Abstract
A comparison of real-time PCR positivity rates for Bordetella pertussis between specimens collected with rayon swabs on an aluminum wire shaft in Amies gel with charcoal and those collected with flocked swabs in universal viral transport medium during an epidemic revealed that their performances were comparable.
TEXT
Since Jules Bordet and Octave Gengou first isolated Bordetella pertussis, the bacillus responsible for whooping cough, in 1906 using their specific medium, named Bordet-Gengou agar (1), the laboratory diagnosis of pertussis has greatly improved with increased sensitivity of detection and a shorter time to diagnosis. The introduction of nucleic acid amplification testing (NAAT), which is faster and more sensitive than culture for the detection of B. pertussis in respiratory specimens, has revolutionized the laboratory diagnosis of pertussis (2). It has also permitted new collecting devices, like flocked swabs in universal transport medium (UTM), to be considered for the collection and transport of specimens for B. pertussis testing. The nylon-flocked swab provides better entrapment during collection and better release of microorganisms than do other swabs (3, 4, 5). However, no studies evaluating the performance of flocked swabs in UTM as collecting devices in the detection of Bordetella by NAAT have been published.
(This study was presented in part at the 113th General Meeting of the American Society for Microbiology, Denver, CO, 18 to 21 May 2013.)
When PCR testing for the detection of B. pertussis in respiratory specimens was introduced in our laboratory, validation studies with spiking experiments, serial dilutions, and determinations of the lower limit of detection were performed to validate the acceptability of flocked swabs in UTM as a collection device (data not shown). To further evaluate the performance of the flocked swabs in UTM, we compared the real-time PCR positivity rate for B. pertussis between specimens collected with the BD BBL CultureSwab Plus Amies gel with charcoal and BD universal viral transport medium with flocked swabs during a pertussis epidemic to assess if one was superior to the other.
The study was initiated during an epidemic of B. pertussis in Minnesota and was conducted over a period of 6 months, from 26 June to 31 December 2012. The specimens were sent mainly from local pediatric and family practice clinics and from a university children's hospital. In general, the transportation time was <24 h. The caregivers had the choice of sending nasopharyngeal wash or nasopharyngeal swab specimens for B. pertussis testing by PCR. If they chose to send a nasopharyngeal swab specimen, they then chose one of two main collection devices, the rayon swab in Amies gel with charcoal or the flocked swab in UTM.
The BD BBL CultureSwab Plus Amies gel with a charcoal unit consists of a regular rayon wound-fiber tip on an aluminum wire shaft and a transport tube with Amies gel enriched with charcoal. The BD universal viral transport system comprises a package containing a peel pouch incorporating a sterile nylon-flocked specimen collection swab and one transport vial of 1 ml UTM with three glass beads. Occasionally, flocked swabs were received in 3-ml UTM vials. The 1-ml vial was recommended to avoid a dilution effect, and the vast majority of the flocked swabs were received in 1-ml vials.
Upon arrival in the laboratory, the specimens were processed. Each specimen collected with a rayon swab in Amies gel with charcoal had the swab tip cut and placed into a 1.5-ml microcentrifuge tube containing 200 μl of phosphate-buffered saline (PBS) solution, was left at room temperature overnight to allow bacteria to elute into the PBS solution, and then was refrigerated at 4°C to 8°C until tested. The specimens collected with the flocked swabs in UTM were kept at 4°C to 8°C until tested.
Nucleic acid was extracted from 200 μl of each of the original specimens by cell lysis, as previously described (6). B. pertussis was detected on the Cepheid SmartCycler by a real-time PCR assay developed in-house using Cepheid (Sunnyvale, CA) analyte-specific reagent primers and probes that targeted the multicopy insertion sequence IS481 of B. pertussis. Validation studies were performed before the clinical specimens were tested (6; data not shown).
From June 2012 to December 2012, the laboratory received 2,750 specimens for B. pertussis testing by PCR. The main collection device used by the health care providers was the flocked swab in UTM, with 1,566 (61.7%) specimens received, compared to the 972 (38.3%) specimens collected by rayon swab in Amies gel with charcoal. The remaining 212 specimens were collected by other methods or had no documentation. The higher rate of flocked-swab utilization is likely because clinicians were more familiar with the collection unit, as they also used it for the collection of respiratory specimens to test for respiratory viruses by rapid testing, PCR, and culture. The overall B. pertussis positivity rate was 5.8% (160/2,750). The specimens collected with the rayon swabs in Amies gel with charcoal had a slightly higher positivity rate of 6.3% (62/972), compared to 5.8% (91/1,566) for the flocked swabs in UTM (Table 1). The difference was not significant (chi-square analysis, P = 0.56). The median age of the patients with a positive result from specimens collected with the rayon swab was 12 years and for the flocked swab was 11 years. Seven of the positive specimens did not have a record of the collection device used.
TABLE 1.
B. pertussis PCR positivity rates per collecting device
| Collection method | Specimen result (no. [%]) |
|
|---|---|---|
| Negative | Positive | |
| BD BBL CultureSwab Plus Amies gel with charcoal, regular aluminum wire | 910 | 62 (6.3) |
| BD UTM with flocked swabs | 1,475 | 91 (5.8) |
| Other | 205 | 7 (3.3) |
| Total | 2,590 | 160 (5.8) |
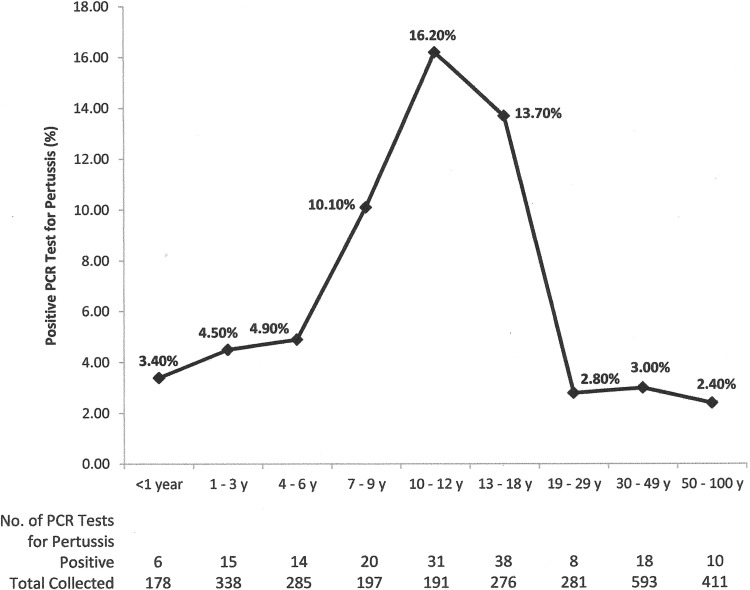
The overall mean cycle threshold (CT) value of the positive specimens collected with the rayon swabs in Amies gel with charcoal was 30.83, compared to 31.32 for specimens collected with the flocked swab in UTM (Table 2). This difference was not significant (unpaired 2-tailed t-test analysis, P = 0.55). The mean CT values of the positive specimens for each collecting device in each age group were also calculated and compared. None of them showed a statistically significant difference (Table 2). However, children 10 to 12 years of age in the group of those with specimens collected in Amies gel with charcoal had the lowest positive mean CT value (28.38) (Table 2), suggesting a higher bacterial load (7). The age groups with the highest positivity rates were preteens (16.2%) and teenagers (13.7%), which is consistent with national data on waning immunity against pertussis in previously immunized individuals (8) (Fig. 1). Another possible reason for the highest positivity rates in preteens and teenagers could be the presence of Bordetella holmesii infection or B. pertussis/B. holmesii coinfection (9–11). This possibility could not be assessed in our study, as the IS481 target does not differentiate between the two species, and B. holmesii-specific testing was not performed.
TABLE 2.
Mean cycle threshold values per age group and collection device
| Age group (yr) (no. of specimens collected) | Data for specimens collected with: |
P valueb | |||
|---|---|---|---|---|---|
| BD BBL CultureSwab Plus Amies gel with charcoal (rayon) |
BD UTM with flocked swab |
||||
| Mean CT (95% CI)a | No. of positive specimens | Mean CT (95% CI) | No. of positive specimens | ||
| <1 (178) | 24.7 (NAf) | 1 | 30.85 (±6.26) | 5 | NA |
| 1–3 (338) | 30.74 (±6.9) | 5 | 30.39 (±2.45) | 10 | 0.91 |
| 4–6 (285) | 30.81 (±4.7) | 6 | 29.78 (±3.57) | 8 | 0.73 |
| 7–9c (197) | 33.31 (±3.76) | 8 | 31.34 (±2.42) | 11 | 0.37 |
| 10–12c (191) | 28.38 (±3.33) | 12 | 31.58 (±2.29) | 18 | 0.11 |
| 13–18d (276) | 31.69 (±2.23) | 21 | 31.16 (±2.28) | 15 | 0.75 |
| 19–29 (281) | 39.1 (NA) | 1 | 32.19 (±2.32) | 7 | NA |
| 30–49e (593) | 29.59 (±4.52) | 6 | 33.29 (±3.19) | 9 | 0.19 |
| 50–100 (411) | 29.45 (±4.61) | 2 | 31.05 (±3.81) | 8 | 0.71 |
| Overall mean CT | 30.83 | 31.32 | 0.55 | ||
CT, cycle threshold; CI, confidence interval.
Rayon versus flocked swabs, by unpaired 2-tailed t-test.
One positive specimen in this age group had no record of collection device.
Two positive specimens in this age group had no record of collection device.
Three positive specimens in this age group had no record of collection device.
NA, not available.
FIG 1.
Prevalence of PCR tests positive for pertussis from June through December 2012, by age.
From the data collected in this study, it appears that the flocked swab in UTM was noninferior to the rayon swab in Amies gel with charcoal for the detection of Bordetella pertussis by PCR. Additional studies to compare the performance characteristics of the two types of transport media/swabs that would involve using both of them in the same patient (i.e., one collected from each naris) will have to be performed to confirm our results.
ACKNOWLEDGMENTS
We are grateful for the excellent technical expertise of the molecular staff in our clinical microbiology laboratory.
We have no financial disclosures or conflicts of interest to report.
Footnotes
Published ahead of print 30 April 2014
REFERENCES
- 1.Guiso N. 2009. Bordetella pertussis and pertussis vaccines. Clin. Infect. Dis. 49:1565–1569. 10.1086/644733 [DOI] [PubMed] [Google Scholar]
- 2.Dragsted DM, Dohn B, Madsen J, Jensen JS. 2004. Comparison of culture and PCR for detection of Bordetella pertussis and Bordetella parapertussis under routine laboratory conditions. J. Med. Microbiol. 53:749–754. 10.1099/jmm.0.45585-0 [DOI] [PubMed] [Google Scholar]
- 3.Daley P, Castriciano S, Chernesky M, Smieja M. 2006. Comparison of flocked and rayon swabs for collection of respiratory epithelial cells from uninfected volunteers and symptomatic patients. J. Clin. Microbiol. 44:2265–2267. 10.1128/JCM.02055-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chernesky M, Castriciano S, Jang D, Smieja M. 2006. Use of flocked swabs and a universal transport medium to enhance molecular detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J. Clin. Microbiol. 44:1084–1086. 10.1128/JCM.44.3.1084-1086.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Horn KG, Audette CD, Tucker KA, Sebeck D. 2008. Comparison of 3 swab transport systems for direct release and recovery of aerobic and anaerobic bacteria. Diagn. Microbiol. Infect. Dis. 62:471–473. 10.1016/j.diagmicrobio.2008.08.004 [DOI] [PubMed] [Google Scholar]
- 6.Arbefeville S, Levi MH, Ferrieri P. 2014. Development of a multiplex real-time PCR assay for the detection of Bordetella pertussis and Bordetella parapertussis in a single tube reaction. Microbiol. Methods 97:15–19. 10.1016/j.mimet.2013.12.003 [DOI] [PubMed] [Google Scholar]
- 7.Nakamura Y, Kamachi K, Toyoizumi-Ajisaka H, Otsuka N, Saito R, Tsuruoka J, Katsuta T, Nakajima N, Okada K, Kato T, Arakawa Y. 2011. Marked difference between adults and children in Bordetella pertussis DNA load in nasopharyngeal swabs. Clin. Microbiol. Infect. 17:365–370. 10.1111/j.1469-0691.2010.03255.x [DOI] [PubMed] [Google Scholar]
- 8.Klein NP, Bartlett J, Rowhani-Rahbar A, Fireman B, Baxter R. 2012. Waning protection after fifth dose of acellular pertussis vaccine in children. N. Engl. J. Med. 367:1012–1019. 10.1056/NEJMoa1200850 [DOI] [PubMed] [Google Scholar]
- 9.Rodgers L, Martin SW, Cohn A, Budd J, Marcon M, Terranella A, Mandal S, Salamon D, Leber A, Tondella ML, Tatti K, Spicer K, Emanuel A, Koch E, McGlone L, Pawloski L, Lemaile-Williams M, Tucker N, Iyer R, Clark TA, DiOrio M. 2013. Epidemiologic and laboratory features of a large outbreak of pertussis-like illnesses associated with cocirculating Bordetella holmesii and Bordetella pertussis—Ohio, 2010-2011. Clin. Infect. Dis. 56:322–331. 10.1093/cid/cis888 [DOI] [PubMed] [Google Scholar]
- 10.Kamiya H, Otsuka N, Ando Y, Odaira F, Yoshino S, Kawano K, Takahashi H, Nishida T, Hidaka Y, Toyoizumi-Ajisaka H, Shibayama K, Kamachi K, Sunagawa T, Taniguchi K, Okabe N. 2012. Transmission of Bordetella holmesii during pertussis outbreak, Japan. Emerg. Infect. Dis. 18:1166–1169. 10.3201/eid1807.120130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Njamkepo E, Bonacorsi S, Debruyne M, Gibaud SA, Guillot S, Nicole Guiso N. 2011. Significant finding of Bordetella holmesii DNA in nasopharyngeal samples from French patients with suspected pertussis. J. Clin. Microbiol. 49:4347–4348. 10.1128/JCM.01272-11 [DOI] [PMC free article] [PubMed] [Google Scholar]