Abstract
The ESwab system (Copan Diagnostics) was evaluated as a nasopharyngeal specimen collection device to be used for methicillin-resistant Staphylococcus aureus (MRSA) detection by the GeneXpert and BD Max MRSA assays. Different MRSA strains and dilutions of each strain were tested in triplicate. ESwabs proved to be a suitable collection system for the two assays tested.
TEXT
Methicillin-resistant Staphylococcus aureus (MRSA) is a major cause of health care-acquired infections (1, 2). Early identification of patients with MRSA nasal carriage can be part of an effective infection prevention program (3–8). Some commercial real-time PCR assays provide MRSA results in a few hours. The Xpert MRSA assay (Cepheid, Sunnyvale, CA), which runs exclusively on the GeneXpert system (Cepheid, Sunnyvale, CA), and the BD Max MRSA assay (BD Diagnostics, Quebec, Canada), which is performed on the BD Max system (BD Diagnostics, Sparks, MD), are examples of such assays (9–12). Both are sample-in/answer-out tests, providing fast results, reducing hands-on time, and improving laboratory efficiency. This is a great improvement in comparison with culture-based methods, which can take up to 72 h to identify MRSA strains (9, 10). However, PCR-based methods require concomitant cultures to recover organisms for epidemiological typing or further susceptibility testing. For these reasons, patients sometimes are subjected to more than one swab collection, with different swabs to be used in different laboratory tests.
The ESwab system (Copan Diagnostics Inc., Murrieta, CA) is a single-swab liquid-based system for collection and transport, with uniquely designed nylon-flocked swabs. With this new swab, the organism inoculum is efficiently released into 1 ml of Amies liquid, making it possible to perform multiple tests (PCR assays and cultures) with the collected sample and avoiding the collection of more than one swab per patient (13–17). The aim of this study was to evaluate and to compare the performance of the ESwab system and traditional swabs (BBL CultureSwab Liquid Stuart; BD Diagnostics, Sparks, MD), as recommended by the assay manufacturers, for the detection of MRSA using two different real-time PCR assays, i.e., the Xpert MRSA assay (Cepheid) and the BD Max MRSA assay (BD Diagnostics).
Two different MRSA strains isolated from patients at Tampa General Hospital (TGH) (Tampa, FL) were used in this study. Strains were previously characterized by strain typing at TGH, using a DiversiLab rep-PCR instrument (bioMérieux, France). Two different clusters were identified, namely, cluster E and cluster AB, both of which are frequently isolated from patients at TGH. Strains were first saved in the Esoteric Testing Laboratory bank of microorganisms and then recovered on blood agar plates (BBL) for testing.
An initial suspension of each strain at 0.5 McFarland standard (1.5 × 108 CFU/ml) was prepared in 5 ml of 0.85% physiological saline solution, followed by seven 10-fold dilutions (1.5 × 107 to 101 CFU/ml) also prepared in saline. All strains and dilutions were tested in triplicate. First, 600 μl of each dilution was distributed into six wells of a microtiter plate (100 μl/well). The ESwab and traditional swab triplicates were inoculated with 100 μl of the dilution by placing each swab in one of the six wells of the prepared microtiter plate and allowing the swab to absorb the suspension for 10 s. After inoculation, the swabs were placed in their respective transport media. Prior to testing, the ESwab tube was vortex-mixed for 5 s and a 200-μl aliquot from the transport medium was transferred either to Xpert MRSA lysis elution buffer or to BD Max MRSA sample buffer. Samples were vortex-mixed again for 5 s before being loaded onto a MRSA cartridge. ESwabs have greater absorption capacity than traditional swabs; thus, a volume >100 μl would have been used if the ESwabs had been transferred directly to the assay buffer. For this reason, use of 200-μl aliquots of the ESwab transport medium was initially chosen for this study. Traditional swabs were transferred directly into the assay buffer, and samples were vortex-mixed for 5 s before being loaded onto a MRSA cartridge. In total, 96 tests were performed for each real-time PCR assay, i.e., 48 tests using ESwabs and 48 tests using traditional swabs.
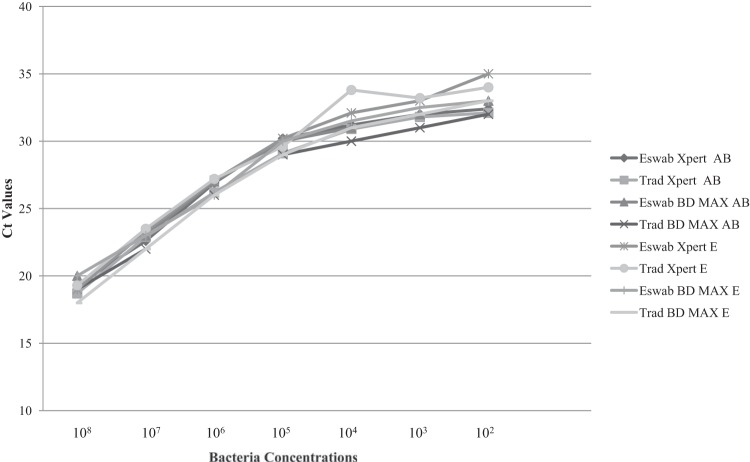
All results from dilutions of 1.5 × 108 to 102 CFU/ml were positive for MRSA, in testing of both real-time PCR assays and swab types. The real-time PCR threshold cycle (CT) values for the same dilutions but different swab types and real-time PCR assays were very similar to each other and, as expected, all CT values increased inversely proportionally to the bacterial concentration. CT values from triplicate tests were averaged, and results are presented in Fig. 1. The dilutions of 1.5 × 101 CFU/ml for cluster E and cluster AB showed positive results for the three traditional swab samples tested in the BD Max MRSA assay and for two of the three traditional swab samples tested in the Xpert MRSA assay. The same dilutions showed negative results for the three ESwab samples tested in the Xpert MRSA assay (cluster E) and for one of the three ESwab samples tested in the BD Max MRSA assay (cluster AB).
FIG 1.
Real-time PCR CT values for bacterial dilutions of 1.5 × 108 to 102 CFU/ml. Trad, traditional swabs.
ESwab transfer to the ESwab medium results in 1:10 dilution of the initial inoculum, and only one-fifth of that solution was initially used for the real-time PCR assays. Therefore, to bring the aliquot concentration to at least one-half of the original inoculum concentration, these negative-result tests were repeated using 500 μl of the ESwab liquid medium instead of 200 μl. Positive results for MRSA were detected in all of the repeated tests (Table 1). Ultimately, the limit of detection observed for ESwab samples using 500 μl of the ESwab liquid medium (1.5 × 101 CFU/ml) was in line with the analytical sensitivities of the Xpert MRSA (10 to 100 CFU/swab) and BD Max MRSA (273 to 645 CFU/swab) assays reported previously by the manufacturers (18, 19).
TABLE 1.
Real-time PCR assay CT values for ESwab samples at bacterial dilutions of 1.5 × 101 CFU/ml
| Volume used (μl) | Result (CT) for: |
|||||
|---|---|---|---|---|---|---|
| Xpert MRSA assay (cluster E) |
BD Max MRSA assay (cluster AB) |
|||||
| Sample 1 | Sample 2 | Sample 3 | Sample 1 | Sample 2 | Sample 3 | |
| 200 | Negative | Negative | Negative | 34.0 | 34.0 | Negative |
| 500 | 28.5 | 27.7 | 29.0 | 32.0 | ||
Rapid accurate identification of MRSA isolates is essential not only for patient care but also for effective infection control programs to limit the spread of MRSA (1, 4, 6, 8, 20, 21). In the past few years, several commercially available rapid tests for the detection of MRSA directly from nasal swabs have been developed for use in clinical laboratories (9–12, 20, 21). Real-Time PCR assays and other molecular tests are gaining popularity as screening tests for MRSA, especially because they are faster than culture methods in identifying patients who are candidates for contact precautions at the time of admission. Currently, there are two automated sample-in/answer-out walk-away real-time PCR assays for MRSA, i.e., the Cepheid Xpert MRSA assay performed on the GeneXpert system and the BD Max MRSA assay performed on the BD Max system. These assays are validated for use only with nasal specimens obtained with BBL CultureSwab Liquid Stuart (BD Diagnostics) or Venturi Transystem Swab Liquid Stuart (Copan Diagnostics) swabs (18, 19). This means that, if further testing of the clinical specimen (strain typing, antibiotic susceptibility testing, or simply repeating the test) is required, then a second swab from the same patient must be collected.
Several studies have demonstrated the superior absorption and releasing capacity of ESwabs, in comparison with traditional swabs (13–17, 22–24). The ESwab is a revolutionary concept because of its ability to offer what standard swabs cannot provide; ESwabs elute the entire sample into 1 ml of transport medium, providing identical aliquots of liquid sample suspension, which enables laboratories to determine and to validate the optimal specimen volumes (and therefore analyte amounts) for use in their assays. This is the first report of the use of the ESwab system as a collection system for the two sample-in/answer-out walk-away real-time PCR assays for MRSA. The results obtained showed that the ESwab system is a suitable alternative sample collection system for both the Xpert MRSA and BD Max MRSA assays. However, it is important to adjust the eluted sample volume to 500 μl in order to obtain sensitivities similar to those obtained with traditional swabs. Moreover, it is possible to perform different tests (PCR assays and cultures) with the same collected samples, avoiding collection of more than one swab sample per patient (from the same site).
Footnotes
Published ahead of print 23 April 2014
REFERENCES
- 1.Klein E, Smith DL, Laxminarayan R. 2007. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg. Infect. Dis. 13:1840–1846. 10.3201/eid1312.070629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pottinger PS. 2013. Methicillin-resistant Staphylococcus infections. Med. Clin. North Am. 97:601–609. 10.1016/j.mcna.2013.02.005 [DOI] [PubMed] [Google Scholar]
- 3.Muto CA, Jernigan JA, Ostrowsky BE, Richel HM, Jarvis WR, Boyce JM, Farr BM. 2003. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and Enterococcus. Infect. Control Hosp. Epidemiol. 24:362–386. 10.1086/502213 [DOI] [PubMed] [Google Scholar]
- 4.van Trijp MJCA, Melles DC, Hendriks DH, Parlevliet GA, Gommans M, Ott A. 2007. Successful control of widespread methicillin-resistant Staphylococcus aureus colonization and infection in a large teaching hospital in The Netherlands. Infect. Control Hosp. Epidemiol. 28:970–975. 10.1086/519210 [DOI] [PubMed] [Google Scholar]
- 5.Jog S, Cunningham R, Cooper S, Wallis M, Marchbank A, Vasco-Knight P, Jenks PJ. 2008. Impact of prospective screening for methicillin-resistant Staphylococcus aureus by real-time polymerase chain reaction in patients undergoing cardiac surgery. J. Hosp. Infect. 69:124–130. 10.1016/j.jhin.2008.02.008 [DOI] [PubMed] [Google Scholar]
- 6.Pohfahl WE, Goettler CE, Ramsey KM, Cochran MK, Nobles DL, Rotondo MF. 2009. Active surveillance screening of MRSA and eradication of the carrier state decreases surgical-site infections caused by MRSA. J. Am. Coll. Surg. 208:981–986. 10.1016/j.jamcollsurg.2008.12.025 [DOI] [PubMed] [Google Scholar]
- 7.Hardy K, Price C, Szczepura A, Gossain S, Davies R, Stallard N, Shabir S, McMurray C, Bradbury A, Hawkey PM. 2010. Reduction in the rate of methicillin-resistant Staphylococcus aureus acquisition in surgical wards by rapid screening for colonization: a prospective, cross-over study. Clin. Microbiol. Infect. 16:333–339. 10.1111/j.1469-0691.2009.02899.x [DOI] [PubMed] [Google Scholar]
- 8.Cookson B, Bonten MJM, MacKenzie FM, Skov RL, Verbrugh HA, Tacconelli E. 2011. Methicillin-resistant Staphylococcus aureus (MRSA) screening and decolonization. Int. J. Antimicrob. Agents 37:195–201. 10.1016/j.ijantimicag.2010.10.023 [DOI] [PubMed] [Google Scholar]
- 9.Rossney AS, Herra CM, Brennan DD, Morgan PM, O'Connell B. 2008. Evaluation of the Xpert methicillin-resistant Staphylococcus aureus (MRSA) assay using the Cepheid GeneXpert real-time PCR platform for rapid detection of MRSA from screening specimens. J. Clin. Microbiol. 46:3285–3290. 10.1128/JCM.02487-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolk DM, Picton E, Johnson D, Davis T, Pancholi P, Ginocchio CC, Finegold S, Welch DF, de Boer D, Fuller D, Solomon MC, Rogers B, Mehta MS, Peterson LR. 2009. Multicenter evaluation of the Cepheid Xpert methicillin-resistant Staphylococcus aureus (MRSA) test as a rapid screening method for detection of MRSA in nares. J. Clin. Microbiol. 47:758–764. 10.1128/JCM.01714-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hombach H, Pfyffer GE, Roos M, Lucke K. 2010. Detection of methicillin-resistant Staphylococcus aureus (MRSA) in specimens from various body sites: performance characteristics of BD GeneOhm MRSA assay, the Xpert MRSA assay, and broth-enriched culture in an area with a low prevalence of MRSA infections. J. Clin. Microbiol. 48:3882–3887. 10.1128/JCM.00670-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalpke AH, Hofko M, Zimmerman S. 2012. Comparison of the BD Max methicillin-resistant Staphylococcus aureus (MRSA) and the BD GeneOhm MRSA achromopeptidase assay with direct- and enriched-culture techniques using clinical specimens for detection of MRSA. J. Clin. Microbiol. 50:3365–3367. 10.1128/JCM.01496-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Human R, Jones G. 2006. A new concept for transporting clinical material on flocked swabs in liquid Amies medium, abstr C-107. Abstr. 106th Gen. Meet. Am. Soc. Microbiol. American Society for Microbiology, Washington, DC [Google Scholar]
- 14.Silbert S, Santos Pereira A, Marques da Silva F, Inoue FM, Martins Bispo PJ, Lamblet LC, Gales AC, Pignatari AC. 2007. Complete MRSA nasal screening using a single, new and novel swab transport system, abstr C-368. Abstr 107th Gen. Meet. Am. Soc. Microbiol. American Society for Microbiology, Washington, DC [Google Scholar]
- 15.Van Horn KG, Audette CD, Sebeck D, Tucker KA. 2008. Comparison of the Copan ESwab system with two Amies agar swab transport systems for maintenance of microorganism viability. J. Clin. Microbiol. 46:1655–1658. 10.1128/JCM.02047-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smismans A, Verhaegen J, Schuermans A, Frans J. 2009. Evaluation of the Copan ESwab transport system for the detection of methicillin-resistant Staphylococcus aureus: a laboratory and clinical study. Diagn. Microbiol. Infect. Dis. 65:108–111. 10.1016/j.diagmicrobio.2009.06.015 [DOI] [PubMed] [Google Scholar]
- 17.De Silva S, Wood G, Quek T, Parrott C, Bennett CM. 2010. Comparison of flocked and rayon swabs for detection of nasal carriage of Staphylococcus aureus among pathology staff members. J. Clin. Microbiol. 48:2963–2964. 10.1128/JCM.01617-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cepheid. 2008. Xpert MRSA package insert. Cepheid, Sunnyvale, CA [Google Scholar]
- 19.BD Diagnostics. 2012. BD MAX MRSA assay package insert. BD Diagnostics, Québec, Canada [Google Scholar]
- 20.Struelens MJ, Denis O. 2006. Rapid molecular detection of methicillin-resistant Staphylococcus aureus: a cost effective tool for infection control in critical care? Crit. Care 10:128–130. 10.1186/cc4855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palavecino EL. 2014. Rapid methods for detection of MRSA in clinical specimens. Methods Mol. Biol. 1085:71–83. 10.1007/978-1-62703-664-1_3 [DOI] [PubMed] [Google Scholar]
- 22.Nys S, Vijgen S, Magerman K, Cartuyvels R. 2010. Comparison of Copan eSwab with the Copan Venturi Transystem for the quantitative survival of Escherichia coli, Streptococcus agalactiae and Candida albicans. Eur. J. Clin. Microbiol. Infect. Dis. 29:453–456. 10.1007/s10096-010-0883-5 [DOI] [PubMed] [Google Scholar]
- 23.Saegeman V, Flamaing J, Muller J, Peetermans WE, Stuyck J, Verhaegen J. 2011. Clinical evaluation of the Copan ESwab for methicillin-resistant Staphylococcus aureus detection and culture of wounds. Eur. J. Clin. Microbiol. Infect. Dis. 30:943–949. 10.1007/s10096-011-1178-1 [DOI] [PubMed] [Google Scholar]
- 24.Moran-Gilad J, Schwartz D, Navon-Venezia S, Carmeli Y. 2012. Laboratory evaluation of the ESwab transport system for the recovery of carbapenem-resistant Acinetobacter baumannii. Eur. J. Clin. Microbiol. Infect. Dis. 31:1429–1433. 10.1007/s10096-011-1460-2 [DOI] [PubMed] [Google Scholar]