Abstract
In the present study, we have investigated the evolution and impact on virulence of a 350-kb genomic duplication present in the most recently evolved members of the Mycobacterium tuberculosis East Asian lineage. In a mouse model of infection, comparing HN878 subclones HN878-27 (no duplication) and HN878-45 (with the 350-kb duplication) revealed that the latter is impaired for in vivo growth during the initial 3 weeks of infection. Furthermore, the median survival time of mice infected with isolate HN878-45 is significantly longer (77 days) than that of mice infected with HN878-27. Whole-genome sequencing of both isolates failed to reveal any mutational events other than the duplication that could account for such a substantial difference in virulence. Although we and others had previously speculated that the 350-kb duplication arose in response to some form of host-applied selective pressure (P. Domenech, G. S. Kolly, L. Leon-Solis, A. Fallow, M. B. Reed, J. Bacteriol. 192:4562–4570, 2010, and B. Weiner, J. Gomez, T. C. Victor, R. M. Warren, A. Sloutsky, B. B. Plikaytis, J. E. Posey, P. D. van Helden, N. C. Gey van Pittius, M. Koehrsen, P. Sisk, C. Stolte, J. White, S. Gagneux, B. Birren, D. Hung, M. Murray, J. Galagan, PLoS One 7:e26038, 2012), here we show that this large chromosomal amplification event is very rapidly selected within standard in vitro broth cultures in a range of isolates. Indeed, subclones harboring the duplication were detectable after just five rounds of in vitro passage. In contrast, the duplication appears to be highly unstable in vivo and is negatively selected during the later stages of infection in mice. We believe that the rapid in vitro evolution of M. tuberculosis is an underappreciated aspect of its biology that is often ignored, despite the fact that it has the potential to confound the data and conclusions arising from comparative studies of isolates at both the genotypic and phenotypic levels.
INTRODUCTION
To date, genetic variation in Mycobacterium tuberculosis, the bacterial agent primarily responsible for the human form of tuberculosis (TB), typically has been associated with the presence of either single-nucleotide polymorphisms (SNPs), deletions, or the insertion sequence IS6110 (1–4). However, growing evidence suggests that gene duplication also is prevalent and represents yet another strategy for the generation of diversity in this globally important pathogen.
Although difficult to detect due to their inherent instability and fitness cost, gene duplications are very common in nature. In bacterial populations, it is estimated that at any given time, approximately 10% of cells growing under nonselective conditions will contain a gene duplication somewhere in their genome. This serves to provide a large reservoir of genetic variation that can favor the growth of specific subpopulations of cells when exposed to various forms of selective pressure. However, in the absence of selection, they typically disappear after a few generations of growth (5). Tandem duplications arise spontaneously via recombination between short repeat sequences in the genome and are the most common form of gene duplication (6–8). Nevertheless, they are relatively unstable and can be eliminated by homologous recombination between the duplicated segments, resulting in deletion of the intervening sequences (5). In some cases, these gene duplications can be stabilized by selection. For example, gene duplications and amplifications may constitute an adaptive response to selective pressure such as growth under certain nutrient conditions or the presence of toxic compounds or antibiotics (9–13), or to compensate for a deleterious mutation (14). In this manner, duplications can act either as a solution by augmenting gene dosage or as an intermediate by providing a transient solution to allow primary expansion of the population to reach levels in which more rare and stable mutations may then occur. Alternatively, they may be a source of genetic innovation and novel biochemical function (5). In Vibrio cholerae (15) and Haemophilus influenzae (16, 17), for example, gene duplications are selected by growth in the host and serve to increase toxin or capsule production in vivo in order to enhance survival and virulence.
Relatively few examples of large chromosomal duplications have been reported in the genus Mycobacterium, including a 56-kb duplication flanked by two copies of IS1096 in M. smegmatis mc2155 (18, 19). Among members of the M. tuberculosis complex, two independent tandem duplications are present in the vaccine strain M. bovis BCG: DU1, a 29-kb duplication that includes oriC and is present only in BCG Pasteur, and DU2, a 36-kb duplication that occurs in different forms and that initially arose through a 141-kb tandem duplication followed by subsequent internal deletions (20). Leung et al. (21) have also reported the presence of additional duplications in the Tice and Birkhaug BCG strains of 22 and 30 kb, respectively. For M. tuberculosis, McEvoy et al. have reported a high rate of molecular evolution at the PPE38 locus and found that the published H37Rv sequence in this region is not representative of M. tuberculosis strains in general (22). More recently, we have identified a 350-kb genome duplication in M. tuberculosis clinical isolates that belong to the most recently evolved (“modern”) sublineages of the East Asian strain family (also referred to as W/Beijing or lineage 2) (23). This very large duplication event spans Rv3128c to Rv3427 and encompasses more than 300 genes. In total, this equates to 8% of the genome being present as two copies. The presence of IS6110 copies at either end of the duplication and also in the junction region suggests that it has evolved through unequal homologous recombination between sister chromatids. Subsequent to our study, Weiner et al. (24) reported the presence of multiple independent large-scale genomic duplications within M. tuberculosis clinical isolates belonging to lineages 2 (East Asian) and 4 (Euro-American). Interestingly, these independent duplications all overlap the same genomic region, although the presence of different boundaries suggests that they all arose through slightly different duplication events. Of the lineage 2 duplications they describe, one was identical to the 350-kb region we had previously reported. Together, these findings suggest that this particular area of the chromosome is relatively unstable and/or there is a selective advantage conferred by having two copies of genes within this region. Interestingly, the four different forms of the DU2 duplication found in BCG strains are also localized around this same part of the genome (20).
Prior to this study, the nature of the selection event(s) giving rise to the 350-kb duplication remained unresolved. Nevertheless, the original identification of the duplication within clinical isolates of the modern East Asian lineage suggested its evolution was associated with enhanced fitness in the face of specific selective pressure(s) encountered within the host. To address this question regarding its origins, in the present study we have utilized both in vivo infection (mouse) and in vitro (liquid culture) models to investigate the evolution, stability, and potential impact on virulence of this large-scale chromosomal rearrangement event.
MATERIALS AND METHODS
Chemicals, bacterial strains, and culture conditions.
All chemicals were supplied by Sigma-Aldrich, Inc., unless otherwise noted. M. tuberculosis strains were grown in Middlebrook 7H9 broth (Difco) supplemented with 10% ADC (8.1 g/liter NaCl, 50 g/liter bovine serum albumin [BSA] fraction V [Calbiochem], 20 g/liter glucose), 0.2% glycerol, and 0.05% Tween 80 or on Middlebrook 7H11 agar (Difco) supplemented with 10% OADC enrichment (ADC plus 0.6 ml/liter oleic acid, 3.6 mM NaOH). M. tuberculosis H37Rv was originally obtained from the American Type Culture Collection (ATCC 27294). The East Asian/Beijing strains HN878 and NHN5 belong to East Asian subgroups 5 and 3, respectively, and were obtained from James Musser (Methodist Hospital Research Institute, Houston, TX) (25), while G4B1.2 (group 4) was originally provided by Sebastien Gagneux and Peter Small (Institute for Systems Biology, Seattle, WA). The HN878 subclones, HN878-27 and HN878-45, were derived in our laboratory as previously described (23). Additional clinical isolates examined in this study were collected over a 6-year period (2001 to 2007) from TB patients resident on the island of Montreal and were initially provided on Lowenstein-Jensen (LJ) agar slants by the Laboratoire de Santé Publique du Quebéc (LSPQ) and scraped into 50% glycerol for storage at −80°C (26).
Nucleic acid techniques.
Deoxynucleoside triphosphates (dNTPs) and Taq polymerase were obtained from Fermentas. PCR and Southern blotting procedures were carried out according to standard protocols (27). Where necessary, 5 or 10% dimethylsulfoxide (DMSO) was also included in the PCR mixtures. Primers used in this study are shown in Table S1 in the supplemental material. For Southern blot analysis, a 1-kb dosR probe was generated by PCR using primers dosR-C and dosRrev(HindIII), while primers alr-F and alr-R were used for generating the 540-bp alr probe. Hybridization was performed using the ECL direct nucleic acid labeling and detection system (GE Healthcare) by following the manufacturer's instructions. Mycobacterial DNA was isolated using the protocol of Pelicic et al. (28).
THP-1 cell infections.
THP-1 monocytes (ATCC TIB-202) were maintained in RPMI modified medium (Wisent Biocenter) containing 10% fetal calf serum (Wisent Biocenter), 0.05 mM 2-mercaptoethanol, and 2 mM l-glutamine. Differentiation into macrophage-like cells was achieved by treatment with PMA (phorbol 12-myristate 13-acetate; 50 ng/ml) over a period of 48 h prior to infection. For infection assays, 24-well plates (Sarstedt) were seeded with 106 cells per well. M. tuberculosis strains were grown in 7H9/ADC to an optical density (OD) of 0.4 (at 600 nm). Medium was removed by centrifugation, and bacterial pellets were resuspended in RPMI modified medium. In order to remove any clumps, the inocula were spun at low speed (200 × g) for 5 min, and the resulting supernatants were passed through a 5-μm-pore-size polyvinylidene difluoride (PVDF) filter (Millex-SV) prior to enumeration of the bacteria in a Petroff-Hausser counting chamber. Infections were carried out at a cell/bacteria ratio of 2:1 for 22 h at 37°C in the presence of 5% CO2, after which time the cells were washed three times and fresh RPMI medium was added. At the specified time points, the cells were lysed with 1% Triton X-100 in PBS, and 10-fold dilutions were prepared from each well and plated onto 7H11/OADC for colony enumeration. For each infection, the starting inocula were also plated to verify that the same numbers of bacteria were added for each M. tuberculosis strain. The statistical significance of the observed differences was calculated using Student's t test (GraphPad Prism v3.0; GraphPad Software, CA).
Mouse infections.
Prior to infection, well-dispersed liquid cultures were adjusted to an OD at 600 nm (OD600) of 0.5 and stored at −70°C as 20% (vol/vol) glycerol stocks. Inocula were prepared by diluting these pretitered stocks to 4 × 106 CFU/ml in phosphate-buffered saline (PBS)-Tween 80 (0.05%). Eight-week-old B6D2/F1 mice (The Jackson Laboratory) were infected using a Lovelace nebulizer (In-Tox) for 10 min. Bacteria were enumerated at 1, 21, 35, 63, and 140 days postinfection (five mice per time point) by homogenizing the lungs and spleens of infected mice in 1 ml of 7H9 medium and plating 10-fold serial dilutions on 7H11/OADC medium containing PANTA antibiotic mixture (12 U/ml polymyxin B, 1.2 μg/ml amphotericin B, 1.2 μg/ml trimethoprim, 1.2 μg/ml azlocillin, and 4.8 μg/ml nalidixic acid; BD) for avoiding contamination of the plates. For the animal survival studies, groups of 21 mice were infected, with 5 mice/group sacrificed on days 1 and 15 postinfection to monitor the infectious dose and early bacterial replication within the lungs. The remaining 11 mice/group were allocated to the survival experiments. Survival proportions were calculated using the Kaplan-Meier method (29), and the Mann-Whitney test was used to determine the statistical significance of the observed survival differences (GraphPad Prism v3.0). All animal experiments were approved and carried out in accordance with the guidelines and regulations of the Animal Care Committee of McGill University.
Whole-genome sequencing (WGS).
Paired-end sequencing was performed at the McGill University and Génome Québec Innovation Centre using the MiSeq 250 system (Illumina). After base trimming (with a Phred-33 score of ≥30) and adaptor clipping using Trimmomatic (v0.25), reads longer than 50 bp were aligned to the reference H37Rv genome (NCBI gi:448814763; NC_000962.3). To generate complete merged binary alignment map (bam) files, a combination of the Burrows-Wheeler aligner (v0.6.2), SAM Tools (v1.8.18), and Picard (v1.82) (30, 31) were used. The average genome coverage was 91% (in reference to H37Rv) with an average read depth of 74× (Genome Analysis Toolkit [GATK] v2.7.4; Broad Institute). In parallel with a visual inspection of the resulting alignments in IGV 2.3 (Integrative Genomics Viewer; Broad Institute), SNPs and insertions/deletions (in-dels) were identified using the Bayesian genotype likelihood model of the GATK UnifiedGenotyper. SNPs with a Phred score lower than 50 were discarded, as were SNPs identified in less than 4 individual reads and/or SNPs located in regions containing >3 SNPs within a 20-bp stretch. SNPs and in-dels identified in this manner were compared with the list of mutations present in the set of strains available in the TB Database (http://www.tbdb.org/). In addition, for SNPs and in-dels not present in the TB Database strains, we also inspected the available genome data for the HN878 (gi:485033518; NZ_CM001043.1) and 210 (gi:261746034; ADAB00000000.1) East Asian strains.
In order to validate the WGS analysis, 30 randomly selected SNPs not previously documented as part of the TB Database project were amplified by PCR and subjected to Sanger sequencing at the McGill University and Génome Québec Innovation Centre. The primers used are listed in Table S1 in the supplemental material.
qRT-PCR.
RNA was purified from cultures at an OD600 of 0.2 as previously described (32). Primers Rv2678c-F-SYB, Rv2678c-rev-SYB, Rv2679-F-SYB, and Rv2679-rev-SYB (see Table S1 in the supplemental material) were used for quantification of expression of genes Rv2678c and Rv2679, while primers SigA1-F and SigA1-R were used to quantify sigA for use as an endogenous control for normalization purposes. The procedures used for first-strand cDNA synthesis and quantitative real-time PCR (qRT-PCR) were as described by Domenech and Reed (33). Two independent biological samples were tested for each strain.
Nucleotide sequence accession number.
Sequence data generated in this study can be obtained through NCBI with the accession number PRJNA242362.
RESULTS
The growth kinetics of M. tuberculosis is altered by the presence of the 350-kb duplication.
We, along with Weiner et al., have previously reported the finding of a massive genomic duplication in a subset of M. tuberculosis clinical isolates belonging to the East Asian strain family (23, 24). This duplication is tandemly arranged and extends more than 350 kb from gene Rv3128c to Rv3427c (Fig. 1). As described in our initial study, we have isolated two distinct subclones of the HN878 strain, one possessing the 350-kb genomic duplication (isolate HN878-45) and one lacking the duplication (isolate HN878-27) (23). In the current study, these two subclones were used in order to examine the potential impact of the duplication on the growth kinetics of M. tuberculosis both in vitro and in vivo.
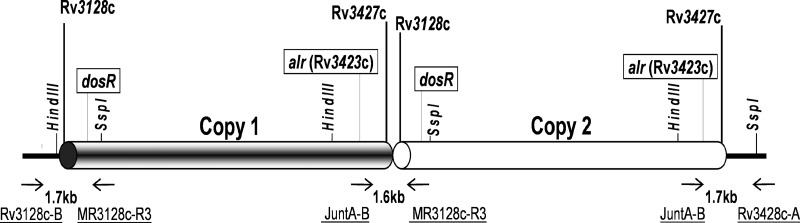
FIG 1.
Schematic representation of the 350-kb M. tuberculosis duplication. Genes Rv3128c and Rv3427c mark the approximate beginning and end of the duplicated segment, respectively. For Southern blotting, the SspI and HindIII restriction sites were used to digest DNA samples prior to hybridization with the dosR and alr gene probes indicated. The relative position of the three sets of primers (underlined) used in PCR to screen isolates for the presence of the duplication are also shown.
Standard in vitro growth assays were performed in 7H9/ADC broth, and bacterial growth was quantified by daily OD600 measurements over a 13-day period (Fig. 2A). A small yet reproducible difference was observed in the growth kinetics of the two clones that reached statistical significance between days 8 and 9 (P = 0.015 by Student's t test). Over the first 8 days, the average doubling time was 29.3 and 24.6 h for isolates HN878-27 and HN878-45, respectively. We reasoned that this minor in vitro growth effect also is reflected in the intracellular growth rates of these isolates, so to address this question we infected THP-1 monocyte-derived macrophages with HN878-27 or HN878-45 at a multiplicity of infection (MOI) of 2:1 (cells to bacteria). Bacterial growth was monitored by plating cell lysates at days 1, 2, 5, and 7 postinfection (Fig. 2B). Contrary to what we expected, the opposite trend was observed in vitro, whereby the number of CFU of HN878-27 was slightly higher than that of HN878-45 at days 5 and 7 postinfection, although this difference did not reach statistical significance (P = 0.06).
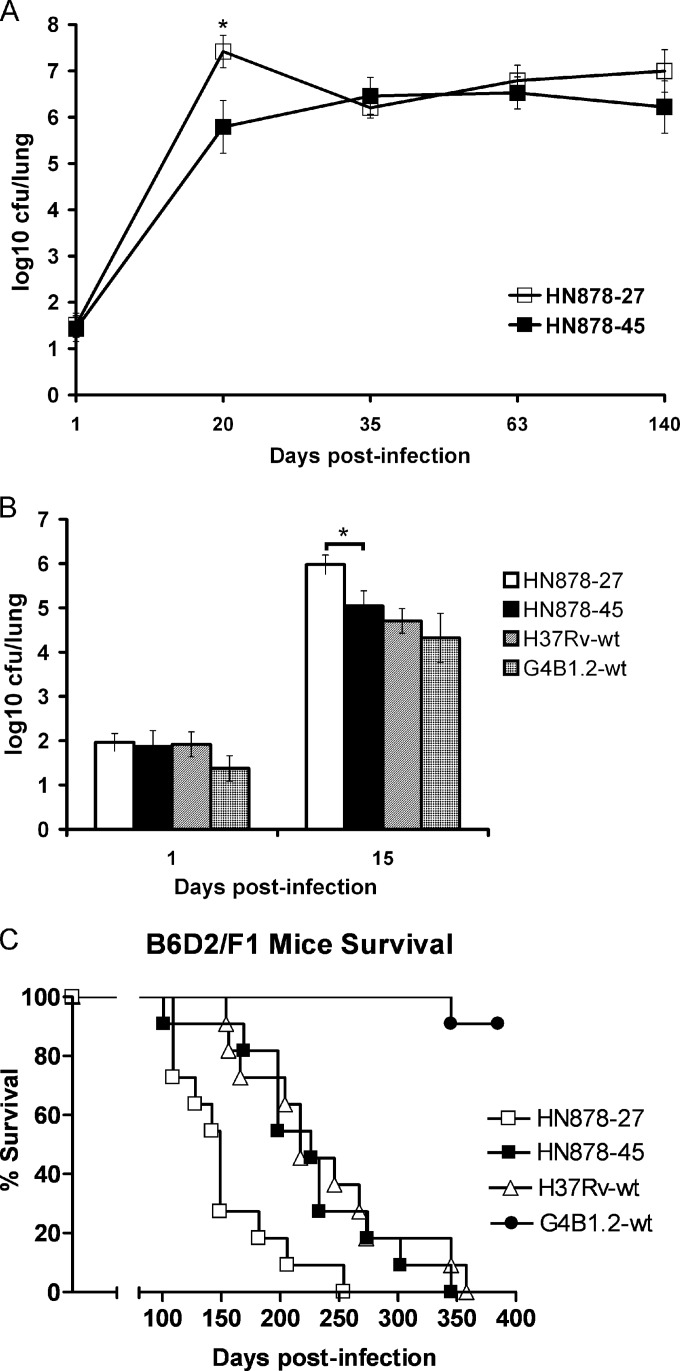
FIG 2.
Growth kinetics of M. tuberculosis isolates with and without the 350-kb duplication. The HN878-45 isolate (filled squares) contains the 350-kb duplication, while HN878-27 (open squares) does not. (A) In vitro growth kinetics in 7H9 broth as determined by OD600 readings (*, P = 0.015 by Student's t test). (B) THP-1 cells were infected at an MOI of 2:1 (cells to bacteria), and intracellular bacterial growth kinetics were measured at days 1, 2, 5, and 7 postinfection through quantification of CFU (P = 0.06 by Student's t test). Experiments were performed in triplicate and repeated twice. Data from single representative experiments are presented.
The presence of the 350-kb duplication impairs growth and virulence of M. tuberculosis in the mouse model of infection.
Bearing in mind that we had initially identified the 350-kb duplication within clinical M. tuberculosis isolates, we speculated that this large genomic rearrangement confers a selective (fitness) advantage upon these bacteria in terms of their ability to grow and/or persist in vivo. In order to test this hypothesis, two groups of B6D2/F1 mice were infected via aerosol with a low dose of either HN878-27 or HN878-45. Bacterial growth was monitored in the lungs and spleens at days 1, 20, 35, 63, and 140 postinfection for 5 mice per group (Fig. 3A). The average initial CFU (day 1) was 37 CFU/lung for the HN878-27-infected mice and 32 CFU/lung for the mice infected with HN878-45. Over the first 3 weeks of infection, HN878-27 showed a doubling time of 24 h, while for isolate HN878-45 the doubling time was 32 h. By 3 weeks postinfection, the average CFU/lung for HN878-27 was 1.5 log10 higher than that for the mice infected with HN878-45 (P < 0.01 by Student's t test). However, once the infection was contained by the host immune response beyond 3 weeks postinfection, the numbers of CFU of both strains recovered from the lungs and spleens (see Fig. S1 in the supplemental material) of infected animals were essentially identical. This finding suggests that compared to HN878-45, the HN878-27 isolate lacking the 350-kb duplication has an advantage for in vivo growth during the acute phase of infection in the mouse model.
FIG 3.
Presence of the 350-kb duplication is associated with attenuation in the mouse model of infection. C57BL/6×DBA2 F1 mice were infected via aerosol with the HN878-27 and HN878-45 strains, the latter of which contains the 350-kb duplication. (A and B) Bacterial numbers were monitored at the indicated time points postinfection by harvesting and plating the lungs of infected mice. Results are expressed as the average log10 CFU (± standard deviations) obtained from five mice at each time point (*, P < 0.01 by Student's t test). In panel B, strains H37Rv and G4B1.2 were also used to infect animals, with only two time points analyzed. Initial CFU/lung were 100, 98, 98, and 51 for H37Rv, HN878-27, HN878-45, and G4B1.2, respectively. (C) Kaplan-Meier survival curves of groups of 11 mice infected with isolates HN878-27 (empty squares), HN878-45 (filled squares), G4B1.2 (filled circles), or the laboratory strain H37Rv (open triangles). The Mann-Whitney test was used to determine the statistical significance of observed differences in survival (GraphPad Prism, v3.0; GraphPad Software, CA). For HN878-27, the median survival time was 149 days versus 226 days for HN878-45 (P = 0.0152). The strains that harbor the 350-kb duplication are HN878-45 and GB41.2 (filled squares and circles, respectively).
In order to confirm the result described above, we performed a second set of mouse infections that also included the laboratory strain H37Rv as well as the group 4 East Asian strain, G4B1.2. Like HN878-45, G4B1.2 contains the 350-kb genomic duplication (23). The four groups of mice were infected with bacterial loads of between 50 and 100 CFU/mouse, with 5 mice per group being sacrificed at days 1 and 15 postinfection for enumeration of the bacterial load in the lungs. As in the first experiment, the doubling time for HN878-27 was shorter (27 h) than the doubling times of the other strains tested, which were 34, 39, and 35 h for HN878-45, H37Rv, and GB1.2, respectively (Fig. 3B). By 15 days postinfection, the level of HN878-27 was, on average, 0.9 log10 higher in the lungs than that of HN878-45 (P < 0.01) and 1.3 log10 higher than that of H37Rv. To further investigate the impact of this growth difference on the relative in vivo virulence of these strains, 11 mice/group were recruited into a long-term animal survival experiment. The Kaplan-Meier survival curves of this experiment are shown in Fig. 3C. Interestingly, the mice infected with isolate HN878-27 (no duplication) presented with a significantly shorter median survival time (149 days) than those infected with HN878-45 (226 days; P = 0.0152 by Mann-Whitney test). The latter was basically identical to the median survival time for the mice infected with the laboratory strain H37Rv (217 days). Quite unexpectedly, after 321 days of infection none of the mice infected with isolate G4B1.2 had succumbed. By this point in time, we were concerned that the mice we had retained in this group were not infected; however, the bacterial load in these mice was determined to be 1.7 × 105 CFU/lung. After 385 days of infection, when we decided to terminate the experiment, only one mouse in this group had died.
In summary, these two mouse infection experiments have demonstrated that in the murine model of infection, the subclone of isolate HN878 that contains the 350-kb duplication (HN878-45) is slightly impaired for replication in the early stages of infection and is significantly less virulent than the HN878-27 subclone that lacks the duplication. Several previous studies have shown that HN878 is hypervirulent in the mouse model (34–37), and in agreement with those studies, here we show that mice infected with isolate HN878-27 have a significantly shorter survival time than those infected with the laboratory strain H37Rv (217 days; P = 0.0025). In contrast, given that the median survival times of the mice infected with isolate HN878-45 and H37Rv were virtually identical (226 versus 217 days), the original HN878 patient isolate probably was more similar to HN878-27 and did not possess the 350-kb duplication.
No other major genetic differences distinguish subclones HN878-27 and HN878-45.
In order to rule out the presence of additional genetic variation between strains HN878-27 and HN878-45 that could account for the observed differences in virulence in the mouse model, genomic DNA (gDNA) was purified from the stocks used to prepare the mouse inocula for WGS. WGS was also carried out for the G4B1.2 strain, and in each case sequencing data were aligned using H37Rv as the reference. The number of SNPs relative to H37Rv among the 3 sequenced isolates is summarized in Table 1. The list of SNPs and in-dels (see Tables S2 to S5 in the supplemental material) were compared to similar data available for strains present in the TB Database (www.tbdb.org) (38). SNPs at 1,490 unique loci were identified among the 3 strains examined in this study. Of these, 207 of 1,312 coding SNPs and 27 of 178 noncoding SNPs were not present in any other strain in the TB Database. In addition, 33 coding and 12 noncoding in-dels were uniquely represented among our set of 3 strains.
TABLE 1.
Number of SNPs detected by WGS
| M. tuberculosis strain | No. ofa: |
|||||||
|---|---|---|---|---|---|---|---|---|
| Coding SNPs (n = 1,312 unique loci) |
Noncoding SNPs (n = 178 unique loci) |
|||||||
| H37Rv | HN878-27 | HN878-45 | B.1.2 | H37Rv | HN878-27 | HN878-45 | B.1.2 | |
| H37Rv | 0 | 0 | ||||||
| HN878-27 | 1,230 | 0 | 167 | 0 | ||||
| HN878-45 | 1,228 | 2 | 0 | 168 | 1 | 0 | ||
| G4B1.2 | 1,230 | 166 | 164 | 0 | 158 | 29 | 30 | 0 |
Relative to M. tuberculosis H37Rv (gi:448814763; NC_000962.3). The total number of coding and noncoding SNPs that distinguish one strain from another is indicated.
Only 3 SNPs (2 coding and 1 noncoding) differentiated subclones HN878-27 and HN878-45 (Table 2), each of which was confirmed by Sanger sequencing. HN878-27 was found to contain two unique SNPs not present in HN878-45 or the HN878 isolate sequenced in another laboratory (39). For HN878-27, the Rv3696c gene which encodes GlpK, a glycerol kinase that catalyzes the Mg2+-ATP-dependent phosphorylation of glycerol to glycerol-3-phosphate (40), contains a G1387A transition that leads to the substitution of alanine for valine (A463V). As this is a conservative substitution to a residue that is not located in a predicted functional domain, we do not anticipate that this change will negatively impact the activity of the enzyme. Indeed, in both Gram-positive and -negative bacteria, this particular residue is often substituted for leucine, an amino acid with an even longer side chain than valine. The second SNP in isolate HN878-27 is a T730C transition that results in the substitution of glutamic acid for glycine (E244G) in Rv1215c, a gene which encodes a hypothetical protein found in the membrane fraction of M. tuberculosis (41, 42). As the function of Rv1215c is unknown at present, it is difficult to assess whether a mutation in this protein could have an effect on virulence. Transposon mutagenesis studies are inconclusive; it seems the gene is essential in H37Rv but not in CDC1551 (43, 44). However, as the residue in question is conserved among orthologs present in the M. tuberculosis complex but not in M. avium, M. paratuberculosis, M. marinum, or M. smegmatis, it seems likely that this amino acid is not critical for protein function.
TABLE 2.
Additional genetic differences between HN878-27 and HN878-45
| SNP type | SNP characteristic |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| HN878a | HN878-27 | HN878-45 | Location | H37Rv | NT coordinate | Gene | Annotation | Amino acid mutation | |
| Coding | Ref.b | C | Ref. | 1358714 | T | 730 | Rv1215c | Conserved hypothetical protein | E244G |
| Ref. | A | Ref. | 4138368 | G | 1387 | Rv3696c | Glycerol kinase, glpK | A463V | |
| Noncodingc | Ref. | Ref. | C | 2995081 | G | ||||
M. tuberculosis HN878 (gi:485033518; NZ_CM001043.1).
Ref., reference allele; i.e., M. tuberculosis H37Rv allele (gi:448814763; NC_000962.3).
For the noncoding SNP, the following characteristics also apply: left gene, Rv2678c (annotation, hemE); right gene, Rv2679 (annotation, echA15); distance from left gene, −19 bp; distance from right gene, +34 bp.
HN878-45 possesses one noncoding SNP not present in HN878-27 or the HN878 clone sequenced by Ioerger et al. (39) that is located 19 bp and 34 bp upstream from the start of Rv2678c and Rv2679, respectively. As this SNP may be located in the promoter region of one of these genes (although we were unable to identify any definitive promoter binding elements or transcriptional start sites), we chose to quantify the expression of these genes by qRT-PCR (see Fig. S2 in the supplemental material). While no change was observed for Rv2679, an ∼30% reduction in the expression of Rv2678c was observed in the HN878-45 strain with the G/C SNP. This gene encodes HemE, the uroporphyrinogen decarboxylase that catalyzes the decarboxylation of four acetate groups of uroporphyrinogen-III to yield coproporphyrinogen-III, and it has been shown to be essential for in vitro growth in M. tuberculosis (45). However, as seen in Fig. 2, the isolate HN878-45 does not shown any impairment for in vitro growth compared to HN878-27, indicating that the decrease in hemE gene expression we observe is not sufficient to impact in vitro growth.
As no other variants were observed between these two isolates (including deletions or insertions), we conclude that the results of the WGS analysis failed to reveal an alternative genetic alteration that can account for the observed difference in virulence in the mouse model among isolates HN878-27 and HN878-45. As such, we believe the WGS data support the attribution of the reduction in virulence in vivo to the presence of the 350-kb duplication in HN878-45.
The situation with the G4B1.2 strain is less straightforward, because aside from the 350-kb duplication that it shares with the HN878-45 strain, there are an additional 194 SNPs and 34 in-dels (including the RD142/RD150 deletions that distinguish group 4 and 5 East Asian/Beijing strains) (see Tables S2 to S5 in the supplemental material). Thus, it is possible that in addition to the duplication, one or more of these mutational events has contributed to the poor virulence of the G4B1.2 strain we observe in the mouse model. In this regard, there is a particular series of mutations in the Rv3877 gene that stands out. Rv3877 encodes the EccD1 transmembrane protein that forms part of the Esx-1 type VII secretion system involved in the secretion of the ESAT-6/CFP-10 complex (46, 47). In addition, the genes encoding EccD1, ESAT-6, and CFP-10 form part of the RD1 region that is deleted in all M. bovis (BCG) vaccine strains and is believed to be largely responsible for their in vivo attenuation (48–50). In the G4B1.2 strain, WGS has identified a 2-bp deletion at position 987 of Rv3877 that initially causes a frameshift. The reading frame is restored shortly thereafter by a 1-bp deletion at position 1024. With respect to H37Rv, this series of mutations leads to a protein sequence mismatch between amino acids 329 and 343 which forms part of an intracytoplasmic domain. The possible effects of the G4B1.2 mutations on the secretion of ESAT-6/CFP-10 are not yet clear and will be the subject of future investigations.
In vitro growth favors acquisition of the 350-kb duplication.
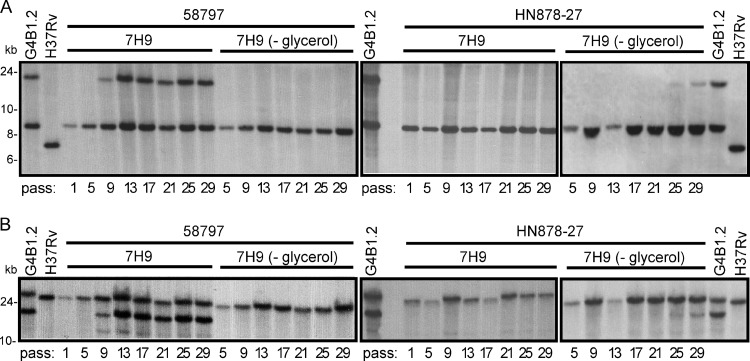
The results of the mouse experiments raised a question about whether the evolution of the 350-kb duplication has occurred in vitro rather than in vivo, which was our original suspicion given that we and others (24) had identified this large genomic duplication within “clinical” M. tuberculosis isolates. In order to address this question, we explored the effect that standard in vitro passaging has on the generation of this 350-kb genetic rearrangement. Glycerol stocks that had been prepared directly from LJ slants provided by the Quebec Public Health Laboratory (LSPQ) were obtained for a range of isolates belonging to different subgroups of the East Asian strain family. We had previously determined that none of these isolates contained the 350-kb duplication (23). Isolates 58797 and 59852 (group 3; defined by the deletions RD105, RD207, and RD181 [1]), 64130 (group 4; deletions RD105, RD207, RD181, and RD150), and HN878-27 (group 5; deletions RD105, RD207, RD181, and RD142) were included in this set of experiments, as was NHN5, a group 3 isolate that does not have the specific IS6110 insertions necessary for the duplication to form (23). Ten-ml cultures were inoculated from the glycerol stocks, and after 3 weeks (passage 1), gDNA was purified and cultures were further passaged by diluting 1:25 in 7H9/ADC either with or without 0.2% glycerol. The latter was done to test if the presence of glycerol was associated with the appearance of the 350-kb duplication, as it was for the evolution of the BCG DU2 duplication (20). Cultures were then passaged every week and gDNA purified every 4 weeks for screening by PCR (Fig. 4). No PCR product was detected in the DNA samples from the 1st passage for any of the isolates, indicating that they were initially devoid of the duplicated sequence. However, after just 5 weekly passages a faint band was observed, and the intensity of this product increased with the number of passages, suggesting that all of the isolates are able to acquire the duplication in vitro. An interesting pattern was also observed for isolates 64130 and HN878-27, in that the intensity of the PCR product increased initially and was then seen to decrease. This finding suggests that these particular cultures went through two distinct phases: (i) an initial adaptive phase that involves the formation of the duplication, followed by (ii) a resolution phase that appears to involve the loss of the duplication from the cell population over time. It is also worth noting that HN878-27 shows a clearly visible PCR product only between passages 9 and 13 for the cultures in regular 7H9, while for the cultures without glycerol, the appearance of the duplicated segment occurs much later (passage 17) and increases in intensity until the end of the experiment. Only for strains 58797 and 64130 are some transient PCR products detected in the cultures without glycerol (Fig. 4; also see Fig. S3 in the supplemental material).
FIG 4.
In vitro passaging of M. tuberculosis cultures selects for the 350-kb duplication. Isolates representing the East Asian lineage were cultivated in vitro for up to 7 months with passages every week. Genomic DNA of these samples was purified every 4 weeks, and the presence of the 350-kb duplication was analyzed by PCR using primers JuntA-B and MR3128c-R3. DNA of isolate GB41.2 was included as a positive control for the presence of the duplication, and DNA from the 29th passage of isolate NHN5 was include as a negative control. The number of passages (pass) corresponding to each of the DNA samples is indicated.
In order to further validate the results obtained by PCR screening, Southern blotting of HindIII/SspI-restricted gDNA was performed and hybridized with probes specific to dosR (Fig. 5A) and alr (Fig. 5B). These two probes were used as markers of either end of the 350-kb duplicated sequence. We were once again able to observe variable patterns among the isolates studied. For example, with isolates 58797 (Fig. 5) and 59852 (see Fig. S4 in the supplemental material), only a single DNA fragment was detected in the 1st-passage samples, indicating that one copy of the dosR and alr genes was present initially. After 9 passages, an additional faint band was observed, suggesting that two copies of these genes now were present in a subset of the bacterial population. By 13 passages, the signal intensity of the two dosR and alr bands was equivalent, suggesting that approximately 100% of the population contained the duplication. When these same isolates were grown without glycerol, only one copy of both genes was detected throughout the experiment. A second pattern was observed with isolate HN878-27; one fragment was detected in cultures grown in regular 7H9 when hybridized to either the dosR or alr probes. As described above, when these same DNA samples were analyzed by PCR, a product representing the duplication was clearly visible between passages 9 and 13. One logical interpretation of this result is that the 350-kb duplication existed in only a small portion of the bacteria (below the detection limit of Southern blotting) and was subsequently lost from the population, possibly due to the acquisition of a second compensatory mutation(s). For the cultures without glycerol, a second band was detected between passages 25 and 29, indicating that the duplication can evolve in this strain in the absence of glycerol, albeit after a much longer period of time. Finally, a third pattern of behavior was able to be distinguished for isolate 64130 (see Fig. S4). Initially, only a single hybridization signal was detected with the dosR and alr probes. Between passages 9 and 17, a transitional phase occurred where two copies of dosR and alr were detected, following which the bacterial population appeared to revert to a single-copy, nonduplicated form. Surprisingly, additional hybridization signals were also observed at passages 9 and 13, suggesting the presence of novel genomic rearrangements spanning this region. For the cultures without glycerol, strain 64130 showed two copies of the dosR-containing segment by passage 21 (see Fig. S4).
FIG 5.
Confirmation by Southern blotting of the rapid in vitro selection of the 350-kb duplication in East Asian M. tuberculosis isolates. DNAs obtained after being passaged in vitro for different amounts of time (Fig. 4) were digested with HindIII and SspI, and Southern blots were hybridized to either dosR (A)- or alr (B)-specific probes. In each case, the presence of two bands is indicative of the presence of the 350-kb duplication. DNA of isolate GB41.2 was included as a positive control for the presence of the duplication and from H37Rv as a negative control. The number of passages (pass) corresponding to each of the DNA samples is indicated.
To accurately quantify the proportion of bacteria that contain the 350-kb duplication after passaging, we analyzed individual clones obtained after plating the cultures of isolates HN878-27 and 58797 from the first and last passages. Isolated colonies were picked into 7H9/ADC and grown for 3 weeks, at which point the individual subclones were tested for the presence of the duplication by PCR. In good agreement with the Southern blotting results, the vast majority of subclones did not have the 350-kb duplication at the first passage (Table 3). By the end of the experiment, for isolate 58787 100% of the bacterial population was positive for the duplication, while for isolate HN878-27 the cells were still negative. In the passages without glycerol, 33% of HN878-27 subclones and 4% of 58787 subclones had acquired the duplication.
TABLE 3.
In vitro growth favors the presence of the 350-kb duplication
| Isolate and pass | % positivea |
nb | |
|---|---|---|---|
| Junctionc | Endd | ||
| HN878-27 | |||
| 1st pass | 0 | 100 | 24 |
| 29th pass | 0 | 100 | 24 |
| 29th pass, no glycerol | 33 | 100 | 24 |
| 58797 | |||
| 1st pass | 0 | 100 | 24 |
| 29th pass | 100 | 100 | 24 |
| 29th pass, no glycerol | 4 | 100 | 24 |
The percentage of positive isolates is given.
Number of isolates tested by PCR.
PCR of the junction region of the duplication using primers JuntA-B and MR3128c-R3.
PCR of the end region of the duplication using primers JuntA-B and Rv3428c-A was used as a quality control of the template DNA.
It is evident from these experiments that in vitro growth under standard culture conditions is sufficient to rapidly select for the 350-kb duplication. When viewed together, the results of the PCR and Southern blot analysis also clearly demonstrate the varied and dynamic nature of the genomic rearrangements that can occur in this region of the chromosome. For some isolates the acquisition of the duplication appears transient and resolves quite quickly, while in others the duplication seems fixed and is maintained over a much longer time frame.
The 350-kb genomic duplication of M. tuberculosis is highly unstable within the mouse model of infection.
Given that the duplication appears to evolve within in vitro cultures, we next questioned the stability of the duplicated segment when M. tuberculosis is growing in vivo within the lungs of infected mice. To look at this, we analyzed individual clones isolated from the initial infection (glycerol) stocks, as well as clones isolated from lung tissue postinfection. As shown in Table 4, the majority of the CFU isolated from the initial stocks for strains HN878-45 (100%) and G4B.1.2 (96%) had the 350-kb duplication. After 63 days of infection this percentage deceased to 83% in the mice infected with HN878-45 and 88% in those infected with G4B1.2. Quite striking is the fact that this percentage kept decreasing as the infections progressed, with only 4% of bacteria from the HN878-45-infected mice and 72% from G4B1.2-infected mice conserving the 350-kb duplication by 190 and 321 days, respectively. In regard to the infections carried out with HN878-27, a strain lacking the duplication, none of the CFU isolated from either the glycerol stocks used for infection or from the lungs after 9 or 20 weeks of infection showed evidence of the 350-kb duplication.
TABLE 4.
The 350-kb duplication is highly unstable during in vivo growth
| Isolate and source | % positivea |
Nb | |
|---|---|---|---|
| Junctionc | Endd | ||
| HN878-27 | |||
| Glycerol stock for mice infection | 0 | 100 | 24 |
| Infected mice, 63 days | 0 | 100 | 24 |
| Infected mice, 140 days | 0 | 100 | 24 |
| HN878-45 | |||
| Glycerol stock for mice infection | 100 | 100 | 24 |
| Infected mice, 63 days | 83 | 100 | 24 |
| Infected mice, 190 days | 4 | 100 | 24 |
| G4B1.2 | |||
| Glycerol stock for mice infection | 96 | 100 | 48 |
| Infected mice, 63 days | 88 | 100 | 48 |
| Infected mice, 321 days | 72 | 100 | 47 |
Percentage of positive isolates.
Number of isolates tested by PCR.
PCR of the junction region of the duplication using primers JuntA-B and MR3128c-R3.
PCR of the end region of the duplication using primers JuntA-B and Rv3428c-A was used as a quality control of the template DNA.
We conclude that the duplication is not selected during in vivo growth within the lungs of infected mice, and it is not stable when bacteria are replicating under these conditions. Interestingly, the mice infected with strain HN878-45, in which the duplication appears much less stable, showed a greatly reduced survival time (226 days) compared to those infected with G4B.1.2 (undefined). This observation is consistent with there being an association between the presence of the duplication and a reduction in virulence in vivo within the mouse model.
DISCUSSION
In the present study, we have characterized two simultaneously isolated, in vitro-derived subclones of East Asian lineage strain HN878, one which harbors a 350-kb duplication that we and others have previously reported (HN878-45) (23, 24) and one that lacks this duplication (HN878-27). While the presence of the duplication appears to confer an advantage for in vitro growth upon HN878-45, it is a disadvantage for in vivo growth within either THP-1 cells or in the mouse model of infection. As well as impaired bacterial replication in the lungs over the initial 3-weeks of infection, mice infected with this isolate also survive significantly longer than those infected with the HN878-27 subclone without the duplication. To put it another way, HN878-27 is more virulent than HN878-45 (and H37Rv), which fits well with the original description of the parental HN878 isolate that is reportedly hypervirulent within both mouse and rabbit models of TB infection (34–37). Hence, in the absence of any original, patient-derived stocks of the HN878 strain that was collected pre-1997 (25), our data suggest that it did not contain the 350-kb duplication when initially isolated. This is also supported by our current finding that the duplication is rapidly selected within in vitro cultures.
WGS analysis of isolates HN878-27 and HN878-45 did not reveal additional mutational events that are likely to have contributed to the striking virulence difference we observe. Even if a 30% reduction in hemE expression in HN878-45 was the result of an SNP in the putative promoter region of this gene that is reported to be essential in vitro, no impairment for in vitro growth was detected. Although we do not know if this reduction in hemE expression also occurs in vivo, it is expected that the heme content of M. tuberculosis would not be significantly affected due to the fact that M. tuberculosis possesses a heme uptake system that enables it to acquire heme directly from the host (51). Importantly, the WGS data did not reveal any mutations within known virulence associated pathways, including the locus associated with phthiocerol dimycocerosate (PDIM) biosynthesis that has previously been shown to be negatively selected through in vitro growth (33).
The fact that we have witnessed such a strong selection against the duplication or, alternatively, the selective enrichment of an initially minor subpopulation of nonduplicated cells (it is difficult to distinguish between these two possibilities) in the lungs of infected animals for two independent strains (HN878-45 and G4B1.2), seems to argue in favor of the duplication being a major contributor to reducing the in vivo virulence of these strains. Moreover, these data serve to highlight the potential for dynamic population changes in vivo even at late time points, when it is generally assumed that very little bacterial replication is occurring. If there were no selective advantage afforded by the 350-kb gene segment in vivo, then there would almost certainly be a fitness/virulence cost associated with maintaining an extra 300 (superfluous) genes within a nutrition-limited and hostile intracellular environment. This appears to have been the case for both the HN878-45 and G4B1.2 strains, for which we saw progressive loss of the duplication from the cells isolated from lungs late in infection. This behavior is typical for bacterial duplications; where the selective advantage is removed, clones bearing the duplication are eliminated from the population (5). The situation is clearly reversed in vitro, where the acquisition of this extra 350 kb of DNA appears to give the bacteria an adaptive advantage for growth under standard 7H9-based laboratory conditions. Aside from the relative in vitro growth rates of HN878-45 versus HN878-27, evidence supporting this comes from the finding that the full-length duplication is rapidly selected in multiple independent isolates grown in vitro. The appearance of the duplication within in vitro cultures is surprisingly fast; after only 5 weeks of broth culture we could detect the duplication in clinical isolates that were initially shown to be devoid of this large-scale gene rearrangement. For a number of the isolates we tested, approximately 100% of cells had the duplication by 13 weeks.
Our observation of accelerated in vitro evolution is in agreement with a report by Turcios et al. (52), who were able to detect rapidly induced, in vitro-generated duplications in the dnaA-dnaN intergenic region that give rise to two copies of oriC in M. tuberculosis clinical isolates. Interestingly, as seen with the 350-kb duplication, the presence of IS6110 at both ends of this region again appears to be the driving force behind this particular duplication, confirming the important role of this insertion element in generating strain variability in M. tuberculosis both in vivo and in vitro (53). Although initially we suspected that the presence of 0.2% glycerol within 7H9/ADC media is the stimulus that selects for the 350-kb duplication in vitro, in our hands it occurs regardless of the presence of glycerol. Nevertheless, when glycerol is present in the media, the duplication is detected at a much earlier time point. This could simply be due to the fact that M. tuberculosis grows faster in the presence of glycerol, such that there is an increased number of cell divisions and an increased chance for mutations to occur over a given time frame. Additionally, it could be that a specific combination of energy and carbon sources (of which glycerol is one) present in 7H9/ADC is what affects the frequency of the duplication event. While on the subject of glycerol, it is interesting that the WGS led to the identification of a glpK mutation unique to the HN878-27 strain. This SNP may be yet another example of in vitro-acquired mutation and may help to explain why this strain did not stably maintain the duplication in the presence of glycerol in vitro.
In the present study, we were able to distinguish two general patterns of behavior during the in vitro passaging experiments in regard to the apparent stability of the duplicated sequence. In strains 64130 and HN878-27, acquisition of the 350-kb duplication was transient, while for strains 58797 and 59852 it appeared to be a more permanent fixture over the time frame of the experiments described here. This finding is likely related to the inherent instability of gene duplications (due to their high fitness cost), which serve to provide an initial adaptive response in the face of changing or hazardous environmental conditions (e.g., the presence of antibiotics or nutritional stress). In this manner, duplications represent a transient evolutionary intermediate that allows expansion of the population to a level that supports the occurrence of rare, yet genetically stable, point mutations. The latter can affect genes within as well as outside the amplified region (5). Presumably, therefore, the transient group noted above has evolved certain compensatory changes that have allowed the duplication structure to be resolved and lost from the population. Why the dynamics of these mutational events appear to vary among the set of strains we have examined is not clear at this point, although given the very large size of the duplication involved in this case (350 kb), it is possible that multiple, sequential point mutations are necessary in order to overcome the need for the duplication in vitro. It is also possible that distinct yet functionally equivalent mutations are being acquired at different rates within the individual strains.
Although the current study has focused on strains of the East Asian genotype, the in vitro generation of genetic and phenotypic variability has been reported previously for the H37Rv laboratory strain. For example, we have published that in vitro growth selects for the rapid and spontaneous loss of PDIM in H37Rv cultures due to a wide variety of mutations, including SNPs and deletions in genes that are involved in the synthesis of this lipid. This selection process creates temporarily heterogeneous cultures of bacteria that are PDIM positive and PDIM negative, but ultimately, due to a slight growth advantage, the cells devoid of PDIM overtake the culture (33). In a more recent report, Ioerger et al. (39) describe the continuous in vitro evolution of the H37Rv genome even in a controlled environment. Through whole-genome sequencing of six H37Rv strains maintained in different laboratories, these authors identified several polymorphisms that are either unique or shared among different H37Rv clones. Along with SNPs and insertions/deletions, they also found variation in the insertion sites of the IS6110 element. The fact that large gene amplification events are not limited to the modern branch of the East Asian strain family was also recently highlighted by Weiner et al., who described a unique 54-kb duplication that exists between Rv1756c and Rv1800 in their version of H37Rv (24). As mentioned above, these authors reported multiple (distinct) large-scale duplications within clinical isolates of lineages 2 (East Asian) and 4 (Euro-American), the largest of which was a massive 532 kb. Given that each of these independent events overlap the same genomic region that is also shared by the 350-kb duplication detailed here, it seems highly likely that every one of these duplication events has evolved within in vitro cultures through slightly different recombination mechanisms.
In conclusion, if we accept that (i) in vitro evolution occurs across multiple M. tuberculosis strain backgrounds through a variety of mechanisms that are highly dynamic, (ii) genetic changes occur very rapidly after placing patient isolates into in vitro culture, and (iii) some of these in vitro events may impact fitness or virulence in vivo, then the results we describe in this study have several important implications that are relevant to all TB researchers, in addition to those who study non-TB mycobacterial diseases whose research depends upon the adaptation of patient-derived isolates to in vitro culture conditions. For example, quite a number of recent studies have demonstrated variability among what were assumed to be clinical isolates in terms of virulence in animal models (54–59). Without pointing to any study in particular, it seems reasonable to assume that all of the isolates in question (including a number that were East Asian/Beijing isolates) would have been held in liquid culture for at least the length of time it takes to prepare gDNA, genotype the strains, and then prepare suitable inocula for carrying out the infections. Given the apparent rapidity and frequency with which genetic variants appear and establish themselves within in vitro cultures, the potential is clearly there for at least some of the phenotypic variation that has been reported to date, even among isolates of the same lineage, to be a function of in vitro-acquired mutations rather than a product of the original patient isolates themselves. In a similar vein, it also seems reasonable to expect that a portion of the genetic heterogeneity (SNPs and in-dels) that has been reported at the individual strain level (4, 60–63) also has been derived during in vitro culture, particularly where isolates have been shared and passaged multiple times by multiple laboratories.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Canadian Institutes of Health Research (CIHR) grants MOP82931 and MOP115133 (awarded to M.R.). M.R. was also supported by a Peter Lougheed/CIHR New Investigator Award.
We are grateful to Fiona McIntosh (Research Institute–McGill University Health Centre) for providing several of the M. tuberculosis strains used in this study.
Footnotes
Published ahead of print 28 April 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01791-14.
REFERENCES
- 1.Gagneux S, DeRiemer K, Van T, Kato-Maeda M, de Jong BC, Narayanan S, Nicol M, Niemann S, Kremer K, Gutierrez MC, Hilty M, Hopewell PC, Small PM. 2006. Variable host-pathogen compatibility in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 103:2869–2873. 10.1073/pnas.0511240103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flores L, Van T, Narayanan S, DeRiemer K, Kato-Maeda M, Gagneux S. 2007. Large sequence polymorphisms classify Mycobacterium tuberculosis strains with ancestral spoligotyping patterns. J. Clin. Microbiol. 45:3393–3395. 10.1128/JCM.00828-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menendez MC, Buxton RS, Evans JT, Gascoyne-Binzi D, Barlow RE, Hinds J, Hawkey PM, Colston MJ. 2007. Genome analysis shows a common evolutionary origin for the dominant strains of Mycobacterium tuberculosis in a UK South Asian community. Tuberculosis 87:426–436. 10.1016/j.tube.2007.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hershberg R, Lipatov M, Small PM, Sheffer H, Niemann S, Homolka S, Roach JC, Kremer K, Petrov DA, Feldman MW, Gagneux S. 2008. High functional diversity in Mycobacterium tuberculosis driven by genetic drift and human demography. PLoS Biol. 6:e311. 10.1371/journal.pbio.0060311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersson DI, Hughes D. 2009. Gene amplification and adaptive evolution in bacteria. Annu. Rev. Genet. 43:167–195. 10.1146/annurev-genet-102108-134805 [DOI] [PubMed] [Google Scholar]
- 6.Anderson P, Roth J. 1981. Spontaneous tandem genetic duplications in Salmonella typhimurium arise by unequal recombination between rRNA (rrn) cistrons. Proc. Natl. Acad. Sci. U. S. A. 78:3113–3117. 10.1073/pnas.78.5.3113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haack KR, Roth JR. 1995. Recombination between chromosomal IS200 elements supports frequent duplication formation in Salmonella typhimurium. Genetics 141:1245–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicoloff H, Perreten V, Levy SB. 2007. Increased genome instability in Escherichia coli lon mutants: relation to emergence of multiple-antibiotic-resistant (Mar) mutants caused by insertion sequence elements and large tandem genomic amplifications. Antimicrob. Agents Chemother. 51:1293–1303. 10.1128/AAC.01128-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonti RV, Roth JR. 1989. Role of gene duplications in the adaptation of Salmonella typhimurium to growth on limiting carbon sources. Genetics 123:19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reams AB, Neidle EL. 2003. Genome plasticity in Acinetobacter: new degradative capabilities acquired by the spontaneous amplification of large chromosomal segments. Mol. Microbiol. 47:1291–1304. 10.1046/j.1365-2958.2003.03342.x [DOI] [PubMed] [Google Scholar]
- 11.Kondratyeva TF, Muntyan LN, Karavaiko GI. 1995. Zinc- and arsenic-resistant strains of Thiobacillus ferrooxidans have increased copy numbers of chromosomal resistance genes. Microbiology 141:1157–1162. 10.1099/13500872-141-5-1157 [DOI] [PubMed] [Google Scholar]
- 12.Sun S, Berg OG, Roth JR, Andersson DI. 2009. Contribution of gene amplification to evolution of increased antibiotic resistance in Salmonella typhimurium. Genetics 182:1183–1195. 10.1534/genetics.109.103028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vinella D, Cashel M, D'Ari R. 2000. Selected amplification of the cell division genes ftsQ-ftsA-ftsZ in Escherichia coli. Genetics 156:1483–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nilsson AI, Zorzet A, Kanth A, Dahlstrom S, Berg OG, Andersson DI. 2006. Reducing the fitness cost of antibiotic resistance by amplification of initiator tRNA genes. Proc. Natl. Acad. Sci. U. S. A. 103:6976–6981. 10.1073/pnas.0602171103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mekalanos JJ. 1983. Duplication and amplification of toxin genes in Vibrio cholerae. Cell 35:253–263. 10.1016/0092-8674(83)90228-3 [DOI] [PubMed] [Google Scholar]
- 16.Cerquetti M, Cardines R, Ciofi Degli Atti ML, Giufre M, Bella A, Sofia T, Mastrantonio P, Slack M. 2005. Presence of multiple copies of the capsulation b locus in invasive Haemophilus influenzae type b (Hib) strains isolated from children with Hib conjugate vaccine failure. J. Infect. Dis. 192:819–823. 10.1086/432548 [DOI] [PubMed] [Google Scholar]
- 17.Cerquetti M, Cardines R, Giufre M, Castella A, Rebora M, Mastrantonio P, Ciofi Degli Atti ML. 2006. Detection of six copies of the capsulation b locus in a Haemophilus influenzae type b strain isolated from a splenectomized patient with fulminant septic shock. J. Clin. Microbiol. 44:640–642. 10.1128/JCM.44.2.640-642.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang XM, Galamba A, Warner DF, Soetaert K, Merkel JS, Kalai M, Bifani P, Lefevre P, Mizrahi V, Content J. 2008. IS1096-mediated DNA rearrangements play a key role in genome evolution of Mycobacterium smegmatis. Tuberculosis 88:399–409. 10.1016/j.tube.2008.02.003 [DOI] [PubMed] [Google Scholar]
- 19.Zhang C, Anderson AJ. 2012. Multiplicity of genes for aromatic ring-hydroxylating dioxygenases in Mycobacterium isolate KMS and their regulation. Biodegradation 23:585–596. 10.1007/s10532-012-9535-z [DOI] [PubMed] [Google Scholar]
- 20.Brosch R, Gordon SV, Garnier T, Eiglmeier K, Frigui W, Valenti P, Dos Santos S, Duthoy S, Lacroix C, Garcia-Pelayo C, Inwald JK, Golby P, Garcia JN, Hewinson RG, Behr MA, Quail MA, Churcher C, Barrell BG, Parkhill J, Cole ST. 2007. Genome plasticity of BCG and impact on vaccine efficacy. Proc. Natl. Acad. Sci. U. S. A. 104:5596–5601. 10.1073/pnas.0700869104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leung AS, Tran V, Wu Z, Yu X, Alexander DC, Gao GF, Zhu B, Liu J. 2008. Novel genome polymorphisms in BCG vaccine strains and impact on efficacy. BMC Genomics 9:413. 10.1186/1471-2164-9-413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McEvoy CR, van Helden PD, Warren RM, Gey van Pittius NC. 2009. Evidence for a rapid rate of molecular evolution at the hypervariable and immunogenic Mycobacterium tuberculosis PPE38 gene region. BMC Evol. Biol. 9:237. 10.1186/1471-2148-9-237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Domenech P, Kolly GS, Leon-Solis L, Fallow A, Reed MB. 2010. Massive gene duplication event among clinical isolates of the Mycobacterium tuberculosis W/Beijing family. J. Bacteriol. 192:4562–4570. 10.1128/JB.00536-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiner B, Gomez J, Victor TC, Warren RM, Sloutsky A, Plikaytis BB, Posey JE, van Helden PD, Gey van Pittius NC, Koehrsen M, Sisk P, Stolte C, White J, Gagneux S, Birren B, Hung D, Murray M, Galagan J. 2012. Independent large scale duplications in multiple M. tuberculosis lineages overlapping the same genomic region. PLoS One 7:e26038. 10.1371/journal.pone.0026038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sreevatsan S, Pan X, Stockbauer KE, Connell ND, Kreiswirth BN, Whittam TS, Musser JM. 1997. Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc. Natl. Acad. Sci. U. S. A. 94:9869–9874. 10.1073/pnas.94.18.9869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed MB, Pichler VK, McIntosh F, Mattia A, Fallow A, Masala S, Domenech P, Zwerling A, Thibert L, Menzies D, Schwartzman K, Behr MA. 2009. Major Mycobacterium tuberculosis lineages associate with patient country of origin. J. Clin. Microbiol. 47:1119–1128. 10.1128/JCM.02142-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 28.Pelicic V, Jackson M, Reyrat JM, Jacobs WR, Jr, Gicquel B, Guilhot C. 1997. Efficient allelic exchange and transposon mutagenesis in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 94:10955–10960. 10.1073/pnas.94.20.10955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplan EL, Meier P. 1958. Nonparametric estimation for incomplete observations. J. Am. Stat. Assoc. 53:457–481. ]? 10.1080/01621459.1958.10501452 [DOI] [Google Scholar]
- 30.Li H, Durbin R. 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760. 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25:2078–2079. 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sherman DR, Voskuil M, Schnappinger D, Liao R, Harrell MI, Schoolnik GK. 2001. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding alpha-crystallin. Proc. Natl. Acad. Sci. U. S. A. 98:7534–7539. 10.1073/pnas.121172498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Domenech P, Reed MB. 2009. Rapid and spontaneous loss of phthiocerol dimycocerosate (PDIM) from Mycobacterium tuberculosis grown in vitro: implications for virulence studies. Microbiology 155:3532–3543. 10.1099/mic.0.029199-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manca C, Tsenova L, Barry CE, III, Bergtold A, Freeman S, Haslett PA, Musser JM, Freedman VH, Kaplan G. 1999. Mycobacterium tuberculosis CDC1551 induces a more vigorous host response in vivo and in vitro, but is not more virulent than other clinical isolates. J. Immunol. 162:6740–6746 [PubMed] [Google Scholar]
- 35.Barczak AK, Domenech P, Boshoff HI, Reed MB, Manca C, Kaplan G, Barry CE., III 2005. In vivo phenotypic dominance in mouse mixed infections with Mycobacterium tuberculosis clinical isolates. J. Infect. Dis. 192:600–606. 10.1086/432006 [DOI] [PubMed] [Google Scholar]
- 36.Manca C, Tsenova L, Bergtold A, Freeman S, Tovey M, Musser JM, Barry CE, III, Freedman VH, Kaplan G. 2001. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha/beta. Proc. Natl. Acad. Sci. U. S. A. 98:5752–5757. 10.1073/pnas.091096998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reed MB, Domenech P, Manca C, Su H, Barczak AK, Kreiswirth BN, Kaplan G, Barry CE., III 2004. A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature 431:84–87. 10.1038/nature02837 [DOI] [PubMed] [Google Scholar]
- 38.Reddy TB, Riley R, Wymore F, Montgomery P, DeCaprio D, Engels R, Gellesch M, Hubble J, Jen D, Jin H, Koehrsen M, Larson L, Mao M, Nitzberg M, Sisk P, Stolte C, Weiner B, White J, Zachariah ZK, Sherlock G, Galagan JE, Ball CA, Schoolnik GK. 2009. TB database: an integrated platform for tuberculosis research. Nucleic Acids Res. 37:D499–D508. 10.1093/nar/gkn652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ioerger TR, Feng Y, Ganesula K, Chen X, Dobos KM, Fortune S, Jacobs WR, Jr, Mizrahi V, Parish T, Rubin E, Sassetti C, Sacchettini JC. 2010. Variation among genome sequences of H37Rv strains of Mycobacterium tuberculosis from multiple laboratories. J. Bacteriol. 192:3645–3653. 10.1128/JB.00166-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zwaig N, Kistler WS, Lin EC. 1970. Glycerol kinase, the pacemaker for the dissimilation of glycerol in Escherichia coli. J. Bacteriol. 102:753–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Souza GA, Leversen NA, Malen H, Wiker HG. 2011. Bacterial proteins with cleaved or uncleaved signal peptides of the general secretory pathway. J. Proteomics 75:502–510. 10.1016/j.jprot.2011.08.016 [DOI] [PubMed] [Google Scholar]
- 42.Xiong Y, Chalmers MJ, Gao FP, Cross TA, Marshall AG. 2005. Identification of Mycobacterium tuberculosis H37Rv integral membrane proteins by one-dimensional gel electrophoresis and liquid chromatography electrospray ionization tandem mass spectrometry. J. Proteome Res. 4:855–861. 10.1021/pr0500049 [DOI] [PubMed] [Google Scholar]
- 43.Sassetti CM, Boyd DH, Rubin EJ. 2003. Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 48:77–84. 10.1046/j.1365-2958.2003.03425.x [DOI] [PubMed] [Google Scholar]
- 44.Lamichhane G, Zignol M, Blades NJ, Geiman DE, Dougherty A, Grosset J, Broman KW, Bishai WR. 2003. A postgenomic method for predicting essential genes at subsaturation levels of mutagenesis: application to Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 100:7213–7218. 10.1073/pnas.1231432100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Griffin JE, Gawronski JD, Dejesus MA, Ioerger TR, Akerley BJ, Sassetti CM. 2011. High-resolution phenotypic profiling defines genes essential for mycobacterial growth and cholesterol catabolism. PLoS Pathog. 7:e1002251. 10.1371/journal.ppat.1002251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guinn KM, Hickey MJ, Mathur SK, Zakel KL, Grotzke JE, Lewinsohn DM, Smith S, Sherman DR. 2004. Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol. Microbiol. 51:359–370. 10.1046/j.1365-2958.2003.03844.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stanley SA, Raghavan S, Hwang WW, Cox JS. 2003. Acute infection and macrophage subversion by Mycobacterium tuberculosis require a specialized secretion system. Proc. Natl. Acad. Sci. U. S. A. 100:13001–13006. 10.1073/pnas.2235593100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Behr MA, Wilson MA, Gill WP, Salamon H, Schoolnik GK, Rane S, Small PM. 1999. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284:1520–1523. 10.1126/science.284.5419.1520 [DOI] [PubMed] [Google Scholar]
- 49.Lewis KN, Liao R, Guinn KM, Hickey MJ, Smith S, Behr MA, Sherman DR. 2003. Deletion of RD1 from Mycobacterium tuberculosis mimics bacille Calmette-Guerin attenuation. J. Infect. Dis. 187:117–123. 10.1086/345862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pym AS, Brodin P, Brosch R, Huerre M, Cole ST. 2002. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol. Microbiol. 46:709–717. 10.1046/j.1365-2958.2002.03237.x [DOI] [PubMed] [Google Scholar]
- 51.Tullius MV, Harmston CA, Owens CP, Chim N, Morse RP, McMath LM, Iniguez A, Kimmey JM, Sawaya MR, Whitelegge JP, Horwitz MA, Goulding CW. 2011. Discovery and characterization of a unique mycobacterial heme acquisition system. Proc. Natl. Acad. Sci. U. S. A. 108:5051–5056. 10.1073/pnas.1009516108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Turcios L, Casart Y, Florez I, de Waard J, Salazar L. 2009. Characterization of IS6110 insertions in the dnaA-dnaN intergenic region of Mycobacterium tuberculosis clinical isolates. Clin. Microbiol. Infect. 15:200–203. 10.1111/j.1469-0691.2008.02107.x [DOI] [PubMed] [Google Scholar]
- 53.McEvoy CR, Falmer AA, Gey van Pittius NC, Victor TC, van Helden PD, Warren RM. 2007. The role of IS6110 in the evolution of Mycobacterium tuberculosis. Tuberculosis 87:393–404. 10.1016/j.tube.2007.05.010 [DOI] [PubMed] [Google Scholar]
- 54.Kato-Maeda M, Shanley CA, Ackart D, Jarlsberg LG, Shang S, Obregon-Henao A, Harton M, Basaraba RJ, Henao-Tamayo M, Barrozo JC, Rose J, Kawamura LM, Coscolla M, Fofanov VY, Koshinsky H, Gagneux S, Hopewell PC, Ordway DJ, Orme IM. 2012. Beijing sublineages of Mycobacterium tuberculosis differ in pathogenicity in the guinea pig. Clin. Vaccine Immunol. 19:1227–1237. 10.1128/CVI.00250-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hernandez Pando R, Aguilar D, Cohen I, Guerrero M, Ribon W, Acosta P, Orozco H, Marquina B, Salinas C, Rembao D, Espitia C. 2010. Specific bacterial genotypes of Mycobacterium tuberculosis cause extensive dissemination and brain infection in an experimental model. Tuberculosis 90:268–277. 10.1016/j.tube.2010.05.002 [DOI] [PubMed] [Google Scholar]
- 56.Lopez B, Aguilar D, Orozco H, Burger M, Espitia C, Ritacco V, Barrera L, Kremer K, Hernandez-Pando R, Huygen K, van Soolingen D. 2003. A marked difference in pathogenesis and immune response induced by different Mycobacterium tuberculosis genotypes. Clin. Exp. Immunol. 133:30–37. 10.1046/j.1365-2249.2003.02171.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aguilar D, Hanekom M, Mata D, Gey van Pittius NC, van Helden PD, Warren RM, Hernandez-Pando R. 2010. Mycobacterium tuberculosis strains with the Beijing genotype demonstrate variability in virulence associated with transmission. Tuberculosis 90:319–325. 10.1016/j.tube.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 58.Krishnan N, Malaga W, Constant P, Caws M, Tran TH, Salmons J, Nguyen TN, Nguyen DB, Daffe M, Young DB, Robertson BD, Guilhot C, Thwaites GE. 2011. Mycobacterium tuberculosis lineage influences innate immune response and virulence and is associated with distinct cell envelope lipid profiles. PLoS One 6:e23870. 10.1371/journal.pone.0023870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Palanisamy GS, DuTeau N, Eisenach KD, Cave DM, Theus SA, Kreiswirth BN, Basaraba RJ, Orme IM. 2009. Clinical strains of Mycobacterium tuberculosis display a wide range of virulence in guinea pigs. Tuberculosis 89:203–209. 10.1016/j.tube.2009.01.005 [DOI] [PubMed] [Google Scholar]
- 60.Gagneux S. 2013. Genetic diversity in Mycobacterium tuberculosis. Curr. Top. Microbiol. Immunol. 374:1–25. 10.1007/82_2013_329 [DOI] [PubMed] [Google Scholar]
- 61.Comas I, Chakravartti J, Small PM, Galagan J, Niemann S, Kremer K, Ernst JD, Gagneux S. 2010. Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyperconserved. Nat. Genet. 42:498–503. 10.1038/ng.590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Niemann S, Koser CU, Gagneux S, Plinke C, Homolka S, Bignell H, Carter RJ, Cheetham RK, Cox A, Gormley NA, Kokko-Gonzales P, Murray LJ, Rigatti R, Smith VP, Arends FP, Cox HS, Smith G, Archer JA. 2009. Genomic diversity among drug sensitive and multidrug resistant isolates of Mycobacterium tuberculosis with identical DNA fingerprints. PLoS One 4:e7407. 10.1371/journal.pone.0007407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kato-Maeda M, Ho C, Passarelli B, Banaei N, Grinsdale J, Flores L, Anderson J, Murray M, Rose G, Kawamura LM, Pourmand N, Tariq MA, Gagneux S, Hopewell PC. 2013. Use of whole genome sequencing to determine the microevolution of Mycobacterium tuberculosis during an outbreak. PLoS One 8:e58235. 10.1371/journal.pone.0058235 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.