE mutant versus S2308), a value of >1 indicates that the gene is overexpressed in the ΔrfbE mutant, and a value of <1 indicates that the gene is expressed less in the mutant.
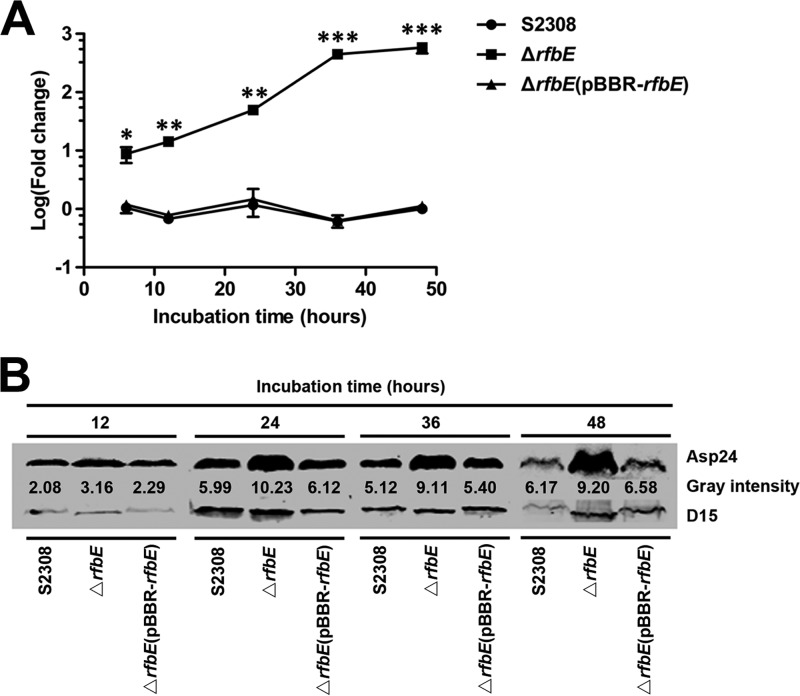
The expression of asp24 in the ΔrfbE mutant was then investigated at 6, 12, 24, 36, and 48 h of bacterial growth in TSB to determine whether the increase in asp24 expression depended on different bacterial growth phases. qRT-PCR showed that asp24 expression was upregulated in the ΔrfbE mutant throughout its entire growth cycle. At 6, 12, 24, 36, and 48 h of growth, 8.7-, 14.3-, 49.6-, 447.8-, and 582.1-fold increases, respectively, were observed (Fig. 1A). Furthermore, enhanced Asp24 protein levels in the ΔrfbE mutant were also determined by Western blotting, using the surface antigen D15 as a reference protein (Fig. 1B). asp24 expression in the ΔrfbE(pBBR-rfbE) complemented strain exhibited levels similar to those in B. abortus S2308 for the entire growth cycle (Fig. 1A and B). Overall, the results indicated that disruption of the O-antigen export system rfbE gene in B. abortus progressively upregulated bacterial asp24 gene expression and its protein level throughout the bacterial growth cycle.
FIG 1.
asp24 expression is significantly upregulated in the ΔrfbE mutant. (A) asp24 expression in the ΔrfbE mutant was significantly upregulated at the indicated incubation times in TSB compared to that in B. abortus S2308, as measured using qRT-PCR. The ΔrfbE(pBBR-rfbE) complemented strain had recovered asp24 expression. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (B) Western blotting revealed an enhanced Asp24 protein level in the ΔrfbE mutant compared to that in B. abortus S2308. The ΔrfbE(pBBR-rfbE) complemented strain had a recovered Asp24 protein level. Protein levels are indicated by gray-scanning intensity values.
asp24-upregulated expression in the ΔrfbE mutant is associated with intracellular O-antigen synthesis and accumulation.
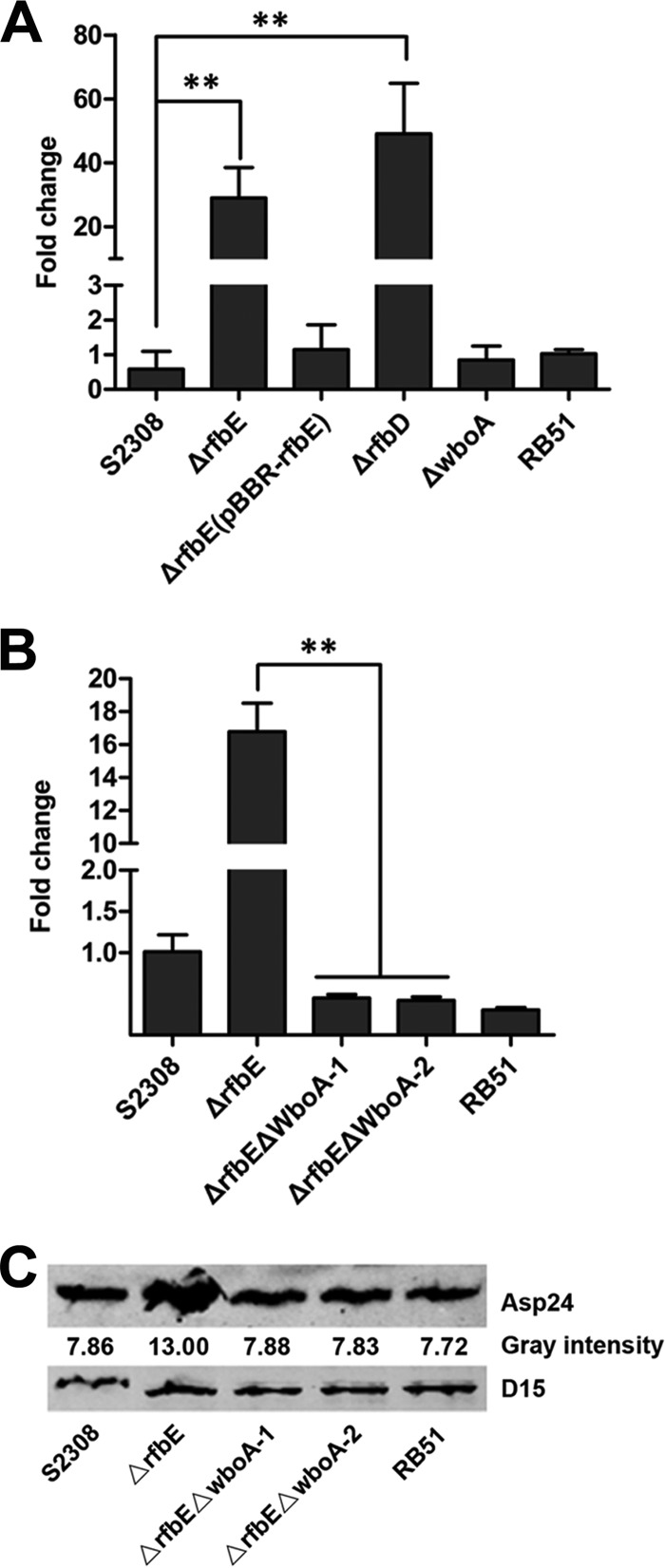
To determine whether asp24 upregulation depends on the Brucella rough phenotype, the level of asp24 expression was determined by qRT-PCR analysis of different rough phenotype strains, including the ΔrfbE, ΔrfbD, ΔwboA, and RB51 strains. Interestingly, asp24 was upregulated only in the ΔrfbE and ΔrfbD mutants, the O-antigen export system-disrupted mutants, with 29-fold and 49-fold increases, respectively (Fig. 2A). asp24 expression was unchanged in the ΔwboA and RB51 strains (Fig. 2A). The results suggested that asp24 upregulation is not associated with the bacterial rough phenotype.
FIG 2.
Intracellular O-antigen synthesis and accumulation are responsible for asp24 upregulation in the ΔrfbE mutant. (A) qRT-PCR showed enhanced asp24 expression in the ΔrfbE and ΔrfbD mutants, but not in the ΔwboA and RB51 strains, compared to WT strain S2308. The ΔrfbE(pBBR-rfbE) complemented strain had recovered asp24 expression. **, P < 0.01. (B) Deletion of the wboA gene from the ΔrfbE mutant resulted in recovered asp24 expression as determined using qRT-PCR. **, P < 0.01. (C) Western blot showing the recovered Asp24 protein level in the wboA gene-deleted ΔrfbE mutant (lanes ΔrfbEΔwboA-1 and ΔrfbEΔwboA-2). Protein levels are indicated by gray-scanning intensity values.
The Brucella O-antigen export system is an ATP-binding cassette (ABC) transporter system, encoded by the rfbE and rfbD genes, which flips the O-antigen from the cytoplasmic face to the periplasmic face of the inner membrane; disruption of this system affects the flipping of O-antigen, resulting in an accumulation of intracellular O-antigen (20). Western blotting was further used to detect intracellular O-antigen synthesis in the rough phenotype strains and showed that O-antigen was detected in the ΔrfbE and ΔrfbD mutants but absent in the ΔwboA and RB51 strains (Fig. 3A), suggesting that disruption of the O-antigen export system does not abolish intracellular O-antigen synthesis.
FIG 3.
Western blotting identifies O-antigen synthesis in bacteria. (A) O-antigen was synthesized in the ΔrfbE and ΔrfbD mutants but not the ΔwboA and RB51 strains. (B) Deletion of the wboA gene from the ΔrfbE mutant abolished O-antigen synthesis; no O-antigen was detected in lanes ΔrfbEΔwboA-1 and ΔrfbEΔwboA-2.
To determine whether asp24-upregulated expression is associated with the accumulation of intracellular O-antigen, a glycosyltransferase gene involved in O-antigen synthesis, wboA (20, 24), was knocked out of the ΔrfbE mutant, thereby generating a double-knockout strain (ΔrfbE ΔwboA). Western blotting showed that no O-antigen was detected in the ΔrfbE ΔwboA strain, suggesting that O-antigen synthesis was abolished due to wboA deletion (Fig. 3B). Furthermore, qRT-PCR and Western blotting results showed that asp24/Asp24 expression in the ΔrfbE ΔwboA strain was restored to levels comparable to those in B. abortus S2308 (Fig. 2B and C). This indicates that asp24 upregulation in the ΔrfbE mutant is associated with the intracellular accumulation of O-antigen.
asp24 upregulation is not the cause of ΔrfbE mutant-induced oncotic and necrotic macrophage death.
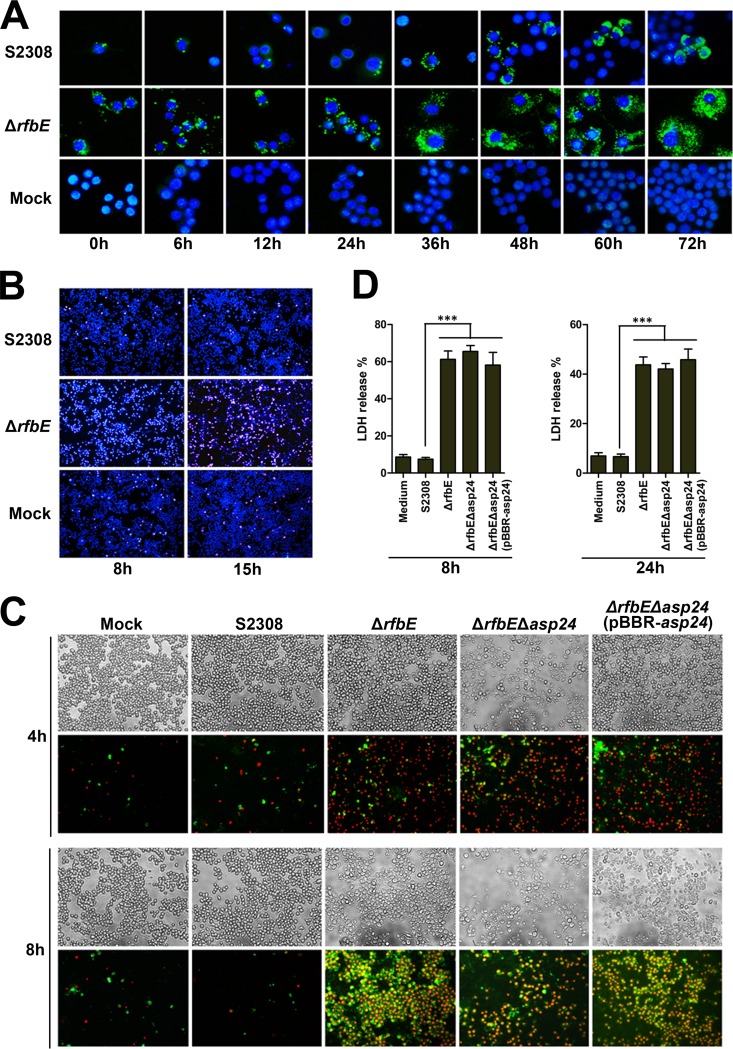
The number of CFU recovered from infected RAW264.7 cells was dramatically reduced for the ΔrfbE mutant compared to WT strain S2308, as previously reported (23); however, the ΔrfbE mutant did multiply in macrophages, as suggested by enhanced bacterial staining postinfection by fluorescence microscopy (Fig. 4A). This result is consistent with that for the rough mutant CA180 infecting RAW264.7 cells (29). Hoechst 33342 (blue) and PI (red) staining showed that more than 60% and 80% of the cells infected by the ΔrfbE mutant were dead at 8 and 15 hpi, respectively (Fig. 4B). A marked cytopathic effect was observed in the infected cells, as evaluated by phase-contrast microscopy (Fig. 4C).
FIG 4.
Brucella rough mutants induce necrosis in RAW264.7 cells. (A) RAW264.7 cells were infected with S2308 or the ΔrfbE mutant at an MOI of 30. Intracellular bacteria were stained at different time points with primary antibody (rabbit anti-Brucella polyclonal antibody) and secondary antibody (Alexa Fluor 488-conjugated goat anti-rabbit IgG) and then observed under a fluorescence microscope (magnification, ×10,000). Uninfected RAW264.7 cells were used as negative controls (Mock). (B) RAW264.7 cells cultured in a 24-well plate were infected with S2308 or the ΔrfbE mutant at an MOI of 200. The cells were stained at 8 and 15 hpi with Hoechst 33342 (blue) and PI (red) and then observed using fluorescence microscopy (magnification, ×100). Uninfected RAW264.7 cells were used as negative controls (Mock). (C) RAW264.7 cells cultured in a 24-well plate were infected with S2308, the ΔrfbE mutant, the double-knockout ΔrfbE Δasp24 strain, or the ΔrfbE Δasp24(pBBR-asp24) complemented strain at an MOI of 200. The cells were stained at 4 and 8 hpi with FITC-annexin (green) and PI (red) and observed by phase-contrast (upper panels) or fluorescence (lower panels) microscopy at a magnification of ×200. Uninfected RAW264.7 cells were used as negative controls (Mock). (D) RAW264.7 cells were infected with S2308 or the ΔrfbE, ΔrfbE Δasp24, or ΔrfbE Δasp24(pBBR-asp24) mutant at an MOI of 200. The supernatants were collected at 8 and 24 hpi, and LDH release was detected using the CytoTox 96 nonradioactive cytotoxicity assay. The supernatants of uninfected RAW264.7 cells were used as negative controls (medium). ***, P < 0.001.
Brucella strains with the rough phenotype can induce macrophage oncosis and necrosis but not apoptosis (29). Apoptosis is a process of programmed cell death which leads to cell death with karyorrhexis and cell shrinkage; necrosis is a form of traumatic cell death that results from acute cellular injury; and oncosis precisely means cell death by swelling and leads to necrosis with karyolysis, standing in contrast to apoptosis. To determine whether asp24 upregulation affects ΔrfbE mutant-induced macrophage death, the asp24 gene was knocked out of the ΔrfbE mutant to produce the ΔrfbE Δasp24 strain, and a complemented strain [ΔrfbE Δasp24(pBBR-asp24)] was constructed using the pBBR-asp24 plasmid. RAW264.7 cells were infected with the S2308, ΔrfbE, ΔrfbE Δasp24, and ΔrfbE Δasp24(pBBR-asp24) strains, and cell death was analyzed by annexin V (green) and PI (red) staining. Annexin V staining was used to detect translocation of phosphatidylserine from the inner cell membrane to the outer cell membrane of cells during the early stage of apoptosis. PI stains the DNA of necrotic cells and/or cells at the late stage of apoptosis. The results showed that infections with the ΔrfbE, ΔrfbE Δasp24, and ΔrfbE Δasp24(pBBR-asp24) strains exhibited similar characteristics of necrosis, indicating that these mutants induced macrophage oncosis and necrosis but not apoptosis (Fig. 4C). In addition, levels of LDH released from RAW264.7 cells infected with the ΔrfbE, ΔrfbE Δasp24, and ΔrfbE Δasp24(pBBR-asp24) mutants showed no significant differences (Fig. 4D). These results indicated that asp24 upregulation is not the cause of ΔrfbE mutant-induced oncotic and necrotic macrophage death.
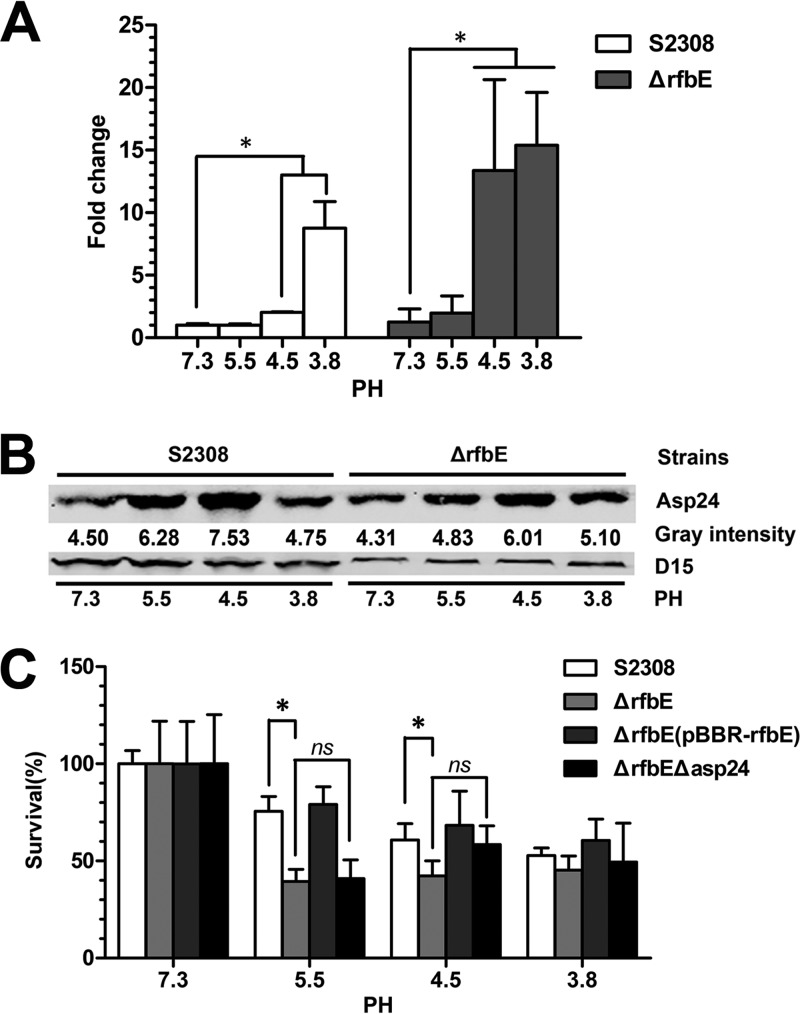
asp24 expression is induced under acidic conditions but not associated with bacterial acid resistance.
To determine asp24 expression under acidic conditions, S2308 and the ΔrfbE mutant were grown to log phase and exposed to TSB at pH 7.3, 5.5, 4.5, and 3.8 for 2 h. qRT-PCR and Western blotting were performed to determine asp24 expression, revealing that asp24 upregulation was induced in both strains at pH 4.5 and 3.8 (Fig. 5A and B).
FIG 5.
asp24/Asp24 expression is induced under acidic conditions but not associated with acid resistance. (A) asp24 expression in the S2308 and ΔrfbE strains was upregulated at pH 4.5 and 3.8 compared to that at pH 7.3, as determined by qRT-PCR. *, P < 0.05. (B) Western blot showing enhanced Asp24 protein levels in S2308 at pH 5.5 and 4.5 and in the ΔrfbE mutant at pH 5.5, 4.5, and 3.8. Protein levels are indicated by gray-scanning intensity values. (C) The survival percentages for the ΔrfbE Δasp24 mutant showed no significant changes for TSB at pH 7.3, 5.5, 4.5, and 3.8 compared to those for the ΔrfbE mutant. ns, no significant difference. The survival percentages for S2308 were significantly higher at pH 5.5 and 4.5 than those for the ΔrfbE mutant. *, P < 0.05.
To determine whether asp24-upregulated expression in the ΔrfbE mutant enhances bacterial acid resistance, the S2308, ΔrfbE, ΔrfbE(pBBR-rfbE), and ΔrfbE Δasp24 strains were recovered after exposure to TSB at pH 7.3, 5.5, 4.5, and 3.8 for 2 h. The survival ratio of the ΔrfbE mutant was decreased significantly at pH 5.5 and 4.5 (P < 0.05) compared to that of WT strain S2308, but it recovered when the strain was complemented with the plasmid pBBR-rfbE (Fig. 5C). However, asp24 deletion in the ΔrfbE mutant (ΔrfbE Δasp24) did not weaken its acid resistance (Fig. 5C). This implies that the lack of O-antigen in B. abortus significantly decreases bacterial acid resistance in an asp24-independent manner.
Altered asp24 expression in B. abortus strains reduces intracellular survival within RAW264.7 and HeLa cells.
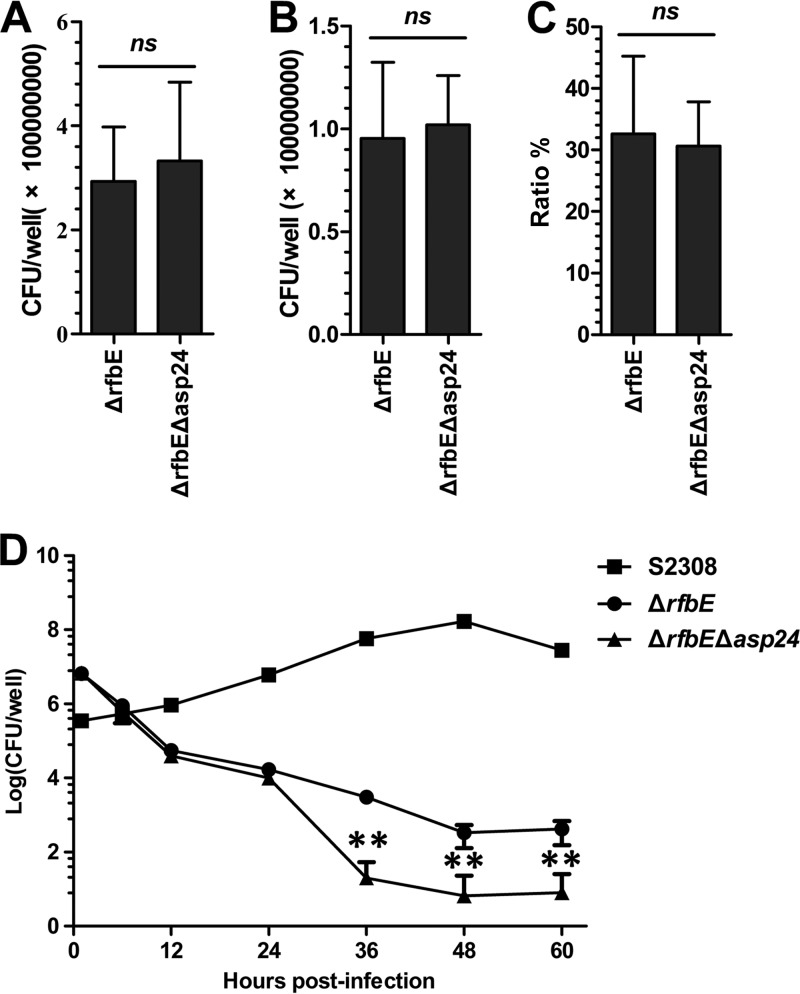
RAW264.7 cells were infected with the ΔrfbE or ΔrfbE Δasp24 mutant to determine the functions of asp24 in bacterial adherence and invasion capacities. The results showed that no significant difference between the two mutants was found for bacterial adherence (Fig. 6A), invasion (Fig. 6B), or invasion ratio (number of invasive bacteria/number of adherent bacteria) (Fig. 6C). Thus, asp24 does not appear to have a role in B. abortus adherence to and invasion into macrophages. An intracellular survival assay demonstrated reduced survival of both the ΔrfbE and ΔrfbE Δasp24 mutants in RAW264.7 cells compared to that of S2308. Intracellular survival of the ΔrfbE Δasp24 mutant was significantly worse than that of the ΔrfbE mutant after 36 hpi (Fig. 6D), indicating a role of asp24 in intracellular survival within macrophages.
FIG 6.
asp24 deletion from the ΔrfbE mutant affects intracellular survival, but not adherence and invasion capacities, in RAW264.7 cells. (A to C) Adherence (A), invasion (B), and invasion ratios (C) for the ΔrfbE and ΔrfbE Δasp24 mutants in RAW264.7 cells, with no significant changes seen for infections at an MOI of 200. The invasion ratio was evaluated as the number of internalized bacteria/number of adherent bacteria. ns, no significant difference. (D) Bacterial intracellular survival of the ΔrfbE Δasp24 mutant was significantly decreased compared to that of the ΔrfbE mutant at 36, 48, and 60 hpi, for infections at an MOI of 50. **, P < 0.01.
HeLa cells were also used to evaluate mutant intracellular survival, in addition to using macrophages. The S2308 and ΔrfbE strains were made to overexpress asp24 through electroporation of the pBBR-asp24 plasmid, generating strains S2308(pBBR-asp24) and ΔrfbE(pBBR-asp24), respectively (Fig. 7A). Both asp24-overexpressing strains showed attenuated survival in HeLa cells compared with that of the respective S2308 (24 and 48 hpi) and ΔrfbE (12, 24, and 48 hpi) strains (Fig. 7B and C). The results implied that appropriate expression of asp24 plays a pivotal role in maximizing virulence for B. abortus. Deletion or overexpression of asp24 in the S2308 and ΔrfbE strains affects their intracellular survival within host cells.
FIG 7.
asp24 deletion or overexpression in the S2308 and ΔrfbE strains results in reduced intracellular survival. (A) asp24 expression in the S2308(pBBR-asp24) and ΔrfbE(pBBR-asp24) strains was upregulated compared to that in the respective S2308 and ΔrfbE strains, as determined using qRT-PCR. *, P < 0.05. (B) The intracellular survival of S2308(pBBR-asp24) (asp24-overexpressing S2308 strain) was significantly decreased at 24 and 48 hpi compared to that of S2308 in HeLa cells infected at an MOI of 500. *, P < 0.05. (C) The intracellular survival of the ΔrfbE Δasp24 (asp24 deletion ΔrfbE strain) and ΔrfbE(pBBR-asp24) (asp24-overexpressing ΔrfbE strain) strains was significantly decreased at 12, 24, and 48 hpi compared to that of the ΔrfbE strain in HeLa cells infected at an MOI of 500. **, P < 0.01.
DISCUSSION
Brucella LPS is an important virulence determinant with important roles in host immune system evasion and bacterial survival (30–32). LPS with a complete O-antigen is crucial for Brucella virulence in humans (33). Disruption of the rfbE or rfbD gene induces the transformation of smooth-type Brucella strains into rough-type strains due to the creation of an incomplete LPS lacking O-antigen (19). The ΔrfbE mutant shows a rough phenotype, which enhances internalization into the host cell and induces macrophage oncosis and necrosis, like the case with other Brucella rough mutants (29, 34, 35). The mutant also has reduced survival in the host cell and fails to establish chronic infection in a mouse model (23). However, the mechanisms behind attenuated infection by the rough mutant have not been deciphered completely. In a previous study, artificial Brucella rough mutants were found to be cytopathic for macrophages and dependent on the type 4 secretion system (T4SS) (36, 37), but the effectors delivered by the T4SS are still unknown.
In this study, asp24-upregulated expression was found in rough mutants with a disrupted O-antigen export system ABC transporter, and further study confirmed that intracellular O-antigen synthesis and accumulation induced asp24 upregulation in the mutant. In Brucella, O-antigen is synthesized via a Wzy-independent mechanism, and after its assembly onto bactoprenol phosphate at the cytoplasmic face of the inner membrane, the complete lipid-linked O-antigen is transported across the inner membrane by an ABC transporter system. rfbD and rfbE were identified and predicted to form the transmembrane and ATPase domains, respectively, of an ABC transporter required for the translocation of the full-length homopolymeric O-antigen from the cytoplasmic to the periplasmic face of the inner membrane (38). In the study, the deletion of rfbD/rfbE resulted in the accumulation of intracellular synthesized O-antigen, which may induce asp24 upregulation by increasing intracellular pressure. The Asp24 protein was initially identified from its enhanced expression in macrophages and under low-pH conditions in vitro (21); its exact role in pathogenesis is unknown. In B. melitensis or B. abortus, asp24 deletion mutants showed attenuated persistence in mice compared to the WT strains, and the B. melitensis Δasp24 strain failed to cause abortion or to colonize fetal tissues in pregnant goats (26, 39). This suggests that asp24 plays an important role in ensuring Brucella's full virulence in infecting mice and goats.
asp24 expression is stimulated under acidic conditions. Asp24 contains an EF-hand motif found in a large family of calcium-binding proteins (40, 41). This motif appears to have a specific role in acid shock adaptation; however, the function of Asp24 has not been defined completely. In a previous report, rough mutants were cytopathic toward macrophages; the ΔrfbE mutant was also found to have this characteristic. In this study, we report that asp24 expression is upregulated in rough mutants with a compromised O-antigen export system, but this upregulation is not the cause of macrophage cytotoxicity. In addition, the acid resistance assay revealed that asp24 deletion did not affect ΔrfbE mutant survival at low pH, even though survival of both the ΔrfbE and ΔrfbE Δasp24 mutants was significantly lower than that of WT strain S2308 under acidic conditions.
asp24 upregulation is induced not only under acidic conditions but also inside macrophages (21). In this study, asp24 upregulation was found in the ΔrfbE and ΔrfbD mutants. To explore the function of asp24 in B. abortus intracellular survival, mutants with altered asp24 expression were constructed, and intracellular survival was assessed using a gentamicin protection assay. In RAW264.7 cells, asp24 deletion from the ΔrfbE mutant reduced its intracellular survival, but both the ΔrfbE and ΔrfbE Δasp24 mutants were cytopathic to the macrophages. To circumvent this cytopathic effect, the experiments were repeated in HeLa cells, because neither strain was cytopathic to HeLa cells. Interestingly, both abolished and overexpressed asp24 reduced ΔrfbE mutant intracellular survival in HeLa cells. Similar results were obtained with HeLa cells infected with the WT strain S2308 overexpressing asp24.
Nonopsonized Brucella entry into macrophages is mediated through lipid rafts (29, 42–44). Once inside cells, Brucella organisms reside in the Brucella-containing vacuole (BCV), which interacts with components of endocytic pathways to ensure bacterial survival (45–48). Inhibition of BCV acidification at the early stages of infection completely abolishes Brucella intracellular survival (49). We hypothesized that Asp24 plays a role in BCV acidification, but the role may not be indispensable at this stage, because asp24 deletion in the ΔrfbE mutant did not completely abolish intracellular survival. Intriguingly, overexpressing asp24 in the ΔrfbE and S2308 strains also reduced intracellular survival, indicating that proper and moderate asp24 expression is necessary to ensure intracellular survival. How the asp24 gene is precisely regulated and expressed during infection inside host cells is still unknown.
In conclusion, asp24 expression is induced in rough mutants with a disrupted O-antigen export system ABC transporter. Its upregulation is not the cause of rough mutant-induced oncosis and necrosis in macrophages, but irregular asp24 expression in WT or mutant strains affects B. abortus intracellular survival within host cells. In the present study, we (i) illustrated that Brucella asp24 upregulation in specific mutants is associated with intracellular O-antigen synthesis and accumulation, (ii) discovered a novel asp24 upregulation mechanism, and (iii) indicated the role of proper Asp24 expression in bacterial intracellular survival. Further studies will investigate the possible mechanism relating Asp24 expression to O-antigen secretion during the normal course of infection.
Supplementary Material
ACKNOWLEDGMENTS
We thank Qingmin Wu for providing B. abortus vaccine strain RB51. We are also very grateful to Jean-Jacques Letesson for kindly providing the anti-O-antigen antibody A76 12G12 F12.
This work was supported by the Chinese National Programs for Fundamental Research and Development (grant 2010CB530202), by the 948 Project of the Chinese Agricultural Ministry (grant 2012-Z9), and by the National Basic Fund for Research Institutes of the Chinese Academy of Agricultural Sciences (grant 2013JB04).
Footnotes
Published ahead of print 21 April 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01765-14.
REFERENCES
- 1.Boschiroli ML, Foulongne V, O'Callaghan D. 2001. Brucellosis: a worldwide zoonosis. Curr. Opin. Microbiol. 4:58–64. 10.1016/S1369-5274(00)00165-X [DOI] [PubMed] [Google Scholar]
- 2.Atluri VL, Xavier MN, de Jong MF, den Hartigh AB, Tsolis RM. 2011. Interactions of the human pathogenic Brucella species with their hosts. Annu. Rev. Microbiol. 65:523–541. 10.1146/annurev-micro-090110-102905 [DOI] [PubMed] [Google Scholar]
- 3.Yang X, Skyberg JA, Cao L, Clapp B, Thornburg T, Pascual DW. 2013. Progress in Brucella vaccine development. Front. Biol. 8:60–77. 10.1007/s11515-012-1196-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang WY, Guo WD, Sun SH, Jiang JF, Sun HL, Li SL, Liu W, Cao WC. 2010. Human brucellosis, Inner Mongolia, China. Emerg. Infect. Dis. 16:2001–2003. 10.3201/eid1612.091081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turse JE, Pei J, Ficht TA. 2011. Lipopolysaccharide-deficient Brucella variants arise spontaneously during infection. Front. Microbiol. 2:54. 10.3389/fmicb.2011.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He Y. 2012. Analyses of Brucella pathogenesis, host immunity, and vaccine targets using systems biology and bioinformatics. Front. Cell. Infect. Microbiol. 2:2. 10.3389/fcimb.2012.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moreno E, Moriyon I. 2002. Brucella melitensis: a nasty bug with hidden credentials for virulence. Proc. Natl. Acad. Sci. U. S. A. 99:1–3. 10.1073/pnas.022622699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fugier E, Pappas G, Gorvel JP. 2007. Virulence factors in brucellosis: implications for aetiopathogenesis and treatment. Expert Rev. Mol. Med. 9:1–10. 10.1017/S1462399407000543 [DOI] [PubMed] [Google Scholar]
- 9.Erridge C, Bennett-Guerrero E, Poxton IR. 2002. Structure and function of lipopolysaccharides. Microbes Infect. 4:837–851. 10.1016/S1286-4579(02)01604-0 [DOI] [PubMed] [Google Scholar]
- 10.Lapaque N, Forquet F, de Chastellier C, Mishal Z, Jolly G, Moreno E, Moriyon I, Heuser JE, He HT, Gorvel JP. 2006. Characterization of Brucella abortus lipopolysaccharide macrodomains as mega rafts. Cell. Microbiol. 8:197–206. 10.1111/j.1462-5822.2005.00609.x [DOI] [PubMed] [Google Scholar]
- 11.Pei J, Turse JE, Ficht TA. 2008. Evidence of Brucella abortus OPS dictating uptake and restricting NF-kappaB activation in murine macrophages. Microbes Infect. 10:582–590. 10.1016/j.micinf.2008.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardoso PG, Macedo GC, Azevedo V, Oliveira SC. 2006. Brucella spp noncanonical LPS: structure, biosynthesis, and interaction with host immune system. Microb. Cell Fact. 5:13. 10.1186/1475-2859-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seleem MN, Boyle SM, Sriranganathan N. 2008. Brucella: a pathogen without classic virulence genes. Vet. Microbiol. 129:1–14. 10.1016/j.vetmic.2007.11.023 [DOI] [PubMed] [Google Scholar]
- 14.Lapaque N, Moriyon I, Moreno E, Gorvel JP. 2005. Brucella lipopolysaccharide acts as a virulence factor. Curr. Opin. Microbiol. 8:60–66. 10.1016/j.mib.2004.12.003 [DOI] [PubMed] [Google Scholar]
- 15.Caroff M, Bundle DR, Perry MB, Cherwonogrodzky JW, Duncan JR. 1984. Antigenic S-type lipopolysaccharide of Brucella abortus 1119-3. Infect. Immun. 46:384–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bundle DR, Cherwonogrodzky JW, Perry MB. 1987. The structure of the lipopolysaccharide O-chain (M antigen) and polysaccharide B produced by Brucella melitensis 16M. FEBS Lett. 216:261–264. 10.1016/0014-5793(87)80702-0 [DOI] [PubMed] [Google Scholar]
- 17.McQuiston JR, Vemulapalli R, Inzana TJ, Schurig GG, Sriranganathan N, Fritzinger D, Hadfield TL, Warren RA, Lindler LE, Snellings N, Hoover D, Halling SM, Boyle SM. 1999. Genetic characterization of a Tn5-disrupted glycosyltransferase gene homolog in Brucella abortus and its effect on lipopolysaccharide composition and virulence. Infect. Immun. 67:3830–3835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winter AJ, Schurig GG, Boyle SM, Sriranganathan N, Bevins JS, Enright FM, Elzer PH, Kopec JD. 1996. Protection of BALB/c mice against homologous and heterologous species of Brucella by rough strain vaccines derived from Brucella melitensis and Brucella suis biovar 4. Am. J. Vet. Res. 57:677–683 [PubMed] [Google Scholar]
- 19.Moriyon I, Grillo MJ, Monreal D, Gonzalez D, Marin C, Lopez-Goni I, Mainar-Jaime RC, Moreno E, Blasco JM. 2004. Rough vaccines in animal brucellosis: structural and genetic basis and present status. Vet. Res. 35:1–38. 10.1051/vetres:2003037 [DOI] [PubMed] [Google Scholar]
- 20.Godfroid F, Cloeckaert A, Taminiau B, Danese I, Tibor A, de Bolle X, Mertens P, Letesson JJ. 2000. Genetic organisation of the lipopolysaccharide O-antigen biosynthesis region of Brucella melitensis 16M (wbk). Res. Microbiol. 151:655–668. 10.1016/S0923-2508(00)90130-X [DOI] [PubMed] [Google Scholar]
- 21.Lin J, Ficht TA. 1995. Protein synthesis in Brucella abortus induced during macrophage infection. Infect. Immun. 63:1409–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian M, Qu J, Han X, Zhang M, Ding C, Ding J, Chen G, Yu S. 2013. Microarray-based identification of differentially expressed genes in intracellular Brucella abortus within RAW264.7 cells. PLoS One 8:e67014. 10.1371/journal.pone.0067014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang M, Han X, Liu H, Tian M, Ding C, Song J, Sun X, Liu Z, Yu S. 2013. Inactivation of the ABC transporter ATPase gene in Brucella abortus strain 2308 attenuated the virulence of the bacteria. Vet. Microbiol. 164:322–329. 10.1016/j.vetmic.2013.02.017 [DOI] [PubMed] [Google Scholar]
- 24.Vemulapalli R, He Y, Buccolo LS, Boyle SM, Sriranganathan N, Schurig GG. 2000. Complementation of Brucella abortus RB51 with a functional wboA gene results in O-antigen synthesis and enhanced vaccine efficacy but no change in rough phenotype and attenuation. Infect. Immun. 68:3927–3932. 10.1128/IAI.68.7.3927-3932.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovach ME, Phillips RW, Elzer PH, Roop RM, 2nd, Peterson KM. 1994. pBBR1MCS: a broad-host-range cloning vector. Biotechniques 16:800–802 [PubMed] [Google Scholar]
- 26.Kahl-McDonagh MM, Ficht TA. 2006. Evaluation of protection afforded by Brucella abortus and Brucella melitensis unmarked deletion mutants exhibiting different rates of clearance in BALB/c mice. Infect. Immun. 74:4048–4057. 10.1128/IAI.01787-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian M, Zhao Y, Shi M, Lin Y, Zou N, Liu P, Wen X, Cao S, Huang Y. 2010. A novel protein-coding ORF72.2 gene was identified from Marek's disease virus strain CVI988. Virol. J. 7:371. 10.1186/1743-422X-7-371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garin-Bastuji B, Bowden RA, Dubray G, Limet JN. 1990. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting analysis of smooth-lipopolysaccharide heterogeneity among Brucella biovars related to A and M specificities. J. Clin. Microbiol. 28:2169–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pei J, Ficht TA. 2004. Brucella abortus rough mutants are cytopathic for macrophages in culture. Infect. Immun. 72:440–450. 10.1128/IAI.72.1.440-450.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gross A, Terraza A, Ouahrani-Bettache S, Liautard JP, Dornand J. 2000. In vitro Brucella suis infection prevents the programmed cell death of human monocytic cells. Infect. Immun. 68:342–351. 10.1128/IAI.68.1.342-351.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martirosyan A, Moreno E, Gorvel JP. 2011. An evolutionary strategy for a stealthy intracellular Brucella pathogen. Immunol. Rev. 240:211–234. 10.1111/j.1600-065X.2010.00982.x [DOI] [PubMed] [Google Scholar]
- 32.Martirosyan A, Gorvel JP. 2013. Brucella evasion of adaptive immunity. Future Microbiol. 8:147–154. 10.2217/fmb.12.140 [DOI] [PubMed] [Google Scholar]
- 33.Young EJ. 1995. An overview of human brucellosis. Clin. Infect. Dis. 21:283–289. 10.1093/clinids/21.2.283 [DOI] [PubMed] [Google Scholar]
- 34.He Y, Vemulapalli R, Zeytun A, Schurig GG. 2001. Induction of specific cytotoxic lymphocytes in mice vaccinated with Brucella abortus RB51. Infect. Immun. 69:5502–5508. 10.1128/IAI.69.9.5502-5508.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, He Y. 2012. Caspase-2-dependent dendritic cell death, maturation, and priming of T cells in response to Brucella abortus infection. PLoS One 7:e43512. 10.1371/journal.pone.0043512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pei J, Wu Q, Kahl-McDonagh M, Ficht TA. 2008. Cytotoxicity in macrophages infected with rough Brucella mutants is type IV secretion system dependent. Infect. Immun. 76:30–37. 10.1128/IAI.00379-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong Z, Wang Y, Qiao F, Wang Z, Du X, Xu J, Zhao J, Qu Q, Dong S, Sun Y, Huang L, Huang K, Chen Z. 2009. Cytotoxicity of Brucella smooth strains for macrophages is mediated by increased secretion of the type IV secretion system. Microbiology 155:3392–3402. 10.1099/mic.0.030619-0 [DOI] [PubMed] [Google Scholar]
- 38.Haag AF, Myka KK, Arnold MF, Caro-Hernandez P, Ferguson GP. 2010. Importance of lipopolysaccharide and cyclic beta-1,2-glucans in Brucella-mammalian infections. Int. J. Microbiol. 2010:124509. 10.1155/2010/124509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kahl-McDonagh MM, Elzer PH, Hagius SD, Walker JV, Perry QL, Seabury CM, den Hartigh AB, Tsolis RM, Adams LG, Davis DS, Ficht TA. 2006. Evaluation of novel Brucella melitensis unmarked deletion mutants for safety and efficacy in the goat model of brucellosis. Vaccine 24:5169–5177. 10.1016/j.vaccine.2006.04.005 [DOI] [PubMed] [Google Scholar]
- 40.Ikura M, Osawa M, Ames JB. 2002. The role of calcium-binding proteins in the control of transcription: structure to function. Bioessays 24:625–636. 10.1002/bies.10105 [DOI] [PubMed] [Google Scholar]
- 41.Zhou Y, Yang W, Kirberger M, Lee HW, Ayalasomayajula G, Yang JJ. 2006. Prediction of EF-hand calcium-binding proteins and analysis of bacterial EF-hand proteins. Proteins 65:643–655. 10.1002/prot.21139 [DOI] [PubMed] [Google Scholar]
- 42.Naroeni A, Porte F. 2002. Role of cholesterol and the ganglioside GM1 in entry and short-term survival of Brucella suis in murine macrophages. Infect. Immun. 70:1640–1644. 10.1128/IAI.70.3.1640-1644.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim S, Watarai M, Suzuki H, Makino S, Kodama T, Shirahata T. 2004. Lipid raft microdomains mediate class A scavenger receptor-dependent infection of Brucella abortus. Microb. Pathog. 37:11–19. 10.1016/j.micpath.2004.04.002 [DOI] [PubMed] [Google Scholar]
- 44.Watarai M. 2004. Interaction between Brucella abortus and cellular prion protein in lipid raft microdomains. Microbes Infect. 6:93–100. 10.1016/j.micinf.2003.11.002 [DOI] [PubMed] [Google Scholar]
- 45.Pizarro-Cerda J, Meresse S, Parton RG, van der Goot G, Sola-Landa A, Lopez-Goni I, Moreno E, Gorvel JP. 1998. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect. Immun. 66:5711–5724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gorvel JP, Moreno E. 2002. Brucella intracellular life: from invasion to intracellular replication. Vet. Microbiol. 90:281–297. 10.1016/S0378-1135(02)00214-6 [DOI] [PubMed] [Google Scholar]
- 47.Starr T, Child R, Wehrly TD, Hansen B, Hwang S, Lopez-Otin C, Virgin HW, Celli J. 2012. Selective subversion of autophagy complexes facilitates completion of the Brucella intracellular cycle. Cell Host Microbe 11:33–45. 10.1016/j.chom.2011.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Bargen K, Gorvel JP, Salcedo SP. 2012. Internal affairs: investigating the Brucella intracellular lifestyle. FEMS Microbiol. Rev. 36:533–562. 10.1111/j.1574-6976.2012.00334.x [DOI] [PubMed] [Google Scholar]
- 49.Porte F, Liautard JP, Kohler S. 1999. Early acidification of phagosomes containing Brucella suis is essential for intracellular survival in murine macrophages. Infect. Immun. 67:4041–4047 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.