Abstract
The ability of Clostridium perfringens type C to cause human enteritis necroticans (EN) is attributed to beta toxin (CPB). However, many EN strains also express C. perfringens enterotoxin (CPE), suggesting that CPE could be another contributor to EN. Supporting this possibility, lysate supernatants from modified Duncan-Strong sporulation (MDS) medium cultures of three CPE-positive type C EN strains caused enteropathogenic effects in rabbit small intestinal loops, which is significant since CPE is produced only during sporulation and since C. perfringens can sporulate in the intestines. Consequently, CPE and CPB contributions to the enteropathogenic effects of MDS lysate supernatants of CPE-positive type C EN strain CN3758 were evaluated using isogenic cpb and cpe null mutants. While supernatants of wild-type CN3758 MDS lysates induced significant hemorrhagic lesions and luminal fluid accumulation, MDS lysate supernatants of the cpb and cpe mutants caused neither significant damage nor fluid accumulation. This attenuation was attributable to inactivating these toxin genes since complementing the cpe mutant or reversing the cpb mutation restored the enteropathogenic effects of MDS lysate supernatants. Confirming that both CPB and CPE are needed for the enteropathogenic effects of CN3758 MDS lysate supernatants, purified CPB and CPE at the same concentrations found in CN3758 MDS lysates also acted together synergistically in rabbit small intestinal loops; however, only higher doses of either purified toxin independently caused enteropathogenic effects. These findings provide the first evidence for potential synergistic toxin interactions during C. perfringens intestinal infections and support a possible role for CPE, as well as CPB, in some EN cases.
INTRODUCTION
Clostridium perfringens ranks among the most important pathogens of humans and livestock, causing both histotoxic diseases and infections originating in the intestines (1–3). The pathogenicity of this anaerobic bacterium is largely due to its production of ∼16 different toxins, although no single C. perfringens strain expresses this complete toxin repertoire. Based upon their production of alpha (CPA), beta (CPB), epsilon (ETX), or iota toxin, C. perfringens isolates are routinely classified into five (A, B, C, D, or E) types (1, 3). By definition, type C strains must express CPA and CPB; however, these isolates sometimes produce additional toxins, e.g., C. perfringens enterotoxin (CPE), that are medically important but not used in the typing classification scheme.
C. perfringens type C strains cause disease in livestock, particularly neonatal animals, when this bacterium grows in the small intestine and produces toxins (1, 4, 5). Type C animal infections include both necro-hemorrhagic enteritis, which can result in death due to direct intestinal damage, and enterotoxemia, which can also cause death following absorption of the toxins from the small intestine into the circulation. Among the more notable type C livestock infections are necro-hemorrhagic enteritis in lambs, piglets, foals, and calves. In adult sheep, type C strains cause “struck,” an enterotoxemia where the infected animals die so quickly that they appear to have been struck by lightning; disease in adult individuals of other animal species is also occasionally observed. In the absence of vaccination, outbreaks of type C infections of newborn animals, especially piglets, can cause herd mortality rates of over 30%, resulting in significant economic losses (1, 4).
In humans, type C isolates cause food-borne enteritis necroticans (EN). Currently, this type C illness occurs sporadically throughout the developing world, with the highest incidence in Southeast Asia and Oceania (6–8). EN is also occasionally observed in developed countries, mainly in people with pancreatic disease (9). Historically, EN (known locally as Darmbrand) affected many malnourished people in northern Germany after World War II (10, 11). Prior to vaccination campaigns conducted during the 1970s and 1980s, EN (known locally as pigbel) was also the most common cause of death in children greater than 1 year old in the Papua New Guinea (PNG) highlands (6, 7). The risk factors for developing human EN include low trypsin levels due to protein-poor diets or pancreatic disease, often coupled with consumption of foods (such as sweet potato) containing trypsin inhibitor. Without surgical intervention, severe EN cases can be fatal within a few hours of the consumption of contaminated foods, particularly meats.
Molecular Koch's postulate analyses demonstrated that CPB production is essential for type C animal disease strain CN3685 to cause either necro-hemorrhagic enteritis or fatal enterotoxemia in animal models (12). However, it is noteworthy that CN3685 does not carry the gene encoding CPE (cpe), which is a potent enterotoxin important for the pathogenesis of several human gastrointestinal (GI) diseases caused by type A strains, including C. perfringens type A food poisoning and CPE-associated non-food-borne GI diseases, such as antibiotic-associated diarrhea (13). Although absent from CN3685, the enterotoxin gene (cpe) is carried by a significant number of type C strains, including many human EN strains (14). For example, older studies using relatively insensitive assays demonstrated that four of eight pigbel strains produced detectable levels of CPE (15, 16), and a more recent study demonstrated CPE production by all five surveyed type C Darmbrand strains (11).
The established ability of many pigbel and Darmbrand strains to express CPE have led to suggestions that CPE, as well as CPB, might contribute to EN (6, 15, 16). Since CPE is expressed only during sporulation by type C strains (14), our current study first evaluated whether supernatants from sporulating culture lysates of CPE-positive type C strains are enteropathogenic in a rabbit small intestinal loop model of type C necro-hemorrhagic enteritis. An isogenic cpe null mutant of type C Darmbrand isolate CN3758 was then prepared and used to directly test whether, at natural production levels, CPE contributes to the enteropathogenicity of sporulating culture lysates in rabbit small intestinal loops. In addition, an isogenic cpb mutant of CN3758 was prepared and used to evaluate possible additive or synergistic interactions between CPB and CPE in the small intestine. Finally, conclusions suggested by studies using sporulating culture lysates were explored further by assessing the in vivo activities of purified CPB and CPE, alone and in combination.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
C. perfringens strains CN3758 and CN2067 are previously characterized, CPE-positive type C Darmbrand isolates from post-World War II Germany (11), while CN5388 is a previously characterized CPE-positive type C strain isolated in 1963 from a pigbel case in Papua New Guinea (17). Each strain was streaked for isolation onto Shahidi-Ferguson perfringens (SFP) agar (Difco Laboratories) containing 0.04% d-cycloserine (Sigma-Aldrich) and then grown overnight at 37°C under anaerobic conditions. Fluid thioglycolate (FTG) medium (Difco Laboratories) broth and TGY broth (3% tryptic soy broth [Becton, Dickinson], 2% glucose [Sigma-Aldrich], 1% yeast extract [Becton, Dickinson], and 0.1% thioglycolate [Sigma-Aldrich]) were routinely used for growing vegetative cultures of these strains while modified Duncan-Strong (MDS) medium (11) was used to obtain sporulating cultures of the three strains. For the growth of CN3758 isogenic toxin null mutants and their respective complementing or reversed mutant strains, MDS medium was supplemented with 15 μg ml−1 of chloramphenicol.
Toxins.
Purified CPB was obtained from BEI Resources (Manassas, VA) or prepared as described previously (12). Purified CPE was prepared using previously described techniques (18).
Construction of a CN3758 cpb null mutant using TargeTron gene knockout technology and preparation of a reversed cpb mutant strain.
The cpb gene in CN3758 was insertionally inactivated using the Clostridium-modified TargeTron gene knockout system, as described previously for type C strain CN3685 (12). Briefly, the mutagenesis plasmid pJIR750bi-s, which carries the cpb-targeted intron (12), was electroporated into CN3758. Transformants were then plated onto brain heart infusion (BHI) agar (Difco) plates containing 15 μg ml−1 of chloramphenicol. Colonies were PCR screened using cpb-specific primers (Table 1, cpbF and cpbR) to detect the presence of a 0.9-kb intron insertion (in the sense orientation) in the cpb gene. A putative cpb mutant, named CN3758cpbko, was further characterized by PCR analyses and Southern blotting (see Results). That cpb null mutant was then subcultured daily in antibiotic-free FTG broth for ∼10 days to cure the intron delivery plasmid.
TABLE 1.
Primers used in this study
| Primer name | Primer sequence (5′–3′) |
|---|---|
| IBS | AAAAAAGCTTATAATTATCCTTAACCTGCTTCATTGTGCGCCCGATAGGGTG |
| EBS1d | CAGATTGTACAAATGTGGTGATAACAGATAAGTCTTCATTAGTAACTTACCTTTCTTTGT |
| EBS2 | TGAACGCAAGTTTCTAATTTCGATTCAGGTTCGATAGAGGAAAGTGTCT |
| cpbF | TTTCATTAGTTATAGTTAGTTCAC |
| cpbR | GGGGTATCAAAAGCTAGCGTGG |
| cpeF | GGAGATGGTTGGATATTAGG |
| cpeR | GGACCAGCAGTTGTAGATA |
Also constructed was CN3758cpbrev, a strain in which the cpb null mutation of CN3758 was reversed by electroporating the LtrA-encoding pJIR750bi-s plasmid back into CN3758cpbko. As described previously (12), growth of CN3758cpbrev at 30°C resulted in LtrA-mediated splicing of the intron from cpb mRNA, thus restoring CPB expression to the reversed mutant.
Construction of a CN3758 cpe null mutant using TargeTron technology and preparation of a cpe complementing strain.
To construct a CN3758 mutant carrying an inactivated cpe gene, the wild-type cpe gene sequence was first inputted into the intron prediction program (Sigma-Aldrich, St. Louis, MO). This program identified eight potential insertion sites across the 957-bp cpe open reading frame (ORF). Based upon that analysis, the site between nucleotides 195 and 196 of the CN3758 cpe ORF was chosen for initial intron targeting. The primers listed in Table 1 (IBS, EBS1d, and EBS2) were used to generate a 350-bp intron targeting sequence to this cpe ORF site. This 350-bp sequence was digested with HindIII and BsrGI (New England BioLabs) restriction enzymes according to the manufacturer's instructions and then ligated into pJIR750ai (a plasmid carrying the TargeTron vector [Sigma]), which had been digested similarly with the same two restriction enzymes. The resultant donor plasmid, named pJIR750cpei, was electroporated into wild-type CN3758, which would cause inactivation of the cpe gene by insertion, in an antisense orientation, of the ∼0.9-kb group II intron from the donor plasmid. Transformants were plated onto BHI agar plates containing 15 μg ml−1 of chloramphenicol. Colonies were PCR screened using the cpe-specific primers (cpeF and cpeR) listed in Table 1. A transformant carrying the intron-disrupted cpe gene was then subcultured daily in FTG broth without antibiotics for ∼10 days to cure the intron-carrying plasmid. This putative cpe null mutant, named CN3758cpeko, was further characterized by Southern blotting and PCR analyses (see Results).
To prepare a complementing strain, named CN3758cpecomp, the cpe null mutant was transformed by electroporation with plasmid pJRC200 (13), which is a shuttle plasmid carrying the wild-type cpe gene.
PCR screening for an intron insertion into the cpb or cpe gene of toxin null mutants.
Each PCR mixture contained 2 μl of template DNA, 10 μl of Taq 2× master mix (Gene Choice), and 1 μl of each primer pair (1 μM final concentration). The primers listed in Table 1 were used to screen for intron insertions into the cpb and cpe genes of CN3758cpbko and CN3758cpeko, respectively. PCRs were performed in a Techne (Burkhardtsdorf, Germany) thermocycler using the following PCR amplification conditions: 94°C for 2 min, followed by 35 cycles of 94°C for 1 min, 55°C for 1 min, and 68°C for 2 min, with a 5-min extension at 68°C. PCR products were run on a 1.5% agarose gel, and this gel was then stained with ethidium bromide for visualization.
Southern hybridization.
C. perfringens genomic DNA was isolated from wild-type, toxin null mutants, complementing, or reversed mutant strains using a MasterPure Gram-positive DNA purification kit (Epicentre Biotechnologies, WI). A 2.5-μg aliquot of each purified DNA sample was then digested overnight with NcoI at 37°C, according to the manufacturer's (New England BioLabs) instructions. The digested DNA samples were electrophoresed on a 1% agarose gel, and the separated DNA digestion products were then transferred onto nylon membranes (Roche) for hybridization with a digoxigenin (DIG)-labeled probe specific for the intron sequence. The probe was prepared by PCR using a PCR DIG Probe synthesis kit (Roche) and primers listed in Table 1 (IBS and EBS2). After hybridization with the probe, the Southern blot was developed using reagents from a DIG DNA labeling and detection kit (Roche), according to the manufacturer's instructions.
Preparation of rabbit polyclonal CPB antiserum.
CPB-specific polyclonal antiserum was produced in rabbits by Pocono Rabbit Farm and Laboratory (Canadensis, PA), a Public Health Service (PHS)-approved, USDA-registered and AAALAC-accredited facility, under their approved IACUC protocol (protocol PRF2A). Briefly, two amino acid sequences (CIEENKPLASIESEYA and CGRYTNVPATENIIPDYQMSK) in the CPB protein were selected for peptide synthesis based on predicted surface exposure, antigenicity, and peptide stability. After synthesis, the peptides were conjugated to keyhole limpet hemocyanin. Two New Zealand White rabbits received subcutaneous injections containing 200 μg of a 50-50 mixture of the peptide conjugate and Freund's complete adjuvant. Booster injections consisting of a 100-μg mixture of both antigens with Freund's incomplete adjuvant were administered at 14, 28, 56, and 77 days after primary injection. Rabbits were terminally bled 14 days after the final injection.
Western blot analysis of CPB and CPE production by CN3758 and its isogenic derivatives.
C. perfringens wild-type CN3758, the toxin null mutants CN3758cpbko and CN3758cpeko, the reversed cpb strain CN3758cpbrev, and the cpe complementing strain CN3758cpecomp were inoculated into FTG broth. After a 20-min heat shock, the cultures were incubated for 18 h at 37°C. An aliquot (0.2 ml) of each FTG culture was then inoculated into 10 ml of TGY broth (for evaluation of CPB production under vegetative growth conditions). Similarly, a 0.8-ml aliquot of the FTG culture was inoculated into 10 ml of fresh MDS medium (for evaluation of CPB and CPE production by sporulating cultures). TGY cultures were grown for 8 h at 37°C. Except for CN3758cpbrev, MDS cultures were grown for 10 to 12 h at 37°C; CN3758cpbrev was first grown for 8 h at 37°C, followed by 8 h of additional growth at 30°C. Culture sporulation was then assessed by phase-contrast microscopy.
To release intracellular CPE, which naturally accumulates cytoplasmically until mother cells lyse at the completion of sporulation (19), each MDS culture was sonicated until >95% cell lysis was reached, producing MDS lysates. These lysates were then centrifuged at 8,000 × g for 5 min, and equal volumes of each culture supernatant were electrophoresed on a 12% polyacrylamide gel containing SDS. Separated proteins on the gels were then transferred onto a nitrocellulose membrane. The membrane was blocked with Tris-buffered saline (TBS)-Tween 20 (0.05%, vol/vol) and nonfat dry milk (5%, wt/vol) for 1 h before it was probed overnight at 4°C with either rabbit polyclonal CPE antiserum (20) or rabbit CPB antiserum, prepared as described earlier. Bound antibody was then detected by incubating the membrane with a horseradish peroxidase-conjugated secondary anti-species-specific antibody, followed by addition of SuperSignal West Pico chemiluminescent substrate (Pierce).
Quantification of CPB and CPE production by MDS cultures of CN3758 and its isogenic derivatives.
MDS culture lysates were prepared as described above and then centrifuged at 8,000 × g for 10 min. A 25-μl aliquot of each resultant supernatant was loaded onto two 12% acrylamide gels containing SDS; the first gel was used for CPB quantification, while the second gel was used for CPE quantification. Serially diluted, purified CPB (0.5 to 64 μg ml−1) or CPE (1 to 200 μg ml−1) was also loaded onto the first or second gel, respectively. After electrophoresis, Western blotting was performed with a CPB or a CPE polyclonal antibody, respectively, as described above. Densitometry of the Western blot bands was performed using ImageJ software, version 1.44p. The results were used to quantify the amount of CPB or CPE in each culture lysate against standard curves obtained using a range of concentrations of purified CPB or CPE.
Quantification of spore formation.
To quantify the number of spores made by each C. perfringens strain, an aliquot (0.8 ml) of an 8-h FTG culture was inoculated into 10 ml of fresh MDS medium, which was then incubated for 10 to 12 h at 37°C. Each MDS culture was heat shocked for 20 min at 70°C to kill the vegetative cells and promote spore germination. Ten-fold serial dilutions (from 10−2 to 10−7) of each culture were made using sterile water, and each of those dilutions was plated onto BHI agar plates. The plates were incubated overnight at 37°C under anaerobic conditions, and colonies on each plate were then counted. Each experiment was repeated three times.
Preparation of supernatants from MDS lysates for rabbit small intestinal loop experiments.
The three C. perfringens wild-type strains, CN3758cpbko, CN3758cpeko, the cpe complementing strain CN3758cpecomp, and the cpb reversed mutant CN3758cpbrev were each grown at 37°C for 18 h in FTG broth. A 0.2-ml aliquot of each FTG culture was then transferred into 10 ml of fresh FTG broth hand grown for 8 h at 37°C. A 0.8-ml aliquot of that second FTG culture was transferred to 10 ml of MDS medium and grown for 10 to 12 h at 37°C, a time point when phase-refractile spores became visible. For the cpb reversed mutant CN3758cpbrev, the strain was grown in the presence of 15 μg ml−1 chloramphenicol to retain the LtrA-encoding plasmid. After growing in MDS for 8 h at 37°C, this strain was transferred to 30°C for another 8 h of growth.
After sonication of each MDS culture, the lysates were centrifuged, and equal volumes of each resultant supernatant were then assayed for the presence of CPB and CPE by Western immunoblotting, as described above. As described previously, trypsin inhibitor ([TI] 1 mg/ml; Sigma) was added to an equal volume of each MDS lysate supernatant (or purified CPB) to inhibit CPB degradation, and these samples were stored at −80°C until use in rabbit loops.
Rabbit small intestinal loops.
Fasted young adult, male or female, New Zealand White rabbits (Charles River, CA) were anesthetized and used to prepare small intestinal loops as described previously (21). Loops were inoculated with 1-ml samples containing one of the following: purified CPB (3 or 10 μg, as specified in the figures or tables) plus TI (1 mg/ml) dissolved in phosphate-buffered saline (PBS), purified CPE (15 or 100 μg, as specified in the figures or tables) dissolved in PBS, a combination of both CPB (3 or 10 μg, as specified in the figures or tables) and CPE (15 or 100 μg, as specified in the figures or tables) plus TI (1 mg/ml) dissolved in PBS, sterile MDS medium plus TI (1 mg/ml), or a 1-ml aliquot of lysate supernatant prepared from equal volumes of MDS cultures of wild-type CN2067, CN5388, or CN3758, the CN3758 cpb null mutant (CN3758cpbko), the CN3758 cpb reversed mutant (CN3758cpbrev), the CN3758 cpe null mutant (CN3758cpeko), or the CN3758 cpe complementing strain (CN3758cpecomp), each with added TI (1 mg/ml).
Once the abdominal incision was sutured, the animals were kept deeply anesthetized until euthanasia by an overdose of sodium barbiturate (Beuthanasia; Schering-Plough Animal Health, Kenilworth, NJ) given 6 h after inoculation. The abdominal cavity was then reopened, and the loops were excised following the same order in which they had been inoculated. Loops were weighed before and after the fluid was removed. They were also examined grossly, and their lengths were measured. Fluid content was expressed as the loop weight-to-length ratio (g/cm). For histological analysis, all tissues were fixed by immersion in 10% buffered, pH 7.4, formalin for a minimum of 24 h, followed by dehydration through graded alcohols to xylene before being embedded in paraffin wax, sectioned at 4 μm, and stained with hematoxylin and eosin.
Tissue sections were examined by a pathologist in a blinded fashion, using a quantitative scoring system as described previously (12, 21). The degree of histologic lesions was scored using a scale of 1 to 5, with 1 indicating no histologic damage and values between 2 and 5 indicating increasingly severe damage. An overall histologic score was calculated based on the following parameters: mucosal necrosis, desquamation of the epithelium, inflammation, villous blunting, edema, and hemorrhage. All procedures were approved by the University of California, Davis, Committee for Animal Care and Use (permit 16383).
Statistical analyses.
For statistical analysis, each in vivo experiment was performed using two replicates of each treatment in four to six different rabbits (8 to 12 total loops). Statistical significance of fluid accumulation and histologic scores was determined using the Friedman test with Dunn's multiple-comparison test post hoc using GraphPad Prism, version 6, software.
RESULTS
Enteropathogenic effects of supernatants from sporulating culture lysates of CPE-positive type C human disease strains.
CPE-positive type C strains produce CPE only during sporulation (14). Therefore, the current study first evaluated whether sporulating cultures of CPE-positive type C human disease strains cause enteropathogenic effects in a rabbit small intestinal loop model of type C necro-hemorrhagic enteritis. For this purpose, three CPE-positive type C strains were used, including CN3758, CN2076, and CN5388. Note that the origins of these strains varied considerably, with CN2076 and CN3758 originating from Darmbrand outbreaks in post-World War II Germany, while CN5388 was isolated from a 1960s pigbel case in Papua New Guinea.
For this enteropathogenicity analysis, the samples tested were supernatants obtained from sonicated lysates of sporulating MDS cultures. Sonicated MDS lysates were used for this analysis because CPE accumulates intracellularly until the mother cell lyses at the completion of sporulation (19); this lysis does not occur until ∼12 h postinoculation in sporulation medium at 37°C (19), so it cannot be mimicked in our 6-h rabbit small intestinal loop model. Instead, using supernatants from MDS culture lysates bypasses mother cell lysis and thus represents a standard inoculum for studying the in vivo effects of CPE-positive type A strains (13). Note that because supernatants of sonicated MDS lysates were used, each sample contained both intracellular proteins, like CPE, and any secreted proteins, including CPB. Trypsin inhibitor was added to all supernatants prior to their inoculation into rabbits to protect CPB, if present in these samples, from proteolysis.
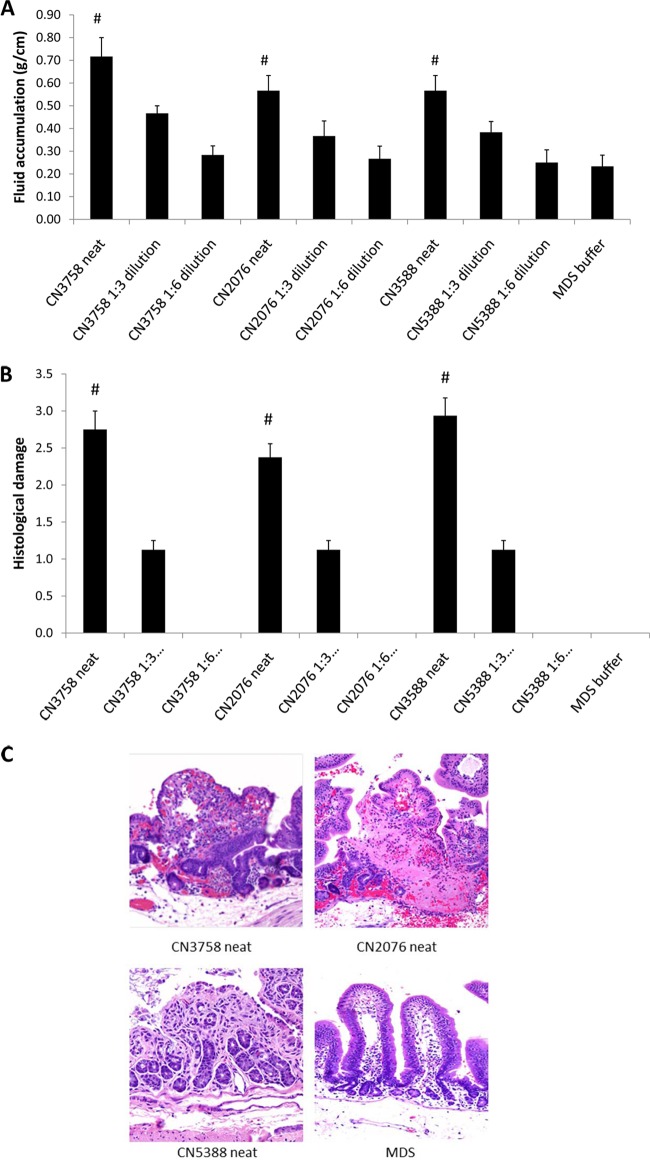
When tested for their enteropathogenicity, undiluted supernatants from sonicated MDS lysates of all three CPE-positive type C EN strains caused similar, statistically significant levels of luminal fluid accumulation in rabbit small intestinal loops (Fig. 1A). Furthermore, there was a similar reduction in luminal fluid accumulation upon equivalent dilutions of MDS lysate supernatants from all three surveyed CPE-positive type C EN strains (Fig. 1A).
FIG 1.
Enteropathogenic effects of supernatants from MDS sporulating cultures of CPE-positive type C EN strains. (A) Luminal fluid accumulation. An equal volume (1 ml) of neat or diluted supernatant from each MDS sporulating culture lysate plus TI was inoculated into separate rabbit small intestinal loops, which were then incubated for 6 h. Shown are mean results from 4 rabbits, each with duplicate samples. Error bars depict the standard errors of the means. #, significant (P < 0.05) difference in fluid accumulation compared to MDS medium alone. (B) Histologic damage scores in rabbit small intestinal loops after 1 ml of neat or diluted supernatant from each MDS culture lysate plus TI was inoculated into separate rabbit small intestinal loops, which were then incubated for 6 h. Shown are mean results from 4 rabbits, each with duplicate samples. The degree of lesions was scored using a scale of 1 to 5, with 1 indicating no histologic damage and values between 2 and 5 indicating increasingly severe damage. An overall histologic score was calculated based on the following parameters: mucosal necrosis, desquamation of the epithelium, inflammation, villus blunting, edema, and hemorrhage. Error bars depict the standard errors of the means. #, significant (P < 0.05) difference in fluid accumulation compared to MDS medium alone. (C) Photomicrographs of representative histologic damage after 6 h of treatment of rabbit small intestinal loops with neat supernatant from each MDS culture lysate plus TI. Magnification, ×200.
In addition to causing significant luminal fluid accumulation, the MDS lysates from each CPE-positive type C EN strain also induced similar gross and histologic lesions in the rabbit small intestinal loops. At the gross level, these MDS lysate supernatants caused rabbit small intestinal loops to become distended with hemorrhagic fluid and severe mucosal and serosal hemorrhage and congestion, all of which could be observed from both the mucosal and serosal sides (data not shown). The intestinal wall appeared thin and had lost its natural tone. At the histologic level, the similar, statistically significant lesions caused by each CPE-positive type C strain included severe diffuse mucosal necrosis with desquamation of the epithelium, villous blunting, edema, and hemorrhage (Fig. 1B). Diffuse infiltration of the lamina propria with neutrophils and mucosal and submucosal edema were also observed. There was a similar reduction in development of histologic lesions after equivalent dilution of MDS lysate supernatants from all three CPE-positive type C strains (Fig. 1B).
CPB and CPE production by wild-type CN3758 grown in MDS.
The Fig. 1 results demonstrating that sporulating culture lysates from multiple CPE-positive type C strains induce similar, statistically significant histologic lesions and luminal fluid accumulation are consistent with the possibility of CPE contributing to the enteropathogenicity of CPE-positive type C strains. Therefore, this study next directly evaluated whether, at natural production levels, CPE can contribute to the enteropathogenic effects of MDS lysates of CPE-positive type C strains, either alone or in combination with CPB.
To address this issue would involve constructing isogenic toxin mutants in CN3758, which is the only one of the three surveyed CPE-positive type C EN strains that is transformable. Before construction of these mutants, Western blotting for CPE and CPB detection was performed (Fig. 2) using supernatants of MDS sporulating culture lysates (MDS lysates) of this strain to assess the presence of both CPE and CPB in supernatants of wild-type CN3758 MDS lysates. When the amounts of the toxins present in the CN3758 MDS lysate supernatants were quantified by densitometric analysis of Western blot bands, these samples were found to contain ∼3 μg/ml of CPB and ∼17 μg/ml of CPE.
FIG 2.
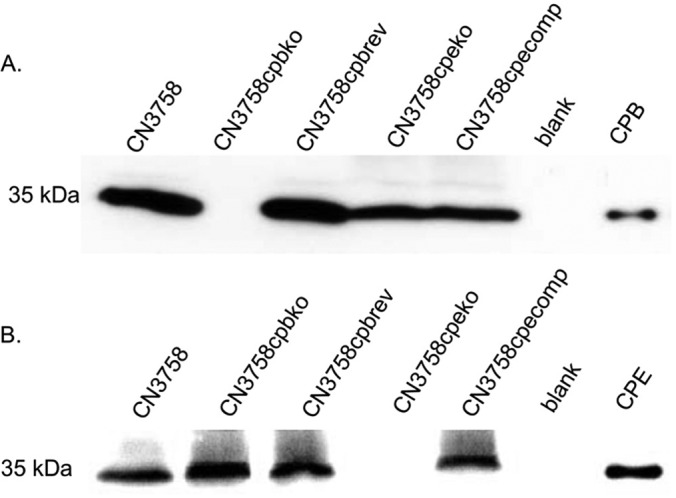
Western blot analysis of toxin production by MDS sporulating cultures of wild-type CN3758, the CN3758cpbko or CN3758cpeko isogenic toxin null mutant, the CN3758cpecomp cpe complementing strain, or the CN3758cpbrev cpb reversed mutant. The bacteria were grown in MDS medium at 37°C for 10 to 12 h; CN3758cpbrev was grown at 37°C for 8 h and then at 30°C for another 8 h to remove the intron from cpb mRNA (see Results). All cultures were then sonicated to produce culture lysates. After centrifugation, equal volumes of each MDS culture lysate supernatant were subjected to SDS-PAGE, followed by Western blotting to detect the presence of CPB (A) or CPE (B). Shown are representative results that were reproducible over at least three repetitions. The molecular mass of each toxin is indicated on the right side of the blots, and purified CPB or CPE was electrophoresed as a positive control in the last lane of each blot.
Construction and characterization of CN3758 cpb and cpe null mutants, a cpe complementing strain, and a reversed cpb mutant.
The presence of both CPB and CPE in supernatants of MDS lysates of CN3758 allowed us to address whether, at their natural levels present in MDS cultures, one or both of these toxins cause the enteropathogenic effects shown in Fig. 1. To evaluate toxin contributions to CN3758 sporulating culture enteropathogenicity, the cpb and cpe genes in the EN strain were individually insertionally inactivated using the Clostridium-modified TargeTron gene knockout system (22), which produced a putative cpb null mutant (named CN3758cpbko) and a putative cpe null mutant (named CN3758cpeko).
The presence of a 0.9-kb intron insertion in the cpb or cpe gene of CN3758cpbko or CN3758cpeko, respectively, was then confirmed by PCR (Fig. 3A). Using a pair of internal primers for the cpb gene, PCR amplified an ∼1.8-kb product from CN3758cpbko DNA but only an ∼900-bp product from wild-type CN3758 DNA. Similarly, using a pair of internal primers for the cpe gene, PCR amplified an ∼1.5-kb product from CN3758cpeko DNA but only an ∼600-bp product from CN3758 DNA.
FIG 3.
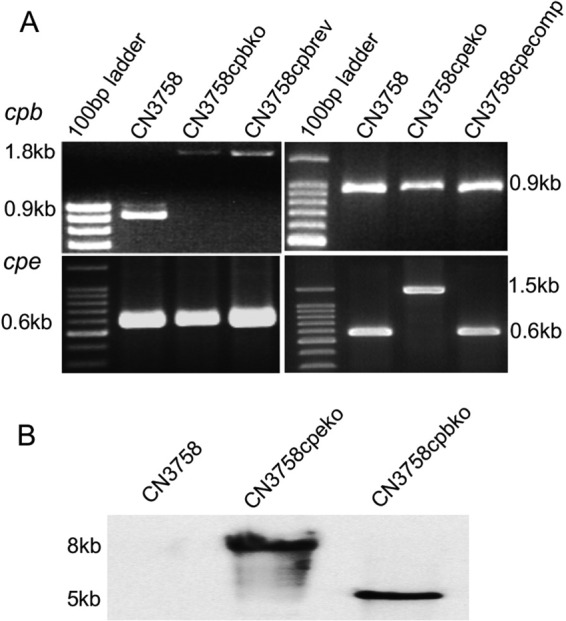
Generation and characterization of the CN3758 wild-type parent, the CN3758cpbko and CN3758cpeko toxin null mutants, the CN3758cpecomp cpe complementing strain, and the CN3758cpbrev cpb reversed mutant. (A) PCR amplification of internal cpb or cpe sequences from wild-type CN3758 and its derivatives. Using DNA extracted from wild-type CN3758, the CN3758cpeko mutant, or the CN3758cpecomp complementing strain, an ∼900-bp product was PCR amplified using primers for internal cpb sequences. However, using DNA from the CN3758cpbko cpb mutant or the CN3758cpbrev reversed cpb mutant, which contain an ∼900-bp intron insertion into the cpb gene, the same PCR assay amplified an ∼1.8-kb PCR product. Similarly, using DNA extracted from wild-type CN3758, the CN3758cpbko mutant, or the CN3758cpbrev reversed cpb mutant, an ∼600-bp product was PCR amplified using primers for internal cpe sequences. However, using DNA from CN3758cpeko, which contains an ∼900-bp intron insertion in the cpe gene, this same PCR assay amplified an ∼1.5-kb PCR product (bottom panel). Purified DNA from the CN3758cpecomp strain supported PCR amplification of the same-sized (∼600-bp) PCR product as amplified from the wild-type strain (see Results for explanation). The migration of a 100-bp molecular weight marker is shown in the first lane of each DNA agarose gel. (B) Southern blot hybridization of a DIG-labeled, intron-specific probe with DNA extracted from wild-type CN3758, CN3758cpbko, or CN3758cpeko. Purified DNA from each strain was digested with EcoRI and electrophoresed on a 1% agarose gel prior to blotting and hybridization with the intron-specific probe.
Complementation of cpb null mutants has not yet been achieved due to difficulty in stably cloning the cpb gene (12). However, as shown previously for strain CN3685 (12), the cpb null mutation created with pJIR750bi-s can be reversed at the mRNA level by reintroducing this plasmid back into the cpb gene null mutant. At 30°C, this allows the pJIR750bi-s-encoded LtrA to remove, by splicing, the sense-oriented intron from cpb mRNA, thus restoring a functional cpb transcript and CPB production.
Therefore, to rule out the possibility that any phenotypic differences observed in later studies between CN3758 and its isogenic cpb mutant were due to unwanted secondary mutations, pJIR750bi-s was reintroduced back into CN3758cpbko, creating a reversed cpb mutant named CN3758cpbrev. As shown in Fig. 3A, PCR using DNA from CN3758cpbrev and primers amplifying internal cpb gene sequences still detected an ∼1.8-kb product, consistent with intron removal in the reversed mutant occurring by splicing at the transcriptional level, rather than by splicing removal of the intron from the disrupted cpb gene.
The cpe null mutant was complemented to restore CPE production. This was achieved by electroporating CN3758cpeko with a shuttle plasmid vector carrying a cloned copy of the native cpe gene, creating CN3758cpecomp. As shown in Fig. 3A, a PCR using internal cpe-specific primers detected the presence of a wild-type cpe gene in trans in CN3758cpecomp. The failure of this PCR to amplify detectable amounts of the larger intron-disrupted cpe gene using CN3758cpecomp DNA is due to preferential PCR amplification of the smaller wild-type cpe gene, as noted previously for other complemented TargeTron mutants of C. perfringens (23).
To confirm that only a single intron had inserted into the CN3758cpeko or CN3758cpbko mutant, Southern blot hybridization was performed using a DIG-labeled, intron-specific probe and genomic DNA isolated from wild-type strain CN3758, the cpb null mutant, or the cpe null mutant. These Southern blot analyses detected the presence of a single intron insertion in both toxin null mutant strains (Fig. 3B).
Comparison of toxin production levels by CN3758, the isogenic toxin null mutants, the reversed cpb mutant, and the cpe complementing strain.
As mentioned earlier, quantitative Western blot results had indicated that ∼3 μg/ml of CPB and ∼17 μg/ml of CPE are present in supernatants of MDS lysates from the parent CN3758 strain. Similar Western blotting demonstrated that no CPB and ∼25 μg/ml of CPE was detectable in supernatants from MDS lysates of the CN3758cpbko cpb null mutant (Fig. 2). These experiments also showed that reversal of the cpb null mutation restored CPB production to ∼3 μg/ml in MDS cultures of CN3758cpbrev, which also contained ∼20 μg/ml of CPE.
Similar Western blot analyses (Fig. 2) further revealed that supernatants from MDS lysates of the cpe null mutant CN3758cpeko contained no CPE and ∼2 μg/ml of CPB. However, ∼16 μg/ml of CPE, along with ∼2 μg/ml of CPB, was detected in MDS lysate supernatants of the cpe complementing strain CN3758cpecomp.
Quantitative comparison of sporulation levels by CN3758, the isogenic toxin null mutants, the reversed cpb mutant, or the cpe complementing strain.
To assess whether all strains were sporulating at similar levels when grown in MDS medium, spore numbers were determined by plate count analysis after each MDS culture had been heat shocked for 20 min at 70°C to kill vegetative cells and facilitate spore germination. As indicated in Fig. 4, wild-type CN3758 exhibited spore formation of 9 × 107 ± 2 × 107 spores/ml when grown in MDS medium. All other CN3758-derived strains formed statistically similar (P > 0.05) numbers of spores (range, 5 × 107 to 7 × 107 ± 1 × 107 to 4 × 107 spore/ml) under these culture conditions.
FIG 4.
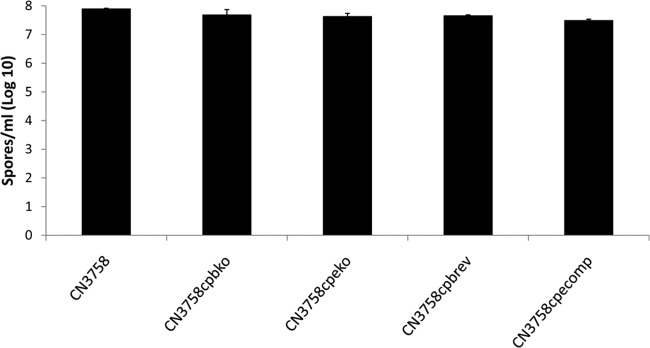
Spore formation by CN3758, CN3758cpbko, CN3758cpbrev, CN3758cpeko, and CN3758cpecomp in MDS medium. The bacteria were grown in MDS medium for 10 to 12 h at 37°C for sporulation, and the sporulating cultures were then heat shocked for 20 min at 70°C to kill vegetative cells and promote spore germination. After a 10-fold serial dilution of these samples with sterile distilled water, the bacteria were plated onto BHI agar plates and grown anaerobically overnight at 37°C for colony counting. Shown here are the mean results on a log10 scale from three independent repetitions. Error bars depict standard deviations.
Gross pathology of rabbit small intestinal loops after treatment with supernatants from MDS lysates of wild-type strain CN3758 or its isogenic derivatives.
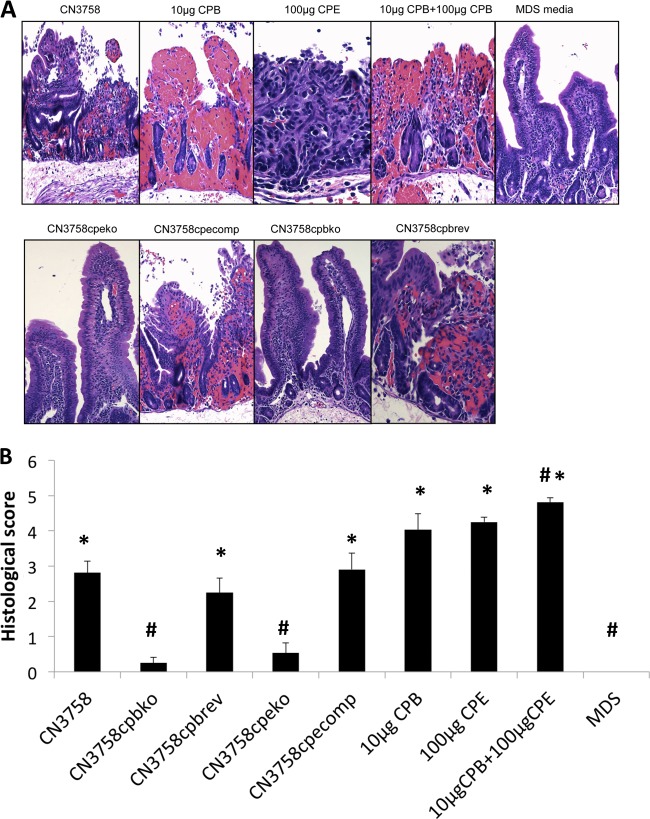
In vivo experiments were then performed to assess the ability of supernatants from MDS lysates of the wild-type strain versus toxin null mutants to induce pathology in rabbit small intestinal loops. Gross pathology after 6 h of treatment with supernatants from MDS lysates of wild-type CN3758 involved distension of the loops with hemorrhagic fluid and severe mucosal and serosal hemorrhage and congestion, all visible from both the serosal (Fig. 5) and mucosal sides (data not shown). The intestinal wall appeared thin and had lost its natural tone. In comparison, no significant gross abnormalities were observed in any of the loops inoculated with either of the toxin mutants or with MDS medium alone. Loops inoculated with supernatants from MDS lysates of CN3758cpbrev or CN3758cpecomp showed similar gross changes to those observed in loops inoculated with wild-type CN3758 (Fig. 5). At the gross level, the loops challenged with MDS lysates containing ∼3 μg/ml of CPB were more hemorrhagic than those containing either no CPB or slightly smaller amounts of CPB, consistent with previous reports that CPB induces hemorrhaging in rabbit small intestinal loops (12, 21).
FIG 5.
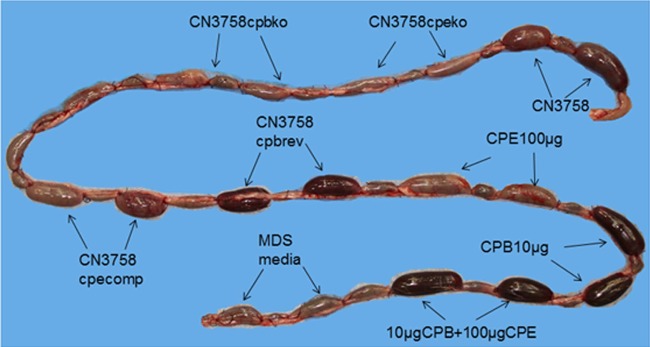
Gross pathology in rabbit small intestinal loops after challenge with supernatants from MDS sporulating culture lysates of wild-type CN3758 or its isogenic derivatives CN3758cpbko, CN3758cpbrev, CN3758cpeko, and CN3758cpecomp. An equal volume (1 ml) of each MDS culture lysate was inoculated into separate rabbit intestinal loops, which were then incubated for 6 h. As positive controls, a 10-μg sample of purified CPB plus TI, a 100-μg sample of purified CPE, or a mix of 10 μg of purified CPB plus TI and 100 μg of purified CPE was similarly injected into loops. As a negative control, 1 ml of sterile, nontoxic MDS medium was injected into a loop. Shown are representative results that were reproducible in six rabbits.
For comparison as positive controls, these initial studies also challenged some loops with 10 μg of purified CPB or 100 μg of purified CPE since these toxin concentrations are known to induce enteropathogenic effects in rabbit small intestinal loops (21, 24). Other loops were instead treated with a combination of both 10 μg of purified CPB and 100 μg of purified CPE. All loops challenged with purified toxins showed distension due to fluid; the loops treated with samples containing 10 μg of purified CPB, whether in the presence or absence of CPE, also appeared bloody.
Luminal fluid accumulation in rabbit small intestinal loops after treatment with supernatants of MDS culture lysates of CN3758 or its isogenic derivatives.
After a 6-h challenge with supernatants from wild-type CN3758 MDS lysates, small intestinal loops accumulated significantly more luminal fluid than control loops similarly treated with MDS medium alone or with supernatants of MDS culture lysates from the cpb or cpe null mutant (Fig. 6). In contrast, loops challenged for 6 h with supernatants from MDS culture lysates of CN3758cpbko or CN3758cpeko showed luminal fluid accumulation that was not significantly different from that of loops treated with MDS medium alone (Fig. 6). Furthermore, restoring CPB or CPE production to the toxin mutants resulted in greater luminal fluid accumulation, with loops challenged with CN3758cpbrev or CN3758cpecomp MDS culture lysates containing significantly higher luminal fluid levels than loops treated with MDS medium alone or with MDS culture lysates of the cpb or cpe null mutant (Fig. 6).
FIG 6.
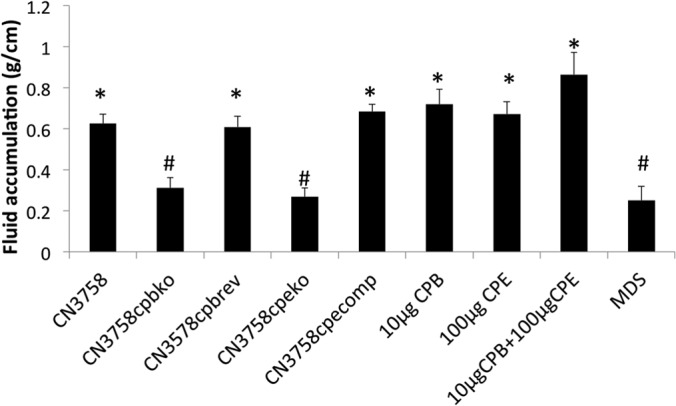
Fluid accumulation in rabbit small intestinal loops challenged for 6 h with supernatants from MDS sporulating culture lysates of wild-type CN3758 and its isogenic derivatives CN3758cpbko, CN3758cpbrev, CN3758cpeko, and CN3758cpecomp. An equal volume (1 ml) of supernatants from each MDS culture lysate was inoculated into separate rabbit intestinal loops, which were then incubated for 6 h. Also shown as positive controls are the effects of high doses of purified CPB (10 μg) plus TI, purified CPE (100 μg), or both toxins combined (CPB/CPE, plus TI). Shown are mean results from 6 rabbits, each with duplicate samples. Error bars depict the standard errors of the means. *, significant (P < 0.05) difference in fluid accumulation compared to MDS medium alone; #, significant (P < 0.05) difference in fluid accumulation compared to wild-type CN3758 MDS lysate.
As positive controls, loops challenged with 10 μg of purified CPB, 100 μg of purified CPE, or a combination of 10 μg of CPB and 100 μg of CPE also showed significant luminal fluid accumulation relative to buffer-treated loops (data not shown).
Histological damage in rabbit small intestinal loops treated with supernatants from MDS lysates of CN3758 or its isogenic derivatives.
Small intestinal loops challenged for 6 h with supernatants from wild-type CN3758 MDS lysates showed severe mucosal necrosis, characterized by desquamation of superficial epithelium and severe villus blunting (Fig. 7A and B and Table 2). The necrotic epithelium was occasionally lined by a pseudomembrane comprised of red blood cells, sloughed necrotic epithelial cells and cell debris, and neutrophils. Mucosal hemorrhage was an almost constant finding in these loops. In contrast, loops treated for 6 h with MDS medium alone or with supernatants from MDS lysates of cpb or cpe null mutants showed very minor or no histological changes. However, loops challenged for 6 h with supernatants from MDS lysates of CN3758cpbrev or CN3758cpecomp showed histological changes that were similar to those observed in loops inoculated with MDS lysate supernatants of the wild-type strain CN3758.
FIG 7.
Histopathology in rabbit small intestinal loops after challenge with supernatants from MDS sporulating culture lysates of wild-type CN3758 or its isogenic derivatives CN3758cpbko, CN3758cpbrev, CN3758cpeko, and CN3758cpecomp. An equal volume (1 ml) of supernatants from each MDS culture lysate was inoculated into separate rabbit intestinal loops, which were then incubated for 6 h. As positive controls, a 10-μg sample of purified CPB plus TI, a 100-μg sample of purified CPE, and a mix of 10 μg of purified CPB plus TI and 100 μg of purified CPE were similarly injected into loops. As a negative control, 1 ml of sterile, nontoxic MDS medium was injected into a loop. After 6 h, samples were processed for hematoxylin and eosin staining (A). Shown are representative results that were reproducible in 6 rabbits. Magnification, ×200. (B) Histologic score of the treated rabbit small intestinal loops described in panel A. The degree of lesions was scored using a scale of 1 to 5, with 1 indicating no histologic damage and values between 2 and 5 indicating increasingly severe damage. An overall histologic score was calculated based on the following parameters: mucosal necrosis, desquamation of the epithelium, inflammation, villus blunting, edema, and hemorrhage. Error bars depict the standard errors of the means. *, significant (P < 0.05) difference in fluid accumulation compared to MDS medium alone; #, significant (P < 0.05 difference in fluid accumulation compared to wild-type CN3758 MDS lysate.
TABLE 2.
Rabbit small intestinal loop histopathology
| Strain and/or treatment | Histological score |
|||||
|---|---|---|---|---|---|---|
| Epithelial necrosis | Lamina propria necrosis | Inflammation | Hemorrhage | Villous blunting | Overall severity of lesions | |
| CN3758 | 2.56 ± 0.30 | 2.22 ± 0.38 | 1.66 ± 0.28 | 2.20 ± 0.10 | 2.56 ± 0.20 | 2.81 ± 0.24 |
| CN3758cpbko | 0.25 ± 0.11 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.81 ± 0.13 | 0.25 ± 0.11 |
| CN3758cpeko | 0.56 ± 0.22 | 0.31 ± 0.22 | 0.25 ± 0.17 | 0.00 ± 0.00 | 0.44 ± 0.16 | 0.53 ± 0.20 |
| CN3578cpbrev | 2.22 ± 0.29 | 1.50 ± 0.31 | 1.31 ± 0.25 | 1.50 ± 0.14 | 2.06 ± 0.08 | 2.25 ± 0.29 |
| CN3758cpecomp | 2.78 ± 0.36 | 2.35 ± 0.40 | 1.63 ± 0.26 | 1.90 ± 0.09 | 2.66 ± 0.22 | 2.91 ± 0.32 |
| MDS medium | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 3 μg of CPB | 0.40 ± 0.24 | 0.27 ± 0.18 | 0.07 ± 0.07 | 0.13 ± 0.13 | 0.53 ± 0.22 | 0.93 ± 0.24 |
| 15 μg of CPE | 0.38 ± 0.12 | 0.17 ± 0.08 | 0.00 ± 0.00 | 0.00 ± 0.00 | 2.13 ± 0.19 | 1.94 ± 0.15 |
| 3 μg of CPB + 15 μg of CPE | 2.00 ± 0.53 | 2.06 ± 0.60 | 0.56 ± 0.16 | 2.06 ± 0.57 | 3.75 ± 0.3 | 3.81 ± 0.31 |
| Buffer | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
As positive controls, loops treated for 6 h with 10 μg of purified CPB, 100 μg of purified CPE, or a combination of 10 μg of purified CPB and 100 μg of purified CPE also showed significantly more severe damage than loops treated with MDS (data not shown).
Development of gross lesions, luminal fluid accumulation, and histologic damage in rabbit small intestinal loops challenged with purified CPB or CPE at the same concentrations present in CN3758 MDS lysates.
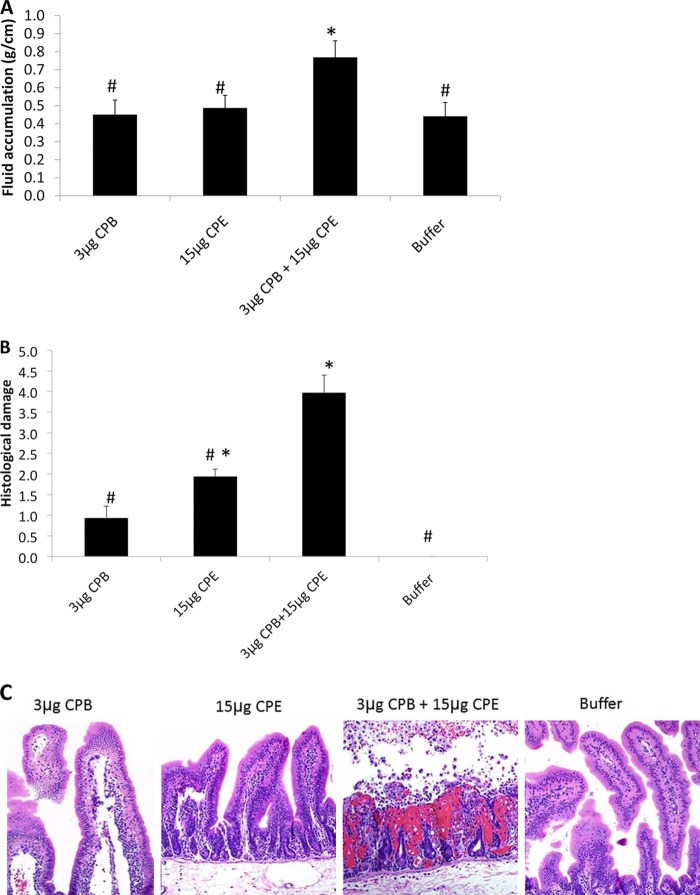
As positive controls for the MDS lysate experiments shown in Fig. 5 to 7, purified CPB or CPE was tested at high concentrations (10 μg or 100 μg, respectively) known to induce enteropathogenic effects (12, 21, 24). However, the results shown in Fig. 5 to 7 also indicated that, at the lower levels naturally present in CN3758 MDS lysates, CPB and CPE act together synergistically to cause luminal fluid accumulation and histologic damage in rabbit small intestinal loops. To further test this conclusion and to assess whether other lysate factors might contribute to the CPB and CPE synergy observed in the experiments shown in Fig. 5 to 7, rabbit small intestinal loops were treated for 6 h with purified CPB or CPE, alone or in combination, at the concentrations present in CN3758 MDS lysates.
After a 6-h challenge, no significant difference in luminal fluid accumulation (Fig. 8A) or gross appearance (data not shown) was detected between loops treated with buffer and loops treated with 3 μg/ml of purified CPB or 15 μg/ml of purified CPE. However, a 6-h cotreatment of loops with both 3 μg/ml of CPB and 15 μg/ml of CPE significantly increased luminal fluid accumulation (Fig. 8A); this fluid was hemorrhagic, and the loops had severe mucosal and serosal hemorrhage and congestion (data not shown). Loops treated for 6 h with 3 μg/ml of purified CPB or 15 μg/ml of purified CPE showed, respectively, no or mild histologic damage, while loops treated for 6 h with a combination of 3 μg/ml of pure CPB and 15 μg/ml of pure CPE showed significant histologic damage (Fig. 8B and C).
FIG 8.
Development of luminal fluid accumulation and histologic damage in rabbit small intestinal loops challenged with purified CPB, CPE, or CPB and CPE at the concentrations present in CN3758 MDS lysate supernatants. Rabbit small intestinal loops were treated for 6 h with 1 ml of buffer containing 3 μg of purified CPB plus TI, 15 μg of purified CPE, or a combination of 3 μg of purified CPB plus TI and 15 μg of purified CPE. (A) Luminal fluid accumulation. Results shown are for 6 rabbits. Error bars depict the standard errors of the means. *, significant (P < 0.05) difference in fluid accumulation compared to buffer alone; #, significantly different (P < 0.05) fluid accumulation compared to all other samples. (B) Overall histologic damage. The degree of lesions was scored using a scale of 1 to 5, with 1 indicating no histologic damage and values between 2 and 5 indicating increasingly severe damage. An overall histologic score was calculated based on the following parameters: mucosal necrosis, desquamation of the epithelium, inflammation, villus blunting, edema, and hemorrhage. Error bars depict the standard errors of the means. Results shown are for 6 rabbits. *, significant (P < 0.05) difference in fluid accumulation compared to buffer alone; #, significantly different (P < 0.05) fluid accumulation compared to all other samples. (C) After 6 h, samples were processed for hematoxylin and eosin staining. Shown are representative results that were reproducible in 6 rabbits. Magnification, ×200.
DISCUSSION
EN caused by C. perfringens type C strains is one of the most serious human intestinal infections, with reported mortality rates of ∼40% (6–8, 15). Treatment of this illness remains problematic since it involves surgery performed soon after the onset of symptoms, which include abdominal pain, vomiting, and bloody diarrhea (7). Although no vaccine is currently available for use in humans, EN can be prevented by vaccination. The pigbel vaccine once used in PNG was prepared using inactivated crude supernatants of a type C strain, leading to the belief that CPB is responsible for this illness (6, 8). However, the repertoire of toxin production by the type C strain used to prepare that pigbel vaccine was not characterized. Since type C strains often produce many toxins, that vaccine may have induced immune responses against multiple toxins. To optimize preparation of future EN vaccines, it is important to use modern approaches to identify which toxins contribute to enteropathogenic effects of EN strains.
As mentioned in the introduction, previous molecular Koch's postulate analyses demonstrated that CPB is essential for type C strain CN3685 pathogenicity (12). However, CN3685 does not produce CPE, which is significant since some investigators have proposed that CPE can also contribute to EN symptoms, particularly diarrhea (6, 15, 16). Several indirect observations support this hypothesis. For example, all type C Darmbrand EN strains characterized in a recent study carried the cpe gene, as well as the cpb gene; furthermore, each of these EN strains could produce CPE (11). In addition, 50% of pigbel strains reportedly also produce CPE (15); the percentage of CPE-positive pigbel strains may actually be higher since that older study used a relatively insensitive CPE detection assay. Furthermore, a second study from the 1970s (16) reported that cell extracts of CPE-positive type C pigbel strains remained enteropathogenic after being incubated with neutralizing alpha toxin (CPA) or CPB antiserum although that previous work did not clearly demonstrate that the activities of these two toxins had actually been blocked.
Since CPE is produced only by sporulating cultures of CPE-positive type C EN strains (11, 14), the current study first evaluated whether CPE could contribute to type C EN strain enteropathogenicity by testing the effects in rabbit small intestinal loops of supernatants from MDS lysates of sporulating cultures from CPE-producing type C EN strains. Supernatants from MDS sporulating culture lysates of all three tested CPE-positive type C EN strains caused similar histologic lesions and luminal fluid accumulation in this animal model. Furthermore, the enteropathogenic activity of supernatants from all three CPE-positive type C EN strains was similarly sensitive to dilution, consistent with the involvement of similar pathogenic mechanisms. Supporting these results as being representative of many CPE-positive type C EN strains, the strains surveyed in this study had two distinct origins; i.e., two of these strains originated from Darmbrand outbreaks in the 1940s and 1950s in post-World War II Germany, while the other strain was isolated from a 1960s pigbel case in Papua New Guinea.
Both CPB and CPE are present in sporulating cultures of these CPE-positive type C EN strains (11, 14) (Fig. 2). Note that the presence of CPB in MDS cultures does not necessarily imply that expression of this toxin is sporulation associated since CN3758 MDS cultures contain a mix of both sporulating cells and vegetative cells. In fact, CN3758 produces slightly more CPB during vegetative growth in TGY broth than MDS sporulation medium (data not shown), indicating that sporulating conditions are not the most favorable for CPB production.
Since both CPE and CPB are present in supernatants of MDS culture lysates of CPE-positive type C EN strains (Fig. 2) (11, 14; also data not shown), the current study then constructed CPE and CPB null mutants of type C EN strain CN3758 to definitively dissect the relative enteropathogenic contributions of these two toxins when present together. Studies treating rabbit small intestinal loops with CN3758, its isogenic CPB null mutant, or a reversed mutant confirmed the long-held view that CPB is important for enteropathogenicity of type C strains. However, results from challenges using supernatants prepared from MDS culture lysates of wild-type CN3758, the isogenic CPE null mutant, or a cpe complementing strain also clearly demonstrated that CPE is required for these MDS lysates to cause histologic damage or fluid accumulation in small intestinal loops.
Having established that both CPB and CPE must be present for CN3758 MDS lysates to cause fluid accumulation or histologic damage in rabbit small intestinal loops, we next challenged some loops with purified CPB or CPE. Consistent with the lysate results, purified CPB or CPE alone caused limited or no enteropathogenic effects when used at the concentrations found naturally in MDS lysates. However, when loops were treated with these same doses of purified CPB and CPE in combination, both significant fluid accumulation and histologic damage were observed. These results indicated that other lysate factors are not necessary when CPB and CPE, at levels present in MDS lysates, act synergistically to cause fluid accumulation or histologic damage.
Findings from the current study also confirmed previous reports (12, 21, 24) that, at larger amounts than present in CN3758 MDS lysates, either CPB or CPE alone is sufficient to cause extensive enteropathogenic effects in rabbit small intestinal loops. The finding that these toxins can act synergistically at low doses but independently at high toxin doses is consistent with previous studies observing enteropathogenic effects in rabbit small intestinal loops challenged with either CPE-negative C. perfringens type C strain CN3685 (12) or supernatants of sporulating culture lysates from CPE-positive, CPB-negative C. perfringens type A strain SM101 or F4969 (13). It is notable that in the previous study testing the intestinal pathogenicity of the CPE-negative strain CN3685 (12), the sample inoculum contained ∼13 μg ml−1 of CPB, in contrast to the ∼3 μg ml−1 of this toxin present in supernatants of CN3758 MDS lysates. Similarly, while the supernatants of CN3758 MDS lysates contained only ∼17 μg ml−1 of CPE, the supernatants from sporulating culture lysates of CPB-negative strain F4969 or SM101 had been concentrated so that loops were challenged with CPE doses of >100 μg ml−1 (13).
The amounts of CPB and CPE present during natural infection by CPE-positive type C infection have never been determined because they would be extremely difficult to measure accurately. The simplest approach would seem to involve measuring fecal toxin levels, but this approach would produce unreliable findings for type C infections since fecal CPB and CPE levels can be affected by many factors. Both toxins are sensitive to fecal proteases (25, 26), so the amounts of CPB and CPE measured in feces would depend upon how soon after the start of illness a fecal sample was collected, how effectively protease degradation could be stopped after feces collection, how quickly the sample was assayed, etc. The questionable reliability of quantitative fecal toxin testing results for type C infections is supported by similar studies measuring CPE levels in feces from patients with C. perfringens type A food poisoning (25), where the values obtained ranged from nanograms of CPE/ml to >100 μg of CPE/ml. The range in fecal CPB levels measured from people or animals with type C infections would likely be even greater since CPB is exceptionally sensitive to proteases (26). Furthermore, measuring CPE or CPB levels in feces (or even in the intestinal lumen) would not accurately reflect toxin levels in a natural type C infection since this would not account for toxins bound to intestinal cells or absorbed into the circulation, where they could then be either free in the blood or bound to other organs.
Nonetheless, several observations support potential pathogenic relevance for the synergistic effects of small amounts of CPE and CPB, as described in this study. First, CN3758 is a human EN disease isolate that, as shown in the current study, retains the ability to cause enteropathogenic effects similar to those produced by other CPE-positive type C EN strains in animal models. Second, previous studies have shown that among CPE-positive type C human EN strains, CN3758 produces typical amounts of CPE and CPB (11, 14). Therefore, establishing that CPE can contribute to the enteropathogenic effects of lysates from this representative type C EN strain strongly suggests that these findings are not specific for CN3758 and are generalizable to other CPE-positive type C EN strains. The experimental association of CPE with the enteropathogenicity of CPE-positive type C EN strains, as shown in our current study, potentially offers insights into EN pathogenesis and, by extension, EN vaccine design. To test these suggestions, future studies should determine whether food-borne C. perfringens type C strains sporulate in vivo during human EN and whether CPE can be present in feces from people with EN. Finally, it should be emphasized that our results do not preclude the possibility that some CPE-positive type C EN strains could produce sufficient amounts of either CPE or CPB for production of one of these toxins alone to be sufficient to cause disease.
Establishing that MDS lysates of EN strain CN3758 must contain the natural concentrations of both CPB and CPE to cause enteropathogenic effects also provides the first evidence supporting possible additive or synergistic toxin interactions during C. perfringens human intestinal infections. Precedent already exists for C. perfringens toxins acting synergistically during histotoxic infections since studies using isogenic mutants of type A strain 13 demonstrated that perfringolysin O (PFO) and CPA exert synergistic effects in a mouse model of C. perfringens gas gangrene (27). Whether PFO and CPA act individually or synergistically during intestinal infections remains unsettled. A recent study (28) using mutants of strain 13, which is not an intestinal disease strain, reported that PFO and CPA have synergistic effects in bovine intestinal loops. In contrast, studies with type C animal intestinal disease strain CN3685 determined that inactivating the cpb gene eliminated the pathogenicity of that strain in mice, goats (a natural disease host), and rabbit small intestinal loops (12, 29, 30). Furthermore, eliminating expression of both CPA and PFO by CN3685 had little or no effect on virulence in rabbit small intestinal loops or mouse models (12, 29). These differences may suggest the importance of using natural intestinal disease strains for dissecting the intestinal pathogenicity of C. perfringens strains.
Determining whether CPA and PFO have synergistic intestinal effects is not highly germane for understanding many human intestinal infections. The majority of CPE-positive type A strains causing food poisoning and many CPE-positive type C EN strains, including CN3758, do not even carry the pfoA gene (11). In addition, little or no CPA production was detectable in the supernatants from MDS cultures that were used as inocula in the current study (data not shown). The absence of CPA and PFO from the inocula used in our study further highlights the importance of CPB and CPE for the enteropathogenic properties of CN3758 under the conditions assayed.
Demonstrating that lower concentrations of CPB and CPE can work together synergistically in the small intestine is also thought provoking since these two toxins share similar molecular actions; i.e., CPB and CPE form pores that induce a strong potassium efflux and calcium influx into sensitive cells, effects which trigger cell death pathways (31–33). However, one explanation for the ability of these toxins to act synergistically may involve their apparent use of different receptors; i.e., some cell lines, such as Vero cells, that respond to CPE are insensitive to CPB (33–35). Furthermore, CPE preferentially affects enterocytes in the upper parts of the villi, while CPB possibly affects both endothelial cells and enterocytes (5, 36). These cell target variations could help to explain the synergistic intestinal effects noted using lower concentrations of these two toxins.
Finally, the discovery that some concentrations of CPB and CPE can act together synergistically may have broader implications. Many other C. perfringens intestinal disease strains produce combinations of toxins proven to be important for intestinal disease. For example, type B strains produce both CPB and ETX, either of which can be important for enteropathogenicity when expressed alone by type C and D strains, respectively (12, 37). Similarly, type D strains often express both CPE and ETX, so it is possible that these two toxins also exert synergistic effects during infection. Future studies will examine the relative enteropathogenic contributions and potential synergism of these toxins in animal models.
ACKNOWLEDGMENTS
This work was generously supported by grants R01 AI056177, AI19844, and T32 AI060525 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Published ahead of print 28 April 2014
REFERENCES
- 1.McClane BA, Uzal FA, Miyakawa MF, Lyerly D, Wilkins TD. 2006. The enterotoxic clostridia, p 688–752 In Dworkin M, Falkow S, Rosenburg E, Schleifer H, Stackebrandt E. (ed), The prokaryotes, 3rd ed. Springer Press, New York, NY [Google Scholar]
- 2.McClane BA, Lyerly DM, Wilkins TD. 2006. Enterotoxic clostridia: Clostridium perfringens type A and Clostridium difficile, p 703–714 In Fischetti VA, Novick RP, Ferretti JJ, Portnoy DA, Rood J. (ed), Gram-positive pathogens, 3rd ed. ASM Press, Washington, DC [Google Scholar]
- 3.Li J, Adams V, Bannam TL, Miyamoto K, Garcia JP, Uzal FA, Rood JI, McClane BA. 2013. Toxin plasmids of Clostridium perfringens. Microbiol. Mol. Biol. Rev. 77:208–233. 10.1128/MMBR.00062-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Songer JG. 1996. Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 9:216–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uzal FA, McClane BA. 2011. Recent progress in understanding the pathogenesis of Clostridium perfringens type C infections. Vet. Microbiol. 153:37–43. 10.1016/j.vetmic.2011.02.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawrence GW. 1997. The pathogenesis of enteritis necroticans, p 198–207 In Rood JI, McClane BA, Songer JG, Titball RW. (ed), The clostridia: molecular genetics and pathogenesis. Academic Press, London, United Kingdom [Google Scholar]
- 7.Johnson S, Gerding DN. 1997. Enterotoxemic infections, p 117–140 In Rood JI, McClane BA, Songer JG, Titball RW. (ed), The clostridia: molecular biology and pathogenesis. Academic Press, London, United Kingdom [Google Scholar]
- 8.Walker PD. 1985. Pig-bel, p 93–116 In Borriello SP. (ed), Clostridia in gastrointestinal disease. CRC Press, Boca Raton, FL [Google Scholar]
- 9.Petrillo TM, Beck-Sague CM, Songer JG, Abramowsky C, Fortenberry JD, Meacham L, Dean AG, Lee H, Bueschel DM, Nesheim SR. 2000. Enteritis necroticans (pigbel) in a diabetic child. N. Engl. J. Med. 342:1250–1253. 10.1056/NEJM200004273421704 [DOI] [PubMed] [Google Scholar]
- 10.Zeissler J, Rassfeld-Sternberg L. 1949. Enteritis necroticans due to Clostridium welchii type F. Br. Med. J. 1:267–269. 10.1136/bmj.1.4597.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma M, Li J, McClane B. 2012. Genotypic and phenotypic characterization of Clostridium perfringens isolates from Darmbrand cases in post-World War II Germany. Infect. Immun. 80:4354–4363. 10.1128/IAI.00818-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sayeed S, Uzal FA, Fisher DJ, Saputo J, Vidal JE, Chen Y, Gupta P, Rood JI, McClane BA. 2008. Beta toxin is essential for the intestinal virulence of Clostridium perfringens type C disease isolate CN3685 in a rabbit ileal loop model. Mol. Microbiol. 67:15–30. 10.1111/j.1365-2958.2007.06007.x [DOI] [PubMed] [Google Scholar]
- 13.Sarker MR, Carman RJ, McClane BA. 1999. Inactivation of the gene (cpe) encoding Clostridium perfringens enterotoxin eliminates the ability of two cpe-positive C. perfringens type A human gastrointestinal disease isolates to affect rabbit ileal loops. Mol. Microbiol. 33:946–958. 10.1046/j.1365-2958.1999.01534.x [DOI] [PubMed] [Google Scholar]
- 14.Fisher DJ, Fernandez-Miyakawa ME, Sayeed S, Poon R, Adams V, Rood JI, Uzal FA, McClane BA. 2006. Dissecting the contributions of Clostridium perfringens type C toxins to lethality in the mouse intravenous injection model. Infect. Immun. 74:5200–5210. 10.1128/IAI.00534-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skjelkvale R, Duncan CL. 1975. Enterotoxin formation by different toxigenic types of Clostridium perfringens. Infect. Immun. 11:563–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skjelkvale R, Duncan CL. 1975. Characterization of enterotoxin purified from Clostridium perfringens type C. Infect. Immun. 11:1061–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Miyamoto K, Sayeed S, McClane BA. 2010. Organization of the cpe locus in CPE-positive Clostridium perfringens type C and D isolates. PLoS One 5:e10932. 10.1371/journal.pone.0010932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonel JL, McClane BA. 1988. Production, purification and assay of Clostridium perfringens enterotoxin. Method. Enzymol. 165:94–103. 10.1016/S0076-6879(88)65018-X [DOI] [PubMed] [Google Scholar]
- 19.McClane BA, Robertson SL, Li J. 2013. Clostridium perfringens, p 465–489 In Doyle MP, Buchanan RL. (ed), Food microbiology: fundamentals and frontiers, 4th ed. ASM Press, Washington, DC [Google Scholar]
- 20.Li J, Chen J, Vidal JE, McClane BA. 2011. The Agr-like quorum-sensing system regulates sporulation and production of enterotoxin and beta2 toxin by Clostridium perfringens type A non-food-borne human gastrointestinal disease strain F5603. Infect. Immun. 79:2451–2459. 10.1128/IAI.00169-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vidal JE, McClane BA, Saputo J, Parker J, Uzal FA. 2008. Effects of Clostridium perfringens beta-toxin on the rabbit small intestine and colon. Infect. Immun. 76:4396–4404. 10.1128/IAI.00547-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, McClane BA, Fisher DJ, Rood JI, Gupta P. 2005. Construction of an alpha toxin gene knockout mutant of Clostridium perfringens type A by use of a mobile group II intron. Appl. Environ. Microbiol. 71:7542–7547. 10.1128/AEM.71.11.7542-7547.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vidal JE, Ma M, Saputo J, Garcia J, Uzal FA, McClane BA. 2012. Evidence that the Agr-like quorum sensing system regulates the toxin production, cytotoxicity and pathogenicity of Clostridium perfringens type C isolate CN3685. Mol. Microbiol. 83:179–194. 10.1111/j.1365-2958.2011.07925.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smedley JG, III, Saputo J, Parker JC, Fernandez-Miyakawa ME, Robertson SL, McClane BA, Uzal FA. 2008. Noncytotoxic Clostridium perfringens enterotoxin (CPE) variants localize CPE intestinal binding and demonstrate a relationship between CPE-induced cytotoxicity and enterotoxicity. Infect. Immun. 76:3793–3800. 10.1128/IAI.00460-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartholomew BA, Stringer MF, Watson GN, Gilbert RJ. 1985. Development and application of an enzyme-linked immunosorbent assay for Clostridium perfringens type A enterotoxin. J. Clin. Pathol. 38:222–228. 10.1136/jcp.38.2.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Macias Rioseco M, Beingesser J, Uzal FA. 2012. Freezing or adding trypsin inhibitor to equine intestinal contents extends the lifespan of Clostridium perfringens beta toxin for diagnostic purposes. Anaerobe 18:357–360. 10.1016/j.anaerobe.2012.03.003 [DOI] [PubMed] [Google Scholar]
- 27.Awad MM, Ellemor DM, Boyd RL, Emmins JJ, Rood JI. 2001. Synergistic effects of alpha-toxin and perfringolysin O in Clostridium perfringens-mediated gas gangrene. Infect. Immun. 69:7904–7910. 10.1128/IAI.69.12.7904-7910.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verherstraeten S, Goossens E, Valgaeren B, Pardon B, Timbermont L, Vermeulen K, Schauvliege S, Haesebrouck F, Ducatella R, Deprez P, Van Immerseel F. 2013. The synergistic necrohemorrhagic action of Clostridium perfringens perfringolysin and alpha toxin in the bovine intestine and against bovine endothelial cells. Vet. Res. 44:45. 10.1186/1297-9716-44-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uzal FA, Saputo J, Sayeed S, Vidal JE, Fisher DJ, Poon R, Adams V, Fernandez-Miyakawa ME, Rood JI, McClane BA. 2009. Development and application of new mouse models to study the pathogenesis of Clostridium perfringens type C enterotoxemias. Infect. Immun. 77:5291–5299. 10.1128/IAI.00825-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia JP, Beingesser J, Fisher DJ, Sayeed S, McClane BA, Posthaus H, Uzal FA. 2012. The effect of Clostridium perfringens type C strain CN3685 and its isogenic beta toxin null mutant in goats. Vet. Microbiol. 157:412–419. 10.1016/j.vetmic.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chakrabarti G, McClane BA. 2005. The importance of calcium influx, calpain, and calmodulin for the activation of CaCo-2 cell death pathways by Clostridium perfringens enterotoxin. Cell. Microbiol. 7:129–146. 10.1111/j.1462-5822.2004.00442.x [DOI] [PubMed] [Google Scholar]
- 32.Chakrabarti G, Zhou X, McClane BA. 2003. Death pathways activated in CaCo-2 cells by Clostridium perfringens enterotoxin. Infect. Immun. 71:4260–4270. 10.1128/IAI.71.8.4260-4270.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Autheman D, Wyder M, Popoff M, D'Herde K, Christen S, Posthaus H. 2013. Clostridium perfringens beta-toxin induces necrostatin-inhibitable, calpain-dependent necrosis in primary porcine endothelial cells. PLoS One 8:e64644. 10.1371/journal.pone.0064644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinthorsdottir V, Halldorsson H, Andresson OS. 2000. Clostridium perfringens beta-toxin forms multimeric transmembrane pores in human endothelial cells. Microb. Pathog. 28:45–50. 10.1006/mpat.1999.0323 [DOI] [PubMed] [Google Scholar]
- 35.McClane BA, Wnek AP, Hulkower KI, Hanna PC. 1988. Divalent cation involvement in the action of Clostridium perfringens type A enterotoxin. J. Biol. Chem. 263:2423–2435 [PubMed] [Google Scholar]
- 36.Miclard J, Jaggi M, Sutter E, Wyder M, Grabscheid B, Posthaus H. 2009. Clostridium perfringens beta-toxin targets endothelial cells in necrotizing enteritis in piglets. Vet. Microbiol. 137:320–325. 10.1016/j.vetmic.2009.01.025 [DOI] [PubMed] [Google Scholar]
- 37.Garcia JP, Adams V, Beingesser J, Hughes ML, Poon R, Lyras D, Hill A, McClane BA, Rood JI, Uzal FA. 2013. Epsilon toxin is essential for the virulence of Clostridium perfringens type D infection in sheep, goats and mice. Infect. Immun. 81:2405–2414. 10.1128/IAI.00238-13 [DOI] [PMC free article] [PubMed] [Google Scholar]