Abstract
Trichomonads are obligate protozoan parasites most renowned as venereal pathogens of the reproductive tract of humans and cattle. Recently, a trichomonad highly similar to bovine venereal Tritrichomonas foetus but having a unique tropism for the intestinal tract was recognized as a significant cause of colitis in domestic cats. Despite a high prevalence, worldwide distribution, and lack of consistently effective drugs for treatment of the infection, the cellular mechanisms of T. foetus pathogenicity in the intestinal tract have not been examined. The aims of this study were to determine the pathogenic effect of feline T. foetus on porcine intestinal epithelial cells, the dependence of T. foetus pathogenicity on adhesion of T. foetus to the intestinal epithelium, and the identity of mediators responsible for these effects. Using an in vitro coculture approach to model feline T. foetus infection of the intestinal epithelium, these studies demonstrate that T. foetus promotes a direct contact-dependent activation of intestinal epithelial cell apoptosis signaling and progressive monolayer destruction. Moreover, these pathological effects were demonstrated to be largely dependent on T. foetus cell-associated cysteine protease activity. Finally, T. foetus cysteine proteases were identified as enabling cytopathic effects by promoting adhesion of T. foetus to the intestinal epithelium. The present studies are the first to examine the cellular mechanisms of pathogenicity of T. foetus toward the intestinal epithelium and support further investigation of the cysteine proteases as virulence factors in vivo and as potential therapeutic targets for ameliorating the pathological effects of intestinal trichomonosis.
INTRODUCTION
Trichomonads are ancient eukaryotic protists. They survive by obligate colonization of warm, moist, and anaerobic mucosal environments within their vertebrate hosts. Numerous species of trichomonads exist, including both pathogenic and presumably commensal organisms (1). Among these, trichomonads infecting the reproductive tract are the most widely studied. Trichomonas vaginalis is the most common nonviral sexually transmitted disease, and it infects an estimated 248 million people worldwide (2). Tritrichomonas foetus, a trichomonad causing similar pathology, is a venereal pathogen of cattle and can result in considerable reproductive and economic losses in infected herds. Recently, a trichomonad highly similar to bovine venereal T. foetus but having a unique tropism for the intestinal tract was recognized as a significant cause of diarrhea in domestic cats (3–6). This same organism is also documented in the colon of pigs (7, 8).
In contrast to venereal trichomonosis, there are currently no mechanistic studies using intestinal epithelial cell lines that examine the virulence factors responsible for disease pathogenesis of trichomonads infecting the gastrointestinal tract. Such studies are needed to better understand the pathological significance of these infections and to enable the development of novel treatment strategies to prevent or ameliorate their clinical effects. This is particularly true for feline intestinal T. foetus, where infection causes a lifelong recurrent diarrhea that is difficult to treat (5, 6, 9–14).
A key observation of T. foetus in infected cats is an intimate association of the organisms with the lumen and crypt epithelium of the colonic mucosa and concurrent infiltration of inflammatory cells into the subepithelial lamina propria. Using a coculture assay approach, we have previously demonstrated that feline T. foetus adheres to intestinal epithelial monolayers in vitro by kinetics that suggest a specific interaction of T. foetus with the epithelium (15). In venereal trichomonosis, adherence of trichomonads to the urogenital epithelium and elaboration of proteases are recognized as central events in mediating host cellular pathogenicity (16–18). Therefore, the aims of the present study were to determine if feline T. foetus mediates cytotoxic effects on intestinal epithelial cells, the dependence of T. foetus pathogenicity on adhesion to the epithelium, and the identity of pharmacologically targetable mediators responsible for these effects. Our results support a central role for cysteine proteases in promoting adhesion-dependent cytotoxicity of feline T. foetus to the intestinal epithelium and support further investigation of the cysteine proteases as virulence factors in vivo; therefore, they are potential therapeutic targets for ameliorating the pathological effects of intestinal trichomonosis.
MATERIALS AND METHODS
IPEC-J2 cells.
A nontransformed intestinal epithelial cell line (IPEC-J2) was used for these studies. This line was originally isolated from neonatal piglet jejunum and was obtained as a gift from Helen M. Berschneider. IPEC-J2 cells were grown in a coculture media, which included advanced Dulbecco's modified eagle medium-nutrient mixture F-12 (DMEM-F12) supplemented with 5 μg/ml each of insulin, transferrin, and selenium, 5 ng/ml epidermal growth factor (EGF), 100 IU/ml penicillin, 100 mg/ml streptomycin, and 5% fetal bovine serum, and incubated at 37°C in 5% CO2. Prior to adhesion studies with trichomonads, ∼4 × 105 IPEC-J2 cells/well were seeded onto permeable polycarbonate filters (0.4-μm pore size, 4.67 cm2; Corning Incorporated, Lowell, MA) and cultivated until confluent and averaging 39 × 104 ± 10 × 104 IPEC-J2 cells per well. For microscopic examination of trichomonad-induced cytotoxicity, ∼5 × 104 IPEC-J2 cells/well were seeded onto Laboratory-Tek chamber slides (Nalge Nunc International, Rochester, NY) or 24-well polystyrene plates (Corning Incorporated, Lowell, MA) and grown to confluence at an average of 20 × 104 ± 3 × 104 cells per well. IPEC-J2 cells were used at passage numbers 38 to 60.
Trichomonads.
Isolation and culture of feline T. foetus and Pentatrichomonas hominis isolates were performed as previously described (15). Trichomonads were harvested in mid- to late-logarithmic phase by centrifugation at 250 × g and washed twice in Hanks' balanced salt solution (HBSS). The trichomonads were resuspended in HBSS at desired concentrations for experimental purposes. One P. hominis and four T. foetus (F, Sti, D, and A) isolates from five different naturally infected cats having clinical signs of diarrhea were used for comparative studies of protease activity and cytotoxicity. For use in coculture experiments with intestinal epithelial cells, T. foetus and P. hominis were used at a multiplicity of infection (MOI) of 50:1. This MOI maximum was based on estimates obtained from 9 archival light microscopic photomicrographs of colonic mucosa from 4 naturally infected cats demonstrating an average number of 12.5 trichomonads per epithelial cell (range, 2 to 47) (Fig. 1).
FIG 1.
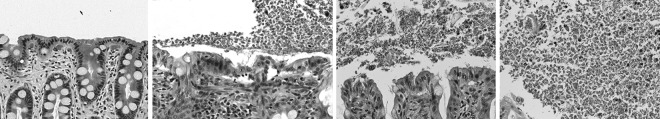
Representative photomicrographs of colonic mucosal biopsy specimens obtained from a normal cat (first panel) and a cat with naturally occurring T. foetus infection on which estimates of the multiplicity of infection in vivo were based (second through fourth panels). Biopsy specimens were formalin fixed, paraffin embedded, sectioned at a thickness of 7 μm, and stained with a modified hematoxylin and eosin stain.
Protein extractions.
Trichomonads (20 × 106) in mid-logarithmic phase were washed twice in HBSS, lysed in radioimmunoprecipitation assay (RIPA) buffer (1× PBS, 1% Igepal, 0.5% sodium deoxycholate, 0.1% SDS), sonicated twice, and incubated for 30 min at 4°C. Supernatants were collected following centrifugation at 15,800 × g for 10 min at 4°C. Protein lysate concentrations were determined by bicinchoninic acid (BCA) assay (Thermo Fisher Scientific, Rockford, IL) using bovine serum albumin as a standard. Lysates were diluted in lithium dodecyl sulfate (LDS) buffer in the absence of a reducing agent and immediately used for substrate-gel electrophoresis or were stored as single-use samples of approximately 400 μg at −80°C. Secreted components for substrate-gel electrophoresis assays were prepared from trichomonads as previously described (19, 20). T. foetus cells (1 × 108) were washed once in HBSS and then incubated in 1 ml Dulbecco's phosphate-buffered solution supplemented with 0.1% l-cysteine hydrochloride and 0.02% ascorbic acid (pH 7.2) at 37°C for 3.5 h. After incubation, trichomonads were centrifuged at 1,000 × g for 10 min, the supernatant was aspirated and filtered through a 0.22-μm-pore-size filter, and filtered supernatants were centrifuged at 15,000 × g for 10 min at 4°C prior to use in substrate-gel electrophoresis. Supernatants were used only when obtained from trichomonads that retained a minimum of 95% motility as assessed by light microscopy.
Substrate-gel electrophoresis.
Trichomonad proteases were separated and analyzed under nondenaturing and nonreducing conditions in 10% Tris-glycine gels containing 0.1% gelatin (Life Technologies, Carlsbad, CA) as the protein substrate. Protein samples were electrophoretically separated at a voltage of 125 V for 90 min. Following electrophoresis, gels were immersed in a nonionic detergent renaturing buffer (Novex zymogram renaturing buffer; Life Technologies, Carlsbad, CA) for 30 min at 25°C to allow proteases to become activated. Following renaturing, gels were equilibrated in a divalent metal cation-repleting developing buffer (Novex zymogram developing buffer; Life Technologies, Carlsbad, CA) for 30 min at 25°C, followed by an overnight incubation at 37°C in fresh developing buffer. After overnight incubation, gels were washed 3 times in deionized water (dH20) for 5 min each and then stained for a minimum of 7 h in Coomassie blue (Coomassie blue staining kit; Life Technologies, Carlsbad, CA). Gels were then incubated in dH2O overnight as suggested by the manufacturer to minimize background staining. Proteolysis was visualized as clear bands against a stained background. Identification of protein classes was determined by pretreatment of live trichomonads or protein lysates with class-specific protease inhibitors immediately prior to electrophoresis. Cysteine (0.015 to 1.0 mM E64), metallo- (0.015 to 5.0 mM EDTA), serine (0.1 to 10 mM phenylmethylsulfonyl fluoride [PMSF], 0.01 to 10 mM diisopropylfluorophosphate [DFP], 0.015 to 1 mM 4-(2-aminoethyl) benzenesulfonyl fluoride [AEBSF]), or aspartic (0.01 to 1.5 mM pepstatin A) protease inhibitor each was applied for 15 min at 37°C. As negative controls, protein extracts were treated identically with the respective protease inhibitor diluents.
Labeling of trichomonads.
Labeling of trichomonads was performed as previously described (15). Briefly, trichomonads were harvested in the late logarithmic phase of growth and inoculated into modified Diamond's media containing 4 μCi/ml [3H]thymidine (17 Ci/mmol) (American Radiolabeled Chemicals). After 36 h, the radiolabeled trichomonads were washed three times by centrifugation (250 × g) and reconstituted in HBSS to remove unassociated radioactive tracer. Trichomonads were counted using a hemocytometer and resuspended in IPEC-J2 media at the desired concentrations.
Coculture adhesion assay.
Adhesion assays were performed as described previously (15). For coculture adhesion assays, 20 × 106 [3H]thymidine-labeled T. foetus cells were inoculated into the apical media of confluent, polarized (transepithelial electrical resistance, ≥2,000 Ω · 4.67 cm2) IPEC-J2 epithelial monolayers seeded onto polycarbonate inserts. Cocultures were incubated at 37°C in 5% CO2 for 6 h. Following adhesion, monolayers were washed twice with sterile HBSS to remove unbound trichomonads. The inserts then were excised using a scalpel blade and placed in 20-ml scintillation vials containing Econo 2 fluid (Fisher Scientific, Pittsburgh, PA). Radioactive emissions were counted using a Wallac 1209 liquid scintillation counter and expressed in counts per minute (cpm). Radioactive emissions measured from serial dilutions of the radiolabeled trichomonads were used for each assay to generate a standard curve of cpm per trichomonad. Numbers of trichomonads adhered to cell monolayers were calculated by applying the cpm measured to the standard curve.
Light microscopy.
For examination of T. foetus-induced epithelial cytotoxicity, IPEC-J2 cells were grown to confluence on chamber slides and inoculated with 10 × 106 T. foetus cells that were pretreated with E64 (300 μM) or vehicle (dH20). Uninfected epithelial cells and cells infected with P. hominis were incubated for up to 36 h at 37°C and used in control experiments. At the end of the incubation period, the wells were gently washed twice with warm HBSS to removed nonadherent trichomonads and cellular debris. The remaining epithelial cells were fixed with 10% neutral buffered formalin for 10 min at room temperature (RT) prior to examination using a Nikon inverted light phase-contrast microscope.
Crystal violet assay.
To provide a quantitative analysis of T. foetus-induced epithelial cytotoxicity, IPEC-J2 cells were grown to confluence on 24-well polystyrene plates and either left uninfected or infected with 10 × 106 T. foetus or P. hominis cells at 37°C for periods ranging from 0 to 36 h. At the end of the desired incubation period, the epithelial monolayers were gently washed with HBSS to remove detached epithelial cells, fixed with 2% paraformaldehyde in PBS for 15 min at RT, washed with HBSS, and stained with 100 μl of 0.13% crystal violet solution dissolved in a 5:2 (vol/vol) ethanol-paraformaldehyde solution. The monolayers were gently washed twice with dH20 and allowed to air dry. Stained cells were solubilized in 100 μl 1% SDS in 50% ethanol and transferred to 96-well plates. The intensity of staining was quantified using a spectrophotometer at a wavelength of 570 nM with a reference wavelength of 650 nM to account for optical interference.
Apoptosis signaling.
For examining the effect of T. foetus on apoptosis signaling by intestinal epithelial cells, monolayers of IPEC-J2 epithelial cells were grown to confluence on polycarbonate inserts and then infected with T. foetus (20 × 106) for durations of 0, 6, 12, 24, and 36 h. T. foetus cells were left untreated or were pretreated with the cysteine protease inhibitor E64 (300 μM) or vehicle (sterile dH20) for 2 h at 37°C and washed with HBSS prior to infection of IPEC-J2 cell monolayers. The isolated effect of T. foetus secretory proteins was determined by coculture of IPEC-J2 cells with 20 × 106 T. foetus cells that were separated at a distance of 2 to 3 mm from the surface of the epithelial monolayer by superimposition of a 10-μm-thick culture plate insert (0.4-μm pore size; Corning Incorporated, Lowell, MA) or by inoculation of epithelial monolayers with conditioned media containing 2 mg/ml secreted proteins of T. foetus. T. foetus conditioned media was prepared as previously described, with minor modifications (21, 22), and using numbers of T. foetus (i.e., 20 × 106 trichomonads) identical to those used for concurrent coculture studies. Briefly, T. foetus cells were washed once in HBSS and then incubated in 250 μl DMEM-F12 supplemented with 10 mM l-cysteine hydrochloride and 10 mM ascorbic acid (pH 7.2) at 37°C for 2 h. After incubation, trichomonads were centrifuged at 1,000 × g for 10 min, and the supernatant was aspirated and filtered through a 0.22-μm-pore-size filter. The filtered supernatant was centrifuged at 15,000 × g for 10 min at 4°C, inoculated into 750 μl IPEC media, and added to the apical surface of the IPEC monolayer. Supernatants were used only when obtained from trichomonads that retained a minimum of 95% motility as assessed by light microscopy. The effect of experimental conditions on trichomonad viability was examined by counting the number of trichomonads present at the endpoint of coculture.
Epithelial monolayers were disrupted with radioimmunoprecipitation assay (RIPA) lysis buffer containing protease inhibitors (halt protease inhibitor; Thermo Fisher Scientific, Rockford, IL). Protein lysates were extracted and quantified as previously described for trichomonads. The lysates were treated with a reducing agent (NuPAGE reducing agent; Life Technologies, Carlsbad, CA) and LDS buffer (Life Technologies, Carlsbad, CA) and heated at 70°C for 10 min prior to electrophoresis using 4 to 12% Bis-Tris polyacrylamide gels (Life Technologies, Carlsbad, CA) at 200 V for 1 h. Proteins were transferred to nitrocellulose membranes at 30 V for 1 h. Following transfer, nitrocellulose membranes were blocked overnight in blocking buffer (Starting Block T20; Thermo Fisher Scientific, Rockford, IL) at 4°C. Immunoblotting was performed using M30 cytodeath primary antibody (1:500; Roche Diagnostics, Indianapolis, IN) in Tris-buffered saline with Tween for 4 h at 25°C, followed by goat anti-mouse horseradish peroxidase-conjugated antibody (1:10,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) for 30 min at 25°C. Immunoblots were developed using an enhanced chemiluminescent substrate (Thermo Fisher Scientific, Rockford, IL) and exposed to radiographic film. Quantitative densitometric analysis of M30 protein bands was performed using SigmaScan software (Systat, Inc., Chicago, IL) and expressed in arbitrary units. In all assays, Cryptosporidium parvum-infected IPEC-J2 cells and untreated cells were used as positive and negative controls, respectively, for M30 expression.
Statistical analysis.
All data were analyzed for normality (Kolmogorov-Smirnov) and variance (Levene median) using a statistical software package and tested for significance using parametric or nonparametric tests as appropriate (Systat, Inc., Chicago, IL). Parametric data were analyzed using Student's t test or one-way analysis of variance (ANOVA) with a post hoc Holm-Sidak test. Nonparametric data were analyzed using Mann-Whitney rank-sum test or Kruskal-Wallis ANOVA on ranks. Results are reported as means ± standard deviations (SD). For all analyses, P ≤ 0.05 was considered significant.
RESULTS
T. foetus induces cytopathic effects on intestinal epithelial cells.
The mechanisms of disease pathogenesis of intestinal trichomonad infection are poorly understood. Therefore, we initially sought to determine if feline T. foetus induces direct cytopathic effects on intestinal epithelial cells. For these studies, confluent monolayers of IPEC-J2 cells were grown in chamber slides, infected with log-phase trichomonads for durations ranging from 0 to 36 h and then examined by means of light microscopy. Infection of IPEC-J2 cells with T. foetus resulted in a progressive destruction of the epithelial monolayer with numerous trichomonads observed adhering to the cells that remained. Compared to T. foetus, an isolate of feline Pentatrichomonas hominis, a presumably nonpathogenic trichomonad, adhered poorly to IPEC-J2 cells; following infection, the epithelial monolayers remained largely intact (Fig. 2).
FIG 2.

Feline T. foetus induces cytopathic effects on intestinal epithelial cells. IPEC-J2 cells were grown to confluence on chamber slides in the absence (A) or presence of coculture with feline T. foetus (B) or feline P. hominis (C) for a duration of 36 h. (B) T. foetus results in extensive destruction of the monolayer. Trichomonads (arrowhead) are observed adhering to the glass slide in areas that have become devoid of epithelial cells. (C) P. hominis infection is not associated with overt monolayer destruction, and trichomonads are observed to adhere to the epithelial cells (arrowheads) (×40 magnification).
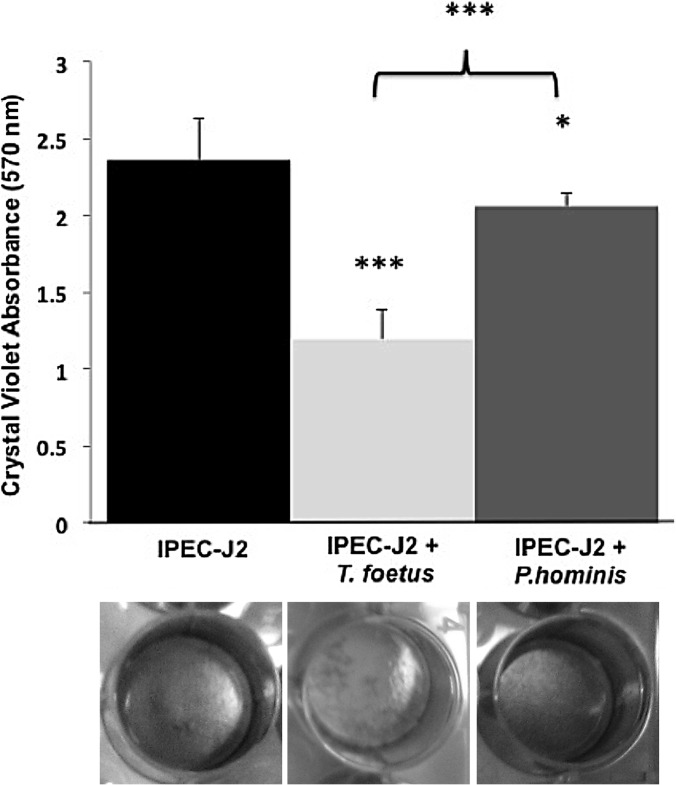
In order to quantitatively evaluate the cytotoxic effect of T. foetus on the intestinal epithelium, monolayers were cultivated in 24-well culture plates and stained with crystal violet after 36 h of coculture with feline T. foetus or P. hominis. Based on loss of crystal violet absorbance, T. foetus severely disrupted IPEC-J2 monolayers compared to uninfected monolayers. In contrast, P. hominis resulted in little destruction of the monolayer (Fig. 3).
FIG 3.
Spectrophotometric analysis of crystal violet absorbance by IPEC-J2 monolayers following coculture in the absence (IPEC-J2) or presence of feline T. foetus (IPEC-J2 + T. foetus) or feline P. hominis (IPEC-J2 + P. hominis) for 36 h. Data represent n = 6 cultures per treatment and are reported as means ± SD. P < 0.05 (*) and P < 0.001 (***) compared to uninfected IPEC-J2 monolayers; ***, P < 0.001 for T. foetus-infected monolayers compared to P. hominis-infected monolayers. (Determined by one-way ANOVA and post hoc Holm-Sidak test.) Representative wells of each treatment condition after staining with crystal violet are below the column of each treatment group.
T. foetus activates apoptosis of intestinal epithelial cells.
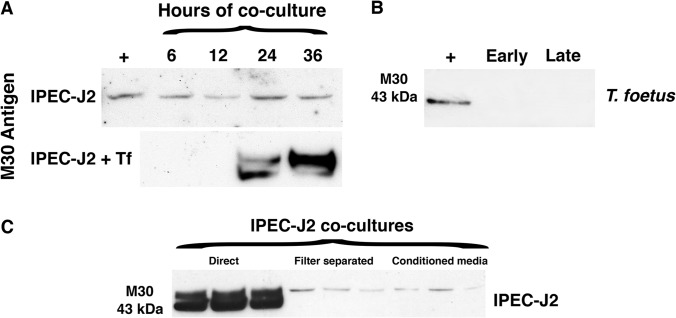
To establish a general and quantifiable mechanism for T. foetus-induced epithelial cytopathogenicity, infected IPEC-J2 cells were further examined for evidence of activation of apoptosis. For these studies, we immunoblotted T. foetus-infected IPEC-J2 monolayers for the presence of a specific epitope of cytokeratin 18 (M30) that is generated by caspase-mediated cleavage. M30 antigen was observed in IPEC-J2 cell lysates beginning at 24 h after infection with T. foetus, with maximal cleavage occurring at 36 h (Fig. 4A). In contrast, M30 antigen was detected at only low levels in uninfected IPEC-J2 cells over the same time period. M30 reactivity was not detected in protein lysates from trichomonads alone at any phase of growth, thereby identifying M30 as being generated by the intestinal epithelial cells under coculture conditions (Fig. 4B).
FIG 4.
T. foetus induces contact-dependent activation of apoptosis in intestinal epithelial cells. (A) Immunoblot of IPEC-J2 cells for the M30 antigen of cleaved cytokeratin 18 after incubation with T. foetus for 6, 12, 24, and 36 h (termed IPEC-J2 + Tf). (B) M30 was not detected in isolated T. foetus trophozoites in either log-phase cultures (early) or overgrown cultures containing large numbers of dead organisms (late). (C) Immunoblot for the M30 antigen following 36 h of coculture of IPEC-J2 monolayers in direct contact with T. foetus, indirect contact with T. foetus (filter separated), or T. foetus conditioned media. Each condition was performed in triplicate. The positive control (+) is IPEC-J2 cells postinfection with Cryptosporidium parvum.
T. foetus cytopathic effects require direct contact with epithelial cells.
Given the presumed importance of trichomonad-host adhesion to disease pathogenesis in venereal infection, we next sought to determine if the cytopathic effects of T. foetus were mediated by direct interaction of trophozoites with intestinal epithelial cells versus a soluble mediator released by T. foetus. In contrast to the proapoptotic effects observed when T. foetus was directly cocultured with IPEC-J2 monolayers, separation of T. foetus from direct contact with the monolayers by imposition of a filter largely prevented apoptosis activation. Separation of T. foetus from the epithelial monolayer had no effect on the viability or number of trichomonads surviving over the 36-h period of coculture (the number of T. foetus cells was 1.1 × 106 ± 0.39 × 106 in direct coculture and 1.0 × 106 ± 0.28 × 106 cells in separated coculture). There was a similar lack of cytokeratin cleavage (M30) when T. foetus-conditioned media alone (containing 2 mg protein in 1 ml) was used to infect IPEC-J2 monolayers (Fig. 4C).
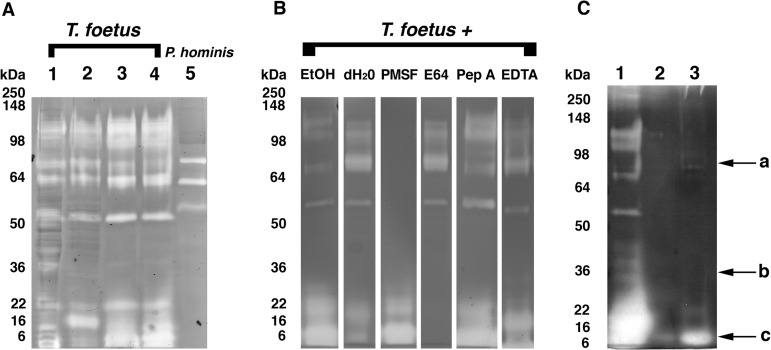
Feline T. foetus expresses multiple protease activities.
Cellular proteases are commonly implicated in the pathogenicity of trichomonads that cause venereal disease; however, their role in mediating cytopathic effects of gastrointestinal trichomonads has not been investigated. To characterize the protease activities of feline intestinal trichomonads, whole-cell protein lysates from 4 different feline T. foetus isolates and 1 feline P. hominis isolate were separated under nondenaturing and nonreducing conditions in 10% Tris-glycine gels containing 0.1% gelatin. The proteolytic activities of each of the feline T. foetus isolates were similar and characterized by at least five different bands of proteolysis, having nondenatured molecular masses of approximately 52, 65, 85, 110, and 120 kDa, and a broader coalescing band of proteolysis with molecular masses of ≤30 kDa (Fig. 5A). Compared to T. foetus, whole-cell lysates of P. hominis revealed weak proteolysis at bands corresponding to nondenatured molecular masses of approximately 54, 64, and 85 kDa. The lower-molecular-mass proteolytic bands observed in feline T. foetus isolates were absent from P. hominis (Fig. 5A).
FIG 5.
Protease activities of feline T. foetus are attributed to serine and cysteine proteases. (A) Representative gelatin zymography of cellular protein lysates (40 μg each) from feline trichomonad isolates. Lanes 1 to 4, T. foetus isolates (A, D, F, and Sti) from four different domestic cats; lane 5, feline P. hominis. (B) The effect of inhibitors on protease activity of cellular protein lysates (40 μg each) from T. foetus (isolate A). Lanes 1 and 2, no inhibitors (vehicle-treated controls; ethanol [EtOH], diluent for pepstatin A and PMSF; dH2O, diluent for E64 and EDTA); lane 3, 5 mM PMSF; lane 4, 300 μM E64; lane 5, 10 μM pepstatin A; lane 6, 5 mM EDTA. (C) Lane 1, 40 μg of cellular protein lysate from T. foetus (isolate A); lane 2, 40 μg of secretory product from the corresponding isolate; lane 3, 275 μg of secretory product from corresponding isolate (arrow a, serine protease activity; arrow b, presumptive location of CP30/CP8; arrow c, cysteine protease activity). Results observed with isolate A are representative of assays performed concurrently with isolates D, F, and Sti.
T. foetus protease activities are attributed to serine and cysteine proteases.
To determine the identity of the cellular protease activities produced by feline T. foetus and P. hominis, protein lysates were treated with class-specific protease inhibitors or their diluents prior to use in substrate-gel electrophoresis. Treatment of both T. foetus and P. hominis protein lysates with PMSF (5 mM) or DFP (1 mM) inhibited proteolytic bands of 50 kDa and larger, thereby identifying these activities as attributed to serine proteases. The molecular mass bands of ≤30 kDa observed in T. foetus and not in P. hominis protein lysates were inhibited by pretreatment with E64, identifying these activities as attributed to cysteine proteases (Fig. 5B). Failure of pepstatin A or EDTA to inhibit the proteolytic activity of either T. foetus or P. hominis suggested the absence of active aspartic proteases or metalloproteases in whole-cell lysates of these trichomonads. When live trichomonads rather than protein lysates were treated with each protease inhibitor, identical inhibitory effects on gel protease activities were observed.
T. foetus cysteine protease activity is predominantly cell associated.
Because T. foetus cytopathic effects were observed to be dependent on direct contact with the intestinal epithelium, we next sought to determine if either the cysteine or serine protease activities of T. foetus were largely cell associated rather than secreted. Accordingly, we compared the protease activities of whole-cell lysates of T. foetus to those obtained using T. foetus conditioned media alone. At equal total protein contents, serine protease activity was demonstrated in both whole-cell-associated and secretory fractions. However, ∼7-fold greater amounts of secreted versus whole-cell protein lysate were required to demonstrate secreted cysteine protease activity (Fig. 5C).
T. foetus cysteine proteases mediate intestinal epithelial cytotoxicity.
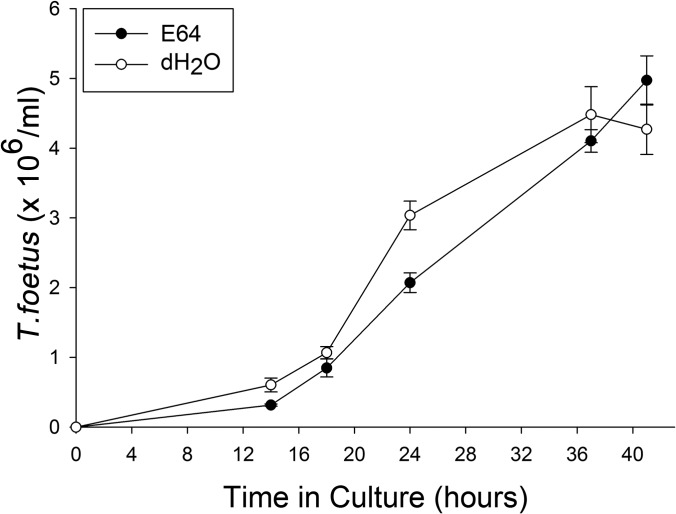
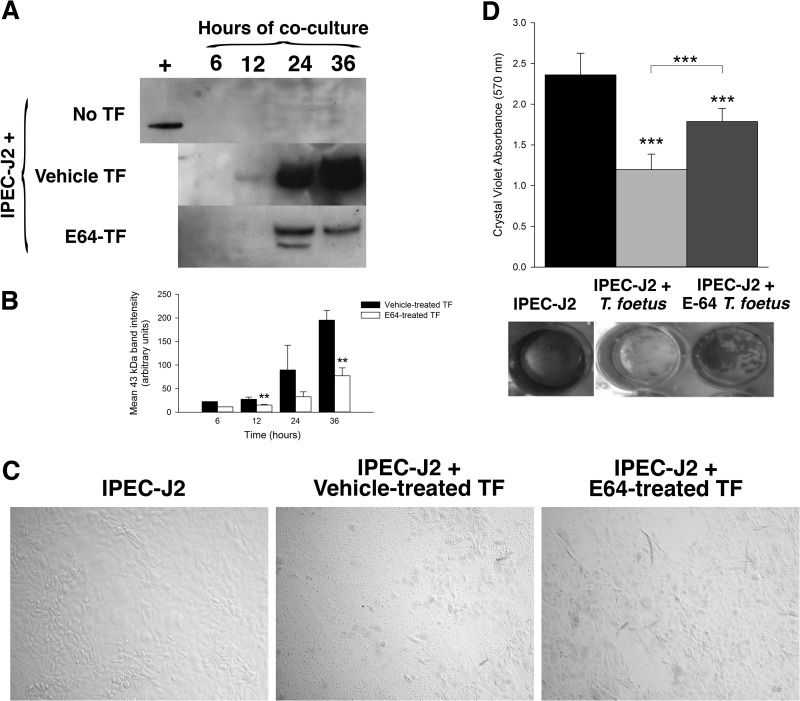
To determine if T. foetus cysteine protease activity mediates intestinal epithelial cytotoxicity, live T. foetus organisms were pretreated with E64 (300 μM) or diluent (dH20) for a duration of 2 h prior to coculture in direct contact with IPEC-J2 cells. Treatment of T. foetus alone with E64 had no adverse effect on motility or growth of the trichomonads compared to treatment with vehicle alone for periods of up to 48 h (Fig. 6). However, inhibition of T. foetus cysteine protease activity prior to coculture with IPEC-J2 cells significantly reduced epithelial cell apoptosis signaling, as demonstrated by diminished cleavage of cytokeratin 18 (Fig. 7A and B). Light microscopic examination of IPEC-J2 cells after infection with E64-treated T. foetus revealed amelioration of monolayer destruction (Fig. 7C). Quantitative examination of monolayer destruction using crystal violet revealed a significant sparing of the monolayers when IPEC-J2 cells were infected with each of three different isolates of E64-treated T. foetus compared to monolayers infected with vehicle-treated T. foetus (Fig. 7D and Fig. 8). The contribution of T. foetus cell-associated serine proteases to epithelial toxicity could not be ascertained in coculture, because treatment of the trichomonads with serine protease inhibitors (5 mM PMSF or 1 mM DFP) at concentrations required to neutralize protease activity was lethal to the trophozoites.
FIG 6.
Growth curve of feline T. foetus (TF) in the presence of the cysteine protease inhibitor E64 (300 μM) or vehicle (deionized water; dH2O) over a period of 48 h. Data points represent the means ± standard deviations from 3 replicate cultures at each time point.
FIG 7.
Cysteine protease activity mediates T. foetus cytopathogenicity. T. foetus was pretreated with the cysteine protease inhibitor E64 (300 μM) or vehicle (dH20) prior to coculture with IPEC-J2 monolayers for 6, 12, 24, or 36 h. (A) Immunoblot of uninfected IPEC-J2 monolayers and monolayers following 36 h of coculture with vehicle-pretreated T. foetus or E64-pretreated T. foetus for the presence of the M30 antigen of cleaved cytokeratin 18. (B) Densitometric analysis of the 3 immunoblots shown in panel A. **, P < 0.01 compared to vehicle-treated T. foetus (one-way ANOVA and post hoc Holm-Sidak test). Data represent n = 3 cultures per treatment and are reported as means ± SD. (C) Representative light microscopy images of uninfected IPEC-J2 monolayers and monolayers following 36 h of coculture with vehicle-treated T. foetus and E64-treated T. foetus (×40 magnification). (D) Spectrophotometric analysis of crystal violet absorbance of IPEC-J2 monolayers following coculture with vehicle-treated T. foetus or E64-treated T. foetus (A isolate) for 36 h. Representative wells are shown below treatment group columns. ***, P < 0.001 for vehicle-treated T. foetus compared to E64-treated T. foetus and compared to uninfected IPEC-J2 monolayers (one-way ANOVA and post hoc Holm-Sidak test). Data represent n = 6 cultures per treatment group and are representative of 3 different feline T. foetus isolates. Data are reported as means ± SD. Data reported from uninfected and T. foetus-infected monolayers are also shown in Fig. 3.
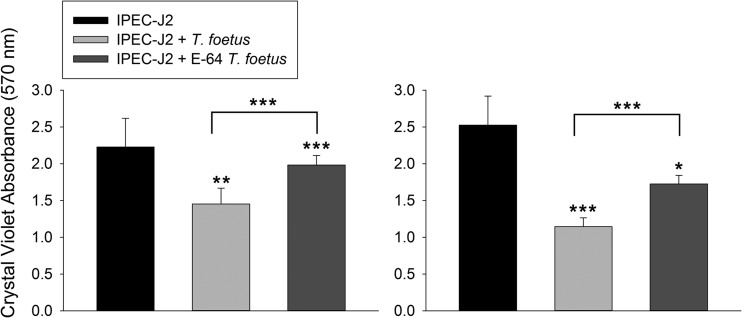
FIG 8.
Cysteine protease activity mediates T. foetus cytopathogenicity. Spectrophotometric analysis of crystal violet absorbance of IPEC-J2 monolayers following coculture for 20 to 28 h with two different strains (F and Sti) of vehicle-treated T. foetus (dH2O) or T. foetus pretreated with E64 (300 μM). Asterisks above bars are comparisons to uninfected IPEC-J2 cells. Comparisons between vehicle-treated T. foetus and T. foetus pretreated with E64 are depicted by overhanging lines. ***, P < 0.001; **, P < 0.01; *, P < 0.05 (one-way ANOVA and post hoc Holm-Sidak test). Data represent n = 4 to 8 cultures per treatment group and are reported as means ± SD.
T. foetus cysteine protease mediates cytopathic effects by promoting adhesion to intestinal epithelial cells.
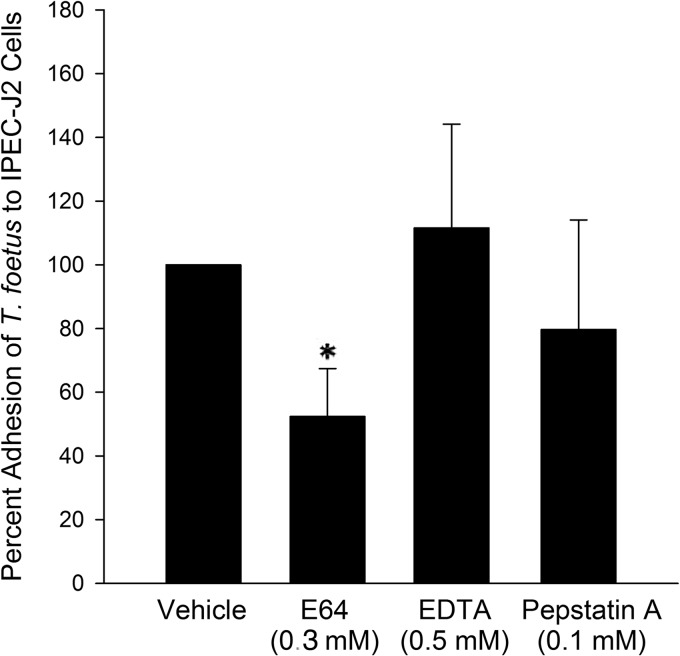
Based on our observations that T. foetus cytotoxicity required direct interaction with the epithelium and was dependent on cysteine protease activity, as well as cysteine protease activity being predominantly cell associated, we hypothesized that cysteine proteases mediate their cytopathic effects by promoting adhesion of T. foetus to the intestinal epithelium. To test this hypothesis, we determined the effect of cysteine protease inhibition on adhesion of [3H]thymidine-labeled T. foetus to IPEC-J2 epithelial monolayers. Inhibition of T. foetus cysteine protease activity by pretreatment with E64 (300 μM) significantly blocked adhesion of trichomonads to the intestinal epithelium (Fig. 9). This effect was specific to cysteine proteases, because pretreatment of T. foetus with metalloprotease (500 μM EDTA) and aspartic protease (100 μM pepstatin A) inhibitors at standard inhibitory concentrations had no significant effect on adhesion compared to that of T. foetus treated with vehicle alone. The contribution of T. foetus cell-associated serine proteases to epithelial adhesion could not be ascertained, because treatment of the trichomonads with serine protease inhibitors PMSF and AEBSF at concentrations required to neutralize protease activity was lethal to the trophozoites.
FIG 9.
Adhesion of T. foetus to intestinal epithelial monolayers is mediated by cysteine protease activity. Adhesion of [3H]thymidine-labeled T. foetus to IPEC-J2 monolayers is significantly reduced following pretreatment of trichomonads with the cysteine protease inhibitor E64 compared to vehicle-treated (dH20) T. foetus. No effect is observed on T. foetus adhesion following pretreatment using protease inhibitors of other classes. *, P < 0.05 compared to vehicle-treated T. foetus (Mann-Whitney rank-sum test). Each data point represents 4 to 11 cultures per treatment.
DISCUSSION
Considerable research has been devoted to understanding the cellular mechanisms of T. vaginalis and T. foetus pathogenicity in the reproductive tract. To the authors' knowledge, this study is the first to investigate the mechanisms of T. foetus pathogenicity in the intestinal tract. We have previously demonstrated both in vivo (6) and using an in vitro coculture model system (15) that feline T. foetus has an affinity for adhering to the intestinal epithelium. In these studies, we used the in vitro coculture model system to investigate the cytotoxic effects of feline T. foetus on intestinal epithelial cells, the dependence of T. foetus pathogenicity on adhesion to the epithelium, and the role of T. foetus cellular proteases in mediating these effects.
These studies were performed using a nontransformed porcine intestinal epithelial cell line (IPEC-J2) to model the intestinal epithelium. Because currently there are no feline intestinal epithelial cell lines with which to conduct these studies, porcine intestinal epithelial cells represented a compelling alternative, as feline T. foetus and the trichomonad of pigs, Tritrichomonas suis, are highly similar organisms that both demonstrate a unique tropism for the gastrointestinal tract (7, 8). Using this approach, we demonstrated that T. foetus causes progressive cytotoxicity to the intestinal epithelium characterized by progressive loss of cells from the monolayer. An ongoing loss of colonic epithelial cells in vivo in cats naturally infected by T. foetus is supported by histological lesions that demonstrate attenuation of the surface epithelium in conjunction with cellular hyperplasia and hypertrophy and increased mitotic activity within the crypts (23).
Further investigation into the cytotoxic effects of feline T. foetus on the intestinal epithelial cells demonstrated activation of apoptosis signaling in association with cell loss. Moreover, these cytotoxic effects were dependent on a direct interaction of T. foetus with the intestinal epithelium and could not be recapitulated using T. foetus conditioned media alone. Although the focus of this study was not to fully characterize the mechanism of epithelial cell death induced by feline T. foetus, activation of apoptosis has also been described in human vaginal and Caco-2 epithelial cells following infection with T. vaginalis (24, 25) and bovine vaginal and oviduct epithelial cells following infection with bovine T. foetus (26, 27). Further work is needed to determine the exact signaling mechanisms of cell death induced by feline T. foetus.
Based on a precedential interest in the role of cellular proteases in mediating the pathological effects of trichomonads that infect the reproductive tract, we focused on characterizing the protease activities of several different feline T. foetus isolates. Feline T. foetus isolates produced multiple and similar protease activities that were functionally attributed to serine and cysteine proteases. In particular, the cysteine proteases are recognized as able mediators of cytotoxicity in coculture models of venereal trichomonosis (16, 24, 28) and can be found in vaginal secretions from women infected with T. vaginalis (29). Cysteine protease inhibitors were recently demonstrated to ameliorate cytotoxicity in a murine model of venereal trichomonosis (19), making this class of proteases an attractive target for investigation as a virulence factor of feline T. foetus. In testing this hypothesis, we pretreated T. foetus with the cysteine protease inhibitor E64 prior to infection of the intestinal epithelium. E64 was chosen in these assays because it is irreversible, cell permeable, nontoxic, and demonstrated an ability to inhibit all feline T. foetus cysteine protease activity based on substrate-gel electrophoresis. Under these conditions, subsequent enterocyte apoptosis activation and monolayer destruction was significantly ameliorated. Using our model system, we were unable to examine the influence of T. foetus cell-associated serine proteases on epithelial adhesion or cytotoxicity, because all tested inhibitors were lethal to T. foetus when used at concentrations required to neutralize serine protease activity.
Adhesion to the host epithelium is a critical first step toward establishing infection and mediating host cell contact-dependent cytotoxicity in venereal trichomonosis (30). Inhibition of T. vaginalis and bovine T. foetus adhesion results in a significant reduction in cytotoxicity to human and bovine vaginal epithelial cell monolayers, respectively (31–33). Two cysteine proteases (CP30 and CP65) have been identified on the plasma membrane surface of T. vaginalis and demonstrated in vitro to promote binding of the organisms directly to the surface of HeLa cell monolayers (28, 34). In bovine T. foetus, the role of cysteine proteases as adhesion proteins has not been shown but is suggested by studies in a mouse model of venereal trichomonosis where inhibition of T. foetus cysteine protease activity resulted in reduced genital colonization (19). To determine if cysteine proteases mediate the cytotoxic effects of feline T. foetus by promoting adhesion of trichomonads to the intestinal epithelium, we examined the effect of cysteine protease inhibition on adhesion of radiolabeled T. foetus to intestinal epithelial monolayers using a well-described coculture assay (15). Inhibition of feline T. foetus cysteine protease activity blocked adhesion of T. foetus to the intestinal epithelium and significantly inhibited activation of apoptosis signaling and monolayer destruction. Cysteine proteases are unlikely to be the only mediators of feline T. foetus adhesion, as E64 inhibited adhesion of approximately 50% of the trophozoites. It remains unknown whether feline T. foetus cysteine proteases directly mediate adhesion to the intestinal epithelium or rather induce the expression of other adhesins responsible for this effect. One could speculate that reduced adherence and cytotoxicity is not due directly to a cysteine protease ligand-dependent binding but altered cell function that reduces the expression of other parasite binding molecules, like sialic acid-binding lectins and lipophosphoglycan-like molecules (35, 36), or reduced production of secreted proteases or toxins close to the surface of the epithelial cell that are dependent on cysteine protease activity. Cysteine proteases of T. vaginalis and bovine T. foetus have been demonstrated in vitro to contribute to extracellular matrix degradation, epithelial cell detachment, induction of host cell apoptosis, complement and antibody destruction, and phagocytosis of host cell elements (22, 28, 37–40). Accordingly, it seems likely that the cytopathic effects of cysteine proteases in feline T. foetus are multifactorial.
Under the present study conditions, the cysteine proteases of feline T. foetus appeared to be predominantly cell associated. Physical separation of T. foetus from the intestinal epithelium or the addition of conditioned media containing 7-fold greater amounts of protein than required to demonstrate soluble cysteine protease activity did not result in demonstrable cytopathic effects toward the intestinal epithelium. Cysteine proteases identified in secretions of venereal trichomonads have been demonstrated to directly contribute to host cell destruction in human and bovine trichomonosis (19, 24). Therefore, while the present study identifies the cell-associated cysteine proteases as largely responsible for the cytopathic effects of feline T. foetus in coculture with intestinal epithelial cells, a pathogenic role for secreted cysteine proteases in alternative models of infection or in vivo cannot be discounted.
It is compelling to speculate that the presence or identity of cysteine proteases could account for differences in pathogenicity between species of trichomonads. While these studies were not designed to answer this question, we found it notable that a feline isolate of Pentatrichomonas hominis, a presumably nonpathogenic intestinal trichomonad with previously reported poor adherence capabilities (15), had no detectable cysteine protease activity and was significantly less destructive to intestinal epithelial cell monolayers. Determining any association between cysteine protease activity and cytotoxicity among the feline T. foetus isolates examined in this study was not possible due to the lack of variation in cysteine protease activity among the isolates. Fifteen cysteine protease genes have been identified within the genome of bovine T. foetus (41). Differential expression levels of these cysteine proteases suggests individualized roles for these proteases in the tactics of the parasite (7). A comparative analysis of 8 different cysteine protease coding regions demonstrated 100% identity among feline isolates. However, Slapeta et al. demonstrated a 1.19% nucleotide difference among the cysteine protease genes of feline compared to bovine strains of T. foetus, suggesting these genes play a role in adaptation to their preferred host or niche within the host (7). Future work will be required to determine the identities and exact molecular masses of the cysteine proteases responsible for mediating the pathogenic effects of feline T. foetus and whether or not they are comparable to those identified for bovine isolates.
Despite a high prevalence, worldwide distribution of infection, and lack of consistently effective drugs for treatment, the cellular mechanisms of feline T. foetus intestinal epithelial pathogenicity have never been studied before (6, 9, 11, 14). In these studies, we have identified a central role for cysteine proteases in promoting adhesion-dependent cytotoxicity of feline T. foetus to the intestinal epithelium. These results support further investigation of the cysteine proteases as virulence factors in vivo and as potential therapeutic targets for ameliorating the clinical signs of feline intestinal trichomonosis in cats with antimicrobial-resistant T. foetus infection.
ACKNOWLEDGMENTS
This work was supported by a grant from the Morris Animal Foundation (grant no. D08FE-04) and the North Carolina Veterinary Medical Foundation's Support for T. foetus Research Innovation and Veterinary Education (STRIVE) fund. M.K.T. was supported by a Ruth L. Kirschstein National Research Service Award (T32 RR024394) as part of North Carolina State University's Comparative Medicine and Translational Research Training Program.
Footnotes
Published ahead of print 21 April 2014
REFERENCES
- 1.Schwebke JR, Burgess D. 2004. Trichomoniasis. Clin. Microbiol. Rev. 17:794–804. 10.1128/CMR.17.4.794-803.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. 2005. Prevalence and incidence of selected sexually transmitted infections, Chlamydia trachomatis, Neisseria gonorrhoeae, syphilis and Trichomonas vaginalis: methods and results used by WHO to generate 2005 estimates. World Health Organization, Geneva, Switzerland [Google Scholar]
- 3.Slapeta J, Craig S, McDonell D, Emery D. 2010. Tritrichomonas foetus from domestic cats and cattle are genetically distinct. Exp. Parasitol. 126:209–213. 10.1016/j.exppara.2010.04.024 [DOI] [PubMed] [Google Scholar]
- 4.Gray SG, Hunter SA, Stone MR, Gookin JL. 2010. Assessment of reproductive tract disease in cats at risk for Tritrichomonas foetus infection. Am. J. Vet. Res. 71:76–81. 10.2460/ajvr.71.1.76 [DOI] [PubMed] [Google Scholar]
- 5.Levy MG, Gookin JL, Poore M, Birkenheuer AJ, Dykstra MJ, Litaker RW. 2003. Tritrichomonas foetus and not Pentatrichomonas hominis is the etiologic agent of feline trichomonal diarrhea. J. Parasitol. 89:99–104. 10.1645/0022-3395(2003)089[0099:TFANPH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 6.Gookin JL, Levy MG, Law JM, Papich MG, Poore MF, Breitschwerdt EB. 2001. Experimental infection of cats with Tritrichomonas foetus. Am. J. Vet. Res. 62:1690–1697. 10.2460/ajvr.2001.62.1690 [DOI] [PubMed] [Google Scholar]
- 7.Slapeta J, Müller N, Stack CM, Walker G, Lew-Tabor A, Tachezy J, Frey CF. 2012. Comparative analysis of Tritrichomonas foetus (Riedmüller, 1928) cat genotype, T. foetus (Riedmüller, 1928) cattle genotype and Tritrichomonas suis (Davaine, 1875) at 10 DNA loci. 1. Int. J. Parasitol. 42:1143–1149. 10.1016/j.ijpara.2012.10.004 [DOI] [PubMed] [Google Scholar]
- 8.Mostegl MM, Richter B, Nedorost N, Maderner A, Dinhopl N, Weissenböck H. 2011. Investigations on the prevalence and potential pathogenicity of intestinal trichomonads in pigs using in situ hybridization. Vet. Parasitol. 178:58–63. 10.1016/j.vetpar.2010.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gookin JL, Stauffer SH, Dybas D, Cannon DH. 2010. Documentation of in vivo and in vitro aerobic resistance of feline Tritrichomonas foetus isolates to ronidazole. J. Vet. Intern. Med. 24:1003–1007. 10.1111/j.1939-1676.2010.0534.x [DOI] [PubMed] [Google Scholar]
- 10.Gookin JL, Stauffer SH, Coccaro MR, Poore MF, Levy MG, Papich MG. 2007. Efficacy of tinidazole for treatment of cats experimentally infected with Tritrichomonas foetus. Am. J. Vet. Res. 68:1085–1088. 10.2460/ajvr.68.10.1085 [DOI] [PubMed] [Google Scholar]
- 11.Kather EJ, Marks SL, Kass PH. 2007. Determination of the in vitro susceptibility of feline Tritrichomonas foetus to 5 antimicrobial agents. J. Vet. Intern. Med. 21:966–970. 10.1111/j.1939-1676.2007.tb03050.x [DOI] [PubMed] [Google Scholar]
- 12.Rosado TW, Specht A, Marks SL. 2007. Neurotoxicosis in 4 cats receiving ronidazole. J. Vet. Intern. Med. 21:328–331. 10.1111/j.1939-1676.2007.tb02968.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gookin JL, Copple CN, Papich MG, Poore MF, Stauffer SH, Birkenheuer AJ, Twedt DC, Levy MG. 2006. Efficacy of ronidazole for treatment of feline Tritrichomonas foetus infection. J. Vet. Intern. Med. 20:536–543. 10.1892/0891-6640(2006)20[536:EORFTO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 14.Gookin JL, Riviere JE, Gilger BC, Papich MG. 1999. Acute renal failure in four cats treated with paromomycin. J. Am. Vet. Med. Assoc. 215:1821–1823 [PubMed] [Google Scholar]
- 15.Tolbert MK, Stauffer SH, Gookin JL. 2013. Feline Tritrichomonas foetus adhere to intestinal epithelium by receptor-ligand-dependent mechanisms. Vet. Parasitol. 192:75–82. 10.1016/j.vetpar.2012.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh BN, Hayes GR, Lucas JJ, Beach DH, Gilbert RO. 2005. In vitro cytopathic effects of a cysteine protease of Tritrichomonas foetus on cultured bovine uterine epithelial cells. Am. J. Vet. Res. 66:1181–1186. 10.2460/ajvr.2005.66.1181 [DOI] [PubMed] [Google Scholar]
- 17.Arroyo R, Alderete JF. 1995. Two Trichomonas vaginalis surface proteinases bind to host epithelial cells and are related to levels of cytoadherence and cytotoxicity. Arch. Med. Res. 26:279–285 [PubMed] [Google Scholar]
- 18.Arroyo R, Alderete JF. 1989. Trichomonas vaginalis surface proteinase activity is necessary for parasite adherence to epithelial cells. Infect. Immun. 57:2991–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cobo ER, Reed SL, Corbeil LB. 2012. Effect of vinyl sulfone inhibitors of cysteine proteinases on Tritrichomonas foetus infection. Int. J. Antimicrobiol. Agents 39:259–262. 10.1016/j.ijantimicag.2011.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomford JW, Talbot JA, Ikeda JS, Corbeil LB. 1996. Characterization of extracellular proteinases of Tritrichomonas foetus. J. Parasitol. 82:112–117. 10.2307/3284125 [DOI] [PubMed] [Google Scholar]
- 21.Yu Y, Chadee K. 1997. Entamoeba histolytica stimulates interleukin 8 from human colonic epithelial cells without parasite-enterocyte contact. Gastroenterology 112:1536–1547. 10.1016/S0016-5085(97)70035-0 [DOI] [PubMed] [Google Scholar]
- 22.Talbot JA, Nielsen K, Corbeil LB. 1991. Cleavage of proteins of reproductive secretions by extracellular proteinases of Tritrichomonas foetus. Can. J. Microbiol. 37:384–390. 10.1139/m91-062 [DOI] [PubMed] [Google Scholar]
- 23.Yaeger M, Gookin JL. 2005. Histologic features associated with Tritrichomonas foetus-induced colitis in domestic cats. Vet. Pathol. 42:797–804. 10.1354/vp.42-6-797 [DOI] [PubMed] [Google Scholar]
- 24.Sommer U, Costello CE, Hayes GR, Beach DH, Gilbert RO, Lucas JJ, Singh BN. 2005. Identification of Trichomonas vaginalis cysteine proteases that induce apoptosis in human vaginal epithelial cells. J. Biol. Chem. 280:23853–23860. 10.1074/jbc.M501752200 [DOI] [PubMed] [Google Scholar]
- 25.Da Costa RF, de Souza W, Benchimol M, Alderete JF, Morgano-Diaz JA. 2005. Trichomonas vaginalis perturbs the junctional complex in epithelial cells. Cell Res. 15:704–716. 10.1038/sj.cr.7290340 [DOI] [PubMed] [Google Scholar]
- 26.Singh BN, Lucas JJ, Hayes GR, Kumar I, Beach DH, Frajblat M, Gilbert RO, Sommer U, Costello CE. 2004. Tritrichomonas foetus induces apoptotic cell death in bovine vaginal epithelial cells. Infect. Immun. 72:4151–4158. 10.1128/IAI.72.7.4151-4158.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Midlej V, Vilela R, Dias AB, Benchimol M. 2009. Cytopathic effects of Tritrichomonas foetus on bovine oviduct cells. Vet. Parasitol. 165:216–230. 10.1016/j.vetpar.2009.07.021 [DOI] [PubMed] [Google Scholar]
- 28.Mendoza-López MR, Becerril-Garcia C, Fattel-Facenda LV, Avila-Gonzalez L, Ruíz-Tachiquín ME, Ortega-Lopez J, Arroyo R. 2000. CP30, a cysteine proteinase involved in Trichomonas vaginalis cytoadherence. Infect. Immun. 68:4907–4912. 10.1128/IAI.68.9.4907-4912.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernández-Gutiérrez R, Avila-González L, Ortega-López J, Cruz-Talonia F, Gómez-Gutierrez G, Arroyo R. 2004. Trichomonas vaginalis: characterization of a 39-kDa cysteine proteinase found in patient vaginal secretions. Exp. Parasitol. 107:125–135. 10.1016/j.exppara.2004.05.004 [DOI] [PubMed] [Google Scholar]
- 30.Alderete JF, Garza GE. 1985. Specific nature of Trichomonas vaginalis parasitism of host cell surfaces. Infect. Immun. 50:701–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bastida-Corcuera FD, Okumura CY, Colocoussi A, Johnson PJ. 2005. Trichomonas vaginalis lipophosphoglycan mutants have reduced adherence and cytotoxicity to human ectocervical cells. Eukaryot. Cell 4:1951–1958. 10.1128/EC.4.11.1951-1958.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilbert RO, Elia G, Beach DH, Klaessig S, Singh BN. 2000. Cytopathogenic effect of Trichomonas vaginalis on human vaginal epithelial cells cultured in vitro. Infect. Immun. 68:4200–4206. 10.1128/IAI.68.7.4200-4206.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh BN, Lucas JJ, Beach DH, Shin ST, Gilbert RO. 1999. Adhesion of Tritrichomonas foetus to bovine vaginal epithelial cells. Infect. Immun. 67:3847–3854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solano-González E, Alvarez-Sánchez ME, Avila-González L, Rodríguez-Vargas VH, Arroyo R, Ortega-López J. 2006. Location of the cell-binding domain of CP65, a 65kDa cysteine proteinase involved in Trichomonas vaginalis cytotoxicity. Int. J. Biochem. Cell Biol. 38:2114–2127. 10.1016/j.biocel.2006.06.003 [DOI] [PubMed] [Google Scholar]
- 35.Babál P, Russell LC. 1999. Sialic acid-specific lectin-mediated adhesion of Tritrichomonas foetus and Tritrichomonas mobilensis. J. Parasitol. 85:33–40. 10.2307/3285696 [DOI] [PubMed] [Google Scholar]
- 36.Singh BN. 1993. Lipophosphoglycan-like glycoconjugate of Tritrichomonas foetus and Trichomonas vaginalis. Mol. Biochem. Parasitol. 57:281–294. 10.1016/0166-6851(93)90204-B [DOI] [PubMed] [Google Scholar]
- 37.Arroyo R, Engbring J, Alderete JF. 1992. Molecular basis of host epithelial cell recognition by Trichomonas vaginalis. Mol. Microbiol. 6:853–862. 10.1111/j.1365-2958.1992.tb01536.x [DOI] [PubMed] [Google Scholar]
- 38.Alvarez-Sánchez ME, Avila-González L, Becerril-García C, Fattel-Facenda LV, Ortega-López J, Arroyo R. 2000. A novel cysteine proteinase (CP65) of Trichomonas vaginalis involved in cytotoxicity. Microb. Pathog. 28:193–202. 10.1006/mpat.1999.0336 [DOI] [PubMed] [Google Scholar]
- 39.Provenzano D, Alderete JF. 1995. Analysis of human immunoglobulin-degrading cysteine proteinases of Trichomonas vaginalis. Infect. Immun. 63:3388–3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dailey DC, Chang TH, Alderete JF. 1990. Characterization of Trichomonas vaginalis haemolysis. J. Parasitol. 101:171–175. 10.1017/S0031182000063204 [DOI] [PubMed] [Google Scholar]
- 41.Huang KY, Shin JW, Huang PJ, Ku FM, Lin WC, Lin R, Hsu WM, Tang P. 2013. Functional profiling of the Tritrichomonas foetus transcriptome and proteome. Mol. Biochem. Parasitol. 187:60–71. 10.1016/j.molbiopara.2012.12.001 [DOI] [PubMed] [Google Scholar]