Abstract
Streptococcal cysteine protease (SpeB), the major secreted protease produced by group A streptococcus (GAS), cleaves both host and bacterial proteins and contributes importantly to the pathogenesis of invasive GAS infections. Modulation of SpeB expression and/or its activity during invasive GAS infections has been shown to affect bacterial virulence and infection severity. Expression of SpeB is regulated by the GAS CovR-CovS two-component regulatory system, and we demonstrated that bacteria with mutations in the CovR-CovS two-component regulatory system are selected for during localized GAS infections and that these bacteria lack SpeB expression and exhibit a hypervirulent phenotype. Additionally, in a separate study, we showed that expression of SpeB can also be modulated by human transferrin- and/or lactoferrin-mediated iron chelation. Accordingly, the goal of this study was to investigate the possible roles of iron and other metals in modulating SpeB expression and/or activity in a manner that would potentiate bacterial virulence. Here, we report that the divalent metals zinc and copper inhibit SpeB activity at the posttranslational level. Utilizing online metal-binding site prediction servers, we identified two putative metal-binding sites in SpeB, one of which involves the catalytic-dyad residues 47Cys and 195His. Based on our findings, we propose that zinc and/or copper availability in the bacterial microenvironment can modulate the proteolytic activity of SpeB in a manner that preserves the integrity of several other virulence factors essential for bacterial survival and dissemination within the host and thereby may exacerbate the severity of invasive GAS infections.
INTRODUCTION
Streptococcus pyogenes, or group A streptococcus (GAS), bacteria can be found on human skin and in the throat, usually causing noninvasive, mild infections, namely, strep throat, cellulitis, erysipelas, and impetigo. Occasionally, however, these bacteria are transformed to the point where they become highly pathogenic and invasive, gaining access to normally sterile sites within the infected host, including blood, muscle, deep tissues, and lungs, and causing severe, life-threatening complications associated with severe and often deadly diseases (1–10). Two of the severe but least common life-threatening infections are necrotizing fasciitis (NF) and streptococcal toxic shock syndrome (STSS) (11, 12). NF is a severe skin infection that results in destruction of muscle, fat, and skin tissues. These severe manifestations have led to the designation of GAS as the “flesh-eating bacterium.” STSS results from uncontrolled and deregulated inflammatory responses that cause rapid loss of blood pressure accompanied by multiple-organ failure, including lungs, liver, and kidneys. Annually, 700 million nonsevere cases are reported worldwide, and approximately 0.1% of these cases emerge as severe infections (13). The various manifestations of GAS infections are mediated by several modes of pathogenic adaptation, where bacteria that are adapted to evade host immune defenses are enriched for, and these successfully colonize and survive in the host and are transmitted to infect another host. We have shown that host factors and host-pathogen interactions also play a pivotal role in modulating and potentiating the severity and manifestations of invasive GAS infections (12, 14–17).
Streptococcal cysteine protease, also known as streptopain or streptococcal pyrogenic exotoxin B (SpeB), is one of the major secreted virulence factors produced by GAS; it is initially synthesized and secreted as a 42-kDa zymogen, which is then self-processed to the 28-kDa mature active form of the protease (18–22). The role of SpeB in GAS infections has been widely studied, yet its contribution to the pathogenesis of invasive GAS infections remains a subject of investigation in several laboratories, and we have shown SpeB to be negatively regulated during severe invasive infections and that active SpeB expression is inversely related to disease severity and outcomes (23–25). However, others have shown that SpeB expression is essential during skin infections (26). SpeB has also been shown to play a role in dispersing the biofilm community, thereby mediating a transition from mild to severe disease states (27, 28). To further understand the variable role of SpeB in GAS infection, we developed a mouse cage model of localized GAS infection in which we found SpeB expression to be irreversibly muted once the bacteria became invasive (25). Loss of SpeB expression spares several bacterial virulence factors from being degraded by this potent protease, thereby promoting a hypervirulent state of the bacteria and leading to very severe and morbid disease outcomes (23, 25).
Subsequent studies from our laboratory to identify key host regulatory factors involved in variable SpeB expression have established a role for human transferrin and lactoferrin mediated by the iron saturation status of these proteins (29). Together, these findings underscored the significance of host-mediated pressure on pathogens to select for those that have acquired novel genes and/or those that can preserve their key virulence factors from being degraded in certain host niches so that they can survive inside the host. The main goal of the present study was to test the hypothesis that host metals may be involved in regulating SpeB expression and/or its activity once the pathogen becomes invasive. We report that zinc inhibits the proteolytic activity of SpeB and that chelating zinc can reverse this inhibition. In trying to identify if any other metals can replicate this inhibitory mechanism, we discovered that copper alone mimics zinc in its ability to inhibit SpeB proteolytic activity. Utilizing online metal-binding site prediction servers, we discovered that SpeB has two putative binding sites for both zinc and copper. Metal-mediated inhibition of SpeB proteolytic activity is a hitherto unrecognized molecular process.
In summary, we have identified a novel molecular mechanism mediating tight control of SpeB activity, a process that is indispensable for maintaining the integrity of several GAS virulence factors. We believe this finding will provide further molecular insights into the preferential selection of SpeB-negative bacteria inside the host.
MATERIALS AND METHODS
Bacteria and culture media.
An invasive GAS isolate, 5448WT (30) (representative of the clonal M1T1 strain), and its isogenic animal-passaged, hypervirulent, SpeB-negative variant 5448AP (25) were used in this study and have been described previously. The bacteria were routinely grown at 37°C in THY medium (Todd-Hewitt broth [Difco] supplemented with 1.5% [wt/vol] yeast extract) without shaking. Sheep blood agar (Becton Dickenson, Franklin Lakes, NJ) was used as a solid medium to test the purity of the cultures.
Reagents.
All the reagents—N-[N-(l-3-trans-carboxyox-irane-2-carbonyl)-l-leucyl]-agmatine (E64), sodium dodecyl sulfate (SDS), high-performance liquid chromatography (HPLC) grade water, methanol, diethylenetriaminepentaacetic acid (DTPA), calcium chloride (CaCl2), magnesium chloride (MgCl2), manganese chloride (MnCl2), zinc sulfate (ZnSO4), cobalt chloride (CoCl2), copper(II) chloride (CuCl2), iron(II) sulfate (FeSO4), iron(III) citrate (FeC6H5O7), and dithiothreitol (DTT)—were bought from Sigma-Aldrich, St. Louis, MO.
Assessment of SpeB production and its activity during growth in the presence of metals.
To assess the roles of transition metals in SpeB production and its proteolytic activity, a preinoculum of GAS isolate 5448WT grown in THY medium for 6 h was added to THY medium supplemented with 1 mM the indicated transition metal. After 18 h of incubation, samples were centrifuged at 2,500 × g for 10 min, and the supernatants were filtered through 0.2-μm filters. The filtered supernatants were then concentrated using 10-kDa centrifugal-filter devices (Ultracel; Amicon, EMD Millipore, Billerica, MA) and analyzed by SDS-PAGE to visualize the secreted proteins using Coomassie staining. Further, aliquots were removed for measuring SpeB proteolytic activity using an EnzChek protease assay kit (Molecular Probes Inc., Eugene, OR) via the hydrolysis of a casein derivative labeled with a green fluorescent dye, Bodipy FL, as instructed by the manufacturer with slight modifications. Briefly, the prepared samples were serially diluted in Tris buffer (pH 7.8), and 100 μl of each dilution was added to the microplate wells. Wells containing samples grown in the presence of E64, a cysteine protease-specific inhibitor (final concentration, ∼28 μM), served as a control for specificity. A 100-μl aliquot of the fluorescent substrate was added to each well; the plates were incubated, according to the manufacturer's instructions, at room temperature for 24 h in the dark; and fluorescence was measured at excitation and emission wavelengths of 485 and 530 nm, respectively. The protease activity obtained with GAS isolate 5448WT grown in THY medium without any supplements was considered the 100% maximum SpeB proteolytic activity.
Proteolytic activity of SpeB in the presence of metals.
To test the abilities of various transition metals to modulate the proteolytic activity of SpeB, GAS isolate 5448WT was grown at 37°C in THY medium with or without addition of E-64 for 18 h, after which the sample was centrifuged at 2,500 × g for 10 min and the supernatants were filtered through 0.2-μm filters. The filtered supernatants were concentrated using 10-kDa centrifugal filter devices (Ultracel; Amicon, EMD Millipore, Billerica, MA). The concentrated supernatant samples were then mixed with (i) no supplements; (ii) individual transition metals alone, (iii) individual transition metals plus DTPA (a divalent metal chelator), and (iv) DTPA alone at appropriate concentrations, as discussed below. These samples were then used for measuring SpeB proteolytic activity using an EnzChek protease assay kit (Molecular Probes Inc., Eugene, OR) as discussed above.
SEC-ICP mass spectrometry (MS) analysis.
The size exclusion chromatography (SEC) separations of samples were carried out in a TSK Gel 3000SW 7.5- by 300-mm SEC column (Tosoh Bioscience, Germany). The mobile phase used was 50 mM ammonium acetate, pH 7, at 0.6 ml · min−1. As the elemental detector, an Agilent 7700x inductively coupled plasma (ICP) mass spectrometer equipped with a plasma frequency-matching RF generator and an octopole collision/reaction system (ORS) coupled with an Agilent 1100 LC series HPLC system, equipped with a binary pump, vacuum membrane degasser, thermostated auto sampler, column oven, and diode array detector with a semimicroflow UV-visible-light (Vis) cell, was used for all chromatographic analysis (Agilent Technologies, Santa Clara, CA). The entire system was controlled using Chemstation software (Agilent Technologies, Santa Clara, CA). Filtered samples (50 μl) were introduced into the HPLC system using the TSK 3000SW column with a precolumn 0.45-μm inline filter. The instrument operating conditions were as follows: forward power, 1,500 W; carrier gas flow rate, 1.1 liter · min−1; make-up gas flow rate, 0.10 liter · min−1; nickel sampling and skimmer cones, collision/reaction cell gas, He at 4 ml · min−1. The isotopes 24Mg, 44Ca, 55Mn, 66Zn, and 63Cu were monitored.
Proteomics.
An Agilent 6300 series HPLC-Chip-electrospray ionization (ESI)-ion trap XCT system (Agilent Technologies, Santa Clara, CA) coupled to an Agilent model 1200 LC was used to carry out protein identifications on the collected SEC-ICP MS fractions. The system uses a capillary binary pump for loading the sample into a microfluidic HPLC-Chip column and a nanoflow binary pump to provide the analytical flow for the reverse-phase (RP) separation on a Zorbax SB 300A C18 column (Agilent Technologies, Santa Clara, CA). Peptide and corresponding protein identifications were conducted with the MASCOT server (Matrix Science).
Expression and purification of rSpeB.
The structural gene of SpeB zymogen was amplified by PCR from genomic DNA of 5448WT without the N-terminal signal sequence (residues 1 to 27) and then cloned into the pET-28a vector. The primer pair used for the PCR was 5′-CGCGGATCCGATCAAAACTTTGCTCGTAAC-3′ (forward) and 5′-CCGCTCGAGAGGTTTGATGCCTACAAC-3′ (reverse). The reverse primer was designed in such a way that recombinant SpeB (rSpeB), when expressed, would harbor a C-terminal His6 tag fusion (the additional amino acids for the affinity tag were LEH6). The recombinant plasmid was transformed into the Escherichia coli BL21(DE3)(pLyS) strain, and the system was inducibly expressed under the control of a strong T7 promoter. Recombinant SpeB was produced by growing cells at 37°C in Luria-Bertani medium for 24 h and purified by Ni2+-chelating chromatography (Pharmacia Biotech). The protein was then concentrated by Amicon ultrafiltration using a 10-kDa-cutoff membrane and exchanged with phosphate-buffered saline (PBS) buffer.
Protein cleavage of rSmeZ by rSpeB.
Recombinant streptococcal mitogenic exotoxin Z (rSmeZ) (200 μg/ml) was incubated with rSpeB (20 μg/ml) in the presence or absence of zinc and/or DTPA at 37°C overnight. The cysteine protease inhibitor E-64 (final concentration, 28 μM) was used to stop the reaction. The samples were frozen at −20°C until further analysis. To check the specificity of the SpeB proteolytic activity, we also included a control whose activity was inhibited by adding E-64 at the start of incubation. The digestion mixtures (rSmeZ with or without rSpeB and/or zinc/DTPA) were separated by SDS-PAGE and transferred onto nitrocellulose paper. We analyzed the proteolytic degradation of rSmeZ following incubation with rSpeB by Western blotting using specific anti-SmeZ antibody as we described previously (31).
Binding site prediction analysis.
We used several online prediction servers, like FINDSITE (32, 33) (http://cssb.biology.gatech.edu/findsite-metal), SeqCHED (34) (http://ligin1.weizmann.ac.il/∼ronenle/Web/SeqCHED), CHED (35) (http://ligin1.weizmann.ac.il/∼lpgerzon/mbs4/mbs.cgi), and FEATURE metal scanning (36) (http://feature.stanford.edu/metals), to predict the binding sites for transition metals. We used 2UZJ as the Protein Data Bank (PDB) model for SpeB. The structure models were generated using MacPyMOL (PyMOL Molecular Graphics System, version 1.5.0.4; Schrödinger, LLC).
Statistical analysis.
Two-way analysis of variance (ANOVA) was used to evaluate differences in SpeB proteolytic activities in the presence of different metal groups and between DTPA treatments. Bonferroni posttests were used after two-way ANOVA where appropriate. A sigmoidal dose-response regression curve was used to fit the zinc-mediated proteolytic inhibition. Statistical analyses were performed using Prism v5.0d for Mac (GraphPad Software, La Jolla, CA) and SigmaPlot v12.3 for Windows (Systat Software, San Jose, CA). The critical significance value (α) was set at 0.05, and if the P values were less than α, we rejected the null hypothesis and reported that the differences observed had a low probability of occurring by chance and were thus significant.
RESULTS
Zinc exposure blocks SpeB maturation and activity in GAS bacteria.
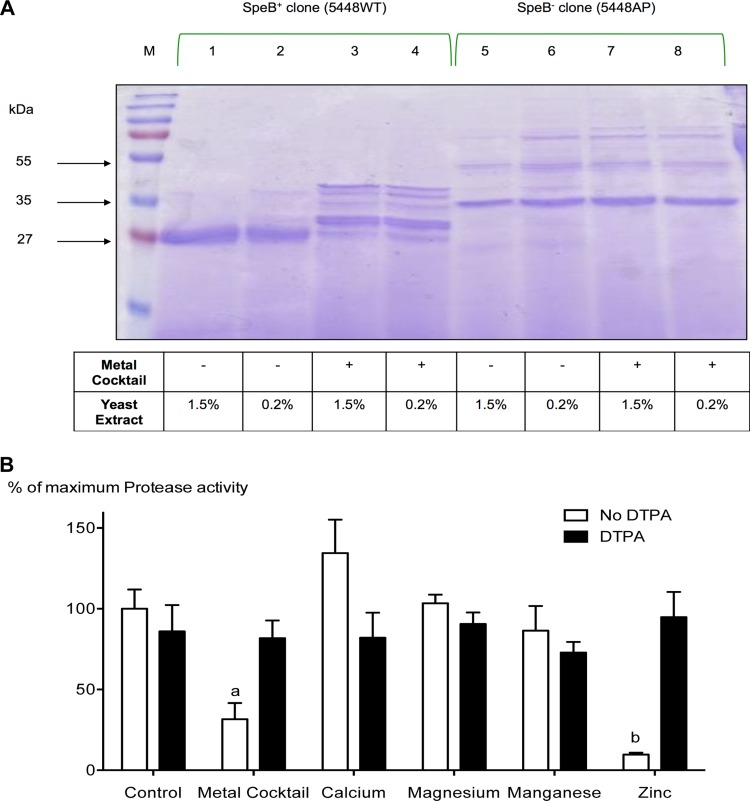
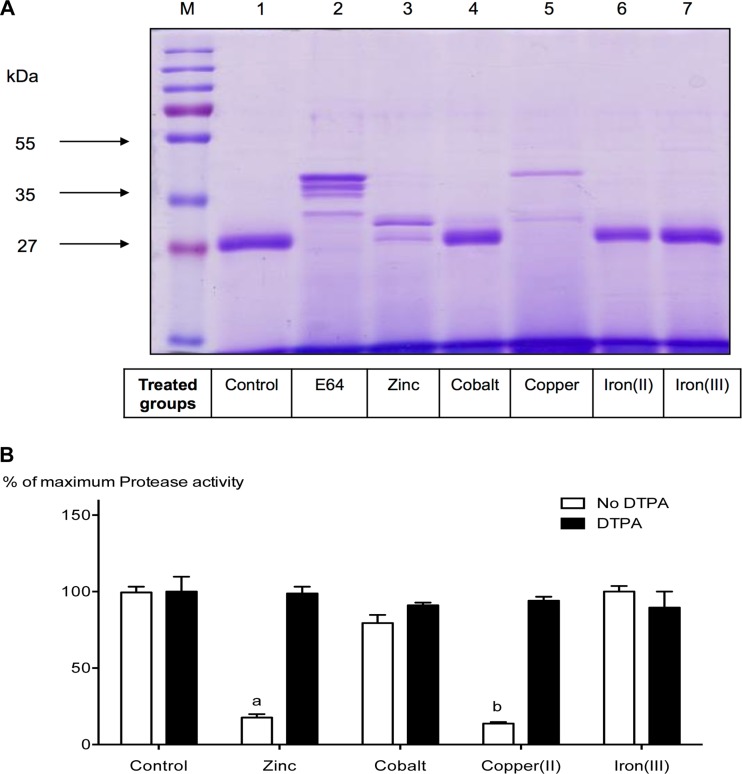
The ability of transition metals to modulate the expression and activity of the cysteine protease SpeB has not been reported so far. However, we have previously shown that transferrin- and lactoferrin-mediated iron depletion inhibits SpeB expression significantly (29). To assess the effects of transition metals on SpeB production and/or its proteolytic activity, a SpeB-positive bacterial isolate (5448WT) and its in vivo-selected isogenic SpeB-negative variant (5448AP) were grown under metal-supplemented conditions as detailed in Materials and Methods, and the secreted proteins were visualized using SDS-PAGE. As a control, yeast extract (YE) was used at two different concentrations (0.2% and 1.5%), since it contained significant amounts of transition metals (37). As shown in Fig. 1A, we observed notable differences in the secreted-protein profiles of 5448WT bacteria grown in media supplemented with a cocktail of transition metals (CaCl2, MgCl2, MnCl2, and ZnSO4) versus bacteria grown without metal supplementation. Irrespective of the yeast extract concentrations, 5448WT bacteria grown in the absence of added metal supplements predominantly secreted the active 28-kDa mature SpeB (Fig. 1A, lanes 1 and 2). In contrast, when the same bacteria were grown in metal-supplemented media, a minimal amount of the mature 28-kDa SpeB was detected. Instead, there were multiple bands representing the SpeB zymogen, along with several immature processed forms of the protease (Fig. 1A, lanes 3 and 4). As expected, SpeB was not detected from 5448AP bacteria irrespective of the metal content of the medium (Fig. 1A, lanes 5 to 8).
FIG 1.
Zinc inhibits SpeB maturation and its activity during growth, and chelating zinc reverses this inhibition. (A) GAS isolate 5448WT was grown in the presence or absence of 1 mM (final concentration) the metal cocktail CaCl2, MgCl2, MnCl2, and ZnSO4. The secreted proteins were separated by SDS-PAGE and visualized using Coomassie staining (lanes 1 to 4). Strain 5448AP, an isogenic mutant that lacks the ability to produce SpeB, was grown under the same conditions and used as a negative control (lanes 5 to 8). Since yeast extract contains appreciable amounts of transition metals (37), we grew both the SpeB-negative (5448AP) and SpeB-positive (5448WT) isolates at 0.2% (even-numbered lanes) or 1.5% (odd-numbered lanes). Irrespective of the yeast extract concentration, there was a notable difference in the secreted-protein profiles of strain 5448WT grown in control medium (lanes 1 and 2) versus medium containing metal supplements (lanes 3 and 4). Lanes 5 to 8, which contain the SpeB-negative control, showed no significant difference in their secreted-protein profiles. (B) GAS isolate 5448WT was grown in the presence of 1 mM individual metal (CaCl2, MgCl2, MnCl2, or ZnSO4) or a cocktail of the metals, with and without the addition of the metal chelator DTPA. After 18 h in culture, the culture supernatants were harvested, concentrated, and used to measure the cysteine protease activity of SpeB using an EnzChek assay, as described in Materials and Methods. The data presented are means and standard deviations (SD) (n = 4). The experiments were repeated four times in triplicate, and each data point represents the percentage of maximum protease activity at 1 mM the specified metal and/or DTPA. The data were normalized to the total protease activity obtained in the absence of metals or chelator (control group). Two-way ANOVA analyses revealed significant interaction (P < 0.001) between bacterial cultures containing different metal groups and their DTPA treatments. a and b indicate significant differences (P < 0.001) within treatments (with and without DTPA) and between cultures containing different metal groups and the specified group by Bonferroni post hoc analysis.
Measurement of the proteolytic activities of the various secreted forms of SpeB from the above-mentioned culture supernatants showed that supernatants recovered from bacteria (5448WT) grown in the presence of a cocktail of metal supplements (CaCl2, MgCl2, MnCl2, and ZnSO4) had significantly reduced proteolytic activity (P < 0.001) (Fig. 1B). Further studies to identify a specific metal(s) responsible for this phenomenon revealed that only zinc was both essential and sufficient to inhibit the proteolytic activity of SpeB (Fig. 1B). Further confirmation came from studies in which addition of the metal chelator DTPA to the cultures restored the proteolytic activity of SpeB (Fig. 1B). Collectively, the above data show that, among the metals tested, the proteolytic activity of SpeB can be modulated by zinc, which blocks the maturation of the SpeB zymogen and thereby the proteolytic degradation of proteins by SpeB.
Zinc inhibits the proteolytic activity of mature SpeB.
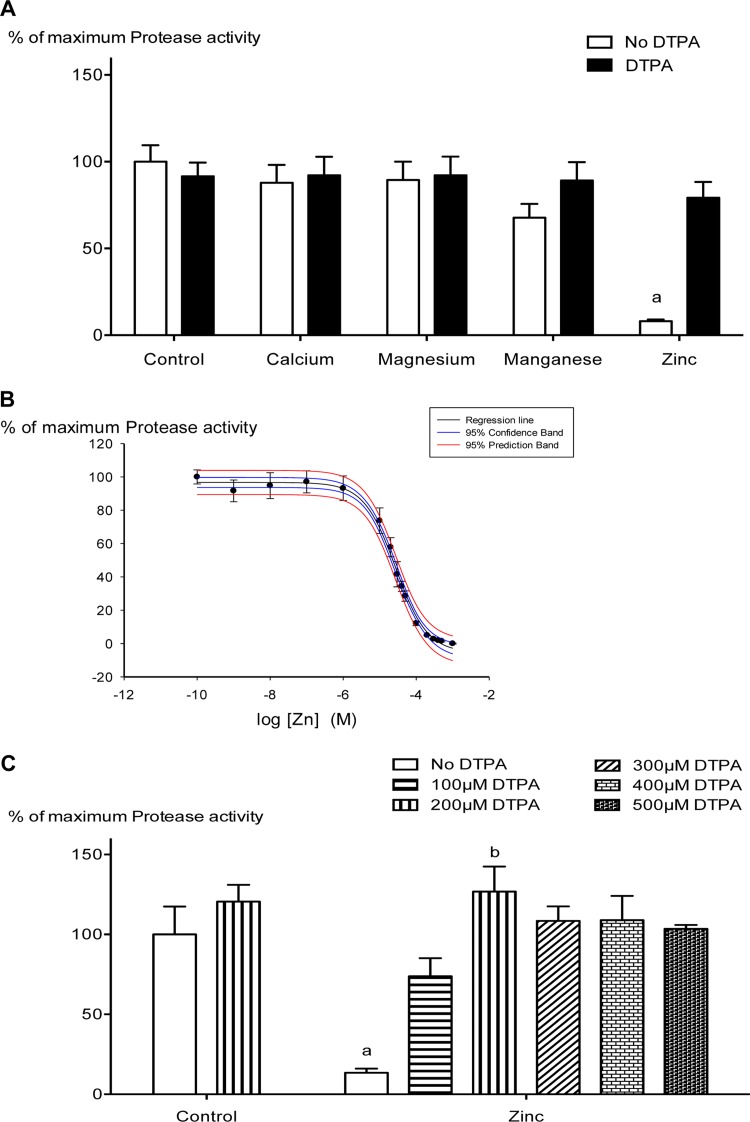
When culture supernatants recovered from 5448WT bacteria grown without any added metal supplement were mixed with individual metals, we observed that zinc was able to inhibit the proteolytic activity of mature SpeB (Fig. 2A), indicating that, in addition to blocking the maturation of SpeB into its active form, zinc also blocks the proteolytic activity of the mature form of SpeB. A dose-response curve for zinc-mediated inhibition revealed a 50% inhibitory concentration (IC50) of 27.29 ± 1.08 μM (Fig. 2B). To ensure that these effects were directly related to zinc, additional experiments were performed in which zinc was added at 10 times its IC50 in the absence or presence of increasing amounts of the metal chelator DTPA. As shown in Fig. 2C, addition of DTPA effectively reversed the ability of zinc to inhibit SpeB proteolytic activity. Together, the above data support an important role for zinc in modulating SpeB activity both before and after SpeB maturation to a potent protease.
FIG 2.
Zinc specifically inhibits SpeB activity at a posttranslational level, and a zinc chelator reverses this process. (A) Concentrated supernatant samples from 5448WT grown in the absence of metals and/or chelator were mixed with 200 μM (final concentration) each of the metals CaCl2, MgCl2, MnCl2, and ZnSO4 in the presence or absence of DTPA. Cysteine protease activity of SpeB was detected using a fluorescent-enzyme assay as described in Materials and Methods. The data presented are means and SD (n = 4; experiments were repeated four times in triplicate), and each data point represents the percentage of maximum protease activity at 200 μM the specified metal and/or DTPA. The data were normalized to the total protease activity obtained in the absence of metals or chelator (control group). Two-way ANOVA analyses revealed significant interaction (P < 0.001) between bacterial cultures containing different metal groups and their DTPA treatments. a indicates a significant difference (P < 0.001) within treatments (with and without DTPA) and between cultures containing different metal groups and the specified group by Bonferroni post hoc analysis. (B) Analysis of protease activity in the presence of increasing concentrations of ZnSO4. The data are means ± standard errors (SE) (n = 4; experiments were repeated four times in triplicate). Ligand binding and a sigmoidal dose-response regression curve fit were used to fit the line (R2 = 0.9950; P < 0.001), and we obtained an IC50 of 27.29 ± 1.08 μM (P < 0.001) for zinc-mediated SpeB inhibition. (C) DTPA can reverse the protease inhibition of SpeB by chelating ZnSO4 (200 μM) when added in increasing concentrations. The data are means and SD (n = 3; experiments were repeated three times in triplicate), and two-way ANOVA revealed an interaction (P < 0.001) between zinc and DTPA. a indicates a significant difference (P < 0.001) within treatments (increasing DTPA concentrations) and between the zinc-treated and control groups, and b indicates a significant difference (P < 0.001) between 200 μM DTPA-treated culture and no treatment (No DTPA) within the zinc group (Bonferroni post hoc analyses).
Zinc mediates inhibition of SpeB proteolytic activity by directly binding to the mature regions of SpeB and not to SpeB propeptide regions.
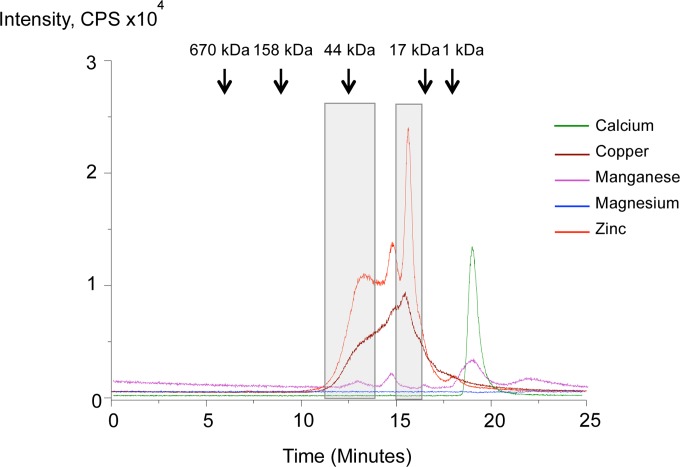
As mentioned above, the enzymatically inactive 42-kDa zymogen form of SpeB is initially secreted by GAS, from which the propeptide region (the first 145 amino acid residues, including the signal peptide) is then self-processed in a stepwise manner involving several intermediate forms to finally create the mature 28-kDa active form of the protease harboring the catalytic dyad, 47Cys and 195His (18–20). To narrow down the regions of SpeB (the propeptide or the mature sequence) essential for zinc binding, we used SEC-ICP MS, where supernatants from bacterial (5448WT) culture mixed with a cocktail of metal supplements, as detailed in Materials and Methods, are separated by SEC, and each fraction was subjected to ICP MS analysis to identify zinc-bound proteins. As shown in Fig. 3, the first zinc-related signal was detected at around 13 min, corresponding to a mass of ∼42 kDa. This was followed by a series of other zinc-related signals. To identify the detected zinc-bound proteins, two fractions of ∼42 kDa and 28 kDa, corresponding, respectively, to the zymogen and mature forms of SpeB, were collected (Fig. 3) for further analyses of proteins by LC-tandem MS (MS-MS).
FIG 3.
Zinc specifically binds to SpeB irrespective of the propeptide region. Shown are the results of SEC followed by ICP MS analyses of zinc (or copper)-bound proteins from bacterial (5448WT) supernatants mixed with 1 mM (final concentration) the metal cocktail CaCl2, MgCl2, MnCl2, CuSO4, and ZnSO4. Molecular mass markers are shown at the top, and the two regions (∼42 and 28 kDa) collected for identifications are boxed. The two regions were found to be the zymogen and the mature forms of SpeB through LC–MS-MS analysis, as shown in Fig. S1 and S2 in the supplemental material.
As shown in Fig. S1 and detailed in the supplemental material, tryptic digests of the collected fractions were analyzed by LC–MS-MS to obtain the full-scan, total ion chromatogram, from which individual peptides were extracted and analyzed further. The identity of each extracted peptide was elucidated using the MASCOT search engine, where the MS-MS spectra were evaluated and compared with the theoretical fragmentation pattern shown in Fig. S2 in the supplemental material. Following LC–MS-MS analyses, one peptide (ENIASFMESYVEQIK) was assigned to the first fraction (42 kDa), corresponding to the SpeB zymogen, whereas two peptides (VGGHAFVIDGADGR and ELSQNQPVYYQGVGK) were assigned to the second fraction (28 kDa), corresponding to the mature, active form of SpeB. These findings were further confirmed through MASCOT search engine analyses, which identified all the peptides as derived from SpeB. The above results confirmed the binding of zinc to mature regions of SpeB and revealed that the propeptide region was dispensable for this binding to occur.
Zinc orchestrates SpeB-mediated SmeZ degradation.
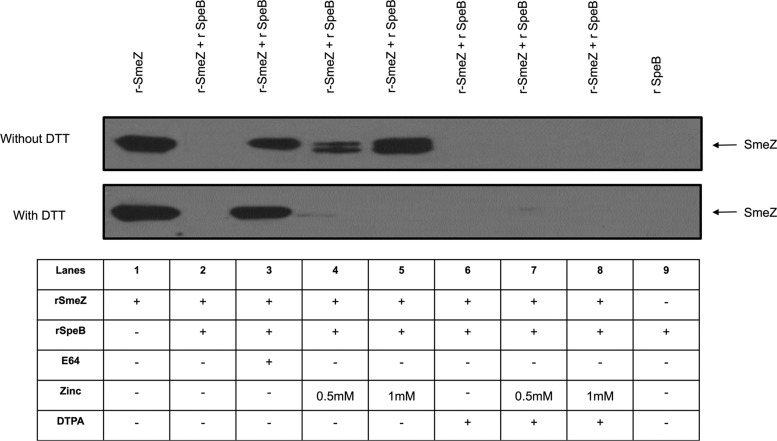
GAS harbor genes encoding several superantigens, including SpeA, SpeF, SpeG, SpeH, and SpeJ, as well as the potent streptococcal mitogenic superantigen SmeZ, which we had found to be highly susceptible to degradation by SpeB, among other GAS superantigens (31). Accordingly, we investigated if zinc and/or DTPA affects the SpeB-mediated degradation of SmeZ. We observed that zinc completely inhibited SpeB's ability to degrade SmeZ, and this inhibition was reversed by the addition of the metal chelator DTPA (Fig. 4, top).
FIG 4.
Zinc inhibits SpeB-mediated proteolytic degradation of the superantigen SmeZ. rSmeZ (200 μg/ml) was mixed with 20 μg/ml of rSpeB and incubated overnight in the presence or absence of zinc and/or DTPA. The proteins in these mixtures were resolved on SDS-12% polyacrylamide gels, and the presence of intact rSmeZ protein was monitored by Western blotting using SmeZ-specific antibodies. (Top) SpeB-mediated complete degradation of SmeZ (lane 2) is blocked by addition of the protease inhibitor E64 (lane 3). As shown in lanes 4 and 5, SpeB-mediated degradation of SmeZ was inhibited at two different concentrations of zinc (0.5 and 1 mM, respectively), and 1 mM DTPA addition reversed this inhibition (lanes 7 and 8). Lanes 1 and 9 were controls for SmeZ antibody specificity. Lane 6 contained DTPA alone as a control. (Bottom) The same experiment was repeated in the presence of 5 mM DTT (reducing agent), and the zinc-mediated inhibition was not observed (lanes 4 and 5), indicating that the ability of zinc to bind SpeB was blocked in a reducing environment.
To further investigate if the binding of zinc to SpeB could be modulated in the presence of reducing agents that block interactions between metal cations and polar amino acid residues, we added the reducing agent DTT to duplicate mixtures of SmeZ with or without SpeB in the presence or absence of added zinc and/or DTPA. We observed that in the presence of DTT, addition of zinc did not block SpeB-mediated degradation of SmeZ (Fig. 4, bottom), suggesting that, in a reducing environment, zinc was unable to bind SpeB and, accordingly, could not inhibit its proteolytic activity. Collectively, the above data prompted us to hypothesize that the binding of zinc to the catalytic dyad (47Cys and 195His) may inhibit the proteolytic activity of SpeB.
Copper mimics zinc in inhibiting SpeB.
To examine the abilities of other transition metals to regulate SpeB expression and/or its activity, we grew the bacteria in the presence of zinc, cobalt, copper, and iron and then collected the culture supernatants, filtered and concentrated them as detailed in Materials and Methods, and analyzed the secreted proteins by SDS-PAGE. As a negative control for SpeB maturation, we also grew the bacteria in the presence of E64 (a protease inhibitor) (Fig. 5A, lane 2). As expected, zinc-treated culture supernatants had minimal amounts of the mature 28-kDa form of SpeB, and a similar effect was observed in the presence of added copper, but not in the presence of any of the other tested metals (Fig. 5A, lanes 3 to 7). In this respect, it was of interest to determine if copper can inhibit the protease activity of SpeB at a postmaturation level, similarly to what we have observed with zinc. SpeB-positive bacteria (5448WT) were grown in the absence of added metal supplements, and the culture supernatants were collected and concentrated. To these concentrated supernatants we added individual metals (zinc, cobalt, copper, or iron) and then measured the proteolytic activity of SpeB. As shown in Fig. 5B, addition of copper inhibited the activity of the mature form of SpeB, and this inhibition was reversed by the addition of DTPA to chelate the copper (Fig. 5B). Further, SEC-ICP MS followed by LC–MS-MS analyses of bacterial supernatants revealed that copper, similarly to zinc, was able to bind the mature regions of SpeB, and in this case, too, the propeptide sequences were not essential for the binding (Fig. 3). These data indicate that copper, like zinc, inhibits the proteolytic activity of SpeB by binding to the mature regions of the protease. We also predict that, similarly to zinc, copper also binds to the catalytic dyad (47Cys and 195His) located within the mature regions of SpeB.
FIG 5.
Copper mimics zinc in its ability to inhibit SpeB proteolytic activity. (A) GAS isolate 5448WT was grown in the presence of 1 mM (final concentration) the specified metal (ZnSO4, CoCl2, FeSO4, or FeC6H5O7) or 500 μM (final concentration) CuCl2. The secreted proteins were separated by SDS-PAGE and visualized using Coomassie staining (lanes 3 to 7). Bacteria grown without any metal supplements (control group) secreted mature 28-kDa SpeB (lane 1). Addition of the protease inhibitor E64 blocked the maturation of SpeB (lane 2). ZnSO4-treated culture supernatants (lane 3) showed a notable difference in their secreted-protein profiles, since ZnSO4 was expected to inhibit SpeB activity. While CuCl2 (lane 5) was the only metal other than Zn able to block SpeB predominantly in its immature zymogen form, other metal treatments (lanes 4, 6, and 7) showed no significant difference compared with the control group (lane 1). (B) Concentrated 5448WT supernatant samples, grown in the absence of metals and/or chelator, were mixed with 200 μM (final concentration) of the metal ZnSO4, CoCl2, CuCl2, or FeC6H5O7 in the presence or absence of DTPA at room temperature. Cysteine protease activity of SpeB was detected by using a fluorescent-enzyme assay as described in Materials and Methods. The data are means and SD (n = 3; experiments were repeated three times in triplicate), and each data point represents the percentage of maximum protease activity at 200 μM the specified metal and/or DTPA. The data were normalized to total protease activity obtained in the absence of metals or chelator (control group). Two-way ANOVA analyses revealed significant interaction (P < 0.001) between bacterial cultures containing different metal groups and their DTPA treatments. a and b indicate significant differences (P < 0.001) within treatments (with and without DTPA) and between cultures containing different metal groups and the specified group by Bonferroni post hoc analysis.
SpeB metal-binding site predictions.
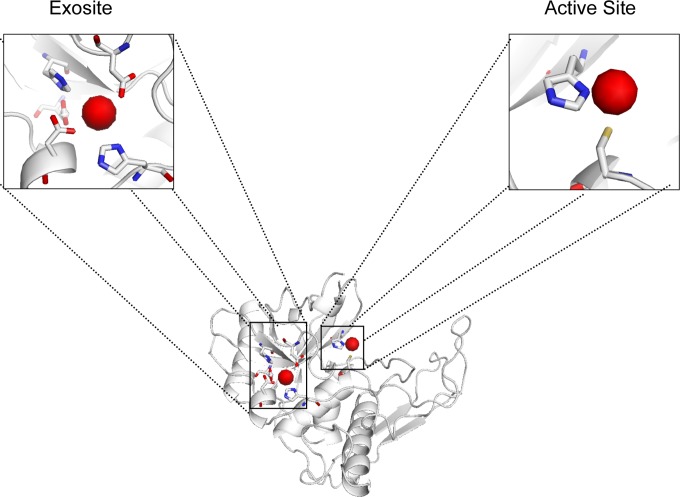
Using 2UZJ (38) as the PDB model for SpeB and the online prediction server FINDSITE (32, 33), we were able to identify potential metal-binding sites for the mature form of SpeB. The server predicted that zinc could potentially bind to SpeB, coordinated by the two active-site residues, namely, 47Cys and 195His (Fig. 6). To screen for any other potential metal-binding sites that may be involved in coordinating zinc and/or other transition metals, we used two other online metal-binding site prediction servers, SeqCHED (34) and CHED (35), which predict sites for any of the transition metals (Zn, Fe, Mn, Cu, Ni, Co, Ca, and Mg) with no specificity for any individual metals. By setting stringent filters, we found a second metal-binding site (exosite) adjacent to the active site of SpeB, which comprises the residues 9Asp, 14His, 200Asp, 209His, and 219Asp and is predicted to be involved in coordinating the transition metals (Fig. 6). We also used FEATURE metal scanning (36), which specifically predicts zinc binding sites, and again, this tool corroborated these metal-binding sites. In summary, we have identified two metal-binding sites for SpeB, both of which can accommodate zinc or copper, and we believe that this modulates SpeB activity.
FIG 6.
SpeB harbors two potential metal-binding sites. 2UZJ was used as the PDB model for SpeB. The potential metal-binding sites are shown in red. Residues involved in the binding of zinc and/or copper include 9Asp, 14His, 200Asp, 209His, and 219Asp in the exosite and 47Cys and 195His in the active site. The hydrogen atoms are colored yellow, oxygen red, and nitrogen blue.
DISCUSSION
In this study, we demonstrated the novel finding that the divalent metals zinc and copper can inhibit SpeB proteolytic activity on a posttranslation level and that chelating these metals can reverse this inhibition. The inhibition was unique to zinc and copper, inasmuch as none of the other metals tested, namely, iron, cobalt, manganese, magnesium, and calcium, had this effect on SpeB proteolytic activity. This finding is of particular clinical relevance, as we have previously shown that SmeZ, one of the potent and major superantigens produced by GAS, is highly susceptible to SpeB degradation (31), and in this study, we showed that SpeB-mediated SmeZ degradation is inhibited in the presence of the metal zinc and that this inhibition can be reversed by chelating the zinc.
Structural bioinformatics tools allowed us to investigate mechanisms by which zinc could mediate its inhibitory effects on SpeB, which, as a widely studied GAS virulence factor, has been crystallized by several groups who aimed to identify the enzyme's catalytic residues, substrate-binding site, protein folding, and maturation process (38–41). In all those cases, SpeB was crystallized either in its mature form or in the inactive zymogen form generated by replacing the active cysteine residue with serine. Using the crystal structure of the mature form of SpeB (2UZJ), which was determined by Olsen et al. (38), we were able to predict metal-binding sites in SpeB. As illustrated in Fig. 6, we predict that SpeB harbors two metal-binding sites, and we propose that both zinc and copper may bind to SpeB via either or both of these sites; however, we predict that inhibition of SpeB proteolytic activity is effected primarily by metal binding to the active site comprising the catalytic-dyad residues (47Cys and 195His). This prediction is supported by our data (Fig. 5B) showing that these metals block the proteolytic activity of the protease. Although the metals can also bind to the second site (exosite), the effect this may have on SpeB activity remains unclear. Whereas the above data are predictions derived from online tools that use multiple other protein templates to predict potential residues involved in metal binding, additional structure studies that can dock each metal within these residues are needed to help in determining the rotamers of the residues.
To our knowledge, metal-mediated regulation of SpeB proteolytic activity has not been reported thus far, and whereas Gryllos et al. reported that expression of SpeB was unaffected at elevated levels of magnesium via CovR-CovS signaling (42), it turned out that the strain used in their study was a GAS type M3 strain (DLS003) in which SpeB regulation was already CovR-CovS independent (43). Nonetheless, several other laboratories reported the regulation of other cysteine proteases by metal ions, including zinc (44–48). For example, human apoptotic caspases, a family of cysteine proteases involved in apoptosis, are a recognized model for studying zinc-mediated inhibition of proteolytic activity (44, 46, 48). These studies revealed that the proteolytic activity of caspase 6 can indeed be inhibited by direct binding of zinc to an exosite distal to the active site (48), whereas caspase 9, which harbors two zinc binding sites, namely, an active site and an exosite, was shown to be inhibited to a greater extent following the biding of zinc to the active site that harbors the catalytic dyad 287Cys and 237His (44). Similar studies done on papain, a cysteine protease derived from the papaya plant, reported that calcium and/or magnesium enhances the proteolytic activity of papain (45), whereas zinc and/or cadmium was shown to inhibit its activity (47). We can conclude that metal (especially zinc)-mediated inhibition of proteolytic activity of cysteine proteases has been a topic of thorough investigation; however, to our knowledge, metal-mediated inhibition of pathogenic bacterial proteases, and GAS in particular, is a novel mode of inhibition that we report here for the first time. Further investigations are warranted to achieve a thorough understanding of the mechanism(s) by which specific metals, namely, zinc and/or copper, may affect host-pathogen interactions.
In summary, the results presented in this study provide direct evidence for metal-mediated, reversible regulation of SpeB proteolytic activity, both before and after its maturation to the active form. This critical observation may, in part, explain the previously observed in vivo selective enrichment for bacteria that lack SpeB expression, especially in localized GAS infections (25). As discussed previously, SpeB has been shown to be essential for skin infections (26) and in biofilm dispersal (27, 28); however, once the bacteria invade to gain access to normally sterile sites, they downregulate expression of SpeB to preserve many of their important secreted virulence proteins that mediate disease pathogenesis, as these proteins are normally degraded by this potent cysteine protease. Thus, the downregulation of SpeB in host niches provides a favorable environment for the bacteria to persist and to preserve their full arsenal, thereby increasing their pathogenic potential (23, 25). Our data suggest that once the bacteria become invasive, the presence of free metals, namely, zinc and/or copper, in the bacterial microenvironment blocks SpeB proteolytic activity, thereby preserving GAS virulence factors that are normally degraded by this potent protease, enabling the pathogen to fight effectively against the host, causing severe disease and pathological complications. Alternatively, if the metals zinc and copper were available during the initial stages of infection or on the skin surface, SpeB activity would be inhibited, and this would block the ability of the bacteria to break through tissues to gain access to normally sterile sites. We propose that the modulation of SpeB activity by these metals plays an important role in pathogen invasiveness and dissemination and, accordingly, in potentiating the severity of GAS infections. Also, we propose that the spatiotemporal bioavailability of zinc and/or copper, which modulate SpeB activity, may further delineate the subsequent effects of these metals on GAS pathogenesis in specific host niches.
Whereas the main source of these metals in the host is currently unknown, we hypothesize that the metals may be released as a result of SpeB-mediated degradation of host proteins. Availability of metals at sites of infection is likely to not only modulate bacterial virulence, but also regulate host immune responses. Future in-depth studies to dissect the effects of SpeB-mediated host protein degradation and subsequent release of zinc and/or copper, as well their spatiotemporal bioavailability, on the severity and outcome of invasive GAS infections will likely inform the development of targeted therapeutic intervention and/or prevention strategies to ameliorate the severity of invasive GAS infections and the ensuing complications.
Supplementary Material
ACKNOWLEDGMENTS
We thank Agilent Technologies for instrumentation, SEC-ICP MS, and nano-HPLC-ESI-IT-MS.
This work was supported by a Merit Review Grant (M.K,) from the Department of Veterans Affairs.
We have no conflicting financial interests.
Footnotes
Published ahead of print 5 May 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01770-14.
REFERENCES
- 1.Johansson L, Thulin P, Low DE, Norrby-Teglund A. 2010. Getting under the skin: the immunopathogenesis of Streptococcus pyogenes deep tissue infections. Clin. Infect. Dis. 51:58–65. 10.1086/653116 [DOI] [PubMed] [Google Scholar]
- 2.Cone LA, Woodard DR, Schlievert PM, Tomory GS. 1987. Clinical and bacteriologic observations of a toxic shock-like syndrome due to Streptococcus pyogenes. N. Engl. J. Med. 317:146–149. 10.1056/NEJM198707163170305 [DOI] [PubMed] [Google Scholar]
- 3.Aziz RK, Kotb M. 2008. Rise and persistence of global M1T1 clone of Streptococcus pyogenes. Emerg. Infect. Dis. 14:1511–1517. 10.3201/eid1410.071660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens DL. 1992. Invasive group A streptococcus infections. Clin. Infect. Dis. 14:2–11. 10.1093/clinids/14.1.2 [DOI] [PubMed] [Google Scholar]
- 5.Cole JN, Barnett TC, Nizet V, Walker MJ. 2011. Molecular insight into invasive group A streptococcal disease. Nat. Rev. Microbiol. 9:724–736. 10.1038/nrmicro2648 [DOI] [PubMed] [Google Scholar]
- 6.Schwartz B, Facklam RR, Breiman RF. 1990. Changing epidemiology of group A streptococcal infection in the U. S. A. Lancet 336:1167–1171. 10.1016/0140-6736(90)92777-F [DOI] [PubMed] [Google Scholar]
- 7.Cunningham MW. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13:470–511. 10.1128/CMR.13.3.470-511.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Low DE, Schwartz B, McGeer A. 1998. The reemergence of severe group A streptococcal disease: an evolutionary perspective, p 93–123 In Scheld WM, Armstrong D, Hughes JM. (ed), Emerging infections 1. ASM Press, Washington, DC [Google Scholar]
- 9.Demers B, Simor AE, Vellend H, Schlievert PM, Byrne S, Jamieson F, Walmsley S, Low DE. 1993. Severe invasive group A streptococcal infections in Ontario, Canada: 1987–1991. Clin. Infect. Dis. 16:792–800. 10.1093/clind/16.6.792 [DOI] [PubMed] [Google Scholar]
- 10.Stevens DL. 1999. The flesh-eating bacterium: what's next? J. Infect. Dis. 179(Suppl 2):S366–S374. 10.1086/513851 [DOI] [PubMed] [Google Scholar]
- 11.Low DE. 2013. Toxic shock syndrome: major advances in pathogenesis, but not treatment. Crit. Care Clin. 29:651–675. 10.1016/j.ccc.2013.03.012 [DOI] [PubMed] [Google Scholar]
- 12.Kotb M, Norrby-Teglund A, McGeer A, El-Sherbini H, Dorak MT, Khurshid A, Green K, Peeples J, Wade J, Thomson G, Schwartz B, Low DE. 2002. An immunogenetic and molecular basis for differences in outcomes of invasive group A streptococcal infections. Nat. Med. 8:1398–1404. 10.1038/nm1202-800 [DOI] [PubMed] [Google Scholar]
- 13.Carapetis JR, Steer AC, Mulholland EK, Weber M. 2005. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 5:685–694. 10.1016/S1473-3099(05)70267-X [DOI] [PubMed] [Google Scholar]
- 14.Kotb M, Norrby-Teglund A, McGeer A, Green K, Low DE. 2003. Association of human leukocyte antigen with outcomes of infectious diseases: the streptococcal experience. Scand. J. Infect. Dis. 35:665–669. 10.1080/00365540310015962 [DOI] [PubMed] [Google Scholar]
- 15.Norrby-Teglund A, Chatellier S, Low DE, McGeer A, Green K, Kotb M. 2000. Host variation in cytokine responses to superantigens determine the severity of invasive group A streptococcal infection. Eur. J. Immunol. 30:3247–3255. [DOI] [PubMed] [Google Scholar]
- 16.Nooh MM, Nookala S, Kansal R, Kotb M. 2011. Individual genetic variations directly effect polarization of cytokine responses to superantigens associated with streptococcal sepsis: implications for customized patient care. J. Immunol. 186:3156–3163. 10.4049/jimmunol.1002057 [DOI] [PubMed] [Google Scholar]
- 17.Norrby-Teglund A, Kotb M. 2000. Host-microbe interactions in the pathogenesis of invasive group A streptococcal infections. J. Med. Microbiol. 49:849–852 [DOI] [PubMed] [Google Scholar]
- 18.Liu T, Elliott S. 1965. Streptococcal proteinase: the zymogen to enzyme transformation. J. Biol. Chem. 240:1138–1142 [PubMed] [Google Scholar]
- 19.Doran JD, Nomizu M, Takebe S, Menard R, Griffith D, Ziomek E. 1999. Autocatalytic processing of the streptococcal cysteine protease zymogen: processing mechanism and characterization of the autoproteolytic cleavage sites. Eur. J. Biochem. 263:145–151. 10.1046/j.1432-1327.1999.00473.x [DOI] [PubMed] [Google Scholar]
- 20.Chen CY, Luo SC, Kuo CF, Lin YS, Wu JJ, Lin MT, Liu CC, Jeng WY, Chuang WJ. 2003. Maturation processing and characterization of streptopain. J. Biol. Chem. 278:17336–17343. 10.1074/jbc.M209038200 [DOI] [PubMed] [Google Scholar]
- 21.Carroll RK, Musser JM. 2011. From transcription to activation: how group A streptococcus, the flesh-eating pathogen, regulates SpeB cysteine protease production. Mol. Microbiol. 81:588–601. 10.1111/j.1365-2958.2011.07709.x [DOI] [PubMed] [Google Scholar]
- 22.Nelson DC, Garbe J, Collin M. 2011. Cysteine proteinase SpeB from Streptococcus pyogenes: a potent modifier of immunologically important host and bacterial proteins. Biol. Chem. 392:1077–1088. 10.1515/BC.2011.208 [DOI] [PubMed] [Google Scholar]
- 23.Aziz RK, Pabst MJ, Jeng A, Kansal R, Low DE, Nizet V, Kotb M. 2004. Invasive M1T1 group A Streptococcus undergoes a phase-shift in vivo to prevent proteolytic degradation of multiple virulence factors by SpeB. Mol. Microbiol. 51:123–134. 10.1046/j.1365-2958.2003.03797.x [DOI] [PubMed] [Google Scholar]
- 24.Kansal RG, McGeer A, Low DE, Norrby-Teglund A, Kotb M. 2000. Inverse relation between disease severity and expression of the streptococcal cysteine protease, SpeB, among clonal M1T1 isolates recovered from invasive group A streptococcal infection cases. Infect. Immun. 68:6362–6369. 10.1128/IAI.68.11.6362-6369.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kazmi SU, Kansal R, Aziz RK, Hooshdaran M, Norrby-Teglund A, Low DE, Halim AB, Kotb M. 2001. Reciprocal, temporal expression of SpeA and SpeB by invasive M1T1 group A streptococcal isolates in vivo. Infect. Immun. 69:4988–4995. 10.1128/IAI.69.8.4988-4995.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lukomski S, Montgomery CA, Rurangirwa J, Geske RS, Barrish JP, Adams GJ, Musser JM. 1999. Extracellular cysteine protease produced by Streptococcus pyogenes participates in the pathogenesis of invasive skin infection and dissemination in mice. Infect. Immun. 67:1779–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Connolly KL, Roberts AL, Holder RC, Reid SD. 2011. Dispersal of Group A streptococcal biofilms by the cysteine protease SpeB leads to increased disease severity in a murine model. PLoS One 6:e18984. 10.1371/journal.pone.0018984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts AL, Connolly KL, Doern CD, Holder RC, Reid SD. 2010. Loss of the group A Streptococcus regulator Srv decreases biofilm formation in vivo in an otitis media model of infection. Infect. Immun. 78:4800–4808. 10.1128/IAI.00255-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kansal RG, Aziz RK, Kotb M. 2005. Modulation of expression of superantigens by human transferrin and lactoferrin: a novel mechanism in host-Streptococcus interactions. J. Infect. Dis. 191:2121–2129. 10.1086/430386 [DOI] [PubMed] [Google Scholar]
- 30.Chatellier S, Ihendyane N, Kansal RG, Khambaty F, Basma H, Norrby-Teglund A, Low DE, McGeer A, Kotb M. 2000. Genetic relatedness and superantigen expression in group A streptococcus serotype M1 isolates from patients with severe and nonsevere invasive diseases. Infect. Immun. 68:3523–3534. 10.1128/IAI.68.6.3523-3534.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nooh MM, Aziz RK, Kotb M, Eroshkin A, Chuang WJ, Proft T, Kansal R. 2006. Streptococcal mitogenic exotoxin, SmeZ, is the most susceptible M1T1 streptococcal superantigen to degradation by the streptococcal cysteine protease, SpeB. J. Biol. Chem. 281:35281–35288. 10.1074/jbc.M605544200 [DOI] [PubMed] [Google Scholar]
- 32.Brylinski M, Skolnick J. 2008. A threading-based method (FINDSITE) for ligand-binding site prediction and functional annotation. Proc. Natl. Acad. Sci. U. S. A. 105:129–134. 10.1073/pnas.0707684105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skolnick J, Brylinski M. 2009. FINDSITE: a combined evolution/structure-based approach to protein function prediction. Brief Bioinform. 10:378–391. 10.1093/bib/bbp017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levy R, Edelman M, Sobolev V. 2009. Prediction of 3D metal binding sites from translated gene sequences based on remote-homology templates. Proteins 76:365–374. 10.1002/prot.22352 [DOI] [PubMed] [Google Scholar]
- 35.Babor M, Gerzon S, Raveh B, Sobolev V, Edelman M. 2008. Prediction of transition metal-binding sites from apo protein structures. Proteins 70:208–217. 10.1002/prot.21587 [DOI] [PubMed] [Google Scholar]
- 36.Ebert JC, Altman RB. 2008. Robust recognition of zinc binding sites in proteins. Protein Sci. 17:54–65. 10.1110/ps.073138508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grant CL, Pramer D. 1962. Minor element composition of yeast extract. J. Bacteriol. 84:869–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olsen JG, Dagil R, Niclasen LM, Sorensen OE, Kragelund BB. 2009. Structure of the mature Streptococcal cysteine protease exotoxin mSpeB in its active dimeric form. J. Mol. Biol. 393:693–703. 10.1016/j.jmb.2009.08.046 [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez-Paez GE, Wolan DW. 2012. Ultrahigh and high resolution structures and mutational analysis of monomeric Streptococcus pyogenes SpeB reveal a functional role for the glycine-rich C-terminal loop. J. Biol. Chem. 287:24412–24426. 10.1074/jbc.M112.361576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kagawa T, Cooney J, Baker H, McSweeney S, Liu M, Gubba S, Musser J, Baker E. 2000. Crystal structure of the zymogen form of the group A Streptococcus virulence factor SpeB: an integrin-binding cysteine protease. Proc. Natl. Acad. Sci. U. S. A. 97:2235–2240. 10.1073/pnas.040549997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang C-C, Houng H-C, Chen C-L, Wang P-J, Kuo C-F, Lin Y-S, Wu J-J, Lin M, Liu C-C, Huang W, Chuang W-J. 2009. Solution structure and backbone dynamics of streptopain: insight into diverse substrate specificity. J. Biol. Chem. 284:10957–10967. 10.1074/jbc.M807624200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gryllos I, Grifantini R, Colaprico A, Jiang S, Deforce E, Hakansson A, Telford JL, Grandi G, Wessels MR. 2007. Mg(2+) signalling defines the group A streptococcal CsrRS (CovRS) regulon. Mol. Microbiol. 65:671–683. 10.1111/j.1365-2958.2007.05818.x [DOI] [PubMed] [Google Scholar]
- 43.Gryllos I, Levin JC, Wessels MR. 2003. The CsrR/CsrS two-component system of group A Streptococcus responds to environmental Mg2+. Proc. Natl. Acad. Sci. U. S. A. 100:4227–4232. 10.1073/pnas.0636231100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huber KL, Hardy JA. 2012. Mechanism of zinc-mediated inhibition of caspase-9. Protein Sci. 21:1056–1065. 10.1002/pro.2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaul P, Sathish HA, Prakash V. 2002. Effect of metal ions on structure and activity of papain from Carica papaya. Nahrung 46:2–6. [DOI] [PubMed] [Google Scholar]
- 46.Perry D, Smyth M, Stennicke H, Salvesen G, Duriez P, Poirier G, Hannun Y. 1997. Zinc is a potent inhibitor of the apoptotic protease, caspase-3. A novel target for zinc in the inhibition of apoptosis. J. Biol. Chem. 272:18530–18533 [DOI] [PubMed] [Google Scholar]
- 47.Sathish HA, Kaul P, Prakash V. 2000. Influence of metal ions on structure and catalytic activity of papain. Indian J. Biochem. Biophys. 37:18–27 [PubMed] [Google Scholar]
- 48.Velazquez-Delgado EM, Hardy JA. 2012. Zinc-mediated allosteric inhibition of caspase-6. J. Biol. Chem. 287:36000–36011. 10.1074/jbc.M112.397752 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.