Abstract
Compared to Cryptococcus neoformans, little is known about the virulence of the molecular types in Cryptococcus gattii. We compared in vitro virulence factor production and survival data using a Drosophila model of infection to further characterize the phenotypic features of different cryptococcal molecular types. Forty-nine different isolates were inoculated into wild-type flies and followed for survival. In vitro, isolates were assessed for growth at 30 and 37°C, melanin production, capsule size, resistance to H2O2, and antifungal susceptibility. A mediator model was used to assess molecular type and virulence characteristics as predictors of survival in the fly model. VGIII was the most virulent molecular type in flies (P < 0.001). At 30°C, VGIII isolates grew most rapidly; at 37°C, VNI isolates grew best. C. gattii capsules were larger than those of C. neoformans (P < 0.001). Mediator model analysis found a strong correlation of Drosophila survival with molecular type and with growth at 30°C. We found molecular-type-specific differences in C. gattii in growth at different temperatures, melanin production, capsule size, ability to resist hydrogen peroxide, and antifungal susceptibility, while growth at 30°C and the VGIII molecular type were strongly associated with virulence in a Drosophila model of infection.
INTRODUCTION
Cryptococcosis is an invasive fungal infection most commonly caused by one of two species of encapsulated yeast, Cryptococcus neoformans and Cryptococcus gattii. C. neoformans is ubiquitous with a worldwide distribution, while C. gattii has been traditionally associated with tropical and subtropical regions (1, 2). However, the geographic distribution of C. gattii continues to expand, following the emergence of this species within the Pacific Northwest (3). Numerous cases have since been described in New Mexico, California (4), North Carolina (5), Georgia, Florida, and Alabama (6), suggesting that this species is already widely distributed within the United States.
Existing guidelines published by the Infectious Disease Society of America do not define differences in management according to Cryptococcus species (7). However, previous reports illustrate significant differences in the epidemiology, susceptibility patterns, and chronicity of infection and a frequency of neurosurgical intervention in C. gattii infections higher than the level in C. neoformans infections, likely due to the propensity of C. gattii to form cryptococcomas (1, 8, 9).
C. gattii has been divided into four distinct molecular types (VGI, VGII, VGIII, and VGIV). VGI and VGII primarily affect immunocompetent patients, while VGIII and VGIV are most frequently found in the immunocompromised (4). Comparative studies between different cryptococcal molecular types have been limited and have primarily focused on the major outbreak strain associated with the Pacific Northwest (VGIIa) and have found VGIIa strains more lethal than VGIIb isolates (10, 11). More recently, VGIIIa and VGIIIb subtypes recovered from HIV/AIDS patients in California were compared in an intranasal murine model (4), and VGIIIa isolates were found to be more virulent than VGIIIb isolates; however, comparisons of cryptococcal molecular types and levels of virulence on a broader scale have not previously been performed. We analyzed the relationship of in vitro production of putative virulence factors with molecular type and virulence in a Drosophila melanogaster model of infection.
(This study was presented in part at IDWeek: a Joint Meeting of IDSA, SHEA, HIVMA, and PIDS, San Francisco, CA, 2013 [12].)
MATERIALS AND METHODS
Isolates.
Forty-nine Cryptococcus isolates obtained from clinical, environmental, and veterinary sources were used (Table 1). Molecular types were identified as described in prior studies (11). Mating type was determined by PCR using two pairs of mating-type-specific primers per cryptococcal species as described previously (13, 14).
TABLE 1.
List of isolates and strain characteristics used in the present study
| Molecular type (no. of isolates) | Name | Alias | Origin | Source | Mating type |
|---|---|---|---|---|---|
| VGI (10) | WM179a | CBS 10078/ | Human | Australia | α |
| 10 | Eucalyptus tree | CA | α | ||
| 88 | Human | Washington, DC | a | ||
| JS66 | Koala | Los Angeles, CA | α | ||
| JS80 | Domestic cat | Gainesville, FL | α | ||
| JS55 | Koala | Los Angeles, CA | α | ||
| JS53 | Domestic cat | Rocklin, CA | α | ||
| JS56 | Parrot | Escondido, CA | α | ||
| JS67 | Koala | Los Angeles, CA | α | ||
| 532 | Koala | Sydney, Australia | α | ||
| VGIIa (9) | WM02.32a | CDC R265 | Human | Duncan, Canada | α |
| CAT | Domestic cat | Sacramento, CA | α | ||
| JS7 | Dog | Sacramento, CA | α | ||
| JS74 | Domestic cat | El Cerrito, CA | α | ||
| WM09.144 | CBS10485 | Human | Herning, Denmark | α | |
| WM06.13 | CBS7750 | Environmental | San Francisco, CA | α | |
| 06-3908 | Human | San Antonio, TX | α | ||
| JS71 | Dog | Sacramento, CA | α | ||
| 432 | Human | Los Angeles, CA | α | ||
| VGIIb (7) | WM06.25a | CDC R272 | Human | Vancouver Island, BC, Canada | α |
| WM1008 | 99-194-1904 | Environmental | Blacktown, Australia | α | |
| WM09.155 | N#10 | Koala | Perth, Australia | α | |
| 535 | Veterinary | Sydney, Australia | α | ||
| 537 | Veterinary | Sydney, Australia | α | ||
| 50513971 | Horse | CA | α | ||
| JS65 | Dog | La Mesa, CA | α | ||
| VGIIc (4) | 08-10290 | Domestic cat | OR | α | |
| 09-10082 | Ovine | OR | α | ||
| 09-11171 | Domestic cat | OR | α | ||
| 11-7650 | Dog | Eugene, OR | α | ||
| VGIII (10) | 82 | Human | Washington, DC | α | |
| 83 | Human | Washington, DC | α | ||
| BR | Human | Sacramento, CA | α | ||
| 50805443 | Horse | CA | α | ||
| B7415 | Alpaca | CA | α | ||
| JS54 | Domestic cat | Sacramento, CA | α | ||
| JS5 | Musk deer | San Diego, CA | α | ||
| JS27 | Domestic cat | American Canyon, CA | α | ||
| JS69 | Domestic cat | Lomita, CA | α | ||
| JS52 | Domestic cat | Davis, CA | a | ||
| VGIV (4) | WM779a | CBS 10101 | Cheetah | Johannesburg, South Africa | α |
| WM2363 | B5742 | Human | Punjab, India | α | |
| WM2364 | B5748 | Human | Himachal Pradesh, India | α | |
| JS26 | Cheetah | Reno, NV | α | ||
| VNI (5) | H99a | Human | NC | α | |
| JS23 | Horse | Williams, CA | α | ||
| JS68 | Domestic cat | Vancouver, WA | α | ||
| JS73 | Dog | San Jose, CA | α | ||
| JS72 | Dog | Tiburon, CA | α |
American Type Culture Collection reference strain—see text for ATCC reference numbers.
In vivo assessment of virulence in Drosophila.
Two- to 4-day-old Oregon-R (OR) flies (60 flies per isolate) were used as wild-type (WT) flies (15). Each isolate was inoculated through the thorax (2 × 109 cells/ml) using a 0.1-μm-inner-diameter needle (approximately 5 × 103 cells per fly). The flies were kept at 29°C and transferred to fresh vials every 2 days. Flies that died <3 h after infection (<5% of the total) were excluded from analysis. Survival to 8 days after infection was assessed. Each experiment was performed in triplicate at different time points.
Quantification of the injection inoculums was performed by transferring cells from the tip of a needle that had been previously dipped into a concentrated Cryptococcus solution into 1 ml of 0.85% normal saline solution. Serial dilutions (100 μl each) were then plated onto yeast extract-peptone-dextrose (YPD) plates and incubated at 37°C for 72 h.
Growth at 30°C and 37°C.
The Cryptococcus isolates used in this study were repopulated in YPD liquid media overnight to mid-log phase. Next, the isolates were washed twice in sterile phosphate-buffered saline (PBS) and resuspended in 2% glucose YNB liquid at a concentration of 1 × 103 yeast cells/ml, and then 100 μl of each isolate was dispensed into 96-well U-bottom plates; medium alone was used as a control. Viability was confirmed by plating a 10-fold dilution of the 1 × 103 yeast cells/ml on a Sabouraud agar plate. The plates were sealed and placed in a plate reader at 30°C or 37°C for 24 h with measurements every hour. Turbidity was measured at 600 nm. Sequential optical density (OD) measurements were used to generate growth curves for each isolate in triplicate.
Susceptibility testing.
Susceptibility testing was done by broth microdilution in accordance with the CLSI M27-A3 methodology (16, 17). Final antifungal concentrations ranged from 0.015 to 8 μg/ml for itraconazole, posaconazole, voriconazole, and amphotericin B and from 0.25 to 64 μg/ml for fluconazole and flucytosine. Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019 were used as controls.
Hydrogen peroxide tolerance.
The ability to resist the fungicidal effects of H2O2 (Fisher Scientific) was measured by broth microdilution using a concentration of 5 × 103 cells and final H2O2 concentrations ranging from 0.1 to 80 mM (18). Isolates were incubated for 3 h at 37°C on a rotary shaker in YPD liquid media, and catalase (Fisher-Scientific) at a concentration of 200 μg/ml was used as an inhibitor of H2O2 activity. Isolates were serially diluted and plated on Sabaroud dextrose for assessment of viability.
Melanin production.
Melanin synthesis was quantified using a modified laccase activity test as previously described (11, 19). Each isolate was grown in YPD broth for 24 h at 30°C. Saturated cultures were then added to 10 ml of melanin induction media (1% yeast nitrogen base [YNB] without amino acid and ammonium sulfate) containing either 10 mM dopamine or 10 mM epinephrine (Sigma) and incubated at 37°C for 48 h. Aliquots were taken and diluted to ensure optical density (OD) readings within the 0.1 to 0.9 absorbance range, and values at 475 and 600 nm were obtained to control for both the quantity of melanin and cell concentrations.
Capsule production.
Isolates were suspended in YPD media, collected in the logarithmic-growth phase by centrifugation, washed twice with PBS, and then placed in six-well plates at a density of 5 × 106 to 5 × 107 cells/ml in RPMI 1640 with MOPS (morpholinepropanesulfonic acid) and HCO3 (pH 7.3) and incubated for 24 h in 5% CO2 at 37°C, as described previously (11, 20). The capsule size of at least 30 yeast cells per isolate (10 μl of cell suspension was mixed with India ink) was measured by light microscopy (Olympus BX51) using the ratio of the diameter of the capsule to the diameter of the cell measured in micrometers.
Statistical analysis.
To identify the relationship between molecular type and survival, we used a mediator model approach. This is a multistep process to test whether Drosophila survival rates differ between molecular types and to investigate the various characteristics that may mechanistically explain differences in survival rates. In the first step, we performed univariate analyses to test if there was a difference in Drosophila survival and the various possible candidate mediators (molecular type and subtype, growth at 30°C and 37°C, antifungal susceptibility, H2O2 tolerance, capsule size, and melanin production). In the second stage, we included all significant mediators in a multiple-regression model to test which candidates remained significant after controlling for others, excluding molecular type. At this stage, a P value of 0.1 or less was considered significant to avoid prematurely removing important explanatory variables.
In the final stage, we examined how P values for the significant candidates from stage 2 changed when the molecular type was included in the model. The reason for performing this last step was that if molecular types became nonsignificant when the significant candidate mediators were included, this would indicate that the effect of molecular type was mediated by the candidate. If molecular type remained significant, this would support the hypothesis that the candidates were mediating survival but that there were other mediating characteristics that had not been included in the model.
Welch's analysis of variance (ANOVA) was used to test for differences in survival among molecular types due to nonhomogeneity. The Kruskal-Wallis test was used to determine the relationship between virulence factors and both molecular type and survival. For normally distributed variables, we used Pearson's correlation test for a relationship with survival. Colinearity was assessed using Pearson's or Spearman's correlation. Mediator model analyses were performed using SAS software version 9.3. P values of less than 0.05 were considered to be statistically significant.
RESULTS
Strain characteristics.
Of the 49 Cryptococcus isolates studied, five were molecular type VNI, 10 VGI, 9 VGIIa, 7 VGIIb, 4 VGIIc, and 10 VGIII, and 4 were VGIV (Table 1). The isolates were chosen to represent a diverse global population in attempt to characterize true molecular-type-specific differences that might exist rather than differences apparent merely in regional subpopulations. Well-characterized isolates available as reference strains through the American Type Culture Collection (ATCC) were also chosen for inclusion.
In vivo assessment of virulence in Drosophila.
Mortality was readily achieved at 5 × 103 cells/fly in our study. The probabilities of flies surviving over time differed significantly among molecular types (P < 0.001) (Fig. 1). VGIII was the most virulent molecular type in flies (P < 0.001 compared to VNI and VGI [the predominant global molecular types], VGIIb, and VGIV). The major subtype observed in the Pacific Northwest outbreak (VGIIa) was also more virulent than other molecular types and subtypes in the fly model (P < 0.001 compared to VGI, VGIIb, and VGIV). Subgroup VGIIc demonstrated virulence similar to that of VGIIa (P < 0.001 compared to VGI and VGIV).
FIG 1.
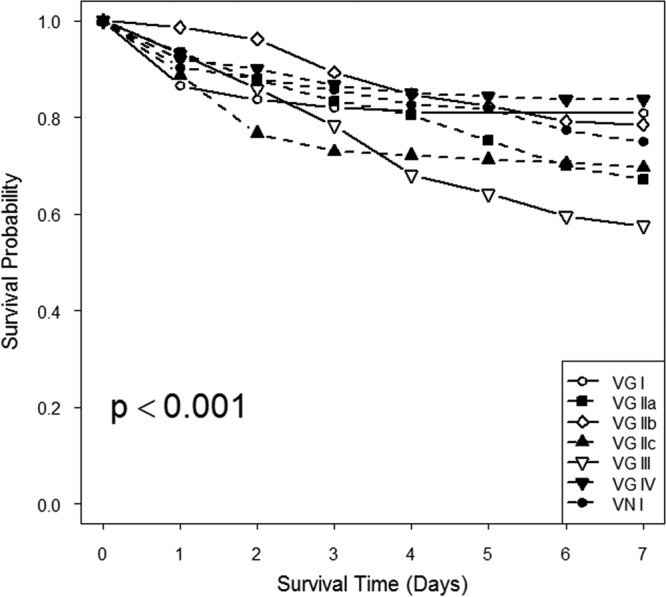
Survival of WT flies infected by injection (5 × 103 cells per fly) of different Cryptococcus molecular types and subtypes. VGIII was the most virulent molecular type observed (P < 0.001 compared to VNI, VGI, VGIIb, and VGIV). VGIIa also demonstrated significant differences from the other molecular types and subtypes (P < 0.001 compared to VGI, VGIIb, and VGIV), and VGIIc demonstrated virulence similar to that of VGIIa (P < 0.001 compared to VGI and VGIV).
Growth at 30°C and 37°C.
Logarithmic-phase growth was reached at different time points. For this reason, doubling time was not used to compare growth curves; instead, overall growth was measured at 24 h as performed in previous studies (11). At 30°C, VGIII isolates grew most rapidly (Fig. 2), with significant increases in growth compared to molecular subtypes VGIIa (P < 0.0001), VGIIb (P = 0.0003), VGIV (P < 0.0001), and VNI (P < 0.0001). Differences in growth were also seen in other types, with VGIIc isolates showing significant increases in growth over 24 h compared to VGIIa isolates (P = 0.027) and VGI isolates showing significant increases in growth over 24 h compared to VGIIa isolates (P = 0.003).
FIG 2.
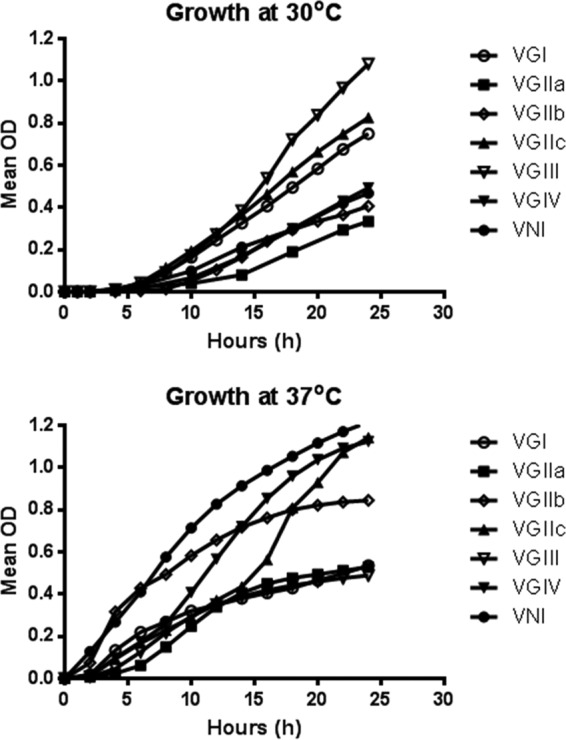
Growth curves of isolates at 30 and 37°C.
At 37°C, VNI isolates grew most rapidly, with significant differences seen compared to molecular types VGI (P = 0.007), VGIIa (P = 0.04), and VGIII (P = 0.014). At 24 h, VGIV isolates also showed growth that was significantly more rapid than that seen with VGIII (P = 0.022) and VGI (P = 0.015) isolates. No other significant differences were found between molecular types and subtypes.
Melanin production.
At 30°C, VNI isolates in dopamine-containing media produced significantly more melanin than VGI (P = 0.004), VGIIa (P = 0.004), VGIIb (P = 0.03), VGIII (P = 0.01), and VGIV (P = 0.04) isolates (Fig. 3). Using epinephrine-containing media, no significant differences in melanin production were seen at 30°C. Isolates grown at 37°C in dopamine- or epinephrine-containing media displayed nonsignificant differences in melanin production between molecular types. All isolates produced more melanin using epinephrine than using dopamine (P < 0.0001). Others have previously observed changes in melanin production between 30 and 37°C (11); however, no significant differences between isolates grown in either dopamine- or epinephrine-containing media at different temperatures were observed in our study.
FIG 3.
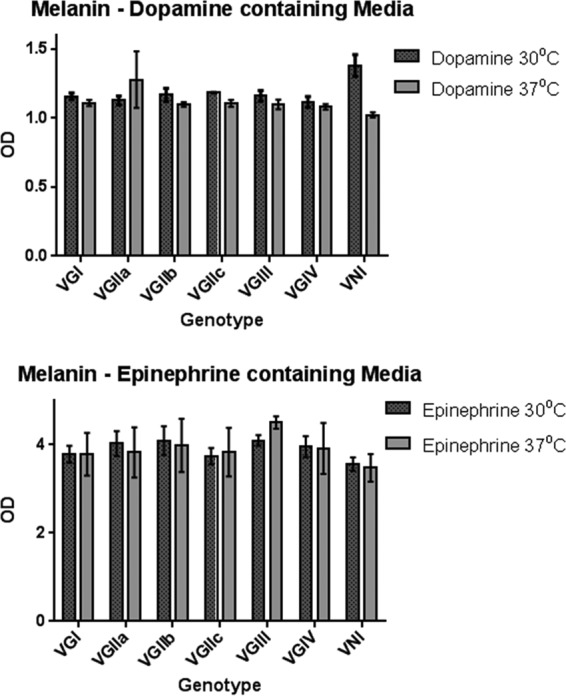
Melanin production of different Cryptococcus molecular types under various growth conditions.
Capsule production.
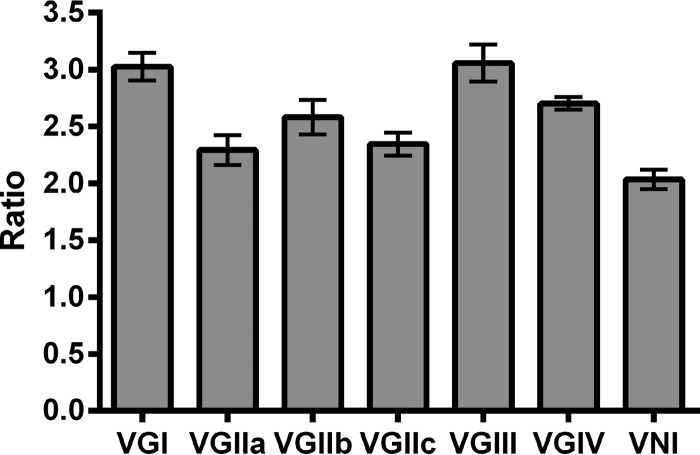
Comparison of all C. gattii capsule sizes to those of C. neoformans revealed highly significant differences, with capsules of C. gattii isolates larger than those of C. neoformans isolates (P = 0.0006). There were also highly significant differences in the capsule ratios between molecular types (Fig. 4)—by ANOVA, VGI isolates produced a capsule larger than that seen with VGIIa isolates (P = 0.0008) or with VNI isolates (P = 0.0001). VGIII isolates similarly produced a larger capsule than isolates of VGIIa (P = 0.002), VGIIc (P = 0.02), or VNI (P = 0.001). No significant differences were seen between other molecular types.
FIG 4.
Capsule sizes of different Cryptococcus molecular types at 37°C. Ratio, mean capsule-capsule/cell wall-cell wall ratio for each isolate.
Hydrogen peroxide tolerance.
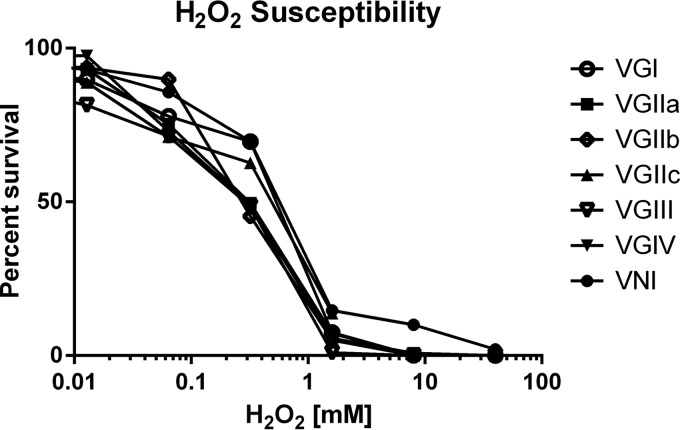
The ability of different cryptococcal molecular types to resist killing by H2O2 was also examined, and at lower H2O2 concentrations, similar findings were observed for all isolates. However, when high concentrations (35 mM) were examined, VNI isolates demonstrated significant differences compared to those of other molecular types (P < 0.05) (Fig. 5).
FIG 5.
Susceptibility to hydrogen peroxide.
Susceptibility testing.
Table 2 summarizes the mean MIC ranges and mean fungicidal concentrations (MFC) of each antifungal. Fluconazole MICs were higher in VGIIc isolates (geometric mean [GM] = 8) than in the other molecular types and subtypes. VGIIc isolates exhibited significantly higher MICs for fluconazole than those of VGI (P = 0.008), VGIII (P = 0.008), and VNI (P = 0.02). Similarly, itraconazole MICs among VGIIc isolates were significantly higher than those for VGI (P = 0.02), VGIIa (P = 0.04), and VGIII (P = 0.001) isolates. Posaconazole MIC differences were significant only for VGIIc > VNI (P = 0.01). Isolates with reduced fluconazole susceptibility exhibited higher posaconazole and voriconazole MICs as well. There were no significant differences in voriconazole, flucytosine, or amphotericin B MICs found between molecular types.
TABLE 2.
Susceptibility testing against different Cryptococcus molecular types and subtypes
| Molecular type (no. of isolates) | Parameter | MIC (μg/ml) |
|||||
|---|---|---|---|---|---|---|---|
| Fluconazole | Itraconazole | Posaconazole | Voriconazole | Amphotericin B | Flucytosine | ||
| VGI (10) | MIC range | 1 to 8 | 1 to 2 | 0.5 to 2 | 0.03 to 1 | 0.25 to 0.5 | 0.125 to 0.5 |
| GM MIC | 2.30 | 1.32 | 0.81 | 0.06 | 0.38 | 0.25 | |
| MFC range | 4 to >64 | 2 to 16 | 1 to 4 | 0.125 to 4 | 0.25 to 0.5 | 2 to >64 | |
| VGIIa (9) | MIC range | 0.5 to 8 | 0.25 to 2 | 0.125 to 2 | 0.03 to 0.125 | 0.125 to 0.5 | 0.125 to 2 |
| GM MIC | 3.08 | 1 | 0.77 | 0.07 | 0.39 | 0.46 | |
| MFC range | 4 to >64 | 2 to >16 | 1 to 8 | 0.5 to 16 | 0.5 to 1 | 1 to >64 | |
| VGIIb (7) | MIC range | 1 to 16 | 1 to 2 | 0.5 to 1 | 0.06 to 0.125 | 0.25 to 0.5 | 0.125 to 0.5 |
| GM MIC | 4 | 1.32 | 0.76 | 0.08 | 0.44 | 0.25 | |
| MFC range | 4 to >64 | 2 to >16 | 2 to 4 | 0.25 to 16 | 0.25 to 1 | 4 to >64 | |
| VGIIc (4) | MIC range | 2 to 16 | 2 to 4 | 1 to 2 | 0.06 to 0.5 | 0.25 to 0.5 | 0.25 to 0.5 |
| GM MIC | 8 | 2.38 | 1.68 | 0.18 | 0.42 | 0.35 | |
| MFC range | 64 to >64 | 2 to 16 | 4 to 8 | 0.25 to >16 | 0.25 to 1 | 8 to >64 | |
| VGIII (10) | MIC range | 2 to 8 | 1 to 2 | 1 | 0.06 to 0.5 | 0.25 to 0.5 | 0.125 to >64 |
| GM MIC | 3.48 | 1.07 | 0.93 | 0.06 | 0.47 | 0.62 | |
| MFC range | 16 to 32 | 2 to 8 | 2 to 4 | 0.125 to 1 | 0.25 to 1 | 4 to >64 | |
| VGIV (4) | MIC range | 2 to 8 | 1 to 2 | 1 | 0.06 to 0.5 | 0.25 to 0.5 | 0.125 to 0.5 |
| GM MIC | 4 | 1.52 | 1 | 0.11 | 0.44 | 0.19 | |
| MFC range | 4 to >64 | 1 to 16 | 2 to 4 | 0.25 to >16 | 0.25 to 0.5 | 4 to >64 | |
| VNI (5) | MIC range | 1 to 2 | 1 to 2 | 0.25 to 1 | 0.03 to 0.125 | 0.5 | 0.125 to 0.5 |
| GM MIC | 1.74 | 1.52 | 0.66 | 0.05 | 0.5 | 0.33 | |
| MFC range | 8 to >64 | 2 to 8 | 2 to 4 | 0.25 to >16 | 0.5 | 2 to >64 | |
Mediator model analysis.
In stage 1 of model selection, we found overwhelming evidence that Drosophila survival was related to the molecular type or subtype (P = 0.0001). The data provided evidence that survival was positively correlated with enhanced growth at 30°C (P = 0.0488), melanin production at 37°C in dopamine-containing media (P = 0.0627), and melanin production at 37°C in epinephrine-containing media (P = 0.0516) and was negatively correlated with isolate growth at 37°C (P = 0.033). The data also provided evidence there were differences between molecular types and subtypes in growth at 30°C (P = 0.0002), growth at 37°C (P = 0.0456), and capsule size (P = 0.0002). In our test for colinearity, we found that no candidate was correlated with any other at 0.6 or higher (results not shown).
In the second stage of model selection, we fitted a multiple-regression model that included voriconazole MIC (P = 0.36), itraconazole MIC (P = 0.22), growth at 30°C (P = 0.0049), growth at 37°C (P = 0.0989), capsule size (P = 0.33), and melanin production at 37°C in dopamine-containing media (P = 0.74) and in epinephrine-containing media (P = 0.61). Only growth at 30°C and 37°C were retained for the final stage.
In the final stage of the mediator model analysis, we fitted a general linear model predicting survival from growth at 30°C and a negative correlation for growth at 37°C. Both sets of data were statistically significant, with P values of 0.045 and 0.027, respectively. When molecular type was added to the model, the effect remained significant (P < 0.0001), and growth variable data became nonsignificant (0.89 and 0.14, respectively). The analysis therefore indicated there was a strong correlation of Drosophila survival with molecular type and that this effect was manifested in vitro as differences among the isolate groups in growth at 30 and 37°C.
DISCUSSION
The recent outbreak of C. gattii infections in the Pacific Northwest illustrates the need to better understand differences in virulence and therapeutic options for infections caused by this emerging pathogen. The majority of isolates in the Pacific Northwest have been of the VGII molecular type (21), which has been found to be more virulent than other cryptococcal molecular type (10, 11) and less susceptible to the triazoles (22, 23). More-recent evidence has suggested that additional C. gattii molecular types and subtypes may also be prevalent, may be associated with substantial morbidity (4), and may represent a previously unrecognized and ongoing epidemic (6, 24, 25). Furthermore, it has been proposed that C. gattii and C. neoformans infections represent distinct clinical syndromes (24, 26), and regimens for treatment of C. gattii infections longer than those used in C. neoformans infections have demonstrated improved outcomes (27).
The mechanisms responsible for these clinical differences remain incompletely defined. Immunopathology, tissue tropism (26), and the patterns of cytokine and chemokine induction might differ between C. gattii and C. neoformans (28, 29), and yet the expression of virulence factors responsible for species-specific differences has not previously been characterized. Prior virulence work, conducted primarily in C. neoformans, was performed using isogenic gene disruption and reconstitution strains or by comparing strains that differ in the expression of the phenotype of interest. These studies are essential, and yet they represent a reductionist approach (30), and it is difficult to extrapolate results from these tightly controlled studies to clinical isolates where multiple virulence determinants are expressed in various quantities in a coordinated and potentially host-dependent fashion (31).
In our Drosophila model of cryptococcal infection, we observed variation in the virulence of isolates within each molecular type, reconfirming the need to evaluate numerous isolates from environmental, veterinary, and human sources. Overall, VGIII isolates were the most virulent, followed by VGIIa, VGIIc, VNI, and VGIIb isolates. These findings are consistent with prior reports using murine models such as the enhanced virulence of VGIIa over VGIIb isolates (11, 32). No mechanism has been proposed for molecular-type-specific virulence differences, and without a known need for a host, Cryptococcus spp. have been termed an “accidental pathogen” in humans (30). As such, ecologic factors have likely played a dominant role in molding these different species over time into the disparate organisms we now face. An improved understanding of these differences in virulence that may relate to morbidity and mortality is therefore of paramount importance (33).
Among these ecologic factors, competition with other organisms and their byproducts is likely intermittent, and yet environmental temperature is an ever-present constraint on and requirement for the ability of Cryptococcus to grow and reproduce. It is therefore not surprising that enhanced growth at different temperatures was a dominant factor in predicting fly death in our model. This is consistent with the results of prior studies, with growth at body temperature found to be highly correlated with infection (30, 34), and the growth of VGIII isolates at 30°C similarly plays a significant role in Drosophila. Further work in other models at different temperatures using these same isolates should provide additional insight.
Few differences in melanin production were seen in our study; at 30°C, however, VNI isolates in dopamine-containing media produced significantly more melanin than the majority of C. gattii molecular types and subtypes. The reasons for this are unclear; however, C. neoformans is frequently found in pigeon guano, while C. gattii is not typically isolated from this source, and pigeon guano has thus been called a “realized ecological niche” for C. neoformans (33). Melanin production in this specific environment may provide a competitive advantage to C. neoformans, and yet the dependence on temperature for virulence factor production and regulation again seems to be the driving factor (35). Furthermore, as melanin production can protect Cryptococcus from a myriad of external insults, including antifungal compounds and xenobiotics, oxidants, UV light, macrophages, and extremes in temperature, this trait is likely to have been selected for environmental survival (36, 37) and thus may account for preferential expression at 30°C rather than 37°C for C. neoformans.
Differences in capsule size were also observed in our study and showed significant variation among species, with C. gattii isolates producing capsules larger than those of C. neoformans. Despite prior reports demonstrating the importance of capsule formation as a protection against fungal recognition and subsequent phagocytosis and as a “sink” for reactive oxygen species (ROS) (38, 39), capsule size did not predict virulence in our model of infection, and yet the larger capsules we noted in C. gattii isolates may have direct clinical relevance. Mitchell et al. reported higher serum cryptococcal antigen (CRAG) titers in C. gattii-infected patients than in those infected with C. neoformans, and this difference was apparent despite the lower incidence of positive cerebrospinal fluid (CSF) cultures in patients with C. gattii (78% versus 100%) (40). Animal models have similarly shown that, at the same inoculum of C. gattii or C. neoformans, CRAG titers are significantly higher in C. gattii-infected mice despite similar and, often, significantly lower organ burdens at the end of study (8)—a mechanism potentially explained by our observation that the capsules in C. gattii strains are larger than the capsules in C. neoformans strains.
Multiple pathways within Cryptococcus spp. augment the protective effect of cryptococcal antigen against reactive oxygen species (ROS), suggesting that defense against oxidative damage is critical for survival. We observed few differences between cryptococcal species and molecular types in relation to hydrogen peroxide tolerance, with the exception of VNI strains at H2O2 concentrations exceeding those found in phagocytic cell phagosomes (low micromolar range) (41). ROS-induced damage to fungal DNA, lipids, and proteins is lethal to a number of fungal organisms (42) and suggests that these mechanisms may be conserved and fundamental to fungal survival following phagocytosis by either the host or environmental amoebae (43). If these mechanisms are uniformly essential for survival, it is therefore not surprising that no differences were observed.
Susceptibility results found in our study confirm those previously reported with respect to higher fluconazole MICs observed in VGII isolates, particularly those of VGIIc (22). The mechanism behind the elevated fluconazole MICs in these isolates has yet to be elucidated. Investigations of ERG11 have found that neither overexpression nor variations within ERG11 coding play a significant role in the elevated MICs found in Pacific Northwest isolates (44), and it is thus likely that efflux pump overexpression is the predominant mechanism. Overexpression (or the development of heteroresistance in the environment) may occur due to an as-yet-unknown unique environmental pressure from xenobiotics.
It is possible our in vitro results failed to find phenotypic differences that may in fact exist in vivo. For example, recent studies have found that Candida albicans and Candida glabrata actively suppress ROS production in phagocytes (45) and our study assessed resistance to exogenous administration of H2O2 and not whole phagocytes. The infection of macrophages by Cryptococcus spp. has also been shown to induce the formation of vesicles containing virulence-associated enzymes, including laccase, urease, and phosphatase, and can lead to fungal melanization (46, 47). Thus, in vivo virulence factor production may differ from even the optimal conditions used for comparative analysis in our study.
This is the first study to have established a correlation between cryptococcal molecular type and virulence in an invertebrate model of infection. The increased recognition of C. gattii infections across the globe accentuates the need to more completely define the epidemiology, susceptibility, and virulence of these organisms, and these differences suggest that identification of all cryptococcal isolates to the species level should be performed on a routine basis. Further work, including validation of molecular-type-specific virulence in murine models of infection, whole-genome evaluation of these organisms, and quantitation of additional virulence factors and their contribution to the predictive model, will need to be undertaken.
In summary, we have conducted a comprehensive assessment of C. gattii molecular type differences through in vitro testing and an invertebrate model of infection and found VGIII the most virulent molecular type assessed followed by VGIIa and VGIIc, with temperature the dominant influence on virulence. The emergence of these types within discrete geographic regions and observed virulence differences may have implications for treatment and outcomes for patients affected by this emerging pathogen.
ACKNOWLEDGMENTS
This project was supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH), through grant UL1 TR000002 and the National Health & Medical Research Council (NH&MRC) of Australia through grant APP1031943 to W.M. D.P.K. acknowledges the Frances King Black endowment.
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print 5 May 2014
REFERENCES
- 1.Sorrell TC. 2001. Cryptococcus neoformans variety gattii. Med. Mycol. 39:155–168. 10.1080/mmy.39.2.155.168 [DOI] [PubMed] [Google Scholar]
- 2.Ellis DH, Pfeiffer TJ. 1990. Natural habitat of Cryptococcus neoformans var. gattii. J. Clin. Microbiol. 28:1642–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacDougall L, Kidd SE, Galanis E, Mak S, Leslie MJ, Cieslak PR, Kronstad JW, Morshed MG, Bartlett KH. 2007. Spread of Cryptococcus gattii in British Columbia, Canada, and detection in the Pacific Northwest, USA. Emerg. Infect. Dis. 13:42–50. 10.3201/eid1301.060827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrnes EJ, III, Li W, Ren P, Lewit Y, Voelz K, Fraser JA, Dietrich FS, May RC, Chaturvedi S, Chaturvedi V, Heitman J. 2011. A diverse population of Cryptococcus gattii molecular type VGIII in southern Californian HIV/AIDS patients. PLoS Pathog. 7:e1002205. 10.1371/journal.ppat.1002205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrnes EJ, III, Li W, Lewit Y, Perfect JR, Carter DA, Cox GM, Heitman J. 2009. First reported case of Cryptococcus gattii in the Southeastern USA: implications for travel-associated acquisition of an emerging pathogen. PLoS One 4:e5851. 10.1371/journal.pone.0005851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris JR, Lockhart SR, Sondermeyer G, Vugia DJ, Crist MB, D'Angelo MT, Sellers B, Franco-Paredes C, Makvandi M, Smelser C, Greene J, Stanek D, Signs K, Nett RJ, Chiller T, Park BJ. 2013. Cryptococcus gattii infections in multiple states outside the US Pacific Northwest. Emerg. Infect. Dis. 19:1620–1626. 10.3201/eid1910.130441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O, Nguyen MH, Pappas PG, Powderly WG, Singh N, Sobel JD, Sorrell TC. 2010. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 50:291–322. 10.1086/649858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson GR, III, Wiederhold NP, Najvar LK, Bocanegra R, Kirkpatrick WR, Graybill JR, Patterson TF. 2012. A murine model of Cryptococcus gattii meningoencephalitis. J. Antimicrob. Chemother. 67:1432–1438. 10.1093/jac/dks060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon-Chung KJ, Bennett JE. 1984. Epidemiologic differences between the two varieties of Cryptococcus neoformans. Am. J. Epidemiol. 120:123–130 [DOI] [PubMed] [Google Scholar]
- 10.Fraser JA, Giles SS, Wenink EC, Geunes-Boyer SG, Wright JR, Diezmann S, Allen A, Stajich JE, Dietrich FS, Perfect JR, Heitman J. 2005. Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature 437:1360–1364. 10.1038/nature04220 [DOI] [PubMed] [Google Scholar]
- 11.Ngamskulrungroj P, Serena C, Gilgado F, Malik R, Meyer W. 2011. Global VGIIa isolates are of comparable virulence to the major fatal Cryptococcus gattii Vancouver Island outbreak genotype. Clin. Microbiol. Infect. 17:251–258. 10.1111/j.1469-0691.2010.03222.x [DOI] [PubMed] [Google Scholar]
- 12.Thompson GR, III, Albert N, Hodge G, Wilson MD, Sykes JE, Bays DJ, Firacative C, Meyer W, Kontoyiannis DP. 2013. IDWeek: Jt. Meet. IDSA, SHEA, HIVMA, PIDS, San Francisco, CA, oral presentation 1824 [Google Scholar]
- 13.Ngamskulrungroj P, Sorrell TC, Chindamporn A, Chaiprasert A, Poonwan N, Meyer W. 2008. Association between fertility and molecular sub-type of global isolates of Cryptococcus gattii molecular type VGII. Med. Mycol. 46:665–673. 10.1080/13693780802210734 [DOI] [PubMed] [Google Scholar]
- 14.Lin X, Litvintseva AP, Nielsen K, Patel S, Floyd A, Mitchell TG, Heitman J. 2007. alpha AD alpha hybrids of Cryptococcus neoformans: evidence of same-sex mating in nature and hybrid fitness. PLoS Genet. 3:1975–1990. 10.1371/journal.pgen.0030186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lionakis MS, Lewis RE, May GS, Wiederhold NP, Albert ND, Halder G, Kontoyiannis DP. 2005. Toll-deficient Drosophila flies as a fast, high-throughput model for the study of antifungal drug efficacy against invasive aspergillosis and Aspergillus virulence. J. Infect. Dis. 191:1188–1195. 10.1086/428587 [DOI] [PubMed] [Google Scholar]
- 16.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard—3rd ed. CLSI document M27-A3. CLSI, Wayne, PA [Google Scholar]
- 17.Thompson GR, III, Wiederhold NP, Fothergill AW, Vallor AC, Wickes BL, Patterson TF. 2009. Antifungal susceptibilities among different serotypes of Cryptococcus gattii and Cryptococcus neoformans. Antimicrob. Agents Chemother. 53:309–311. 10.1128/AAC.01216-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaturvedi V, Wong B, Newman SL. 1996. Oxidative killing of Cryptococcus neoformans by human neutrophils. Evidence that fungal mannitol protects by scavenging reactive oxygen intermediates. J. Immunol. 156:3836–3840 [PubMed] [Google Scholar]
- 19.Pukkila-Worley R, Gerrald QD, Kraus PR, Boily MJ, Davis MJ, Giles SS, Cox GM, Heitman J, Alspaugh JA. 2005. Transcriptional network of multiple capsule and melanin genes governed by the Cryptococcus neoformans cyclic AMP cascade. Eukaryot. Cell 4:190–201. 10.1128/EC.4.1.190-201.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaragoza O, Casadevall A. 2004. Experimental modulation of capsule size in Cryptococcus neoformans. Biol. Proced. Online 6:10–15. 10.1251/bpo68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Byrnes EJ, III, Li W, Lewit Y, Ma H, Voelz K, Ren P, Carter DA, Chaturvedi V, Bildfell RJ, May RC, Heitman J. 2010. Emergence and pathogenicity of highly virulent Cryptococcus gattii genotypes in the northwest United States. PLoS Pathog. 6:e1000850. 10.1371/journal.ppat.1000850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Espinel-Ingroff A, Aller AI, Canton E, Castanon-Olivares LR, Chowdhary A, Cordoba S, Cuenca-Estrella M, Fothergill A, Fuller J, Govender N, Hagen F, Illnait-Zaragozi MT, Johnson E, Kidd S, Lass-Florl C, Lockhart SR, Martins MA, Meis JF, Melhem MS, Ostrosky-Zeichner L, Pelaez T, Pfaller MA, Schell WA, St-Germain G, Trilles L, Turnidge J. 2012. Cryptococcus neoformans-Cryptococcus gattii species complex: an international study of wild-type susceptibility endpoint distributions and epidemiological cutoff values for fluconazole, itraconazole, posaconazole, and voriconazole. Antimicrob. Agents Chemother. 56:5898–5906. 10.1128/AAC.01115-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Datta K, Rhee P, Byrnes E, III, Garcia-Effron G, Perlin DS, Staab JF, Marr KA. 2013. Isavuconazole activity against Aspergillus lentulus, Neosartorya udagawae, and Cryptococcus gattii, emerging fungal pathogens with reduced azole susceptibility. J. Clin. Microbiol. 51:3090–3093. 10.1128/JCM.01190-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marr KA. 2011. Cryptococcus gattii: the tip of the iceberg. Clin. Infect. Dis. 53:1196–1198. 10.1093/cid/cir738 [DOI] [PubMed] [Google Scholar]
- 25.Kunadharaju R, Choe U, Harris JR, Lockhart SR, Greene JN. 2013. Cryptococcus gattii, Florida, USA, 2011. Emerg. Infect. Dis. 19:519–521. 10.3201/eid1903.121399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ngamskulrungroj P, Chang Y, Sionov E, Kwon-Chung KJ. 2012. The primary target organ of Cryptococcus gattii is different from that of Cryptococcus neoformans in a murine model. mBio 3:e00103-12. 10.1128/mBio.00103-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen SC, Korman TM, Slavin MA, Marriott D, Byth K, Bak N, Currie BJ, Hajkowicz K, Heath CH, Kidd S, McBride WJ, Meyer W, Murray R, Playford EG, Sorrell TC, Australia and New Zealand Mycoses Interest Group (ANZMIG) Cryptococcus Study 2013. Antifungal therapy and management of complications of cryptococcosis due to Cryptococcus gattii. Clin. Infect. Dis. 57:543–551. 10.1093/cid/cit341 [DOI] [PubMed] [Google Scholar]
- 28.Schoffelen T, Illnait-Zaragozi MT, Joosten LA, Netea MG, Boekhout T, Meis JF, Sprong T. 2013. Cryptococcus gattii induces a cytokine pattern that is distinct from other cryptococcal species. PLoS One 8:e55579. 10.1371/journal.pone.0055579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leongson K, Cousineau-Cote V, Goupil M, Aumont F, Senechal S, Gaboury L, Jolicoeur P, Kronstad JW, de Repentigny L. 2013. Altered immune response differentially enhances susceptibility to Cryptococcus neoformans and Cryptococcus gattii infection in mice expressing the HIV-1 transgene. Infect. Immun. 81:1100–1113. 10.1128/IAI.01339-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Solache MA, Izquierdo-Garcia D, Smith C, Bergman A, Casadevall A. 2013. Fungal virulence in a lepidopteran model is an emergent property with deterministic features. mBio 4:e00100-13. 10.1128/mBio.00100-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clancy CJ, Nguyen MH, Alandoerffer R, Cheng S, Iczkowski K, Richardson M, Graybill JR. 2006. Cryptococcus neoformans var. grubii isolates recovered from persons with AIDS demonstrate a wide range of virulence during murine meningoencephalitis that correlates with the expression of certain virulence factors. Microbiology 152:2247–2255. 10.1099/mic.0.28798-0 [DOI] [PubMed] [Google Scholar]
- 32.Ma H, Hagen F, Stekel DJ, Johnston SA, Sionov E, Falk R, Polacheck I, Boekhout T, May RC. 2009. The fatal fungal outbreak on Vancouver Island is characterized by enhanced intracellular parasitism driven by mitochondrial regulation. Proc. Natl. Acad. Sci. U. S. A. 106:12980–12985. 10.1073/pnas.0902963106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielsen K, De Obaldia AL, Heitman J. 2007. Cryptococcus neoformans mates on pigeon guano: implications for the realized ecological niche and globalization. Eukaryot. Cell 6:949–959. 10.1128/EC.00097-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu OW, Chun CD, Chow ED, Chen C, Madhani HD, Noble SM. 2008. Systematic genetic analysis of virulence in the human fungal pathogen Cryptococcus neoformans. Cell 135:174–188. 10.1016/j.cell.2008.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobson ES, Compton GM. 1996. Discordant regulation of phenoloxidase and capsular polysaccharide in Cryptococcus neoformans. J. Med. Vet. Mycol. 34:289–291. 10.1080/02681219680000491 [DOI] [PubMed] [Google Scholar]
- 36.Nosanchuk JD, Rudolph J, Rosas AL, Casadevall A. 1999. Evidence that Cryptococcus neoformans is melanized in pigeon excreta: implications for pathogenesis. Infect. Immun. 67:5477–5479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Aisen P, Casadevall A. 1995. Cryptococcus neoformans melanin and virulence: mechanism of action. Infect. Immun. 63:3131–3136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaragoza O, Chrisman CJ, Castelli MV, Frases S, Cuenca-Estrella M, Rodriguez-Tudela JL, Casadevall A. 2008. Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell. Microbiol. 10:2043–2057. 10.1111/j.1462-5822.2008.01186.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Meara TR, Alspaugh JA. 2012. The Cryptococcus neoformans capsule: a sword and a shield. Clin. Microbiol. Rev. 25:387–408. 10.1128/CMR.00001-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitchell DH, Sorrell TC, Allworth AM, Heath CH, McGregor AR, Papanaoum K, Richards MJ, Gottlieb T. 1995. Cryptococcal disease of the CNS in immunocompetent hosts: influence of cryptococcal variety on clinical manifestations and outcome. Clin. Infect. Dis. 20:611–616. 10.1093/clinids/20.3.611 [DOI] [PubMed] [Google Scholar]
- 41.Winterbourn CC, Hampton MB, Livesey JH, Kettle AJ. 2006. Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome: implications for microbial killing. J. Biol. Chem. 281:39860–39869. 10.1074/jbc.M605898200 [DOI] [PubMed] [Google Scholar]
- 42.Lambou K, Lamarre C, Beau R, Dufour N, Latge JP. 2010. Functional analysis of the superoxide dismutase family in Aspergillus fumigatus. Mol. Microbiol. 75:910–923. 10.1111/j.1365-2958.2009.07024.x [DOI] [PubMed] [Google Scholar]
- 43.Steenbergen JN, Shuman HA, Casadevall A. 2001. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc. Natl. Acad. Sci. U. S. A. 98:15245–15250. 10.1073/pnas.261418798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gast CE, Basso LR, Jr, Bruzual I, Wong B. 2013. Azole resistance in Cryptococcus gattii from the Pacific Northwest: investigation of the role of ERG11. Antimicrob. Agents Chemother. 57:5478–5485. 10.1128/AAC.02287-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wellington M, Dolan K, Krysan DJ. 2009. Live Candida albicans suppresses production of reactive oxygen species in phagocytes. Infect. Immun. 77:405–413. 10.1128/IAI.00860-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eisenman HC, Frases S, Nicola AM, Rodrigues ML, Casadevall A. 2009. Vesicle-associated melanization in Cryptococcus neoformans. Microbiology 155:3860–3867. 10.1099/mic.0.032854-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodrigues ML, Nakayasu ES, Oliveira DL, Nimrichter L, Nosanchuk JD, Almeida IC, Casadevall A. 2008. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot. Cell 7:58–67. 10.1128/EC.00370-07 [DOI] [PMC free article] [PubMed] [Google Scholar]