Abstract
Chronic tegumentary leishmaniasis is characterized by a scarcity of parasites in lesions and a heightened inflammatory response. Deregulated and hyperactive inflammation contributes to tissue damage and parasite persistence. The mechanisms by which immune cells are recruited to the lesion and their relationship to clinical outcomes remain elusive. We examined the expression levels of chemokines and their receptors in relation to clinical outcome in dermal leishmaniasis caused by Leishmania (Viannia) panamensis. Primary macrophages from healthy donors were infected with L. panamensis strains isolated from self-healing patients (n = 4) and those presenting chronic disease (n = 5). A consistent pattern of upregulation of neutrophil (cxcl1, cxcl2, cxcl5, and cxcl8/il-8) and monocyte (ccl2, ccl7, ccl8, cxcl3, and cxcl10) chemotactic chemokines and ccr1 and ccr5 receptor genes, evaluated by reverse transcription-quantitative PCR (qRT-PCR), was observed upon infection with strains from patients with chronic dermal leishmaniasis; induction of CXCL5 and CCL8 was corroborated at the protein level. No apparent upregulation was elicited in macrophages infected with strains from self-healing patients. Expression levels of ccl8, cxcl2, cxcl3, and cxcl5 in lesion biopsy specimens from patients with chronic cutaneous leishmaniasis (CL) were compared to those in biopsy specimens from Montenegro skin tests of individuals with asymptomatic infection. Increased expression levels of cxcl5 (P < 0.05), ccl8, and cxcl3 were corroborated in chronic CL lesions. Our study revealed a dichotomy in macrophage chemokine gene expression elicited by L. panamensis strains from patients with self-healing disease and those presenting chronic disease, consistent with parasite-mediated hyperactivation of the inflammatory response driving chronicity. The predominant upregulation of neutrophil and monocyte chemoattractants indicates novel mechanisms of sustained inflammatory activation and may provide new therapeutic targets against chronic dermal leishmaniasis.
INTRODUCTION
Clinical manifestations of Leishmania (Viannia) panamensis infections range from self-healing cutaneous lesions to chronic ulcers and mucosal involvement of several years' duration (1). Murine models of cutaneous leishmaniasis (CL) caused by Leishmania major have revealed that a polarized Th1 response is associated with disease resistance and resolution (2). However, a mixed Th1/Th2 response characterizes human CL caused by Leishmania (Viannia) species, without a clear cytokine profile being linked to the clinical outcome of infection (3).
Chronic dermal disease caused by species of the Leishmania (Viannia) subgenus is characterized by lesions of prolonged evolution, a scarcity of parasites in the lesion, an exacerbated inflammatory response, and refractoriness to chemotherapy (4–6). Conversely, self-healing manifestations have been associated with strong yet limited T cell activation and a low-level antibody response (7). Poorly regulated and hyperactive inflammation evidently contributes to tissue damage, disease progression, and parasite persistence (8, 9). However, the mechanisms by which immune cells are recruited to the lesion site and become activated, and their relationship to clinical outcome, are not well understood.
Activation and migration of immune cells are orchestrated by a fine balance of cytokine and chemokine responses. Infections with New and Old World Leishmania species modulate the early expression of chemokines and chemokine receptors in their host cells (10, 11), potentially benefiting the parasite through recruitment of host cells that allow establishment and perpetuation of infection (12, 13). However, alterations in the chemokine network may contribute to uncontrolled immune responses that can modulate parasite survival and promote or mitigate the associated immunopathology, thereby influencing the outcome of infection (11). Illustrating the potential role of chemokines in the pathogenesis of infection, murine models of L. major infection have shown that higher levels of CCL5 at the infection site correlate with disease resistance in C57BL/6 mice compared to susceptible BALB/c mice (14). During human infection, increased CCL2 expression has been reported in lesions from patients with localized self-healing CL caused by L. mexicana, compared to patients with nonhealing diffuse cutaneous disease (a chronic but hyporeactive presentation of CL), in which CCL3 is upregulated (15).
Previous evidence suggests that Leishmania virulence, based on lesion sizes induced in mice, may be linked to the differential expression of chemokines in murine macrophages. Infection of BALB/c mice with a highly virulent strain of L. braziliensis induced high expression levels of CCL3, CCL2, CCL11, and CXCL1/KC in footpad lesions and correlated with enhanced inflammation compared to infection with a less virulent L. braziliensis strain (16). The contribution of chemokine networks and their differential induction by infecting parasite populations in the outcome of human infection with Leishmania (Viannia) is unknown.
We have exploited the availability of L. panamensis strains isolated from patients with prospectively documented self-healing and chronic dermal leishmaniasis to analyze the modulation and expression profile of chemokines and chemokine receptors in human macrophages in response to infection with these putative pathogenically distinct clinical strains. Furthermore, we have compared this in vitro response to the in vivo chemokine response in biopsy specimens from patients with chronic CL and from the leishmanin skin test site in individuals with asymptomatic infection. Our results reveal a striking contrast in the elicited chemokine responses in these clinical outcomes, potentially opening a new avenue for the design of immunotherapeutic strategies to prevent or resolve chronic and disfiguring clinical manifestations.
MATERIALS AND METHODS
Ethics statement.
This study was approved and monitored by the institutional review board for ethical conduct of research involving human subjects of the Centro Internacional de Entrenamiento e Investigaciones Médicas (CIDEIM), in accordance with national (resolution 008430, República de Colombia, Ministry of Health, 1993) and international (Declaration of Helsinki and amendments, World Medical Association, Seoul, South Korea, October 2008) guidelines. All individuals voluntarily participated in this study, and informed consent was obtained from each participant.
Study design.
In order to evaluate the relationship between chemokine gene expression and the clinical outcome of dermal leishmaniasis caused by L. panamensis, we analyzed the gene expression profile of immunological determinants in primary human macrophages from a single healthy donor. Macrophages were infected in vitro with L. panamensis strains isolated from patients with clinically divergent chronic (nonhealing disease) or self-healing lesions obtained from CIDEIM BioBank. Chronic disease was defined as active confirmed dermal leishmaniasis of >6 months' duration; self-healing infection was defined as confirmed disease that healed without treatment after diagnosis. The use of macrophages from the same donor aimed to maintain the host cell background constant, in order to examine the parasite-specific effect on the host cell response. Expression of selected chemokines elicited by infection of primary human macrophages was also evaluated by using skin biopsy specimens obtained from lesions of patients with chronic CL or the site of delayed-type hypersensitivity response to the leishmanin (Montenegro) skin test in individuals with asymptomatic infection.
Study subjects.
Subjects were recruited into three groups according to the following criteria: (i) healthy volunteers who had not been exposed to leishmaniasis transmission (n = 9); (ii) asymptomatic individuals (n = 5), defined as residents of an area where dermal leishmaniasis is endemic with a positive Montenegro skin test reaction (MSR) and no evidence or history of dermal lesions; and (iii) patients with chronic CL (n = 8), defined as patients with cutaneous lesions with ≥6 months of disease evolution, with parasitological confirmation by microscopic examination of lesion smears or biopsy specimens and/or parasite isolation, who had not received antileishmanial treatment before enrollment. Clinical and demographic characteristics of all infected individuals are listed in Table 1. All subjects had negative serology for HIV and human T cell lymphotropic virus type 1 (HTLV-1).
TABLE 1.
Clinical characteristics and evolution of study subjects from whom biopsy specimens were obtained
| Characteristic | Value for group |
P value | |
|---|---|---|---|
| Asymptomatic (n = 5) | Chronic (n = 8) | ||
| Mean age (yr) (range) | 49 (27–64) | 36 (21–66) | 0.29 |
| % male patients | 100 | 100 | |
| % of patients of ethnic group | |||
| Afro-Colombian | 100 | 50 | |
| Mestizo | 37.5 | ||
| Indigenous | 12.5 | ||
| Mean Montenegro skin test reaction zone (mm) (range) | 14 (13–16) | 13 (9.7–19) | 0.56 |
| Median no. of lesions per subject (range) | 1 (1–8) | ||
| Median duration of older lesion (mo) (range) | 19 (6–240) | ||
| % of patients with lesion type | |||
| Ulcer | 50 | ||
| Plaque | 50 | ||
| % of patients with Leishmania isolated | |||
| L. panamensis | 37.5 | ||
| L. braziliensis | 12.5 | ||
| Not determineda | 100 | 50 | |
Not determined because no strain was isolated.
Buffy coat isolation.
Total blood leukocytes were isolated by centrifugation at 400 × g for 15 min at room temperature (RT). White blood cells (WBCs) were collected from the interface between plasma and red blood cells (RBCs) and incubated in RBC lysis buffer (150 mM NH4CL, 10 mM KHCO3, 0.1 mM Na2EDTA) for 5 min at RT, washed twice with phosphate-buffered saline (PBS), and resuspended in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) for subsequent procedures.
Cell culture and differentiation.
Peripheral blood samples from healthy volunteers were collected and processed to obtain peripheral blood mononuclear cells (PBMCs) by centrifugation over Ficoll-Hypaque gradients (Sigma-Aldrich) according to the manufacturer's instructions. Macrophages were differentiated from PBMCs by adherence to cell culture plastic ware (catalog number 353046; BD) in serum-free RPMI for 2 h, followed by culture for 7 days in RPMI 1640 supplemented with 20% FBS at 37°C in 5% CO2.
Leishmania strains.
Clinical strains were obtained from CIDEIM BioBank. Strains were isolated by needle aspiration of cutaneous lesions or lesion biopsy specimens of mucosal lesions, propagated, and immediately stored in liquid nitrogen until use. Strains were typed by immunoreactivity to monoclonal antibodies and zymodeme analysis. L. panamensis strains MHOM/CO/08/5433 (L.p.5433chr), MHOM/CO/11/5430 (L.p.5430chr), and MHOM/CO/08/5397 (L.p.5397chr) were isolated from patients with chronic CL with >6 months of disease evolution. MHOM/CO/87/1320 (L.p.1320chr) and MHOM/CO/85/2504 (L.p.2504chr) were isolated from lesion biopsy specimens of nasal mucosa from patients presenting with >10 years of disease evolution. Strains MHOM/CO/85/2272 (L.p.2272sh), MHOM/CO/85/2271 (L.p.2271sh), MHOM/CO/89/2189 (L.p.2189sh), and MHOM/CO/83/1022 (L.p.1022sh) were isolated from patients with self-healing CL. A laboratory-derived L. panamensis strain stably transfected with the luciferase reporter gene (L.p.LUC 001) was employed for standardization procedures. For infection, promastigotes were grown at 25°C in Senekjie's biphasic blood agar and passed for a maximum of 2 subpassages into RPMI 1640 supplemented with 10% heat-inactivated FBS.
Infection.
Infection was performed as previously described (17). Briefly, 2 × 106 WBCs/well or 1 × 106 primary macrophages were dispensed into 24- or 6-well plates, respectively. To optimize parasite phagocytosis, stationary-phase promastigotes were opsonized by resuspension in RPMI 1640 containing 10% heat-inactivated human AB+ serum and incubated for 1 h at 34°C. After opsonization, total WBCs were incubated with stationary-phase promastigotes at a Leishmania-to-monocyte ratio of 10:1 for 2 and 24 h at 34°C. Differentiated macrophages were infected at a 10:1 Leishmania-to-macrophage ratio for 24 h at 34°C in 5% CO2. The infection rate under these conditions is an average of 70% ± 14%, with total parasite loads between 200 and 300 parasites/100 macrophages.
PCR arrays and gene expression profiling.
Total RNA was extracted from uninfected and infected cultured cells, as well as biopsy specimens, by using TRIzol (Invitrogen, USA) followed by RNA cleanup with RNeasy minikit columns (Qiagen, USA). For PCR array profiling, RNA was reverse transcribed with an RT First Strand kit (SABiosciences-Qiagen). For confirmatory TaqMan gene expression assays (Applied Biosystems, Foster City, CA), cDNA was synthesized by using a high-capacity cDNA reverse transcription kit (Applied Biosystems). Gene expression evaluation was conducted by reverse transcription-quantitative PCR (qRT-PCR) using SABiosciences-Qiagen PCR arrays on a Bio-Rad CFX-96 detection platform. For total white blood cell samples, we employed chemokine and chemokine receptor arrays (PAHS-022ZD [formerly PAHS-022], where a change in the PCR array configuration by the manufacturer resulted in only 62 out of 84 genes being common to arrays PAHS-022 and PAHS-022ZD; thus, analyses are based on a 62-gene panel). For purified PBMC-derived human macrophages, the human inflammatory cytokine and receptor array (PAHS-011A) was utilized. These analyses included 84 different inflammation-related genes (chemokines, cytokines, and chemokine and cytokine receptors), as detailed by the manufacturer. Gene expression was normalized to a five-gene panel comprised of β2-microglobulin, hypoxanthine phosphoribosyltransferase 1, β-actin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and the ribosomal protein L13a. Data were analyzed by the ΔΔCT method, and fold changes were calculated compared to uninfected macrophages and expressed as 2−ΔΔCT. Data were processed and analyzed on the RT2 Profiler PCR Array Data Analysis online tool provided by the manufacturer. For skin biopsy specimens, chemokine gene expression assays were conducted with TaqMan probes (Applied Biosystems) for CCL8 (Hs00271615_m1), CXCL2 (Hs00601975_m1), CXCL3 (Hs00171061_m1), and CXCL5 (Hs00171085_m1), analyzed by absolute quantitation based on extrapolation to a standard curve, and expressed as a ratio to GAPDH (Hs99999905_m1).
Genotyping of strains by multilocus microsatellite typing (MLMT).
Microsatellite typing was used to examine the heterogeneity of genotypes within and between strains from patients presenting chronic or self-healing disease. Total DNA was extracted from log-phase promastigotes by using DNAzol reagent (Invitrogen, USA). DNA samples from 30 banked L. panamensis strains isolated from patients from the Departments of Nariño, Chocó, and Valle del Cauca in Colombia were analyzed. Fourteen microsatellites distributed in 13 Leishmania chromosomes were amplified by PCR, as previously described (18). The size of the microsatellites was determined by the mobility of the PCR products in 4.5% agarose gels. Genetic distances were estimated by using MSA software, and neighbor-joining trees were constructed by using MEGA 5.
Chemokine secretion determined by ELISA.
Human primary macrophages from six independent donors were cultured in 24-well plates at 0.3 × 106 cells/well in complete RPMI. Macrophages were infected with L. panamensis strains L.p.2272sh, L.p.2504chr, and L.p.5397chr for 8, 24, 48, and 72 h at a parasite-to-macrophage ratio of 10:1. Culture supernatants were collected, and the secretion of CXCL5 and CCL8 was assessed by enzyme-linked immunosorbent assays (ELISAs) using ELISA kits as recommended by the manufacturer (R&D Systems). Absorbance readings at 450 nm were performed on a ChameleonV microplate reader (Hidex).
Statistical analysis.
The Kolmogorov-Smirnov test was applied to determine the parametric or nonparametric distribution of the data. Thereafter, nonparametric data were analyzed by using the Mann-Whitney U test for comparisons. Kruskal-Wallis one-way analysis of variance followed by Dunn's multiple-comparison test was employed for group comparisons. Statistical significance was defined as a P value of <0.05. All data were analyzed by using Prism 5 software (GraphPad Software, Inc., La Jolla, CA).
RESULTS
Dynamics of inflammatory gene expression in human WBCs during infection with L. panamensis.
Gene expression dynamics of inflammation-related genes (chemokines, cytokines, and receptors, as detailed in Materials and Methods) in human WBCs following exposure for 2 h and 24 h to a cloned laboratory-selected luciferase-transfected L. panamensis strain (L.p.LUC 001) were evaluated. Adequate amplification signals and melting peaks were not obtained for 4 out of 62 evaluated genes (ccl1, slit2, cxcl12, and cx3cl1). A marked repression of the inflammatory response early after parasite contact (2 h) was observed, in which 30% (19/58) of inflammation-related genes were downregulated <0.5-fold (see Table S1 in the supplemental material). At 24 h postinfection, gene expression was almost completely restored, with only 10% of genes being downregulated and over 40% of genes being induced >1.5-fold. Consistently upregulated genes at 2 h and 24 h postinfection were tnf-α, CCL chemokine (ccl2, ccl3, ccl4, and ccl7), and CXCL chemokine (cxcl1, cxcl2, and il-8) genes (see Table S1 in the supplemental material). To identify central components of a sustained inflammatory response, experiments were subsequently conducted following 24 h of infection.
Chemokine gene expression is strongly induced in human macrophages by infection with L. panamensis strains isolated from patients with chronic dermal leishmaniasis.
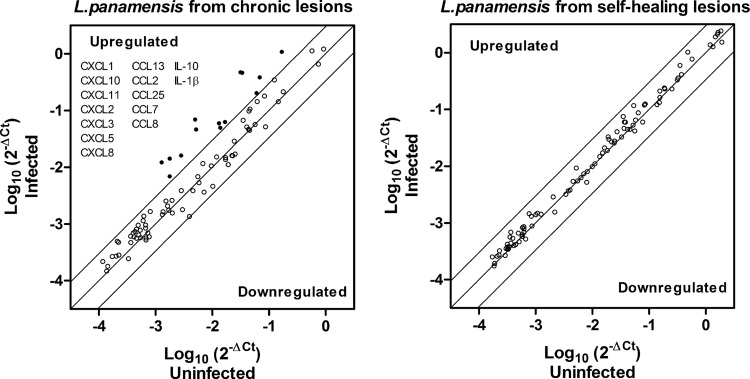
Macrophages are an important source of CCL and CXCL chemokine production and central orchestrators of immune regulation during leishmaniasis (19, 20). Thus, we investigated the relationship between the clinical outcome of tegumentary leishmaniasis and the gene expression of inflammatory mediators in monocyte-derived human macrophages. As shown in Fig. 1, expression analysis of an 84-gene array revealed a dichotomy in the response elicited by infection with strains isolated from patients with chronic (CHR strains) versus self-healing (SH strains) disease (see Table S2 in the supplemental material). Infection with CHR strains, whether isolated from patients with chronic cutaneous leishmaniasis (n = 3) or from those with chronic mucosal involvement (n = 2), strongly (>4-fold) and consistently modulated the chemokine network, inducing a distinctive profile of chemokine gene expression characterized by upregulation of CCL (ccl13, ccl2, ccl25, ccl7, and ccl8) and CXCL (cxcl1, cxcl10, cxcl11, cxcl2, cxcl3, cxcl5, and cxcl8) chemokines, il-10, and il-1b (Fig. 1; see also Table S2 in the supplemental material). The levels of 11 of these 14 genes were significantly higher (P < 0.05) following infection with CHR strains than following infection with SH strains (Table 2). Macrophage gene expression following infection with SH strains (n = 4) was predominantly unaffected (Fig. 1; see also Table S2 in the supplemental material). The profile of chemokine expression induced by CHR strains is consistent with a predominant neutrophil (CXCL1, CXCL2, CXCL5, and CXCL8/interleukin-8 [IL-8]) and monocyte (CCL2, CCL7, CCL8, CCL13, CXCL3, and CXCL10) chemotactic milieu (Table 3).
FIG 1.
Regulation of inflammatory gene expression in human macrophages in response to infection with L. panamensis strains isolated from patients with chronic and self-healing disease. Primary human macrophages were infected with L. panamensis strains isolated from patients with chronic (CHR) (n = 5) or self-healing (SH) (n = 4) dermal leishmaniasis for 24 h. Scatter plots show the average values of gene expression of 84 inflammatory genes (chemokines, cytokines, and receptors) for macrophages infected with CHR (left) and SH (right) strains, assessed by qRT-PCR. Significant modulation was established as a 4.0-fold change in expression levels (up- or downregulation, represented as circles above or below the 4-fold threshold lines) relative to those in uninfected cells.
TABLE 2.
Expression of chemokine genes in human macrophages infected in vitro with L. panamensis
| Gene | Avg fold change in gene expression compared to uninfected macrophages ± SD (range)a |
|
|---|---|---|
| L. panamensis from patients with chronic disease (n = 5) | L. panamensis from patients with self-healing disease (n = 4) | |
| CCL13 | 4.2 ± 2.7 (1.8–7.7) | 1.6 ± 0.7 (0.9–2.5) |
| CCL2 | 6.5 ± 0.9 (5.7–7.9)* | 1.5 ± 0.7 (0.6–2.3) |
| CCL25 | 3.6 ± 1.7 (2.2–6.6) | 2.1 ± 1.3 (0.3–3.5) |
| CCL7 | 4.7 ± 1.7 (3.2–6.9)* | 1.8 ± 1.2 (0.5–3.0) |
| CCL8 | 14.1 ± 4.0 (10.2–19.6)* | 1.8 ± 1.0 (0.9–3.2) |
| CXCL1 | 8.1 ± 1.8 (5.3–10.2)* | 2.0 ± 1.1 (0.8–3.3) |
| CXCL10 | 6.1 ± 2.8 (3.6–10.0)* | 1.1 ± 0.1 (0.9–1.2) |
| CXCL11 | 4.2 ± 2.1 (1.7–6.8)* | 0.8 ± 0.2 (0.6–1.1) |
| CXCL2 | 9.3 ± 2.9 (5.4–16.5)* | 2.3 ± 1.1 (1.2–3.4) |
| CXCL3 | 14.7 ± 5.2 (7.7–21.5)* | 2.4 ± 1.2 (1.1–4.0) |
| CXCL5 | 10.4 ± 4.9 (4.9–17.4)* | 1.6 ± 1.4 (0.5–3.6) |
| CXCL8/IL-8 | 19.4 ± 12.0 (3.1–33.6)* | 3.5 ± 3.3 (0.5–7.3) |
| IL-1β | 8 ± 7.0 (1.7–19.4) | 1.5 ± 1.1 (0.5–3.0) |
| IL-10 | 3.9 ± 1.0 (2.8–5.7)* | 1.7 ± 0.1 (1.5–1.8) |
Values represent averages ± standard deviations (ranges) of fold changes over uninfected macrophages. *, P < 0.05 (P values obtained from independent analyses applying the Mann-Whitney U test).
TABLE 3.
Role of chemokines in the pathogenesis of Leishmania infectiona
| Chemokine | Chemotactic function(s) | Effect(s) during Leishmania infection | Reference(s) |
|---|---|---|---|
| CCL2 (MCP-1) | Monocytes, NK cells, DCs, and T cells (CD45RO+) | Strongly expressed in lesions of patients with self-healing localized CL and stimulates elimination of intracellular Leishmania | 15, 16, 38, 41 |
| CCL2-independent effects of CCR2 are indispensable for control of L. major infection and generation of protective immune responses | |||
| Higher-level induction in mice infected with pathogenic L. braziliensis than in mice infected with nonpathogenic L. braziliensis strains | |||
| CCL8 (MCP-2) | Monocytes, NK cells, DCs, eosinophils, and T cells (CD45RO+ RA+) | Promotes the switch from resolving to persistent infection through hematopoietic progenitor differentiation into regulatory DCs | 36 |
| CCL7 (MCP-3) | Monocytes, NK cells, DCs, eosinophils, and T cells (CD45RO+) | Mediates activated Th2 cell recruitment to infection site in BALB/c mice | 42 |
| CCL13 (MCP-4) | Monocytes, NK cells, DCs, eosinophils, and T cells (CD45RO+) | No information available | |
| CCL25 | Thymocytes, DCs, and NK cells | No information available | |
| CXCL1 (MGSA/GROα) | Neutrophils and lymphocytes | Higher-level induction in mice infected with pathogenic L. braziliensis than in mice infected with nonpathogenic L. braziliensis strains | 16 |
| CXCL10 (IP-10) | Monocytes/macrophages, T cells, NK cells, and DCs | Critical for rendering protective cellular immunity during vaccination with SLA-pulsed CpG-ODN-stimulated DCs, conferring protection against L. donovani infection | 13, 16, 43 |
| Inhibited by L. major infection, potentially preventing NK cell activation | |||
| Higher-level induction in mice infected with pathogenic L. braziliensis than in mice infected with nonpathogenic L. braziliensis strains | |||
| CXCL11 | Activated T cells and NK cells | No information available | |
| CXCL2 (MIP-2α) | Neutrophil chemoattractant | Leishmania-induced CXCL2 expression via NF-κB p65RelA cleavage | 44 |
| CXCL3 (MIP-2β) | Monocyte chemoattractant | CXCL3 gene expression is induced upon infection of human macrophages with L. major | 45 |
| CXCL5 | Neutrophil activator | In iNKT cell-depleted mice infected with L. donovani, hepatic granulomas had lower granulocyte and monocyte infiltration, correlated with lower CXCL5 expression levels | 46 |
| CXCL8 (IL-8) | Recruits and activates neutrophils | Infection of monocytes with L. major induces IL-8 | 39, 47 |
| Plasma levels of IL-8 are increased in patients with VL |
For a comprehensive review, see reference 48. MCP-1, monocyte chemoattractant protein 1; DCs, dendritic cells; VL, visceral leishmaniasis; MGSA/GROα, melanoma growth stimulatory activity/growth-regulated protein alpha; SLA, soluble leishmanial antigen; CpG-ODN, CpG oligodeoxynucleotides; iNKT cells, invariant natural killer T cells.
Expression of ccr2, a common receptor for CCL2, CCL7, and CCL8, was unaffected by L. panamensis infection (see Table S2 in the supplemental material). However, expression of ccr1 (CCL7 receptor) and ccr5 (CCL8 receptor) was significantly higher (P = 0.007) in macrophages infected with CHR strains (see Table S2 in the supplemental material). With the exception of downregulation of ccr7 induced by CHR strains, expression of other macrophage CC and CXC receptors remained unchanged following infections with either CHR or SH strains (see Table S2 in the supplemental material).
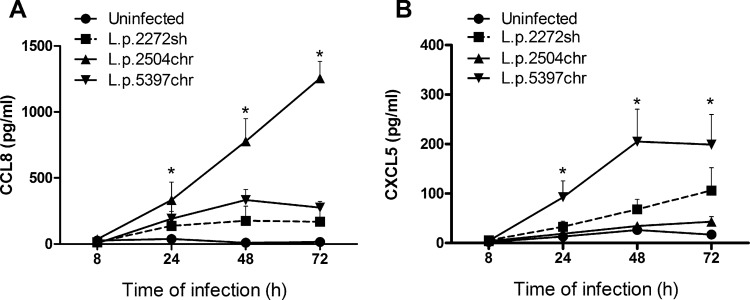
To explore whether the gene induction pattern observed in response to infection with CHR strains was reflected at the protein level, we measured the secretion of CXCL5 and CCL8 in supernatants of human primary macrophage cultures (n = 6) infected with SH strain L.p.2272sh and CHR strains L.p.2504chr and L.p.5397chr. Selection of these strains was based on including the phenotypic spectrum of strains analyzed, from self-healing disease (L.p.2272sh) to long-term chronic disease, with one from a patient with 6 months of disease evolution (L.p.5397chr) and another with >10 years of disease evolution (L.p.2504chr). Selection of CXCL5 and CCL8 chemokines was based on their high levels of expression at the mRNA level (Table 2). Secretion of CCL8 and CXCL5 was detectable as early as 24 h after infection (Fig. 2). CCL8 production was significantly induced by infection with CHR strain L.p.2504chr and accumulated in a time-dependent manner. Although L.p.5397chr also induced higher levels of CCL8 production than did L.p.2272sh, this difference was not statistically significant (Fig. 2). CXCL5 was strongly induced by CHR strain L.p5397chr, and this response was statistically significant (P < 0.05) compared to the response of the uninfected control. In contrast, CHR strain L.p.2504chr did not induce a marked secretion of CXCL5, in line with lower levels of gene expression (13.4-, 4.9-, and 3.6-fold after infection with L.p.5397chr, L.p.2504chr, and L.p.2272sh, respectively, compared to uninfected macrophages). Together, these data show that the direction of the elicited responses was similar at both the mRNA and protein levels.
FIG 2.
Macrophage chemokine secretion induced by infection with SH and CHR strains. Shown are kinetics of CCL8 (A) and CXCL5 (B) secretion in human primary macrophages obtained from healthy donors (n = 6) after 8 to 72 h of infection with L. panamensis strains isolated from patients with self-healing CL (L.p.2272sh) and from those with chronic disease (L.p.5397chr and L.p.2504chr). Data are expressed as means ± standard errors of the means. Statistical significance was estimated by using Kruskal-Wallis one-way analysis of variance followed by Dunn's test for multiple comparisons. *, P < 0.05 compared to the uninfected control.
Genetically diverse strains induce similar pathogenicity phenotypes in human macrophages.
Considering the consistent divergence of macrophage responses elicited by infection with strains from chronic versus self-healing disease, genetic diversity analysis of the Leishmania strains was undertaken to evaluate whether the difference in pathogenicity and the capacity to induce a repertoire of inflammatory response genes would be accompanied by discernible genomic differences. Multilocus microsatellite typing (MLMT) of the 9 L. panamensis strains (5 CHR and 4 SH strains) and 21 additional L. panamensis strains isolated from patients in the Departments of Nariño, Chocó, and Valle del Cauca in Colombia (departments from which the SH and CHR strains originated) and genetic distance analysis revealed genetic diversity at the levels of geographical region and phenotype of the clinical outcome (Fig. 3). CHR and SH strains were distributed within 4 nonoverlapping groups (Fig. 3), indicating that pathogenicity phenotypes were not differentiated by genotype and suggesting that common mechanisms of modulation of the host cell inflammatory response could be shared among genetically diverse parasite populations.
FIG 3.
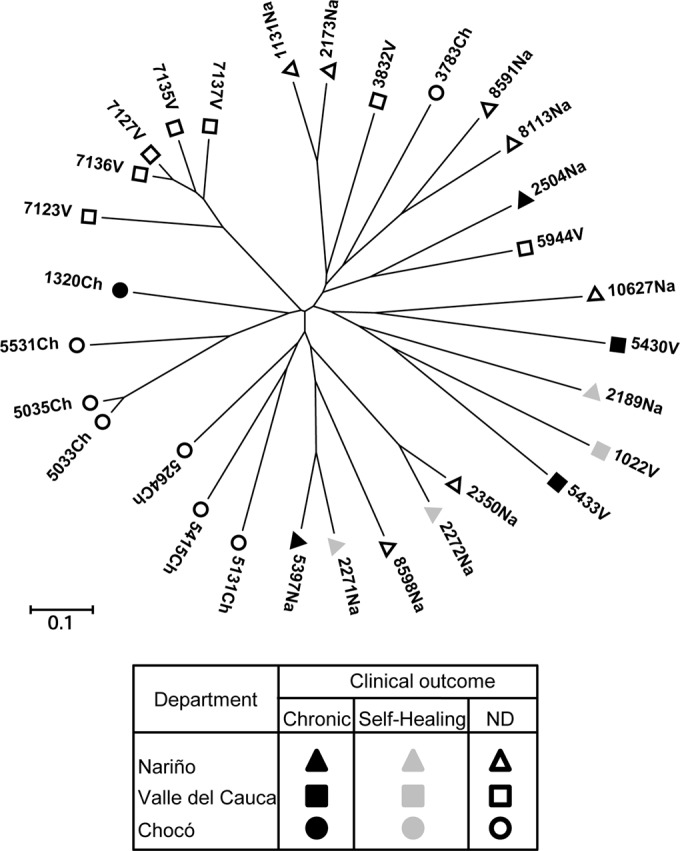
MLMT analysis of L. panamensis strains isolated from patients with self-healing and chronic dermal leishmaniasis. Shown is a neighbor-joining tree generated from the distances calculated for the microsatellite data of L. panamensis strains isolated from patients at the Departments of Valle del Cauca (n = 10), Nariño (n = 12), and Chocó (n = 8), Colombia. Strains isolated from self-healing patients (n = 4) and from patients with chronic disease (n = 5) are shown. ND, not determined; Na, Nariño; V, Valle del Cauca; Ch, Chocó.
CXCL5, CXCL3, and CCL8 are overexpressed in lesion biopsy specimens from patients with chronic cutaneous disease.
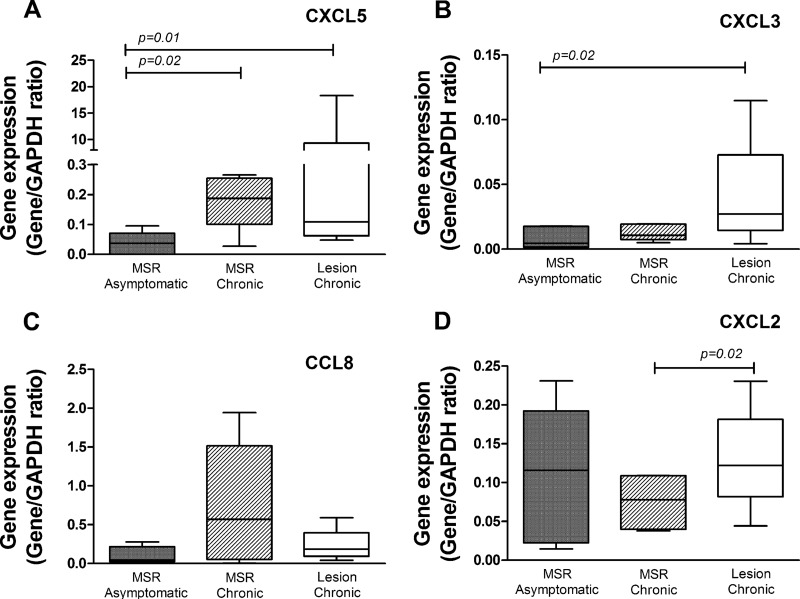
To study the relationship between the macrophage responses elicited ex vivo upon infection and the in vivo inflammatory chemokine response, we evaluated the expression levels of ccl8, cxcl2, cxcl3, and cxcl5 in lesion biopsy specimens from patients with chronic CL (n = 5) and in biopsy specimens of Montenegro skin test reactions (MSR) from patients with chronic lesions (n = 5) and asymptomatically infected individuals (n = 5). Selection of these chemokines was based on their higher expression levels in human macrophages infected with strains isolated from patients with chronic disease (Table 2). MSR biopsy specimens from asymptomatically infected individuals were defined as the comparison group, considering asymptomatic infection to represent the clinical resistance phenotype in the spectrum of Leishmania infection.
Increased expression of cxcl5 (P < 0.05) was observed in lesion and MSR biopsy specimens from chronically infected patients compared to MSR biopsy specimens from individuals with asymptomatic infection (Fig. 4A). Similarly, a trend of increased expression of cxcl3 and ccl8 was observed in lesion and MSR biopsy specimens of patients with chronic CL (Fig. 4B and C), while no difference in the levels of expression of cxcl2 was detected. One out of the four strains isolated from the patients with chronic CL corresponded to L. braziliensis, and the remaining strains were identified as L. panamensis (Table 1). Species-specific differences could contribute to variations in the chemokine expression profiles among patients with similar clinical outcomes of infection. Correlation analysis of gene expression in biopsy specimens from chronic lesions showed a positive, statistically significant correlation between cxcl5 and cxcl2 (P = 0.034; r2 = 0.85), cxcl3 (P = 0.003; r2 = 0.97), and ccl8 (P = 0.005; r2 = 0.95), suggesting coregulation of gene expression. These results demonstrate a consistent inflammatory chemokine expression profile during both in vitro and in vivo responses, substantiating the usefulness of primary macrophages as a surrogate system for the chemokine response in active lesions and supporting the involvement of cxcl2, cxcl3, cxcl5, and ccl8 in the chronicity of cutaneous disease.
FIG 4.
Chemokine gene expression in skin biopsy specimens from individuals with chronic CL and from those withy asymptomatic infection. The expression levels of cxcl5 (A), cxcl3 (B), ccl8 (C), and cxcl2 (D) were measured in biopsy specimens of lesions and intradermal Montenegro skin test reaction (MSR) specimens from chronic patients (n = 5) and in MSR biopsy specimens from asymptomatic individuals (n = 5). P values were calculated by using a Mann-Whitney test for comparisons between groups.
DISCUSSION
This study of the human host cell response to infection with L. panamensis has revealed a dichotomy in the macrophage chemokine responses elicited by infection with strains of distinct pathogenicity in the human host, manifested as either chronic or self-healing disease. Chronic dermal leishmaniasis has been associated with immunological hyperreactivity (5, 6), overproduction of pro- and anti-inflammatory cytokines (21), and scarce but persistent parasites at the lesion site (4). Upregulation of inflammatory functions is accompanied by increased cellular infiltration of lesions, perpetuating the immunopathology (23, 24). We have previously shown that macrophage permissiveness for survival and replication of intracellular Leishmania correlates with the clinical outcome of infection; macrophages from patients with chronic CL were more permissive to infection than were cells from individuals with asymptomatic disease (25). Together, these findings support the participation of both host-specific and parasite-mediated hyperactivation of the inflammatory response as factors contributing to chronicity.
The mediator signature associated with infection of primary macrophages with L. panamensis strains from chronic disease patients was consistent with a strong and poorly regulated inflammatory response, reflected by the concomitant upregulation of the anti-inflammatory cytokine IL-10 and proinflammatory chemokines. This was true for strains isolated from patients having chronic CL or chronic mucosal involvement, suggesting that chronicity (time of evolution), rather than tissue localization, is a defining variable of parasite- and host-mediated immune deregulation, as also suggested for L. braziliensis infections (6, 26). Evidence of poorly regulated responses early in lesion evolution has recently been reported for L. braziliensis infection (26), based on high levels of pro- and anti-inflammatory gene expression in cutaneous lesions with <3 months of disease evolution. Hence, the establishment of infection and subsequent local inflammatory responses promoting pathogenesis may be defined early in the host-pathogen interaction.
Induction of ccl2, ccl7, ccl8, cxcl1, cxcl2, cxcl3, cxcl5, cxcl8, cxcl10, and cxcl11, together with the chemokine receptors ccr5 (ccl8 receptor) and ccr1 (ccl7 and ccl8 receptor), defines a strong and redundant cascade of inflammatory signals for recruitment of monocytes and neutrophils (Table 3). Sustained recruitment and activation of innate immune cells would enable recruitment and activation of adaptive immune cell responses, potentiating chronic inflammation and immunopathology (5, 6, 33). The role of neutrophils during Leishmania infection is complex. They act as temporary hosts and facilitators of macrophage colonization during the early stage of infection with L. major, L. donovani, and L. infantum, thereby promoting establishment of infection (12, 27–29). Conversely, they can cooperate with macrophages in the induction of a protective immune response and parasite elimination (30, 31). Although a neutrophilic infiltrate in the mid-dermis has been described for cutaneous lesions caused by L. panamensis with 0.5 to 8 months of disease evolution, 94% of these patients presented lesions ≤4 months before diagnosis (32). Hence, the participation of neutrophils during chronic phases of disease and the relationship of Leishmania pathogenicity to cell recruitment and activation remain elusive. At early stages of infection, upregulation of neutrophil and monocyte chemoattractants could favor the establishment of infection, and at later stages, it could promote antigen/parasite persistence.
As expected, the expression of cxcr2, the receptor of the polymorphonuclear chemotactic chemokines cxcl1, cxcl2, cxcl3, cxcl5, and cxcl8 (34), was unaffected by L. panamensis infection in human macrophages. Whether infection modulates expression of this receptor in human neutrophils remains to be determined. Genetic analyses previously revealed an association between single nucleotide polymorphisms in cxcr1 and cxcr2 genes and mucosal or cutaneous manifestations of L. braziliensis infection (35), suggesting the participation of these receptors in the outcome of infection with species of the Leishmania (Viannia) subgenus.
To our knowledge, this is the first documentation of the involvement of ccl8, cxcl3, and cxcl5 during human infection with a species of the Leishmania (Viannia) subgenus. Recently, Nguyen et al. showed that infection with L. donovani induces the expression of CCL8 by murine stromal cells promoting regulatory dendritic cell differentiation and suggested that the aberrant production of this chemokine may be associated with persistent infection during chronic inflammation (36). The present findings for human dermal leishmaniasis caused by L. panamensis demonstrate that infection with strains isolated from patients with chronic disease, but not from self-healing individuals, induced CCL8 at the gene and protein levels, in line with maintenance of a chronic inflammatory response.
In the present analyses, ccl8, cxcl3, and cxcl5 gene products were also found to be upregulated in biopsy specimens from chronic lesions, although the difference in expression levels compared to those in MSR biopsy specimens from asymptomatic individuals was not as marked as that observed in primary human macrophages infected with L. panamensis. While RNA extracted from lesion biopsy specimens is enriched in nucleic acids from keratinocytes, fibroblasts, and infiltrating inflammatory cells, analyses of monocyte-derived macrophages evaluate gene expression at the host cell level. The different types and proportions of cells in biopsy specimens and primary macrophage cultures would be expected to impact the observed repertoire and magnitude of gene expression.
Modulation of ccl2 (37, 38), cxcl1, cxcl10 (11), cxcl2 (27), and il-8 (39) was previously shown during infection with L. major and L. braziliensis in mice. Our results show that L. panamensis not only induces the expression of these chemokines in human macrophages but also does so distinctively and preferentially during infection with strains isolated from lesions of patients with chronic disease. Despite genetic heterogeneity, the distinctive responses elicited by infection with SH and CHR L. panamensis strains were consistent in terms of both the magnitude and the pattern of the inflammatory gene profile. These findings support a common mechanism(s) of modulation of the host cell response among genetically diverse L. panamensis strains linked to the outcome of infection. Exploration of such mechanisms through comparative whole-genome sequencing and transcriptome sequencing (RNA-Seq) analyses is in progress.
Finally, caution should be taken in generalizing these results to other Leishmania infections involving other species and/or epidemiological contexts. The profile and magnitude of cytokine and chemokine gene expression during human tegumentary leishmaniasis can vary depending on the infecting parasite species and strain, the time of lesion evolution, host genetics, and other factors (16, 26, 40). Whether a unique gene expression signature defines the clinical outcome of Leishmania (Viannia) infections remains to be determined, yet these findings encourage such a possibility.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge the support of the CIDEIM BioBank personnel, especially Maryori Vidarte and Alejandra Arcos for provision and phenotyping of the clinical strains used in this study. We also thank Rafael Góngora and Mariana Rosales for technical assistance in MLMT genotyping. We thank Emily Adams at the Liverpool School of Tropical Medicine for providing us with the MLMT primer sets.
M.F. was supported by a Wilbur G. Downs fellowship and a Benjamin H. Kean fellowship in tropical medicine (ASTMH). D.A.V. was supported by Colciencias-CIDEIM young investigator award 525 004022012. This work received support from Fogarty/NIH grant D43 TW006589, NIAID/NIH grant R01 AI093775, Swiss National Science Foundation grant IZ70ZD_131421, and Colciencias grant 222945921594, 565-2008.
Footnotes
Published ahead of print 21 April 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01133-13.
REFERENCES
- 1.Weigle KA, Santrich C, Martinez F, Valderrama L, Saravia NG. 1993. Epidemiology of cutaneous leishmaniasis in Colombia: environmental and behavioral risk factors for infection, clinical manifestations, and pathogenicity. J. Infect. Dis. 168:709–714. 10.1093/infdis/168.3.709 [DOI] [PubMed] [Google Scholar]
- 2.Scott P, Natovitz P, Coffman RL, Pearce E, Sher A. 1988. CD4+ T cell subsets in experimental cutaneous leishmaniasis. Mem. Inst. Oswaldo Cruz 83(Suppl 1):256–259 [DOI] [PubMed] [Google Scholar]
- 3.Follador I, Araujo C, Bacellar O, Araujo CB, Carvalho LP, Almeida RP, Carvalho EM. 2002. Epidemiologic and immunologic findings for the subclinical form of Leishmania braziliensis infection. Clin. Infect. Dis. 34:E54–E58. 10.1086/340261 [DOI] [PubMed] [Google Scholar]
- 4.Murray HW, Berman JD, Davies CR, Saravia NG. 2005. Advances in leishmaniasis. Lancet 366:1561–1577. 10.1016/S0140-6736(05)67629-5 [DOI] [PubMed] [Google Scholar]
- 5.Saravia NG, Valderrama L, Labrada M, Holguin AF, Navas C, Palma G, Weigle KA. 1989. The relationship of Leishmania braziliensis subspecies and immune response to disease expression in New World leishmaniasis. J. Infect. Dis. 159:725–735. 10.1093/infdis/159.4.725 [DOI] [PubMed] [Google Scholar]
- 6.Carvalho EM, Johnson WD, Barreto E, Marsden PD, Costa JL, Reed S, Rocha H. 1985. Cell mediated immunity in American cutaneous and mucosal leishmaniasis. J. Immunol. 135:4144–4148 [PubMed] [Google Scholar]
- 7.Carvalho EM, Correia Filho D, Bacellar O, Almeida RP, Lessa H, Rocha H. 1995. Characterization of the immune response in subjects with self-healing cutaneous leishmaniasis. Am. J. Trop. Med. Hyg. 53:273–277 [DOI] [PubMed] [Google Scholar]
- 8.Wortmann GW, Aronson NE, Miller RS, Blazes D, Oster CN. 2000. Cutaneous leishmaniasis following local trauma: a clinical pearl. Clin. Infect. Dis. 31:199–201. 10.1086/313924 [DOI] [PubMed] [Google Scholar]
- 9.Travi BL, Osorio Y, Saravia NG. 1996. The inflammatory response promotes cutaneous metastasis in hamsters infected with Leishmania (Viannia) panamensis. J. Parasitol. 82:454–457. 10.2307/3284085 [DOI] [PubMed] [Google Scholar]
- 10.Matte C, Olivier M. 2002. Leishmania-induced cellular recruitment during the early inflammatory response: modulation of proinflammatory mediators. J. Infect. Dis. 185:673–681. 10.1086/339260 [DOI] [PubMed] [Google Scholar]
- 11.Teixeira MJ, Teixeira CR, Andrade BB, Barral-Netto M, Barral A. 2006. Chemokines in host-parasite interactions in leishmaniasis. Trends Parasitol. 22:32–40. 10.1016/j.pt.2005.11.010 [DOI] [PubMed] [Google Scholar]
- 12.van Zandbergen G, Klinger M, Mueller A, Dannenberg S, Gebert A, Solbach W, Laskay T. 2004. Cutting edge: neutrophil granulocyte serves as a vector for Leishmania entry into macrophages. J. Immunol. 173:6521–6525. 10.4049/jimmunol.173.11.6521 [DOI] [PubMed] [Google Scholar]
- 13.van Zandbergen G, Hermann N, Laufs H, Solbach W, Laskay T. 2002. Leishmania promastigotes release a granulocyte chemotactic factor and induce interleukin-8 release but inhibit gamma interferon-inducible protein 10 production by neutrophil granulocytes. Infect. Immun. 70:4177–4184. 10.1128/IAI.70.8.4177-4184.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santiago HC, Oliveira CF, Santiago L, Ferraz FO, de Souza DG, de-Freitas LA, Afonso LC, Teixeira MM, Gazzinelli RT, Vieira LQ. 2004. Involvement of the chemokine RANTES (CCL5) in resistance to experimental infection with Leishmania major. Infect. Immun. 72:4918–4923. 10.1128/IAI.72.8.4918-4923.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ritter U, Moll H, Laskay T, Brocker E, Velazco O, Becker I, Gillitzer R. 1996. Differential expression of chemokines in patients with localized and diffuse cutaneous American leishmaniasis. J. Infect. Dis. 173:699–709. 10.1093/infdis/173.3.699 [DOI] [PubMed] [Google Scholar]
- 16.Teixeira MJ, Fernandes JD, Teixeira CR, Andrade BB, Pompeu ML, Santana da Silva J, Brodskyn CI, Barral-Netto M, Barral A. 2005. Distinct Leishmania braziliensis isolates induce different paces of chemokine expression patterns. Infect. Immun. 73:1191–1195. 10.1128/IAI.73.2.1191-1195.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rojas R, Valderrama L, Valderrama M, Varona MX, Ouellette M, Saravia NG. 2006. Resistance to antimony and treatment failure in human Leishmania (Viannia) infection. J. Infect. Dis. 193:1375–1383. 10.1086/503371 [DOI] [PubMed] [Google Scholar]
- 18.Oddone R, Schweynoch C, Schonian G, dos Santos de Sousa C, Cupolillo E, Espinosa D, Arevalo J, Noyes H, Mauricio I, Kuhls K. 2009. Development of a multilocus microsatellite typing approach for discriminating strains of Leishmania (Viannia) species. J. Clin. Microbiol. 47:2818–2825. 10.1128/JCM.00645-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basu MK, Ray M. 2005. Macrophage and Leishmania: an unacceptable coexistence. Crit. Rev. Microbiol. 31:145–154. 10.1080/10408410591005101 [DOI] [PubMed] [Google Scholar]
- 20.Handman E, Bullen DV. 2002. Interaction of Leishmania with the host macrophage. Trends Parasitol. 18:332–334. 10.1016/S1471-4922(02)02352-8 [DOI] [PubMed] [Google Scholar]
- 21.Diaz YR, Rojas R, Valderrama L, Saravia NG. 2010. T-bet, GATA-3, and Foxp3 expression and Th1/Th2 cytokine production in the clinical outcome of human infection with Leishmania (Viannia) species. J. Infect. Dis. 202:406–415. 10.1086/653829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reference deleted.
- 23.Faria DR, Souza PE, Duraes FV, Carvalho EM, Gollob KJ, Machado PR, Dutra WO. 2009. Recruitment of CD8(+) T cells expressing granzyme A is associated with lesion progression in human cutaneous leishmaniasis. Parasite Immunol. 31:432–439. 10.1111/j.1365-3024.2009.01125.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guarin N, Palma GI, Pirmez C, Valderrama L, Tovar R, Saravia NG. 2006. Comparative immunohistological analysis of the Montenegro skin test reaction in asymptomatic infection and in acute and chronic cutaneous leishmaniasis. Biomedica 26(Suppl 1):38–48 [PubMed] [Google Scholar]
- 25.Robledo S, Wozencraft A, Valencia AZ, Saravia N. 1994. Human monocyte infection by Leishmania (Viannia) panamensis. Role of complement receptors and correlation of susceptibility in vitro with clinical phenotype. J. Immunol. 152:1265–1276 [PubMed] [Google Scholar]
- 26.Costa-Silva MF, Gomes LI, Martins-Filho OA, Rodrigues-Silva R, de Moura Freire J, Quaresma PF, Pascoal-Xavier MA, Mendes TA, Serakides R, Zauli DA, Campi-Azevedo AC, Melo MN, Gontijo CM, Peruhype-Magalhaes V, Teixeira-Carvalho A. 2014. Gene expression profile of cytokines and chemokines in skin lesions from Brazilian Indians with localized cutaneous leishmaniasis. Mol. Immunol. 57:74–85. 10.1016/j.molimm.2013.08.008 [DOI] [PubMed] [Google Scholar]
- 27.Muller K, van Zandbergen G, Hansen B, Laufs H, Jahnke N, Solbach W, Laskay T. 2001. Chemokines, natural killer cells and granulocytes in the early course of Leishmania major infection in mice. Med. Microbiol. Immunol. 190:73–76. 10.1007/s004300100084 [DOI] [PubMed] [Google Scholar]
- 28.Ritter U, Frischknecht F, van Zandbergen G. 2009. Are neutrophils important host cells for Leishmania parasites? Trends Parasitol. 25:505–510. 10.1016/j.pt.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 29.Peters NC, Egen JG, Secundino N, Debrabant A, Kimblin N, Kamhawi S, Lawyer P, Fay MP, Germain RN, Sacks D. 2008. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science 321:970–974. 10.1126/science.1159194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ribeiro-Gomes FL, Moniz-de-Souza MC, Alexandre-Moreira MS, Dias WB, Lopes MF, Nunes MP, Lungarella G, DosReis GA. 2007. Neutrophils activate macrophages for intracellular killing of Leishmania major through recruitment of TLR4 by neutrophil elastase. J. Immunol. 179:3988–3994. 10.4049/jimmunol.179.6.3988 [DOI] [PubMed] [Google Scholar]
- 31.Novais FO, Santiago RC, Bafica A, Khouri R, Afonso L, Borges VM, Brodskyn C, Barral-Netto M, Barral A, de Oliveira CI. 2009. Neutrophils and macrophages cooperate in host resistance against Leishmania braziliensis infection. J. Immunol. 183:8088–8098. 10.4049/jimmunol.0803720 [DOI] [PubMed] [Google Scholar]
- 32.Palma GI, Saravia NG. 1997. In situ characterization of the human host response to Leishmania panamensis. Am. J. Dermatopathol. 19:585–590. 10.1097/00000372-199712000-00006 [DOI] [PubMed] [Google Scholar]
- 33.Ribeiro-de-Jesus A, Almeida RP, Lessa H, Bacellar O, Carvalho EM. 1998. Cytokine profile and pathology in human leishmaniasis. Braz. J. Med. Biol. Res. 31:143–148. 10.1590/S0100-879X1998000100020 [DOI] [PubMed] [Google Scholar]
- 34.Bonecchi R, Galliera E, Borroni EM, Corsi MM, Locati M, Mantovani A. 2009. Chemokines and chemokine receptors: an overview. Front. Biosci. 14:540–551. 10.2741/3261 [DOI] [PubMed] [Google Scholar]
- 35.Castellucci L, Jamieson SE, Miller EN, Menezes E, Oliveira J, Magalhaes A, Guimaraes LH, Lessa M, de Jesus AR, Carvalho EM, Blackwell JM. 2010. CXCR1 and SLC11A1 polymorphisms affect susceptibility to cutaneous leishmaniasis in Brazil: a case-control and family-based study. BMC Med. Genet. 11:10. 10.1186/1471-2350-11-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen Hoang AT, Liu H, Juarez J, Aziz N, Kaye PM, Svensson M. 2010. Stromal cell-derived CXCL12 and CCL8 cooperate to support increased development of regulatory dendritic cells following Leishmania infection. J. Immunol. 185:2360–2371. 10.4049/jimmunol.0903673 [DOI] [PubMed] [Google Scholar]
- 37.Zaph C, Scott P. 2003. Interleukin-12 regulates chemokine gene expression during the early immune response to Leishmania major. Infect. Immun. 71:1587–1589. 10.1128/IAI.71.3.1587-1589.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ritter U, Moll H. 2000. Monocyte chemotactic protein-1 stimulates the killing of Leishmania major by human monocytes, acts synergistically with IFN-gamma and is antagonized by IL-4. Eur. J. Immunol. 30:3111–3120. [DOI] [PubMed] [Google Scholar]
- 39.Badolato R, Sacks DL, Savoia D, Musso T. 1996. Leishmania major: infection of human monocytes induces expression of IL-8 and MCAF. Exp. Parasitol. 82:21–26. 10.1006/expr.1996.0003 [DOI] [PubMed] [Google Scholar]
- 40.Melby PC, Andrade-Narvaez FJ, Darnell BJ, Valencia-Pacheco G, Tryon VV, Palomo-Cetina A. 1994. Increased expression of proinflammatory cytokines in chronic lesions of human cutaneous leishmaniasis. Infect. Immun. 62:837–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quinones MP, Estrada CA, Jimenez F, Martinez H, Willmon O, Kuziel WA, Ahuja SK, Ahuja SS. 2007. CCL2-independent role of CCR2 in immune responses against Leishmania major. Parasite Immunol. 29:211–217. 10.1111/j.1365-3024.2006.00935.x [DOI] [PubMed] [Google Scholar]
- 42.Katzman SD, Fowell DJ. 2008. Pathogen-imposed skewing of mouse chemokine and cytokine expression at the infected tissue site. J. Clin. Invest. 118:801–811. 10.1172/JCI33174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Majumder S, Bhattacharjee S, Paul Chowdhury B, Majumdar S. 2012. CXCL10 is critical for the generation of protective CD8 T cell response induced by antigen pulsed CpG-ODN activated dendritic cells. PLoS One 7:e48727. 10.1371/journal.pone.0048727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gregory DJ, Godbout M, Contreras I, Forget G, Olivier M. 2008. A novel form of NF-kappaB is induced by Leishmania infection: involvement in macrophage gene expression. Eur. J. Immunol. 38:1071–1081. 10.1002/eji.200737586 [DOI] [PubMed] [Google Scholar]
- 45.Guerfali FZ, Laouini D, Guizani-Tabbane L, Ottones F, Ben-Aissa K, Benkahla A, Manchon L, Piquemal D, Smandi S, Mghirbi O, Commes T, Marti J, Dellagi K. 2008. Simultaneous gene expression profiling in human macrophages infected with Leishmania major parasites using SAGE. BMC Genomics 9:238. 10.1186/1471-2164-9-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robert-Gangneux F, Drogoul AS, Rostan O, Piquet-Pellorce C, Cayon J, Lisbonne M, Herbelin A, Gascan H, Guiguen C, Samson M, Gangneux JP. 2012. Invariant NKT cells drive hepatic cytokinic microenvironment favoring efficient granuloma formation and early control of Leishmania donovani infection. PLoS One 7:e33413. 10.1371/journal.pone.0033413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peruhype-Magalhaes V, Martins-Filho OA, Prata A, Silva LDA, Rabello A, Teixeira-Carvalho A, Figueiredo RM, Guimaraes-Carvalho SF, Ferrari TC, Van Weyenbergh J, Correa-Oliveira R. 2006. Mixed inflammatory/regulatory cytokine profile marked by simultaneous raise of interferon-gamma and interleukin-10 and low frequency of tumour necrosis factor-alpha(+) monocytes are hallmarks of active human visceral Leishmaniasis due to Leishmania chagasi infection. Clin. Exp. Immunol. 146:124–132. 10.1111/j.1365-2249.2006.03171.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oghumu S, Lezama-Davila CM, Isaac-Marquez AP, Satoskar AR. 2010. Role of chemokines in regulation of immunity against leishmaniasis. Exp. Parasitol. 126:389–396. 10.1016/j.exppara.2010.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.