Abstract
Proper development of T cells depends on lineage-specific regulators controlled transcriptionally and posttranslationally to ensure precise levels at appropriate times. Conditional inactivation of F-box protein Fbw7 in mouse T-cell development resulted in reduced thymic CD4 single-positive (SP) and splenic CD4+ and CD8+ cell proportions. Fbw7 deficiency skewed CD8 SP lineage differentiation, which exhibited a higher incidence of apoptosis. Similar perturbations during development of CD8-positive cells were reported with transgenic mice, which enforced GATA3 (T-cell differentiation regulator) expression throughout T-cell development. We observed augmented GATA3 in CD4/CD8 double negative (DN) stage 4, CD4 SP, and CD8 SP lineages in Fbw7-deficient thymocytes. Using overexpressed proteins in cultured cells, we demonstrated that Fbw7 bound to, ubiquitylated, and destabilized GATA3. Two Cdc4 phosphodegron (CPD) candidate sequences, consensus Fbw7 recognition domains, were identified in GATA3, and phosphorylation of Thr-156 in CPD was required for Fbw7-mediated ubiquitylation and degradation. Phosphorylation of GATA3 Thr-156 was detected in mouse thymocytes, and cyclin-dependent kinase 2 (CDK2) was identified as a respondent for phosphorylation at Thr-156. These observations suggest that Fbw7-mediated GATA3 regulation with CDK2-mediated phosphorylation of CPD contributes to the precise differentiation of T-cell lineages.
INTRODUCTION
The F-box protein Fbw7 (also known as Fbxw7, Sel-10, or Cdc4) forms an Skp1-cullin1-F box protein (SCF) complex that mediates the ubiquitylation of substrates. Fbw7 binds to a high-affinity recognition motif termed the Cdc4 phosphodegron (CPD), with a consensus sequence of T/S(PO3)-P-X-X-S/T/D/E (where X indicates an arbitrary residue) (1). Fbw7 often promotes the turnover of substrates via phosphorylation of the CPD. Interestingly, many Fbw7 substrates synergize and/or function to promote specific cell differentiation. Notch1, c-Myc, and mTOR regulate quiescence and storage of hematopoietic stem cells, and Notch1, c-Myc, c-Myb, and MCL1 contribute to the development of the common lymphoid progenitor lineages (2). To investigate the role of Fbw7-mediated ubiquitylation of substrates, Fbw7 conditional knockouts were constructed with tissue-specific expression of Cre recombinase. Using gene targeting mice, some studies have reported that ablation of Fbw7 in T cells resulted in the predisposition to thymic enlargement and thymic lymphoma, which expressed both CD4 and CD8, suggesting their derivation from immature T cells, and the accumulation of c-Myc, Notch1, MCL1, and NF-κB2 (3–5). In this paper, we focused on the reduced thymic CD4 single-positive (SP) and CD8 SP and splenic CD4+ and CD8+ cell proportions in mice, which were conditionally depleted of Fbw7. From further detailed analysis, we found that Fbw7 deficiency also skewed the differentiation of the CD8 SP lineage, which exhibited a higher incidence of apoptosis. Interestingly, similar perturbations during development of CD8-positive cells have been reported with transgenic (Tg) mice in which expression of GATA3 was enforced throughout T-cell development (6).
T-cell progenitors undergo maturation in the thymus and subsequently migrate to the peripheral lymphoid organs. T-cell lineages of thymocytes are classified by the expression pattern of two surface antigens, CD4 and CD8. Most immature T cells do not express CD4 or CD8 and are referred to as double-negative (DN) cells. Maturation of DN cells into double-positive (DP) cells requires expression of both antigens, and further progression leads to the retained expression of CD4 or CD8 in the single-positive (SP) cells (7).
Proper development of T cells depends on lineage-specific regulators, including GATA3, which is one of the factors involved in T-cell specification and commitment. The mammalian GATA family of transcription factors comprises six types, GATA binding protein 1 (GATA1) to GATA6. While each GATA protein has a distinct and restricted tissue expression pattern, GATA1 to GATA3 are classified as the hematopoietic factors. GATA3 is expressed by immune cells. GATA3 is an important regulator of T-cell differentiation and involved in β-selection and CD4 SP T-cell development in the early stage of commitment and T helper 2 (Th2) cell maturation (8–14). GATA3 is upregulated during the development of CD4 but not CD8 SP thymocytes (15, 16). These distinctions act as one of the mediators of the CD4/CD8 lineage decision of thymocytes as overexpression of GATA3 during positive selection inhibited CD8 SP cell development (6). In addition, the increased abundance of GATA3 during the late DN stage disturbs accurate progression from DN to DP and may result in transformed cells, which are characterized as CD4+ CD8+ (6). GATA3 expression is regulated by Notch and NF-κB2 during Th2 differentiation (2, 17–19). We inferred that the protein degradation system might play a critical role in the quantitative regulation of GATA3, similar to a transcriptional regulator. Yamashita et al. reported that extracellular signal-regulated kinase (ERK)–mitogen-activated protein kinase (MAPK) activation stabilized GATA3 through inhibition of the ubiquitin (Ub)-proteasome signaling and that Mdm2 was involved in the ubiquitylation of GATA3 in T cells although the involvement for phosphorylation in regulation by Mdm2 has not been elucidated (20).
We found that GATA3 protein accumulated in T-cell lineages of Fbw7-deficient thymocytes. With the identification of two candidate CPD sequences in GATA3 and given that Fbw7 plays crucial roles in the development of T-cell lineages through the regulation of transcription factors, we hypothesized that Fbw7 targets GATA3 and that its interaction, which is regulated by cyclin-dependent kinase 2 (CDK2)-mediated phosphorylation of CPD, modulates the development of T-cell lineages.
MATERIALS AND METHODS
Conditional knockout mice.
The generation and genotyping of conditional knockout Lck-Cre/Fbw7flox/flox mice were described previously (3). Mice 6 to 10 weeks of age were used for analysis. All mice were treated according to the protocols approved by the Hamamatsu University School of Medicine Animal Care Committees at the Center Animal Care facility.
Cell culture.
Whole thymocytes obtained from Fbw7flox/flox or Lck-Cre/Fbw7flox/flox mice and HUT78 cells (Riken) were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml). HEK293 and HeLa cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml).
Generation of P-T156-GATA3 antibody.
Polyclonal antibodies against phosphorylated Thr-156 of GATA3 (P-T156-GATA3) were raised against a keyhole limpet hemocyanin (KLH)-conjugated chemically synthesized phosphorylated Thr-156 peptide (P-T156 peptide), corresponding to the CPD region of GATA3 (residues 150 to 161) (MBL). Antiserum obtained from an immunized guinea pig was purified using column chromatography conjugated with the P-T156 peptide and then passed through a column conjugated with nonphosphorylated Thr-156 peptide (Peptide Institute) to eliminate antibodies against nonphosphorylated Thr-156 peptide. The antibody specificity was confirmed by enzyme-linked immunosorbent assay and immunoblotting.
Antibodies and fluorescence-activated cell sorting (FACS) analysis.
Phycoerythrin (PE)-Cy5-conjugated anti-CD4 (RM4-5), fluorescein isothiocyanate (FITC)-conjugated anti-CD8a (53-6.7), and PE-labeled annexin V were purchased from BD Pharmingen. PE-Cy7-conjugated anti-CD44 (IM7) and Alexa Fluor 700-conjugated anti-CD25 (PC61) were also purchased from Biolegend. After cell surface labeling, thymic T cells and splenic T cells of mice were scored and sorted by FACS Aria instruments (BD). In addition, anti-Myc 9B11 (Cell Signaling), anti-Myc 9E10 (Roche), anti-FLAG M2 (Sigma), antihemagglutinin (anti-HA) 3F10 (MBL), anti-GATA3 HG3-31 (Santa Cruz), anti-Fbw7 (catalog number A301-720A; Bethyl), anti-CDK2 (TDL), antiphosphothreonine (catalog number 71-8200; Invitrogen), anti-glutathione S-transferase (anti-GST) (B-14; Santa Cruz), anti-HSP90 (catalog number 610419; BD), anti-α-tubulin (clone DM1A; Sigma), anti-β-actin (clone AC15; Sigma), and anti-NF-κB2 (catalog number A301-822A; Bethyl) were also purchased for immunoblot analysis. Alexa Fluor 546-conjugated anti-GATA3 was prepared by an Alexa Fluor 546 monoclonal antibody labeling kit (Zenon).
ICC analysis.
Immunocytochemistry (ICC) was performed on cytospin preparations of the sorted cell subpopulations. Cells were incubated overnight with Alexa Fluor 546-conjugated anti-GATA3 antibody at 4°C, followed by 4′,6′-diamidino-2-phenylindole (DAPI) staining. The relative protein levels were calculated as the means ± standard deviations (SD) from 10 random areas.
Plasmids, recombinant proteins, and protein kinases.
Complementary DNAs encoding wild-type (WT) and mutant GATA3 were cloned into pcDNA3.1/Myc-His (Invitrogen). FLAG-tagged Fbw7 was cloned into pcDNA3.1 (Invitrogen). The expression plasmid for ubiquitin (pCGN-HA-Ub) was previously described (21). All point mutants of GATA3 were constructed using standard recombinant DNA techniques. Glutathione S-transferase (GST) and GST-fused GATA3 proteins were expressed in Escherichia coli BL21 and affinity purified with glutathione-Sepharose 4B (GE Healthcare). The fusion proteins were eluted with 10 mM reduced glutathione. Recombinant protein kinases used in the in vitro phosphorylation assay were cyclin E/CDK2 (Abcam), cyclin D1/CDK4 (Abcam), cyclin D2/CDK4 (Abcam), cyclin A/CDK2 (Abcam), cyclin B/CDK1 (Abcam), ERK1 (Carna Biosciences), p38α (Carna Biosciences), HIPK2 (Carna Biosciences), NLK (Carna Biosciences), and glycogen synthase kinase 3β (GSK3β; NEB). For the in vitro binding assay, recombinant cyclin A/CDK2 (Carna Biosciences) and p38α (Millipore) were used.
Immunoprecipitation and ubiquitylation assay.
Plasmids were transiently transfected into HEK293 cells by the calcium phosphate method. After 43 h, cells were treated with 20 μM MG132 (Peptide Institute) for 5 h and subsequently lysed in lysis buffer containing protease inhibitors. For immunoprecipitation (IP), cell lysates were incubated with antibodies and protein G-Sepharose 4FF (GE Healthcare) at 4°C. Immunocomplexes were washed with lysis buffer. For denaturing and IP analysis, lysates from plasmid-transfected HEK293 or HeLa cells were denatured by the addition of SDS sample buffer and incubation at 100°C for 8 min before being incubated with an anti-Myc antibody and protein G-Sepharose 4FF at 4°C. Immunoprecipitated samples as well as the original cell lysates (input) were separated by SDS-PAGE and transferred from the gel onto a polyvinylidene difluoride (PVDF) membrane (Millipore), followed by immunoblotting. Proteins were visualized using an enhanced chemiluminescence system (PerkinElmer).
Degradation assay.
Plasmids were transfected into HeLa cells. At 24 h after transfection, each transfection was replated into five culture dishes for the chase experiment, and after an additional 24 h, cells were treated with 12.5 mg/ml of cycloheximide for the times indicated in Fig. 6. Cell lysates were subjected to immunoblotting. The intensity of the bands was quantitated using the image analysis software Image Gauge, version 4.21 (Fujifilm), and the signal intensity of each GATA3 protein was normalized using levels of HSP90 as a loading control.
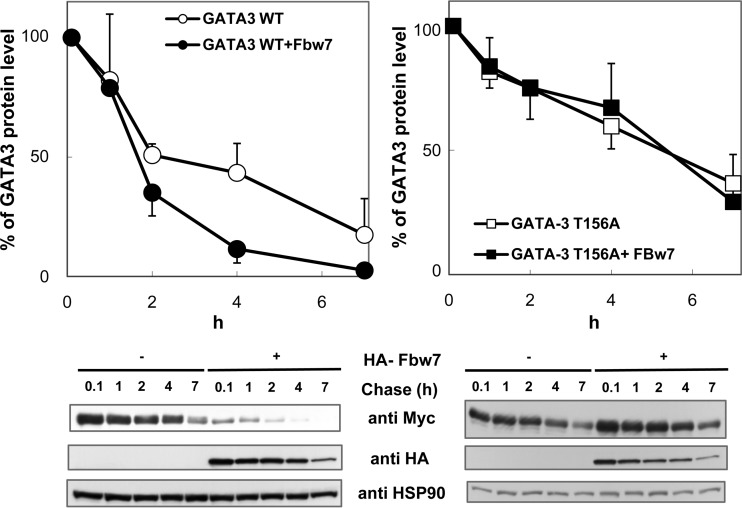
FIG 6.
Fbw7 promotes degradation of GATA3 in a Thr-156-dependent manner. HeLa cells were transfected with Myc-tagged WT (left) or T156A GATA3 (right) in the absence or presence of HA-Fbw7 and treated with 12.5 μg/ml cycloheximide to inhibit protein synthesis for the indicated times. GATA3 levels were analyzed at various time points by immunoblotting (bottom panels). The percentage of GATA3 at each time point was quantitated by image analysis and normalized against HSP90 (top panels). GATA3 protein levels were calculated as the means ± SDs from three independent experiments. Immunoblots show data from one representative experiment.
In vitro phosphorylation assay.
GATA3 WT peptide or GATA3-T156A peptide (0.1875 mM) was incubated with 0.1 μg of recombinant kinase at 30°C for 1 h in reaction buffer containing 50 μM ATP and 3.5 μCi of [γ-32P]ATP (6,000 Ci/mmol), in a final volume of 20 μl. Reactions were terminated by the addition of 10 μl of 250 mM H3PO4. Peptides were trapped on P81 papers (Whatman), which were washed six times with 75 mM H3PO4 and then monitored for radioactivity in a liquid scintillation counter, as previously described (22). A purified GST-fused WT or T156A mutant of GATA3 was incubated with the kinase sources indicated in Fig. 7 and 8 at 30°C for 30 min in reaction buffer containing 63 mM ATP. CDK2 inhibitor (CVT313; Enzo Life Sciences) or competitor (purified recombinant p27) at the indicated dose was added before incubation. The reaction was terminated by boiling mixtures for 5 min. For in vitro phosphorylation and binding assays, instead of boiling, phosphorylated mixtures were incubated for an additional hour at 4°C with lysate from HEK293 cells exogenously expressing Fbw7. GST-fused proteins were then precipitated using glutathione-Sepharose beads. All reaction mixtures were subjected to immunoblot analysis using the indicated antibody.
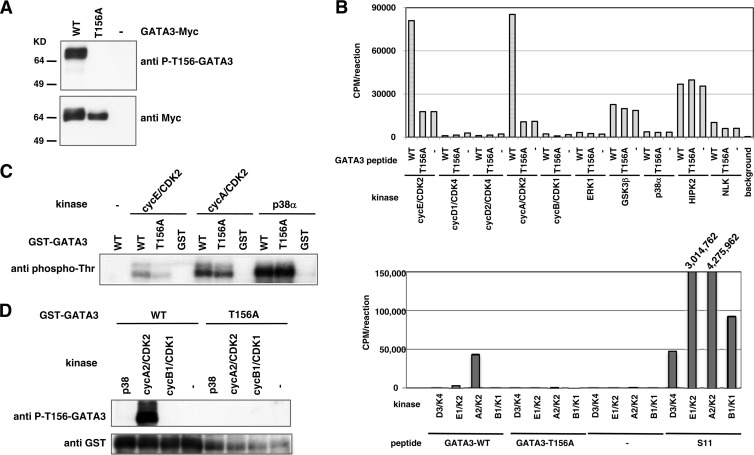
FIG 7.
Thr-156 of GATA3 is phosphorylated by CDK2. (A) Transiently expressed GATA3 is phosphorylated at Thr-156 in HEK293 cells. HEK293 cells were transfected with WT or T156A GATA3 and treated with 20 μM MG132 and 20 nM okadaic acid for 5 h to inhibit proteolysis and dephosphorylation of GATA3. Cell lysates were prepared with lysis buffer containing phosphatase inhibitors and protease inhibitors and subjected to immunoblot analysis using phospho-T156-GATA3 or Myc antibodies. (B) CDK2 phosphorylates GATA3 peptide in a Thr-156-dependent manner in vitro. A synthetic peptide corresponding to aa 150 to 161 of WT or T156A GATA3 was incubated with [γ-32P]ATP and the indicated kinases at 30°C for 30 min (top panel). S11 peptide (KAPLTPKKAK) is efficiently phosphorylated by various CDKs (22). To confirm the activities of the CDKs used in our experiments, we performed in vitro kinase assays using the S11 peptide as a positive control. Wild-type or T156A synthetic GATA3 peptide or S11 peptide was incubated with [γ-32P]ATP along with the indicated CDKs at 30°C for 30 min (bottom panel). The peptides were trapped on P81 papers and monitored for radioactivity using a liquid scintillation counter. (C and D) Recombinant GATA3 is phosphorylated at Thr-156 by CDK2 in vitro. WT or T156A GST-fused GATA3 was expressed in E. coli and affinity purified using glutathione-Sepharose 4B. GST alone was prepared in parallel as a control. The proteins were incubated with the kinases as indicated in reaction buffer at 30°C for 30 min. Reaction products were subjected to immunoblot analysis with the indicated antibodies.
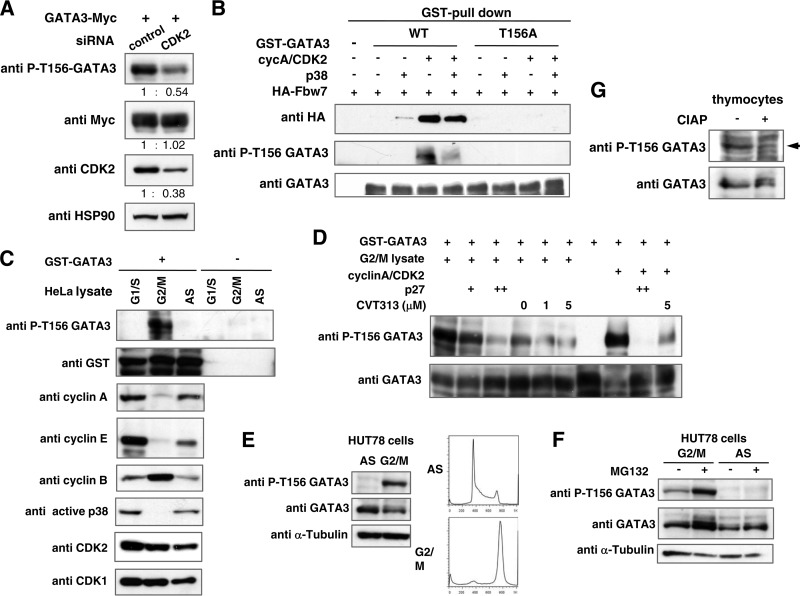
FIG 8.
Phosphorylation at Thr-156 in GATA3 by CDK2 is required for association of Fbw7 and is executed in HUT78 cells during G2/M phase and in thymocytes of mice. (A) Depletion of CDK2 reduced phosphorylation of Thr-156 in GATA3 in HEK293 cells. HEK293 cells were transfected with a WT GATA3 expression plasmid and CDK2 or a control siRNA. After 43 h of transfection, cells were treated with okadaic acid and MG132 for 5 h. Cell lysates were prepared and subjected to immunoblot analysis with the indicated antibodies. The numbers reflect the ratio of the levels of the indicated proteins between the CDK2 siRNA-transfected cells and control cells. (B) GATA3 binding to Fbw7 in vitro is Thr-156 phosphorylation dependent. Purified WT or T156A GST-GATA3 was incubated with the indicated kinases in reaction buffer at 30°C for 30 min. Lysates from HEK293 cells transfected with HA-Fbw7 were immunoprecipitated with HA antibody, and the immunocomplexes containing Fbw7 were incubated with phosphorylated GST-GATA3 in vitro as indicated. To analyze GATA3 and Fbw7 binding, the GST fusion protein complexes were precipitated using glutathione-Sepharose beads and subjected to immunoblotting with the indicated antibodies. (C and D) Phosphorylation of recombinant GATA3 in vitro. Reaction products were then subjected to immunoblot analysis with the indicated antibodies. (C) Phosphorylation of Thr-156 in GATA3 in G2/M-arrested cells. GST-GATA3 was incubated with lysate prepared from either cells arrested in G1/S or G2/M phase or nonsynchronized (AS) HeLa cells. (D) Effects of CDK2 inhibition on Thr-156 phosphorylation of GATA3 in G2/M cell lysate. The responsible kinase in G2/M-phase cell lysate is CDK2. GST-GATA3 was incubated with the indicated kinase sources in the absence or presence of CDK2 inhibitor (CVT313) or competitor (p27). (E and F) Thr-156 phosphorylation of endogenous GATA3 in G2/M phase in T-cell lymphoma HUT78 cells. G2/M-arrested and asynchronous (AS) HUT78 cells were prepared as indicated in Materials and Methods. Cell lysates were prepared and subjected to immunoblot analysis with the indicated antibodies. (F) G2/M-arrested and asynchronous cells were treated with or without MG132 for 5 h before harvest. Cell lysates were prepared and subjected to immunoblot analysis with the indicated antibodies. (G) Cell lysate from whole thymocytes obtained from an Fbw7flox/flox mouse at 6 weeks of age was incubated with or without calf intestinal alkaline phosphatase (CIAP) at 37°C for 30 min and subjected to immunoblot analysis with the indicated antibodies.
RNA interference.
HEK293 cells were transfected with GATA3 expression plasmid and small interfering (siRNA) oligonucleotides for CDK2 using Lipofectamine 2000 reagent (Life Technologies), according to the manufacturer's protocol. After 43 h, cells were treated with 20 nM okadaic acid and 20 μM MG132 for 5 h. The nucleotide sequence of the CDK2 siRNA was 5′-AAGGUGGUGGCGCUUAAGAAA-3′ with 3′ dTdT overhangs (Sigma Genosys). Cell lysates were subjected to immunoblotting.
qRT-PCR analysis.
Total RNA was isolated from cells using RNAiso (TaKaRa) or Isogen-LS (Wako) and subjected to reverse transcription with random hexanucleotide primers and SuperScript II reverse transcriptase (Invitrogen). The resulting cDNA was subjected to quantitative real-time PCR (qRT-PCR) using a Rotor-Gene 3000 system (Corbett Research) and a SYBR premix Ex Taq kit (TaKaRa). The sequences of PCR primers were as follows: 5′-AGGCAGGGAGTGTGTGAACT-3′ (sense) and 5′-TCATAGTCAGGGGTCTGTTA-3′ (antisense) for GATA3; 5′-CAGGGAAACCCAGGAAAAAC-3′ (sense) and 5′-AGTTCCGCACATCCTTCTTG-3′ (antisense) for CCR7; 5′-AGAAGAGGGGATTGATGAAC-3′ (sense) and 5′-AGTGTTGTCATCAGAACCAC-3′ (antisense) for Fbw7; 5′-TGCACCACCAACTGCTTAG-3′ (sense) and 5′-CAGGCAGGGATGATGTTC-3′ (antisense) for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The amount of transcript was normalized against that of GAPDH as an internal standard.
Cell cycle synchronization.
For arrest during G1/S phase, HeLa cells were incubated with 1 μg/ml aphidicolin (Sigma) for 16 h, incubated in aphidicolin-free medium for 10 h, and then incubated with 1 μg/ml aphidicolin for 16 h. For arrest in G2/M phase, HeLa and HUT78 cells were incubated with 1 μg/ml aphidicolin for 16 h, incubated in aphidicolin-free medium for 4 h, and then incubated with 100 ng/ml nocodazole (Sigma) for 16 h. The DNA content of HUT78 cells nonsynchronized or synchronized at G2/M phase was examined by flow cytometry after propidium iodide staining (Beckman Coulter).
RESULTS
Conditional inactivation of Fbw7 in the T-cell lineages impedes T-cell development.
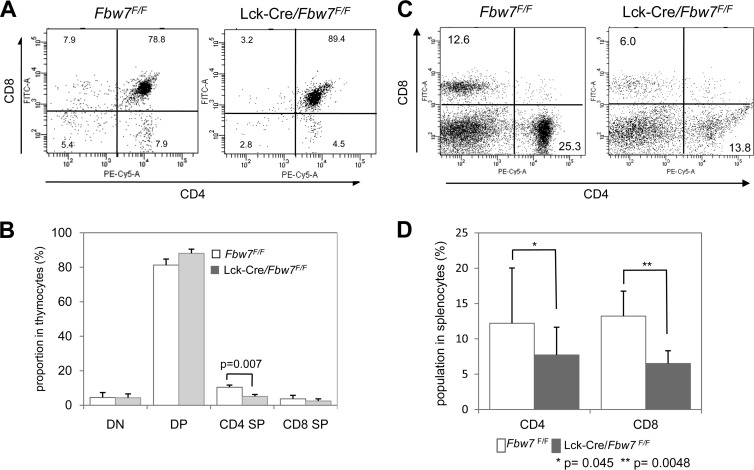
To address the unknown in vivo contribution of Fbw7 to T-cell development, we used conditional knockout Lck-Cre/Fbw7flox/flox mice, which lost Fbw7 expression from genetic deletion by Cre recombinase activity under the control of the Lck promoter (3). We found a significant decrease in the CD4 SP cell proportion in Lck-Cre/Fbw7flox/flox mice compared with Fbw7flox/flox mice (Fig. 1A and B). Moreover, we observed a tendency toward a reduction of the CD8 SP and an increase of the DP cells as a proportion of the thymocyte population (Fig. 1A and B). To determine the influence of the ablation of Fbw7 on the development of peripheral T cells, we examined spleens harboring CD4+ and CD8+ T cells from Fbw7flox/flox and Lck-Cre/Fbw7flox/flox mice. The percentages of both CD4+ and CD8+ cells were significantly reduced in the spleen of Lck-Cre/Fbw7flox/flox mice compared with Fbw7flox/flox control mice (Fig. 1C and D). Because the CD4 SP subtype in the thymus of Lck-Cre/Fbw7flox/flox mice was significantly reduced, it might affect the splenic CD4+ T cells. Our results suggest that the aberrant accumulation of an Fbw7 substrate, which may be GATA3, in CD8 SP cells from Lck-Cre/Fbw7flox/flox mice might be due to enhanced apoptosis and/or perturbed maturation at the final differentiation stage in the thymus.
FIG 1.
The CD4 SP subset in the thymus and CD4+ and CD8+ T-cell subpopulations in the spleen of Lck-Cre/Fbw7flox/flox mice are reduced. (A) Representative flow cytometric analysis of surface expression of CD4 and CD8 on thymocytes from Fbw7flox/flox or Lck-Cre/Fbw7flox/flox mice at 8 weeks of age. The percentages of DN, DP, and SP populations are indicated. (B) Proportions of the T-cell subsets determined in panel A. Data are means ± SD from six mice of each genotype. (C) Representative flow cytometric analysis of surface expression of CD4 or CD8 on splenocytes from Fbw7flox/flox or Lck-Cre/Fbw7flox/flox mice at 9 to 10 weeks of age. The percentages of CD4+ and CD8+ T-cell subpopulations are indicated. (D) Proportions of the T-cell subsets determined in panel C. Data are means ± SD from six mice of each genotype. The P value was determined using the Student t test. F/F, flox/flox.
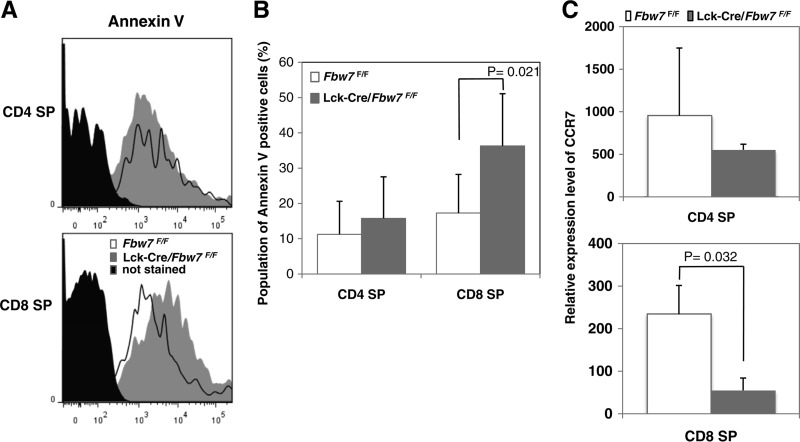
We next examined the fraction of annexin V-positive cells in the SP subpopulation in thymocytes of Lck-Cre/Fbw7flox/flox and Fbw7flox/flox mice. Although no clear differences in annexin V-positive CD4 SP cell populations was observed, we found significantly more annexin V-positive CD8 SP cells in Lck-Cre/Fbw7flox/flox mice than in control mice (Fig. 2A and B). Previously, mice with enforced GATA3 expression throughout T-cell development, driven by the CD2 locus control region (CD2-GATA3 Tg mice), contained higher numbers of apoptotic cells in the thymus, especially in CD8 SP cells, than in the controls and decreased CD8+ T-cell numbers in the periphery although total numbers of CD8 SP cells in the thymus were within normal ranges compared with controls (6). Our findings of aberrations in Lck-Cre/Fbw7flox/flox mice corresponded well with those observed in CD2-GATA3 Tg mice. These data imply that excess GATA3 in the CD8 SP subpopulation in the thymus may cause the distinctive aberrations observed in CD8+ splenocytes.
FIG 2.
Defects in T-cell maturation in Lck-Cre/Fbw7flox/flox mice. Apoptotic levels in SP subpopulations from Fbw7flox/flox and Lck-Cre/Fbw7flox/flox mice were evaluated. (A) Graphs show representative profiles of annexin V staining in CD4 SP and CD8 SP subsets from both groups of mice at 8 to 9 weeks. (B) Percentage of annexin V-positive cells determined from panel A. Data are means ± SD from the percentage of annexin V staining in CD4 SP and CD8 SP subsets from five Fbw7flox/flox and six Lck-Cre/Fbw7flox/flox mice. The P value was determined using the Student t test. (C) The relative expression level of CCR7 in CD4 SP and CD8 SP subsets in the thymus from Fbw7flox/flox or Lck-Cre/Fbw7flox/flox mice at 8 to 9 weeks of age. CCR7 levels were measured by qRT-PCR and normalized against GAPDH as an internal standard. Data are means ± SD from three mice of each genotype.
Newly generated SP thymocytes migrate from the thymic cortex to the medulla, where they undergo functional maturation to acquire immune competence and egress capability (23, 24). CC-chemokine receptor 7 (CCR7) is an important receptor for the medullary positioning of SP cells and an indispensable signal mediator for unperturbed thymic T-cell differentiation and maturation (24, 25). CCR7 remains at low levels in newly generated SP cells and is quickly upregulated and maintained at high levels afterwards (26). CD8+ CCR7− T cells are more sensitive to spontaneous apoptosis than CD8+ CCR7+ T cells (27). This suggests that insufficient CCR7 in CD8+ SP cells suppresses final maturation in the thymus, followed by an induction of apoptosis (27). We speculated that reduced CCR7 expression could be involved in defects in development and survival of CD8 SP cells in the thymus of Lck-Cre/Fbw7flox/flox mice and next evaluated CCR7 expression in SP cells of Lck-Cre/Fbw7flox/flox and Fbw7flox/flox mice. Significant reduction of CCR7 expression was detected in CD8 SP cells from Lck-Cre/Fbw7flox/flox mice compared with expression in Fbw7flox/flox mice although loss of Fbw7 did not affect the relative expression level of CCR7 in CD4 SP cells (Fig. 2C). Normal CD8 SP cells harbor lower GATA3 levels and may be associated with reduced CCR7 transcription. Finally, we speculate that repression of CCR7 caused by the accumulation of GATA3 in CD8 SP cells resulted in the prevention of final maturation and survival in the thymus of the Lck-Cre/Fbw7flox/flox mice.
Loss of Fbw7 causes accumulation of GATA3 during T-cell differentiation in the thymus.
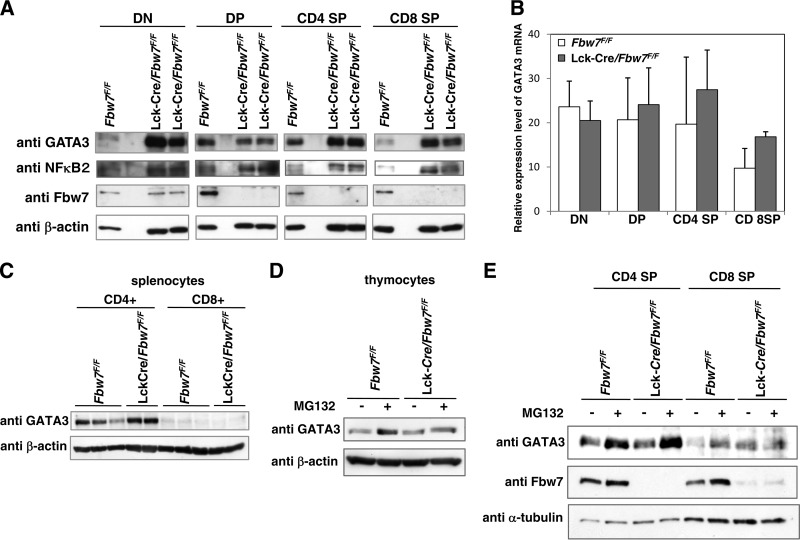
GATA3 gene expression is required during T-cell differentiation from early to late stages in the thymus (28). To examine the in vivo contribution of Fbw7 to GATA3 stability during T-cell development, we examined the expression level of GATA3 in subsets of thymocytes. CD4 SP and especially DN and CD8 SP subsets from Lck-Cre/Fbw7flox/flox mice displayed marked increases in GATA3 protein levels compared with cells from Fbw7flox/flox mice, whereas an increase of GATA3 in comparison with the level in control animals was not observed in the DP lineage of Fbw7-deficient mice (Fig. 3A).
FIG 3.
Loss of Fbw7 stabilizes GATA3 protein in mouse thymocytes. (A) Representative immunoblot analysis of Fbw7 and its target proteins in the subsets of thymocytes from Fbw7flox/flox or Lck-Cre/Fbw7flox/flox mice. DN, DP, CD4 SP, and CD8 SP cells were purified from Fbw7flox/flox or Lck-Cre/Fbw7flox/flox mice at 8 weeks of age using flow cytometry and lysed. The lysates were subjected to immunoblot analysis with the indicated antibodies. (B) qRT-PCR analysis of GATA3 expression in the sorted FACS fractions obtained in panel A. The amount of transcripts was normalized against that of GAPDH as an internal standard. Data are means ± SD of values from three Fbw7flox/flox and three Lck-Cre/Fbw7flox/flox mice. (C) Purification of CD8+ and CD4+ T cells from mouse splenocytes from Fbw7flox/flox or Lck-Cre/Fbw7flox/flox mice at 8 to 9 weeks of age was performed with CD8+ positive selection and CD4+ negative selection by the magnetic bead method, respectively. Immunoblot analysis of whole lysates from each purified splenic T-cell subset was performed with the indicated antibodies. (D) T-cell subsets obtained from Fbw7flox/flox or Lck-Cre/Fbw7flox/flox mice at 8 weeks of age were cultured in RPMI 1640 medium for 2 h and incubated with 20 μM MG132 for 4 h. Cells were lysed and subjected to immunoblot analysis with the indicated antibodies. (E) Whole thymocytes obtained from Fbw7flox/flox or Lck-Cre/Fbw7flox/flox mice at 8 weeks of age were cultured in RPMI 1640 medium for 4 h and incubated with 20 μM MG132 for 5 h. Cells were lysed and subjected to immunoblot analysis with the indicated antibodies.
Fbw7 protein was detected throughout the stages of T-cell differentiation in control mice and was abolished in DP, CD4 SP, and CD8 SP subsets of Lck-Cre/Fbw7flox/flox mice although it was detectable in DN subsets (Fig. 3A). A previous report showed that Lck-Cre-mediated inactivation of the floxed allele started at DN2 (29). Therefore, Fbw7 expression can be manipulated after the transition to the DN2 stage in Lck-Cre/Fbw7flox/flox mice. Although Fbw7 was expressed in the DP subset of the controls, Fbw7 elimination did not result in increased GATA3 in DP cells of Lck-Cre/Fbw7flox/flox mice (Fig. 3A). To more closely evaluate GATA3 protein levels, relative expression levels of GATA3 mRNA during T-cell development were estimated by qRT-PCR. In contrast to protein levels, GATA3 mRNA expression was not significantly influenced by the genetic status of Fbw7 (Fig. 3B). Consequently, we speculated that Fbw7-mediated GATA3 degradation occurs at the DN stage but not at the DP stage. Although CD8 SP subsets in the thymus of the Lck-Cre/Fbw7flox/flox mice retained GATA3 expression, CD8+ T cells in the spleen did not express GATA3 (Fig. 3C).
We demonstrated the involvement of the Fbw7 and proteasome-mediated degradation system in the regulation of GATA3 protein in mouse thymocytes. GATA3 protein accumulated in control thymocytes treated with the proteasome inhibitor MG132, whereas it did not accumulate in MG132-treated Lck-Cre/Fbw7flox/flox mouse thymocytes (Fig. 3D). Meanwhile, GATA3 levels in the absence of MG132 were equivalent in Lck-Cre/Fbw7flox/flox and Fbw7flox/flox thymocytes (Fig. 3D). Because approximately 80% of the thymocyte population consisted of the DP subset, in which we did not observe Fbw7-mediated GATA3 turnover, the influence of Fbw7 genotype on GATA3 levels would not be apparent in the entire thymocyte population. Increasing GATA3 protein in CD4 SP and CD8 SP subsets of Lck-Cre/Fbw7flox/flox mice proposes a role for Fbw7 in regulating GATA3 protein levels in both of these subsets (Fig. 3A). Nevertheless, other E3 ligases might have a partial contribution to GATA3 turnover in these cells because GATA3 accumulation under MG132 treatment was also observed in the CD4 SP subset of Lck-Cre/Fbw7flox/flox mice (Fig. 3E). Taking these observations together, we propose that the significant survival of the CD8 SP lineage is caused by the accumulation of GATA3 in Fbw7-depleted mice.
We also investigated levels of NF-κB2, another substrate of Fbw7 known to accumulate during T-cell development in the thymic subsets of Lck-Cre/Fbw7flox/flox mice (5). Changes in NF-κB2 protein levels were observed during the entire T-cell development stage of Lck-Cre/Fbw7flox/flox mice, showing a distinct pattern from changes in GATA3 levels (Fig. 3A). This implies that Fbw7-mediated GATA3 stability is regulated by signaling pathways distinct from those of NF-κB2.
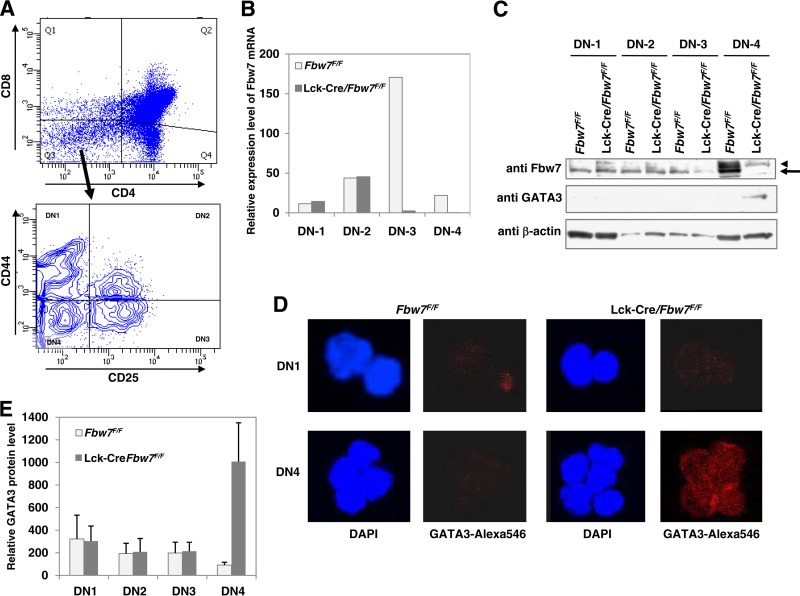
To clarify the correlation of accumulation of GATA3 with depletion of Fbw7 in Lck-Cre/Fbw7flox/flox mice, we performed additional analysis focusing on subpopulations from DN1 to DN4, which were sorted using anti-CD44 and anti-CD25 antibodies, in addition to anti-CD4 and anti-CD8 antibodies (Fig. 4A). We initially observed the elimination of Fbw7 expression at the DN3 and DN4 stages in Lck-Cre/Fbw7flox/flox mice by comparing RNA expression levels to those of control mice (Fig. 4B). It was in accord with the quantitative transition of Fbw7 protein. As shown in Fig. 4C, the depletion of Fbw7 protein in Lck-Cre/Fbw7flox/flox mice started at DN3 and was completed by DN4. Moreover, we found that GATA3 protein was increased in DN4 (Fig. 4C), which was confirmed by immunocytochemical analysis (Fig. 4D and E). These results suggest that Fbw7 participates in the degradation of GATA3 in DN4 cells.
FIG 4.
GATA3 protein is stabilized in the DN4 subset of Lck-Cre/Fbw7flox/flox mice. (A) Thymocytes from Fbw7flox/flox or Lck-Cre/Fbw7flox/flox mice at 9 to 10 weeks of age were incubated with CD4, CD8, CD25, and CD44 for sorting cells from DN1 to DN4 lineages. A representative gating strategy is shown. The sorted FACS fractions obtained in panel A were subjected to qRT-PCR analysis of Fbw7 expression (B), immunoblot analysis (C), and immunocytochemical staining using Alexa Fluor 546 conjugated with anti-GATA3 antibody (D and E). DAPI staining was also examined for detection of the nuclear location (D and E). In panel C, the arrow and arrowhead indicate Fbw7 and nonspecific signal, respectively.
Fbw7 binds to, ubiquitylates, and degrades GATA3 in a Thr-156-dependent manner.
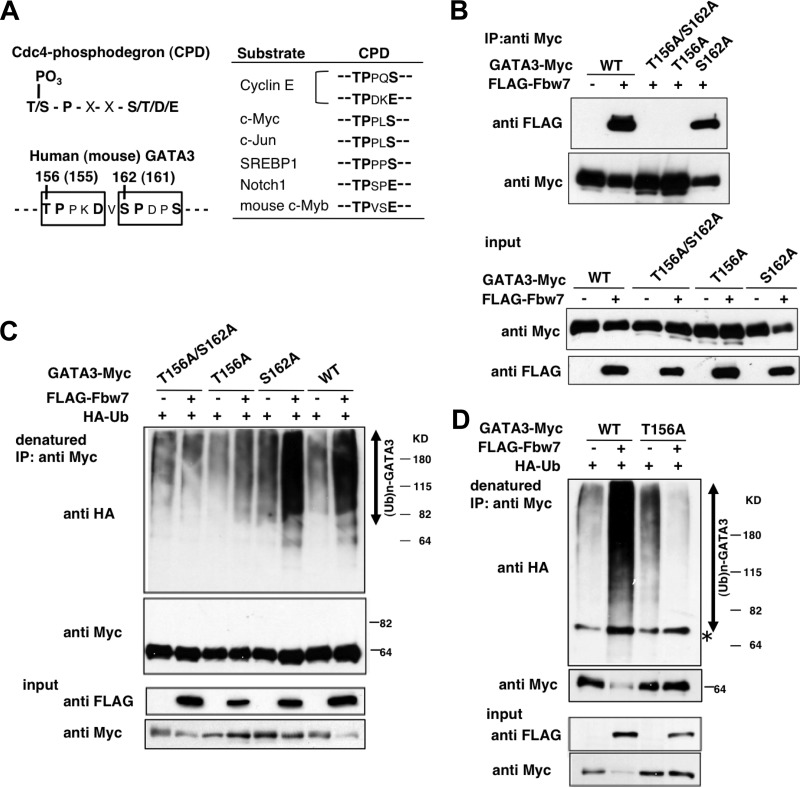
The accumulation in the thymic subsets of Lck-Cre/Fbw7flox/flox mice predicts GATA3 as a novel target for Fbw7 ubiquitin ligase. Fbw7 often interacts with its substrates by binding the phosphorylated CPD in its target proteins (Fig. 5A). We searched the amino acid sequence of GATA3 and noticed two CPD sequences arranged in tandem in both human and mouse GATA3 proteins (Fig. 5A, left). To determine whether Fbw7 interacts with GATA3 protein and whether phosphorylation of CPD is required for recognition by Fbw7 in cultured cells, we prepared a human wild-type (WT) GATA3 expression plasmid and three GATA mutants with amino acid substitutions (T156A/S162A, T156A, and S162A), in which a Thr and/or Ser residue was replaced by Ala. The Myc-tagged WT or mutant GATA3 and FLAG-tagged Fbw7 expression plasmids were cotransfected into HEK293 cells. Cell extracts were subjected to immunoprecipitation (IP) to evaluate the binding between Fbw7 and GATA3. WT GATA3 successfully coimmunoprecipitated Fbw7 (Fig. 5B). Although the S162A mutant retained binding at levels similar to those of the WT, the T156A/S162A and T156A GATA3 mutants completely lost Fbw7 binding ability (Fig. 5B). This result suggests that Thr-156, but not Ser-162, in GATA3 is required for recognition by Fbw7.
FIG 5.
Fbw7 promotes ubiquitylation of GATA3 in a Thr-156-dependent manner. (A) Sequence alignment of known Fbw7 substrates containing the Cdc4 phosphodegron (CPD) consensus sequence for recognition by Fbw7. Conserved residues within the CPD are shown in bold. Two putative CPD sites (open box) in human and mouse GATA3 are indicated. Numbered residues indicate putative phosphorylation sites. (B) Fbw7 binding to GATA3 is dependent on Thr-156 in GATA3 in vivo. HEK293 cells were transfected with Myc-tagged wild-type (WT) or mutant GATA3 along with FLAG-Fbw7, as indicated, and incubated with 20 μM MG132 for 5 h. Cell lysates were immunoprecipitated (IP) with antibodies against Myc, followed by immunoblotting for FLAG and Myc (top panels). Immunoblot analysis of input from each transfection confirmed expression levels of WT GATA3, mutant GATA3, and Fbw7 (bottom panels). (C) HEK293 cells were transfected with Myc-tagged WT or mutant GATA3, FLAG-Fbw7, and HA-ubiquitin as indicated. Cells were incubated with 20 μM MG132 for 5 h, lysed, and denatured with sample buffer containing SDS and 2-mercaptoethanol to dissociate proteins associated with GATA3. Myc-tagged GATA3 was immunoprecipitated and analyzed by immunoblotting with the indicated antibodies (top panels). Whole-cell extracts (input) were subjected to immunoblotting to confirm protein expression (bottom panels). (D) The same experiment as described in panel A was performed in HeLa cells.
We next examined whether Fbw7 promotes ubiquitylation of GATA3 in HEK293 cells by denatured IP analysis. A strongly enhanced ubiquitylation signal was detected on WT GATA3 in the presence of Fbw7 (Fig. 5C). Notably, Fbw7-mediated ubiquitylation of both T156A/S162A and T156A mutants was markedly reduced, while the S162A mutant was successfully ubiquitylated (Fig. 5C). Similar results were obtained in HeLa cells (Fig. 5D).
To address the possibility that the SCFFbw7 ubiquitin ligase targets GATA3 for degradation, the effect of Fbw7 expression on the turnover of exogenous GATA3 was investigated in HeLa cells. Coexpression of Fbw7 significantly facilitated the degradation of WT GATA3 (Fig. 6 left). In contrast, the T156A mutant was relatively stable compared with WT GATA3 in the absence of Fbw7, and its level did not change with Fbw7 (Fig. 6). These results suggest that modification of GATA3 on Thr-156 is one of the key events for recognition by SCFFbw7, which mediates GATA3 ubiquitylation and degradation. Moreover, owing to degradation of WT GATA3 by coexpressed Fbw7, protein levels of WT GATA3 in the presence of HA-Fbw7 were significantly reduced in comparison with levels in the absence of HA-Fbw7, even at the first measurement of this assay (0.1 h).
CDK2 phosphorylates Thr-156 in GATA3.
We speculated that like other substrates of Fbw7, regulation of GATA3 by Fbw7 would be mediated by phosphorylation of Thr-156 in the CPD. To evaluate whether Thr-156 of GATA3 was phosphorylated in intact cells, a phospho-specific antibody (anti-P-T156-GATA3) that recognizes phosphorylated Thr-156 was prepared. Myc-tagged WT GATA3 expressed in HEK293 cells treated with both phosphatase and proteasome inhibitors was detected by the anti-P-T156-GATA3 antibody, but no signal was detected using the GATA3 T156A mutant (Fig. 7A). These data suggest that GATA3 Thr-156 is phosphorylated in vivo.
Fbw7-mediated degradation of substrate is often triggered by the activation of GSK3 (1, 4, 5, 30, 31). GSK3 phosphorylates Ser and Thr residues, after an initial “priming phosphorylation” of a Ser or Thr located four amino acids C-terminal to the site of GSK phosphorylation (32). If Asp and Glu can be considered to mimic this priming phosphorylation, the residues surrounding Thr-156 in GATA3 can be classified as a GSK3 phosphorylation consensus motif (Fig. 5A, left). To examine whether GSK3 phosphorylates Thr-156 of GATA3 in vitro, we prepared two types of synthetic peptides (amino acids [aa] 150 to 161 of GATA3) for the WT and the Thr-156 mutant sequence in which Thr was replaced with Ala (T156A). Unexpectedly, there was no detectable incorporation of 32P in the WT or T156A peptide, indicating that GSK3 does not phosphorylate either peptide (Fig. 7B, top). We examined other Ser/Thr kinases and found that cyclin E/CDK2 and cyclin A/CDK2, but not cyclin B1/CDK1, specifically phosphorylated WT GATA3 peptide (Fig. 7B, top). Their kinase activity was confirmed using the S11 peptide, which is a confirmed substrate for CDK1, CDK2, and CDK4/CDK 6 (Fig. 7B, bottom) (22).
To further confirm the specific phosphorylation on Thr-156, GST fusion recombinant GATA3 proteins, WT and T156A mutant, were examined as substrates. Reduced CDK2-mediated phosphorylation of the T156A mutant compared with WT GATA3 was confirmed by immunoblotting with anti-phospho-Thr and anti-P-T156-GATA3 antibodies (Fig. 7C and D). A previous study reported that GATA3 is also phosphorylated by p38 MAPK (33, 34). Consistent with this report, p38 strongly phosphorylated both recombinant GATA3 proteins (Fig. 7C) although it did not exhibit kinase activity toward Thr-156 in GATA3 (Fig. 7B, top, and D).
Physiological CDK2, which is activated during G2/M phase, works on Thr-156 of GATA3 and regulates its stability.
We further investigated physiological effects on GATA3 of CDK2. Reduction of CDK2 by siRNA inhibited phosphorylation on Thr-156, providing evidence that CDK2 is involved in CPD phosphorylation of GATA3 in vivo (Fig. 8A). To verify the requirement of phosphorylation of GATA3 for binding to Fbw7, we tested the binding ability of phosphorylated GATA3 to Fbw7 in vitro. WT GATA3 phosphorylated by CDK2 was able to bind Fbw7, while the T156A GATA3 mutant did not, regardless of the phosphorylating kinase (Fig. 8B). These results suggest that CDK2-mediated phosphorylation of Thr-156 is essential for binding of GATA3 to Fbw7.
Cyclin E/CDK2 plays a critical role in the G1/S-phase transition (35). Cyclin A then replaces cyclin E to associate with CDK2, and this complex subsequently functions in S-phase progression and G2/M transition (36). We conducted an in vitro phosphorylation assay using cell lysates prepared from G1/S or G2/M synchronized or nonsynchronized HeLa cells as the kinase source. Recombinant GATA3 was phosphorylated only in the presence of G2/M lysate even though both cyclin A and cyclin E protein levels in the lysate were decreased (Fig. 8C). To verify the responsible kinase in the G2/M lysate, we tested the effects of a CDK2 inhibitor (CVT313) and competitor (p27) on kinase activity. Both p27 and CVT313 inhibited the kinase activity on Thr-156 in a dose-dependent manner (Fig. 8D).
Phosphorylation at Thr-156 in GATA3 is executed in HUT78 cells during G2/M phase and in thymocytes of mice.
Cell cycle-dependent phosphorylation of CPD in endogenous GATA3 was also confirmed in the HUT78 cell line, which is a T-cell lymphoma line. Phosphorylated Thr-156 was detected in G2/M arrested cells but not in nonsynchronized cells, which contained a G2/M population of only approximately 15% (Fig. 8E). We further investigated the property of Thr-156-phosphorylated GATA3. MG132 treatment resulted in an accumulation of Thr-156-phosphorylated GATA3 in G2/M (Fig. 8F). This implies that phosphorylation of GATA3 at Thr-156 induces its proteasome-dependent degradation during G2/M. These results suggest that cyclin A/CDK2 regulates GATA3 stability through the phosphorylation of the CPD during G2/M phase in cultured cells. GATA3 Thr-156 phosphorylation was also detected in mouse thymocytes (Fig. 8G). Furthermore, ICC analysis using anti-phospho-Thr-156 antibody detected phosphorylated Thr-156 in GATA3 in the DN subset of thymocytes (data not shown). We found that phosphorylation of Thr-156 in GATA3 in DP subsets was observed at an equal level relative to that in DN subsets (DN/DP, 1:1.16) (data not shown). Because GATA3 accumulates in the DN but not in the DP subset in Lck-Cre/Fbw7flox/flox mice (Fig. 3A), Thr-156 phosphorylation of GATA3 may participate in Fbw7-mediated degradation of GATA3 in the DN subset. In contrast, an unknown mechanism that suppresses Fbw7-mediated degradation of GATA3 may be present in the DP lineage, while Thr-156 phosphorylation of GATA3 was observed.
DISCUSSION
Half of GATA3-overexpressing CD2-GATA3 Tg mice developed thymic lymphoblastoid tumors (6). This may be consistent with a previous study showing that half of Lck-Cre/Fbw7flox/flox mice developed thymic lymphoma (3). We confirmed the accumulation of GATA3 protein at the DN stage in Lck-Cre/Fbw7flox/flox mice. Based on the expression pattern of CD2, which starts at a late DN stage, like Lck, overexpression of GATA3 must occur at this point in CD2-GATA3 Tg mice. We speculate that the induction of GATA3 in the thymus of CD2-GATA3 Tg mice and the accumulation of GATA3 in the thymus of Lck-Cre/Fbw7flox/flox mice begin at the same phase. The increased abundance of GATA3 late in the DN stage may disturb accurate progression from DN to DP and may result in transformed cells, which have been characterized as CD4+ CD8+ in both Lck-Cre/Fbw7flox/flox and CD2-GATA3 Tg mice (3, 37). This may imply that an appropriate amount of GATA3 is essential for the favorable development of T cells not only at the SP phase but also at an earlier phase. We indicated that depletion of Fbw7 protein in Lck-Cre/Fbw7flox/flox mice started at DN3 and was completed at DN4 and that GATA3 accumulated at DN4 in mice. We further investigated whether GATA3 accumulation at DN4 affected the proportion of DN4 subpopulations. The proportion in Lck-Cre/Fbw7flox/flox mice was not significantly different from that in Fbw7flox/flox mice (data not shown). Previous studies suggested that development from DN2 to DN3 might be affected by the deregulation of GATA3 levels. Overexpression of GATA3 in mouse fetal liver progenitors blocked the development of DN2 and DN3 cells in fetal thymus organ culture or in an OP9-DL1 coculture system (14, 38). Xu et al. (39) reported that E2A repressed GATA3 expression at the DN2 stage. In that study, increased GATA3 in E2A−/− DN2 lineages prevented T-cell differentiation to the DN3 stage and caused an aberrant proliferation of DN2 cells. Fbw7 may be involved in development of the DN2-DN3 stage by repression of GATA3 levels. In our study using Lck-Cre/Fbw7flox/flox mice, the effects of Fbw7 depletion were focused on the DN4 stage. To evaluate the contribution of Fbw7 on GATA3 turnover in earlier DN subpopulations, Scl-Cre or Mx1-Cre driver Fbw7 knockout mice, in which depletion of Fbw7 is expected throughout the DN stages, may be useful. However, some confusion may occur when these mice are used because, in addition to GATA3, other Fbw7 targets such as Notch play roles in DN development. Data regarding GATA3-targeting genes, which are active in the DN lineage, have not been reported to date. Consequently, further observations will be required in future studies to identify phenotypes which might be obtained as a result of accumulation of GATA3 in Fbw7-depleted mice.
Aberrant expression of GATA3 was reported in classical Hodgkin lymphoma (cHL) (40). A pathological feature of cHL is the occurrence of a small number of the typical Hodgkin and Reed/Sternberg (HRS) tumor cells among a mixed cellular infiltrate. Whereas HRS cells are derived from germinal center B cells, they ectopically express GATA3. GATA3 contributes to cytokine signaling in HRS cells, which presumably has an essential role in cHL pathogenesis (40). Anomalous GATA3 expression in HRS cells is stimulated by the deregulated activity of NF-κB and Notch1, which bind directly to the GATA3 promoter (40). Intriguingly, both NF-κB and Notch1 are substrates of Fbw7 (5, 41). Therefore, once Fbw7 deficiency occurs in cHL, NF-κB and Notch1 accumulate and subsequently induce GATA3 expression. Further, the repressed degradation of GATA3 would enhance the malignant signal. Finally, the defect of Fbw7 in cHL may promote the development of aggressive disease caused by excess GATA3.
Although CD8 SP subsets in the thymus of the Lck-Cre/Fbw7flox/flox mice retained GATA3 expression, CD8+ T cells in the spleen did not express GATA3 (Fig. 3C). The GATA3-independent pathway may be responsible for the reduced frequency of CD8+ T cells in the spleen of Fbw7-deficient mice. Nonetheless, the decrease in a percentage of CD8+ T cells in splenocytes of the Lck-Cre/Fbw7flox/flox mice compared with control mice was confirmed. This is consistent with results from CD2-GATA3 Tg mice showing that exogenous expression of GATA3 was not detected in splenocytes (37). We observed a significant reduction of CCR7 expression in the CD8 SP cells from Lck-Cre/Fbw7flox/flox mice compared with Fbw7flox/flox mice. Because of inadequate GATA3 levels, proper cell differentiation and exit from the thymus might be disturbed, and/or apoptosis of immature subsets might be induced in CD8 SP subsets of Lck-Cre/Fbw7flox/flox mice. Ultimately, only the normal CD8 SP subpopulation that expresses appropriate GATA3 protein levels may succeed in translocation toward peripheral organs. We detected a decrease in not only CD8+ T cells but also CD4+ T cells in splenocytes of the Lck-Cre/Fbw7flox/flox mice compared with control levels. A previous study showed that the CD4+ T-cell subset in the spleen of CD2-GATA3 Tg mice advanced the Th2-committed phenotype although its rate was comparable to that of control mice (37). Accordingly, we speculate that some aberration, from excess GATA3 during differentiation and maturation in the thymus, persisted throughout development of CD4+ T cells in the spleen. Nevertheless, similar to results in CD2-GATA3 Tg mice, in Lck-Cre/Fbw7flox/flox mice there was no significant effect on CD4 SP cells in the thymus. This might suggest that CD8 SP cells were more sensitive to changes in GATA3 than CD4 SP cells. In our experiments, GATA3 protein was markedly decreased in the CD8 SP subset after maturation of the DP lineage in control mice. In contrast, deficiency of Fbw7 resulted in accumulation of GATA3 protein in CD8 SP subsets, suggesting that Fbw7-mediated degradation plays a key role in regulating GATA3 protein levels in the subsets. Because the forced expression of GATA3 induces apoptosis and inhibits the final maturation of CD8 SP T cells, it is suggested that reduction of GATA3 is required for the satisfactory development of the CD8 SP lineage after positive selection. The proportion of CD8 SP cells in the thymic cells is dependent on the extent of apoptosis and proliferation during differentiation. It is speculated that enhanced apoptosis of CD8 SP cells in Lck-Cre/Fbw7flox/flox mice decreased their percentage in thymocytes. Consequently, GATA3 accumulation caused by the depletion of Fbw7 might induce this decrease in cell populations. However, the same explanation cannot be applied to the reduction of CD4 SP cells, because increased GATA3 was not observed to influence viability of CD4 SP cells. Indeed, it might be an effect of the accumulation of other Fbw7 substrates. Onoyama et al. also speculated that DP thymocytes had lost the ability to undergo positive selection or that the proliferation or survival of SP cells was impaired in Fbw7-deficient mice (3). Nevertheless, they suggested that the participating molecules remained to be elucidated.
In a reference database for gene expression analysis (RefExA), expression of GATA3 is observed not only in the thymus and spleen but also in salivary gland, breast, skin, bladder, kidney, placenta, and blood. RefExA also demonstrated the expression of Fbw7 in brain, breast, skin, heart, adrenal gland, intestine, stomach, and testis, in addition to thymus. Therefore, GATA3 may be regulated by Fbw7-mediated degradation in numerous tissues that express both GATA3 and Fbw7. This possibility should be investigated in the future using a tissue-specific conditional knockout system.
Many substrates of Fbw7 contain a CPD sequence, which is often phosphorylated by GSK3 (1, 4, 5, 30, 31). Although we initially predicted that the CPD in GATA3 is phosphorylated by GSK3, our data indicate that the targeting kinase is CDK2, not GSK3. Consistent with these data, we previously showed that S/T-P-X-K/R, which corresponds to the CPD sequence in GATA3, is the consensus targeting sequence of cyclin A/E-CDK2 (22). Here, we propose GATA3 as the first target of CDK2-mediated phosphorylation for degradation by Fbw7. These observations suggest that regulatory signals in addition to GSK3 participate in regulating Fbw7 substrates. Levels of these proteins, which have various functions in the development of T cells, will be maintained at the appropriate stage for proper differentiation, maturation, and survival of T cells. Because GSK3-independent phosphorylation of CPD in GATA3 is a unique Fbw7 targeting pathway, we predict that GATA3 degradation should be distinct from the other Fbw7 targets in T cells. MAPK-associated phosphorylation of GATA3 is related to the so-called emergent signals in later stages of T-cell development, while the CDK2-associated GATA3 degradation system mediated by Fbw7 may be required in early lineages for constitutive precise differentiation.
We showed that phosphorylation of Thr-156, which was an essential modification for binding of Fbw7, was mediated by CDK2. It is consistent that the phosphorylated level of intrinsic GATA3 varies during G2/M phase in T-cell lymphoma HUT78 cells. We also detected the phosphorylation at Thr-156 of GATA3 in thymocytes of mouse. Furthermore, we clarified that Fbw7 participates in GATA3 degradation in the DN4 lineage. Because CDK2 activity is present during the G1/S border to G2/M stages in proliferating cells, it was speculated that CDK2-dependent phosphorylation of GATA3 and its degradation by Fbw7 in G2/M occur in proliferating DN4 cells and T-cell lymphomas such as HUT78 cells. Our data indicated that Fbw7 also participates in GATA3 degradation in CD8 SP cells. Because cell proliferation is not present in CD8 SP cells, cell cycle-independent CDK2 activity may participate in the phosphorylation of Thr-156. Alternatively, it is possible that unknown kinases and/or other mechanisms are involved in Fbw7-mediated degradation of GATA3 in CD8 SP cells.
Finally, we propose that control of GATA3 levels by Fbw7 contributes to the fine-tuning of T-cell development.
ACKNOWLEDGMENTS
We thank M. Hakamata, M. Matsumoto, and A. Ardiyanti for technical support and N. Minegishi and T. Nakajima for useful discussions.
This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan grants-in-aid 24570151 (K.K.) and 25112508 and 19057005 (M.K.).
Footnotes
Published ahead of print 12 May 2014
REFERENCES
- 1.Welcker M, Clurman BE. 2008. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat. Rev. Cancer 8:83–93. 10.1038/nrc2290 [DOI] [PubMed] [Google Scholar]
- 2.Kitagawa K, Kitagawa M. 2012. The SCF ubiquitin ligases involved in hematopoietic lineage. Curr. Drug Targets 13:1641–1648. 10.2174/138945012803529974 [DOI] [PubMed] [Google Scholar]
- 3.Onoyama I, Tsunematsu R, Matsumoto A, Kimura T, de Alboran IM, Nakayama K, Nakayama KI. 2007. Conditional inactivation of Fbxw7 impairs cell-cycle exit during T cell differentiation and results in lymphomatogenesis. J. Exp. Med. 204:2875–2888. 10.1084/jem.20062299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inuzuka H, Shaik S, Onoyama I, Gao D, Tseng A, Maser RS, Zhai B, Wan L, Gutierrez A, Lau AW, Xiao Y, Christie AL, Aster J, Settleman J, Gygi SP, Kung AL, Look T, Nakayama KI, DePinho RA, Wei W. 2011. SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature 471:104–109. 10.1038/nature09732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukushima H, Matsumoto A, Inuzuka H, Zhai B, Lau AW, Wan L, Gao D, Shaik S, Yuan M, Gygi SP, Jimi E, Asara JM, Nakayama K, Nakayama KI, Wei W. 2012. SCF(Fbw7) modulates the NFκB signaling pathway by targeting NFκB2 for ubiquitination and destruction. Cell Rep. 1:434–443. 10.1016/j.celrep.2012.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nawijn MC, Ferreira R, Dingjan GM, Kahre O, Drabek D, Karis A, Grosveld F, Hendriks RW. 2001. Enforced expression of GATA-3 during T cell development inhibits maturation of CD8 single-positive cells and induces thymic lymphoma in transgenic mice. J. Immunol. 167:715–723. 10.4049/jimmunol.167.2.715 [DOI] [PubMed] [Google Scholar]
- 7.Ellmeier W, Sawada S, Littman DR. 1999. The regulation of CD4 and CD8 coreceptor gene expression during T cell development. Annu. Rev. Immunol. 17:523–554. 10.1146/annurev.immunol.17.1.523 [DOI] [PubMed] [Google Scholar]
- 8.Ting CN, Olson MC, Barton KP, Leiden JM. 1996. Transcription factor GATA-3 is required for development of the T-cell lineage. Nature 384:474–478. 10.1038/384474a0 [DOI] [PubMed] [Google Scholar]
- 9.Hosoya T, Maillard I, Engel JD. 2010. From the cradle to the grave: activities of GATA-3 throughout T-cell development and differentiation. Immunol. Rev. 238:110–125. 10.1111/j.1600-065X.2010.00954.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrar JD, Ouyang W, Lohning M, Assenmacher M, Radbruch A, Kanagawa O, Murphy KM. 2001. An instructive component in T helper cell type 2 (Th2) development mediated by GATA-3. J. Exp. Med. 193:643–649. 10.1084/jem.193.5.643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pai SY, Truitt ML, Ho IC. 2004. GATA-3 deficiency abrogates the development and maintenance of T helper type 2 cells. Proc. Natl. Acad. Sci. U. S. A. 101:1993–1998. 10.1073/pnas.0308697100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pai SY, Truitt ML, Ting CN, Leiden JM, Glimcher LH, Ho IC. 2003. Critical roles for transcription factor GATA-3 in thymocyte development. Immunity 19:863–875. 10.1016/S1074-7613(03)00328-5 [DOI] [PubMed] [Google Scholar]
- 13.Ho IC, Tai TS, Pai SY. 2009. GATA3 and the T-cell lineage: essential functions before and after T-helper-2-cell differentiation. Nat. Rev. Immunol. 9:125–135. 10.1038/nri2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson MK, Hernandez-Hoyos G, Dionne CJ, Arias AM, Chen D, Rothenberg EV. 2002. Definition of regulatory network elements for T cell development by perturbation analysis with PU.1 and GATA-3. Dev. Biol. 246:103–121. 10.1006/dbio.2002.0674 [DOI] [PubMed] [Google Scholar]
- 15.Hendriks RW, Nawijn MC, Engel JD, van Doorninck H, Grosveld F, Karis A. 1999. Expression of the transcription factor GATA-3 is required for the development of the earliest T cell progenitors and correlates with stages of cellular proliferation in the thymus. Eur. J. Immunol. 29:1912–1918. [DOI] [PubMed] [Google Scholar]
- 16.Hernández-Hoyos G, Anderson MK, Wang C, Rothenberg EV, Alberola-Ila J. 2003. GATA-3 expression is controlled by TCR signals and regulates CD4/CD8 differentiation. Immunity 19:83–94. 10.1016/S1074-7613(03)00176-6 [DOI] [PubMed] [Google Scholar]
- 17.Das J, Chen CH, Yang L, Cohn L, Ray P, Ray A. 2001. A critical role for NF-κB in GATA3 expression and TH2 differentiation in allergic airway inflammation. Nat. Immunol. 2:45–50. 10.1038/83158 [DOI] [PubMed] [Google Scholar]
- 18.Amsen D, Antov A, Jankovic D, Sher A, Radtke F, Souabni A, Busslinger M, McCright B, Gridley T, Flavell RA. 2007. Direct regulation of Gata3 expression determines the T helper differentiation potential of Notch. Immunity 27:89–99. 10.1016/j.immuni.2007.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang TC, Yashiro-Ohtani Y, Del Bianco C, Knoblock DM, Blacklow SC, Pear WS. 2007. Notch directly regulates Gata3 expression during T helper 2 cell differentiation. Immunity 27:100–110. 10.1016/j.immuni.2007.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamashita M, Shinnakasu R, Asou H, Kimura M, Hasegawa A, Hashimoto K, Hatano N, Ogata M, Nakayama T. 2005. Ras-ERK MAPK cascade regulates GATA3 stability and Th2 differentiation through ubiquitin-proteasome pathway. J. Biol. Chem. 280:29409–29419. 10.1074/jbc.M502333200 [DOI] [PubMed] [Google Scholar]
- 21.Nakayama K, Nagahama H, Minamishima YA, Matsumoto M, Nakamichi I, Kitagawa K, Shirane M, Tsunematsu R, Tsukiyama T, Ishida N, Kitagawa M, Hatakeyama S. 2000. Targeted disruption of Skp2 results in accumulation of cyclin E and p27Kip1, polyploidy and centrosome overduplication. EMBO J. 19:2069–2081. 10.1093/emboj/19.9.2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitagawa M, Higashi H, Jung HK, Suzuki-Takahashi I, Ikeda M, Tamai K, Kato J, Segawa K, Yoshida E, Nishimura S, Taya Y. 1996. The consensus motif for phosphorylation by cyclin D1-Cdk4 is different from that for phosphorylation by cyclin A/E-Cdk2. EMBO J. 15:7060–7069 [PMC free article] [PubMed] [Google Scholar]
- 23.Hale JS, Fink PJ. 2009. Back to the thymus: peripheral T cells come home. Immunol. Cell Biol. 87:58–64. 10.1038/icb.2008.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehrlich LI, Oh DY, Weissman IL, Lewis RS. 2009. Differential contribution of chemotaxis and substrate restriction to segregation of immature and mature thymocytes. Immunity 31:986–998. 10.1016/j.immuni.2009.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forster R, Davalos-Misslitz AC, Rot A. 2008. CCR7 and its ligands: balancing immunity and tolerance. Nat. Rev. Immunol. 8:362–371. 10.1038/nri2297 [DOI] [PubMed] [Google Scholar]
- 26.Teng F, Zhou Y, Jin R, Chen Y, Pei X, Liu Y, Dong J, Wang W, Pang X, Qian X, Chen WF, Zhang Y, Ge Q. 2011. The molecular signature underlying the thymic migration and maturation of TCRαβ+ CD4+ CD8 thymocytes. PLoS One 6:e25567. 10.1371/journal.pone.0025567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JW, Ferris RL, Whiteside TL. 2005. Chemokine C receptor 7 expression and protection of circulating CD8+ T lymphocytes from apoptosis. Clin. Cancer Res. 11:7901–7910. 10.1158/1078-0432.CCR-05-1346 [DOI] [PubMed] [Google Scholar]
- 28.Hosoya T, Kuroha T, Moriguchi T, Cummings D, Maillard I, Lim KC, Engel JD. 2009. GATA-3 is required for early T lineage progenitor development. J. Exp. Med. 206:2987–3000. 10.1084/jem.20090934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolfer A, Wilson A, Nemir M, MacDonald HR, Radtke F. 2002. Inactivation of Notch1 impairs VDJβ rearrangement and allows pre-TCR-independent survival of early alpha beta lineage thymocytes. Immunity 16:869–879. 10.1016/S1074-7613(02)00330-8 [DOI] [PubMed] [Google Scholar]
- 30.Kitagawa K, Hiramatsu Y, Uchida C, Isobe T, Hattori T, Oda T, Shibata K, Nakamura S, Kikuchi A, Kitagawa M. 2009. Fbw7 promotes ubiquitin-dependent degradation of c-Myb: involvement of GSK3-mediated phosphorylation of Thr-572 in mouse c-Myb. Oncogene 28:2393–2405. 10.1038/onc.2009.111 [DOI] [PubMed] [Google Scholar]
- 31.Busino L, Millman SE, Scotto L, Kyratsous CA, Basrur V, O'Connor O, Hoffmann A, Elenitoba-Johnson KS, Pagano M. 2012. Fbxw7α- and GSK3-mediated degradation of p100 is a pro-survival mechanism in multiple myeloma. Nat. Cell Biol. 14:375–385. 10.1038/ncb2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen P, Goedert M. 2004. GSK3 inhibitors: development and therapeutic potential. Nat. Rev. Drug Discov. 3:479–487. 10.1038/nrd1415 [DOI] [PubMed] [Google Scholar]
- 33.Chen CH, Zhang DH, LaPorte JM, Ray A. 2000. Cyclic AMP activates p38 mitogen-activated protein kinase in Th2 cells: phosphorylation of GATA-3 and stimulation of Th2 cytokine gene expression. J. Immunol. 165:5597–5605. 10.4049/jimmunol.165.10.5597 [DOI] [PubMed] [Google Scholar]
- 34.Maneechotesuwan K, Xin Y, Ito K, Jazrawi E, Lee KY, Usmani OS, Barnes PJ, Adcock IM. 2007. Regulation of Th2 cytokine genes by p38 MAPK-mediated phosphorylation of GATA-3. J. Immunol. 178:2491–2498. 10.4049/jimmunol.178.4.2491 [DOI] [PubMed] [Google Scholar]
- 35.Hinds PW. 2003. Cdk2 dethroned as master of S phase entry. Cancer Cell 3:305–307. 10.1016/S1535-6108(03)00084-9 [DOI] [PubMed] [Google Scholar]
- 36.Woo RA, Poon RY. 2003. Cyclin-dependent kinases and S phase control in mammalian cells. Cell Cycle 2:316–324. 10.4161/cc.2.4.468 [DOI] [PubMed] [Google Scholar]
- 37.Nawijn MC, Dingjan GM, Ferreira R, Lambrecht BN, Karis A, Grosveld F, Savelkoul H, Hendriks RW. 2001. Enforced expression of GATA-3 in transgenic mice inhibits Th1 differentiation and induces the formation of a T1/ST2-expressing Th2-committed T cell compartment in vivo. J. Immunol. 167:724–732. 10.4049/jimmunol.167.2.724 [DOI] [PubMed] [Google Scholar]
- 38.Taghon T, Yui MA, Rothenberg EV. 2007. Mast cell lineage diversion of T lineage precursors by the essential T cell transcription factor GATA-3. Nat. Immunol. 8:845–855. 10.1038/ni1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu W, Carr T, Ramirez K, McGregor S, Sigvardsson M, Kee BL. 2013. E2A transcription factors limit expression of Gata3 to facilitate T lymphocyte lineage commitment. Blood 121:1534–1542. 10.1182/blood-2012-08-449447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stanelle J, Doring C, Hansmann ML, Kuppers R. 2010. Mechanisms of aberrant GATA3 expression in classical Hodgkin lymphoma and its consequences for the cytokine profile of Hodgkin and Reed/Sternberg cells. Blood 116:4202–4211. 10.1182/blood-2010-01-265827 [DOI] [PubMed] [Google Scholar]
- 41.Hubbard EJ, Wu G, Kitajewski J, Greenwald I. 1997. sel-10, a negative regulator of lin-12 activity in Caenorhabditis elegans, encodes a member of the CDC4 family of proteins. Genes Dev. 11:3182–3193. 10.1101/gad.11.23.3182 [DOI] [PMC free article] [PubMed] [Google Scholar]