Abstract
A differential time to positivity (DTP) of ≥120 min is useful for diagnosing catheter-related bacteremia, but data on diagnosing catheter-related candidemia (CRC) in this way are limited. We wished to evaluate the usefulness of the DTP for diagnosing CRC. All adult patients who had the same Candida species isolated from blood cultures drawn simultaneously from a central venous catheter (CVC) and a peripheral vein were included at a tertiary care hospital over an 18-month period. A total of 105 patients with candidemia who had positive simultaneous CVC and peripheral vein blood cultures were included in our study. Sixty-one patients (58%) had CRC (47 definite and 14 probable), and 38 (36%) had candidemia from another source (non-CRC). The remaining 6 patients (6%) with indeterminate candidemia were excluded from the final analysis. The overall sensitivity and specificity of a DTP of ≥120 min for diagnosing CRC were 85% (95% confidence interval [CI], 74% to 93%) and 82% (95% CI, 66% to 92%), respectively, and for neutropenic patients, they were 75% (95% CI, 19% to 99%) and 100% (95% CI, 75% to 100%), respectively. For Candida glabrata infections, the optimal DTP cutoff was ≥6 h, with a sensitivity of 63% (95% CI, 35% to 85%) and a specificity of 75% (95% CI, 35% to 97%). In summary, DTP is useful for diagnosing CRC, and a DTP of ≥120 min appears to be the optimal cutoff except for CRC caused by C. glabrata. For neutropenic patients, DTP may be useful as an adjunct test to rule in CRC and to decide whether a catheter should be removed.
INTRODUCTION
Candidemia is a common nosocomial bloodstream infection associated with high mortality (1). Central venous catheters (CVCs) are usually present in patients with candidemia and constitute an increased risk for developing candidemia (2, 3). Catheter removal is currently recommended when the catheter is the source of candidemia (4). However, this recommendation is controversial because of the difficulty of establishing the origin of candidemia before catheter removal and because of the potential complications and increased costs of inserting new catheters. In addition, the role of catheters in neutropenic patients is less clear because the gastrointestinal tract is a likely source of candidemia in these patients. Therefore, accurate tools for diagnosing catheter-related candidemia without having to remove a catheter are required.
Although semiquantitative catheter tip culture has been regarded as the mainstay of diagnosing catheter-related bloodstream infections (5), this method requires catheter removal. Taking simultaneous quantitative blood cultures from the catheter and a peripheral vein may be useful for diagnosing catheter infection without having to remove a catheter (6, 7), but this method is labor-intensive, time-consuming, and expensive and therefore not widely used in routine clinical practice. Given the limitations of these two methods, the measurement of the differential time to positivity (DTP) between blood cultures drawn through the CVC and a peripheral vein has been used as an important diagnostic indicator. It has been reported that a DTP of ≥120 min is a sensitive and specific diagnostic marker for catheter-related bacteremia in patients with short- and long-term catheters (8, 9). However, limited data are available on the optimal cutoff value and diagnostic performance of DTP for diagnosing catheter-related infection in patients with candidemia. Therefore, we evaluated the diagnostic usefulness of the DTP for diagnosing CRC in patients with CVCs.
MATERIALS AND METHODS
Study design and setting.
This retrospective cohort study was conducted at the Asan Medical Center, Seoul, Republic of Korea. This 2,700-bed university-affiliated teaching hospital has an average of approximately 124,000 annual patient discharges. Between July 2012 and December 2013, we included all adult (≥16 years of age) patients with a CVC for whom blood cultures drawn simultaneously from the CVC and a peripheral vein grew the same Candida species. Bloodstream infections with multiple organisms were excluded from the analysis.
Definitions.
We defined the differential time to positivity (DTP) as the difference in the time to positivity (TTP) between blood cultures drawn simultaneously from the CVC and a peripheral vein. If more than one blood culture bottle grew Candida species on the same date, the bottle with the shortest TTP was chosen. Significant colonization of the catheter tip was defined as semiquantitative catheter culture by the roll-plate method in which ≥15 CFU of an organism were cultured from the catheter tip (5).
We defined four groups according to their likelihood of catheter-related candidemia (CRC). A case was defined as definite CRC if a removed catheter tip revealed the growth of ≥15 CFU of Candida species and the same Candida species was identified from the catheter tip and peripheral blood (5). A case was defined as probable CRC if, (i) in a patient receiving antifungal agents active against the Candida species recovered from the catheter, the catheter tip culture revealed growth of 1 to 14 CFU of Candida species, and the symptoms and signs of sepsis disappeared within 48 h after catheter removal, or (ii) the infection was refractory to antifungal therapy alone but improved within 48 h after catheter removal. A case was defined as non-CRC if any of the following conditions were satisfied: (i) catheter tip cultures were negative or not available and a noncatheter source of candidemia was established by microbiological culture, (ii) the catheter tip cultures made within 24 h before the start of effective antifungal therapy were negative, or (iii) the symptoms and signs of candidemia improved before or without catheter removal. Cases that did not meet any of the criteria described above were classified as indeterminate candidemia and were excluded from the final analysis.
Short-term CVCs were those with a dwell time of <30 days, and long-term CVCs were those with a dwell time of ≥30 days. Neutropenia was defined as an absolute neutrophil count of <500 cells/μl at the time of obtaining the paired blood cultures.
Microbiological tests.
Blood cultures were obtained by doctors (interns) on the basis of clinical suspicion of infection. They were instructed to draw 16 to 20 ml of blood through the CVC and aseptically inject 8 to 10 ml of the specimen into two bottles, according to the recommendation of the manufacturer (Bactec Plus Aerobic/F and Bactec Lytic/10 Anaerobic/F; Becton, Dickinson DIS, Sparks, MD, USA). All blood culture bottles were taken promptly to the microbiology laboratory and placed in an automatic culture detector (Bactec FX, Becton, Dickinson DIS). The catheters were removed at the discretion of the primary care physicians when a catheter infection was suspected. A 5-cm segment of the catheter tip was cut off and delivered to the microbiology laboratory for culture by the semiquantitative roll-plate method (5). Yeasts were identified using a Vitek 2 YST card (bioMérieux, Marcy l'Étoile, France).
Data collection.
Electronic medical records were reviewed for age, sex, underlying disease, length of hospitalization, intensive unit stay, duration of stay in the intensive unit, status of neutropenia, duration of neutropenia, recent surgery and receipt of corticosteroids, chemotherapy, parenteral nutrition, type of catheter, duration of catheter stay, and Candida species causing bloodstream infection.
Statistical analysis.
We determined the significance of the differences between the patient groups using SPSS 18.0 for Windows (SPSS, Inc., Chicago, IL). Continuous variables were compared using the Mann-Whitney U test or Student's t test, as appropriate. Categorical variables were compared using the chi-square test or Fisher's exact test, as appropriate. All tests of significance were 2-tailed, and a P value of ≤0.05 was considered to be significant. The DTPs were plotted using GraphPad Prism 5.0 for Windows (GraphPad Software, San Diego, CA, USA). The diagnostic values of the DTPs were calculated and the corresponding receiver operating characteristic (ROC) curves plotted using MedCalc 11.5 (MedCalc Software, Ostend, Belgium). The sensitivities, specificities, and likelihood ratios, along with associated 95% confidence intervals (CIs), were determined for several DTP cutoffs. We constructed a receiver operating characteristic (ROC) curve by plotting the true-positive rate (sensitivity) against the false-positive rate (1 − specificity) over a range of cutoff values for DTP. We estimated the ROC curve area using a nonparametric procedure.
RESULTS
Between July 2012 and December 2013, we analyzed 177 pairs of simultaneously drawn blood cultures that tested positive for Candida species. Of these, 33 pairs (19%) had positive CVC blood cultures and negative peripheral vein blood cultures, and 36 pairs (20%) had negative CVC blood cultures and positive peripheral vein blood cultures. Another 108 pairs of cultures (61%) had positive results on both the CVC and peripheral vein blood cultures. We excluded 3 of these pairs because bacteria and Candida grew in the same blood bottle, and the TTP for the Candida species was therefore not determined. We included in the analysis the remaining 105 pairs, which were simultaneous blood cultures that were positive for the same Candida species. Of these 105 cases, 47 were definite CRC (45%), 14 probable CRC (13%), and 38 non-CRC (36%), according to the predefined case definitions. The remaining 6 cases (6%) did not meet any criteria for CRC or non-CRC. The cultures from these 6 patients had no microbiological evidence of catheter infection and no other source of candidemia, but the patients died within 48 h after the onset of candidemia, without the catheters being removed. Excluding these 6 cases of indeterminate candidemia, 99 cases were included in the final analysis (Fig. 1).
FIG 1.

Flow diagram of blood cultures during the study period. CBC, central blood culture; PBC, percutaneous blood culture; CRC, catheter-related candidemia.
Patient characteristics.
The catheters were removed and catheter tip cultures were performed in 91 of the 99 patients (92%). In the other 8 patients (8%), the catheters were retained, and catheter tip cultures were not performed. Of the 91 patients who underwent catheter tip culture, significant colonization (≥15 CFU) by Candida species was found in 47 (51%), insignificant colonization (1 to 15 CFU) in 8 (9%), and no growth in 36 (40%).
Of the 52 patients with no growth (n = 36) or insignificant colonization (n = 8), or whose catheters were not removed (n = 8), 14 were classified as having probable CRC and 38 as having non-CRC. Of the 14 patients with probable CRC, 8 had nonsignificant catheter tip colonization (1 to 15 CFU) while receiving effective antifungal therapy, with no alternative sources of candidemia, and the symptoms and signs of catheter infection disappeared by 48 h after catheter removal. In the other six patients, the infections were refractory to antifungal therapy but improved within 48 h after catheter removal. A noncatheter source of candidemia was documented by positive culture results in 15 of the 38 patients with non-CRC, as follows: postoperative abdominal infection (n = 6), peritonitis due to bowel perforation (n = 5), urinary tract infection (n = 2), peritonitis associated with continuous ambulatory peritoneal dialysis (CAPD) (n = 1), and arteriovenous fistula infection (n = 1). Another 8 patients met the criteria of non-CRC because of negative results of the catheter tip cultures performed within 24 h after the initiation of effective antifungal therapy. The remaining 15 patients met the criteria for non-CRC because of the resolution of symptoms and signs of catheter sepsis before or without catheter removal.
Utility of DTP for diagnosing CRC.
Table 1 presents the characteristics of patients with CRC and non-CRC. Patients with non-CRC were more likely to have underlying hematologic malignant conditions, be receiving chemotherapy, and have infections due to Candida tropicalis, as well as a higher frequency and longer duration of neutropenia. In contrast, patients with CRC were more likely to a have longer intensive care unit (ICU) stay. The median TTPs in the peripheral blood according to Candida species were as follows: 17 h (interquartile range [IQR], 13 to 22) for C. tropicalis, 28 h (IQR, 21 to 33) for C. parapsilosis, 29 h (IQR, 19 to 40) for C. albicans, and 37 h (IQR, 31 to 47) for C. glabrata. The TTP for C. glabrata was significantly longer than for the other Candida species (median, 37 versus 22 h, respectively; P < 0.001). C. tropicalis was a more frequent cause of candidemia in neutropenic patients than in nonneutropenic patients (72% [12/17] versus 22% [18/82], respectively; P < 0.001). The TTP in the peripheral blood was shorter in neutropenic patients than in nonneutropenic patients (median, 19 versus 21 h, respectively; P = 0.009). We found a TTP of <55 h in the peripheral blood for diagnosing CRC to be an optimal cutoff from the ROC curve. The sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio for this cutoff were 97% (95% CI, 89 to 100), 5% (95% CI, 1 to 18), 1.02 (95% CI, 0.93 to 1.11), and 0.62 (95% CI, 0.09 to 4.24), respectively (see Table S1 in the supplemental material).
TABLE 1.
Characteristics of 99 patients with suspected catheter-related candidemia
| Characteristica | Patients with CRC (n = 61) | Patients with non-CRC (n = 38) | P value |
|---|---|---|---|
| Male sex | 30 (49) | 26 (68) | 0.06 |
| Age (median [IQR]) (yr) | 65 (54–73) | 62 (53–71) | 0.28 |
| Underlying disease | |||
| Solid tumor | 24 (40) | 16 (42) | 0.78 |
| Hematologic malignancy | 6 (10) | 13 (34) | 0.003 |
| Diabetes mellitus | 17 (28) | 7 (18) | 0.29 |
| End stage renal disease | 5 (8) | 3 (8) | >0.99 |
| Chronic liver disease | 5 (8) | 2 (5) | 0.70 |
| Chronic lung disease | 2 (3) | 3 (8) | 0.37 |
| Solid organ transplantation | 4 (7) | 2 (5) | >0.99 |
| Hematologic stem cell transplantation | 1 (2) | 2 (5) | 0.56 |
| Length of hospitalization (median [IQR]) (days) | 27 (12–54) | 18 (9–33) | 0.11 |
| ICU stay | 27 (44) | 15 (40) | 0.64 |
| Duration of stay in ICU (median [IQR]) (days) | 24 (11–38) | 10 (5–17) | 0.005 |
| Neutropeniab | 4 (7) | 13 (34) | <0.001 |
| Duration of neutropenia (median [IQR]) (days) | 6 (2–7) | 11 (7–21) | 0.047 |
| Recent surgery | 29 (48) | 13 (34) | 0.19 |
| Receipt of corticosteroids | 8 (13) | 8 (21) | 0.30 |
| Chemotherapy | 13 (21) | 18 (47) | 0.007 |
| Parenteral nutrition | 37 (61) | 22 (58) | 0.79 |
| Catheter type | |||
| Nontunneled CVC | 38 (62) | 17 (45) | 0.09 |
| Tunneled CVC | 19 (31) | 18 (47) | 0.11 |
| PICC | 4 (7) | 3 (8) | >0.99 |
| Duration of catheter placement (median [IQR]) (days) | 16 (9–35) | 13 (6–20) | 0.18 |
| Long-term catheterization (≥30 days) | 17 (28) | 6 (16) | 0.17 |
| Candida species infection | |||
| C. albicans | 22 (36) | 8 (21) | 0.11 |
| C. tropicalis | 12 (20) | 18 (47) | 0.004 |
| C. glabrata | 16 (26) | 8 (21) | 0.56 |
| C. parapsilosis | 10 (16) | 3 (8) | 0.36 |
| C. krusei | 1 (2) | 0 (0) | >0.99 |
| C. guilliermondii | 0 (0) | 1 (3) | 0.38 |
Data are the no. (%) of patients, unless otherwise indicated. IQR, interquartile range; ICU, intensive care unit; CVC, central venous catheter; PICC, peripherally inserted central venous catheter.
Defined as an absolute neutrophil count of <500 cells/μl.
Patients with non-CRC were more likely to have a shorter DTP (median, 0 h) than those with definite CRC (median, 8 h; P < 0.001) and probable CRC (median, 10 h; P < 0.001) (Fig. 2). Table 2 gives the different DTP threshold values and the corresponding diagnostic values. When we selected a DTP cutoff of ≥120 min, the accepted criterion for catheter-related bacteremia, the sensitivity and specificity for diagnosing CRC were 85% (95% CI, 74% to 93%) and 82% (95% CI, 66% to 92%), respectively. When the analysis was restricted to the 85 patients with definite CRC and non-CRC, they were 83% (95% CI, 69% to 92%) and 82% (95% CI, 66% to 92%), respectively. When the analysis was restricted to the 75 patients with non-glabrata Candida infections, the sensitivity and specificity of a DTP of ≥120 min for diagnosing CRC were 89% (95% CI, 76% to 96%) and 90% (95% CI, 73% to 98%), respectively.
FIG 2.
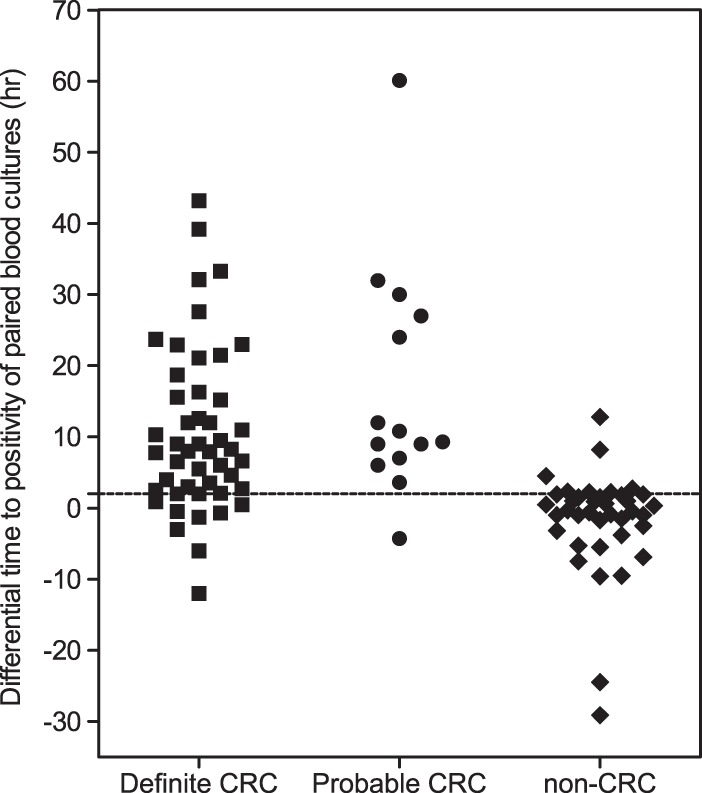
Differential time to positivity among patient populations with different likelihoods of catheter-related candidemia (CRC). The horizontal dashed line marks the 120-min cutoff.
TABLE 2.
Diagnostic utility of differential time to positivity at the different cutoff points in patients with candidemia according to diagnostic category groups and Candida species types
| Diagnosis and differential times to positivity (h) | No. with CRC status of: |
Sensitivity (% [95% CI]) | Specificity (% [95% CI]) | Positive likelihood ratio (95% CI) | Negative likelihood ratio (95% CI) | |
|---|---|---|---|---|---|---|
| Yes | No | |||||
| Definite and probable CRC (n = 61) vs non-CRC (n = 38)a | ||||||
| ≥13.0 | 19 | 0 | 32 (20–44) | 100 (91–100) | NAb | 0.69 (0.58–0.82) |
| 9.0–13.0c | 13 | 1 | 52 (39–65) | 97 (86–100) | 19.93 (2.84–139.94) | 0.49 (0.37–0.64) |
| 3.5–9.0 | 14 | 2 | 75 (63–86) | 92 (79–98) | 9.55 (3.19–28.56) | 0.27 (0.17–0.42) |
| 3.0–3.5 | 1 | 0 | 77 (65–87) | 92 (79–98) | 9.76 (3.27–29.16) | 0.25 (0.16–0.40) |
| 2.5–3.0d | 2 | 1 | 80 (68–89) | 89 (75–97) | 7.63 (3.00–19.44) | 0.22 (0.13–0.37) |
| 2.0–2.5e | 3 | 3 | 85 (74–93) | 82 (66–92) | 4.63 (2.35–9.11) | 0.18 (0.10–0.34) |
| 1.5–2.0 | 0 | 5 | 85 (74–93) | 68 (51–83) | 2.70 (1.67–4.36) | 0.22 (0.11–0.41) |
| 1.0–1.5 | 0 | 2 | 85 (74–93) | 63 (46–78) | 2.31 (1.51–3.55) | 0.23 (0.12–0.45) |
| 0.5–1.0 | 2 | 3 | 89 (78–96) | 55 (38–71) | 1.98 (1.37–2.85) | 0.21 (0.10–0.44) |
| <0.5 | 7 | 21 | NA | NA | NA | NA |
| Definite CRC (n = 47) vs non-CRC (n = 38) | ||||||
| ≥13.0 | 14 | 0 | 30 (17–45) | 100 (91–100) | NA | 0.70 (0.58–0.85) |
| 9.0–13.0c | 8 | 1 | 47 (32–62) | 97 (86–100) | 17.79 (2.51–126.01) | 0.55 (0.42–0.72) |
| 3.5–9.0 | 11 | 2 | 70 (55–83) | 92 (79–98) | 8.89 (2.95–26.77) | 0.32 (0.21–0.51) |
| 3.0–3.5 | 1 | 0 | 72 (57–84) | 92 (79–98) | 9.16 (3.05–27.54) | 0.30 (0.19–0.48) |
| 2.5–3.0d | 2 | 1 | 77 (62–88) | 90 (75–97) | 7.28 (2.84–18.63) | 0.26 (0.15–0.44) |
| 2.0–2.5e | 3 | 3 | 83 (69–92) | 82 (66–92) | 4.50 (2.28–8.90) | 0.21 (0.11–0.40) |
| 1.5–2.0 | 0 | 5 | 83 (69–92) | 68 (51–83) | 2.63 (1.62–4.27) | 0.25 (0.13–0.48) |
| 1.0–1.5 | 0 | 2 | 83 (69–92) | 63 (46–78) | 2.25 (1.46–3.48) | 0.27 (0.14–0.53) |
| 0.5–1.0 | 2 | 3 | 87 (74–95) | 55 (38–71) | 1.95 (1.35–2.82) | 0.23 (0.10–0.51) |
| <0.5 | 6 | 21 | NA | NA | NA | NA |
| Definite and probable CRC (n = 45) vs non-CRC (n = 30) in patients with non-glabrata Candida infections | ||||||
| ≥3.5 | 35 | 0 | 78 (63–89) | 100 (88–100) | NA | 0.22 (0.13–0.38) |
| 3.0–3.5 | 1 | 0 | 80 (65–90) | 100 (88–100) | NA | 0.20 (0.11–0.36) |
| 2.5–3.0c,d | 1 | 0 | 82 (68–92) | 100 (88–100) | NA | 0.18 (0.09–0.33) |
| 2.0–2.5e | 3 | 3 | 89 (76–96) | 90 (73–98) | 8.89 (3.02–26.14) | 0.12 (0.05–0.28) |
| 1.5–2.0 | 0 | 5 | 89 (76–96) | 73 (54–88) | 3.33 (1.83–6.09) | 0.15 (0.06–0.36) |
| 1.0–1.5 | 0 | 2 | 89 (76–96) | 67 (47–83) | 2.67 (1.59–4.47) | 0.17 (0.07–0.40) |
| 0.5–1.0 | 1 | 3 | 91 (79–98) | 57 (37–75) | 2.10 (1.38–3.20) | 0.16 (0.06–0.42) |
| <0.5 | 4 | 17 | NA | NA | NA | NA |
CRC, catheter-related candidemia.
NA, not available.
Area under the curve-derived cut point defining utility as a rule-in test (i.e., high specificity at the expense of suboptimal sensitivity).
Area under the curve-derived cut point by the Youden index, which allows for the selection of an optimal best-fit specificity and sensitivity cut point from the receiver operating characteristic curve.
Cut point used for diagnosing catheter-related bacteremia.
From the ROC curve, we determined that the optimal cutoff for diagnosing CRC was ≥150 min. When we used this cutoff value, the sensitivity and specificity for diagnosing CRC were 80% (95% CI, 68% to 89) and 89% (95% CI, 75% to 97%), respectively. Figure 3 shows the ROC curve, and we estimated the area under the curve to be 0.87 (95% CI, 0.79 to 0.93). In addition, we determined a clinically useful cutoff value with high specificity while sacrificing sensitivity (selection of a rule-in test). When we selected a DTP cutoff of ≥9 h, the sensitivity and specificity for diagnosing CRC were 52% (95% CI, 39% to 65%) and 97% (95% CI, 86% to 100%), respectively (Table 2). For non-glabrata Candida infection, when we selected a DTP cutoff of ≥150 min, the sensitivity and specificity for diagnosing CRC were 82% (95% CI, 68% to 92%) and 100% (95% CI, 88% to 100%), respectively, and it was a good rule-in test for diagnosing CRC (Table 2).
FIG 3.
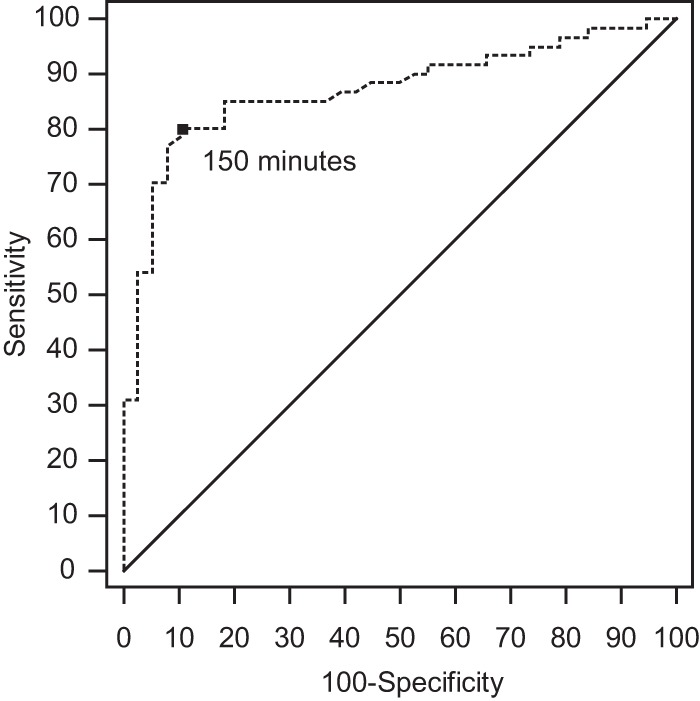
A receiver operating characteristic curve showing the accuracy of differential time to positivity as a diagnostic marker for catheter-related candidemia.
Utility of DTP for diagnosing CRC among selected subgroups of patients.
Table 3 presents the diagnostic utilities of DTPs of ≥120 min in selected subgroups of patients. Although the optimal DTP cutoff was ≥150 min, based on ROC curve analysis, we selected a cutoff of ≥120 min for diagnosing CRC; this cutoff value has been widely used for diagnosing catheter-related bacteremia, and the diagnostic performances of DTP were similar using these two cutoff values.
TABLE 3.
Utility of differential time to positivity of ≥120 min in determining catheter-related candidemia among selected subgroups of patients
| Patient status and subgroups | No. of patients with DTP of ≥120 min/no. of patients with DTP tested | Sensitivity (% [95% CI]) | No. of patients with DTP of <120 min/no. of patients with DTP tested | Specificity (% [95% CI]) | Positive likelihood ratio (95% CI) | Negative likelihood ratio (95% CI) |
|---|---|---|---|---|---|---|
| Candida species infection | ||||||
| C. albicans | 20/22 | 90 (71–99) | 7/8 | 88 (47–100) | 7.27 (1.16–45.71) | 0.10 (0.03–0.40) |
| C. tropicalis | 11/12 | 92 (62–100) | 16/18 | 89 (65–99) | 8.25 (2.21–30.81) | 0.09 (0.01–0.62) |
| C. parapsilosis | 8/10 | 80 (44–97) | 3/3 | 100 (29–100) | NCa | 0.22 (0.06–0.69) |
| C. glabrata | 10/13 | 77 (46–95) | 4/8 | 50 (16–84) | 1.54 (0.72–3.27) | 0.46 (0.14–1.55) |
| All but C. glabrata | 40/45 | 89 (76–96) | 27/30 | 90 (73–98) | 8.89 (3.02–26.14) | 0.12 (0.05–0.28) |
| All species | 52/61 | 85 (74–93) | 31/38 | 82 (66–92) | 4.63 (2.35–9.11) | 0.18 (0.10–0.34) |
| Status of neutropenia | ||||||
| Nonneutropenic | 49/57 | 86 (74–94) | 18/25 | 72 (51–88) | 3.07 (1.62–5.81) | 0.19 (0.10–0.39) |
| Neutropenic | 3/4 | 75 (19–99) | 13/13 | 100 (75–100) | NC | 0.25 (0.05–1.36) |
| Catheter duration | ||||||
| Short-term (<30 days) | 37/44 | 84 (70–93) | 25/32 | 78 (60–91) | 3.84 (1.97–7.49) | 0.20 (0.10–0.41) |
| Long-term (≥30 days) | 15/17 | 88 (64–99) | 6/6 | 100 (54–100) | NC | 0.12 (0.03–0.43) |
| Antibiotic status | ||||||
| Did not receive antifungal agents | 45/53 | 85 (72–93) | 26/32 | 81 (64–93) | 4.53 (2.18–9.40) | 0.19 (0.10–0.36) |
| Received antifungal agents | 7/8 | 88 (47–100) | 5/6 | 83 (36–100) | 5.25 (0.86–32.03) | 0.15 (0.02–0.97) |
NC, not calculated.
Because the TTP differed depending on the Candida species, we evaluated the diagnostic usefulness of a DTP of ≥120 min according to the Candida species. For C. glabrata, the sensitivity and specificity were 77% (95% CI, 46% to 95%) and 50% (95% CI, 16% to 84%), respectively. Based on the ROC curve analysis, we determined that the optimal cutoff for C. glabrata was ≥6 h. When we used this cutoff, the sensitivity and specificity were 63% (95% CI, 35% to 85%) and 75% (95% CI, 35% to 97%), respectively. For non-glabrata Candida infections, the sensitivity and specificity of a DTP of ≥120 min were 89% (95% CI, 76% to 96%) and 90% (95% CI, 73% to 98%), respectively. Based on the ROC curve analysis, we determined that the optimal cutoff for non-glabrata Candida infections was ≥150 min. The sensitivity and specificity of a DTP of ≥150 min for non-glabrata Candida infections were higher, at 82% (95% CI, 68% to 92%) and 100% (95% CI, 88% to 100%), than the sensitivity (63%) and specificity (75%) of a DTP of ≥6 h for C. glabrata (P = 0.03 and 0.04, respectively).
We evaluated the usefulness of a DTP of ≥120 min for diagnosing CRC among selected subgroups of the patients with candidemia. The sensitivity and specificity of the DTP for nonneutropenic patients were 86 (95% CI, 74% to 94%) and 72% (95% CI, 51% to 88%), respectively, and for neutropenic patients, they were 75% (95% CI, 19% to 99%) and 100% (95% CI, 75% to 100%), respectively. The sensitivity and specificity for short-term catheters (<30 days) were 84% (95% CI, 70% to 93%) and 78% (95% CI, 60% to 91%), respectively, and for long-term catheters (≥30 days), they were 88% (95% CI, 64% to 99%) and 100% (95% CI, 54% to 100%), respectively. In patients who received effective antifungal agents, they were 85% (95% CI, 72% to 93%) and 81% (95% CI, 64% to 93%), respectively, while for patients receiving effective antifungal agents when the simultaneous blood cultures were set up, they were 88% (95% CI, 47% to 100%) and 83% (95% CI, 36% to 100%), respectively.
DISCUSSION
We found that the DTP cutoff value of ≥120 min, the accepted criterion for diagnosing catheter-related bacteremia, provided the optimal values of 85% sensitivity and 82% specificity for diagnosing CRC. A recent study analyzing 24 patients with candidemia reported that the sensitivity and specificity of a DTP of ≥120 min for diagnosing CRC were 95% and 40%, respectively (10). The high sensitivity and low specificity in that study may be attributed to two factors: the small number of patients with positive simultaneous blood cultures (19 patients with CRC and 5 patients with non-CRC) and a difference in the definitions of CRC and non-CRC between that study and ours. In the study by Bouza et al. (10), patients with candidemia were classified as having CRC or non-CRC according to the results of catheter tip culture alone. However, since catheter tip culture alone is not sensitive enough for diagnosing CRC, we classified the patients on the basis of clinical features, including alternative sites of candidemia and exposure to antifungal agents, in addition to the catheter tip culture results.
When using the cutoff of 120 min, the sensitivity and specificity of DTP for C. glabrata were 77% and 50%, respectively. For other Candida species, however, the sensitivity and specificity of a DTP of ≥120 min were 89% and 90%, respectively. In this study, the TTP of C. glabrata was significantly longer than those of other Candida species, consistent with the results of previous studies (10–12). Because of the longer TTP for C. glabrata, we hypothesized that the optimal DTP cutoff for C. glabrata might be longer than that of other Candida species. We found that based on the ROC curve analysis, the optimal cutoff for C. glabrata was ≥6 h, with 63% sensitivity and 75% specificity, which was still unsatisfactory. In addition, it can be partially explained by the fact that C. glabrata displays the lowest biofilm metabolic activity compared with those of the other Candida species (13). In this context, the biologic features of C. glabrata might be different from those of other Candida species. Therefore, our findings have important implications for clinicians who encounter patients with candidemia who have CVCs. First, in settings in which C. glabrata is not prevalent, a DTP of ≥120 min is useful for diagnosing CRC, the same as for diagnosing catheter-related bacteremia. Second, in settings in which C. glabrata is prevalent, the diagnostic performance of DTP may not be satisfactory, so clinicians should interpret the DTP results in accord with the responsible Candida species.
It is generally recommended that the CVC be removed in patients with candidemia (4, 14) because of studies suggesting that CVC removal is associated with improved outcomes (15–17). However, these recommendations are controversial for neutropenic patients because the gastrointestinal tract has been reported to be an important source of candidemia in such patients (18). In a retrospective study, the CVC was identified as a source of candidemia in only 27% of neutropenic cancer patients (19). In addition, in a recent analysis of 842 adults with candidemia, early CVC removal was not associated with any clinical benefit (20). Furthermore, CVC removal in neutropenic patients often creates significant intravenous access problems. Given the complexity of CVC management in neutropenic patients, a reliable tool for diagnosing CRC is urgently needed to help clinicians decide whether to remove the CVC. Our study showed that when the DTP of ≥120 min was applied to neutropenic patients, the specificity was 100% (95% CI, 75% to 100%). Therefore, this method may be helpful to rule in CRC and to decide whether the catheter should be removed. Another consideration is that diagnostic tools sensitive enough to rule out the CVC as the source of candidemia are needed to select those patients in whom the CVC can be safely retained without compromising mycological success. We found that the sensitivity of a DTP of ≥120 min was 70% (95% CI, 19% to 99%) in neutropenic patients, but the significance of that result is limited because of the small number of CRCs in such patients, and because of the wide range of confidence intervals. Recently, peripheral blood TTP gave promising results as a tool for ruling out CRC (11). Ben-Ami et al. (11) reported that a TTP in the peripheral blood of ≥30 h ruled out the CVC as the source of candidemia (a TTP of ≤30 h displayed a sensitivity and specificity of 100% and 51%, respectively, for detecting definite CRC), but most patients in that study were nonneutropenic (11). The diagnostic performance of TTP among neutropenic patients may differ from that among nonneutropenic patients because of the higher proportion of C. tropicalis coming from the gastrointestinal tract in neutropenic patients (21) and the shorter TTP of C. tropicalis (10–12). Further studies with a large number of neutropenic patients should be performed to evaluate this issue.
The use of peripherally inserted central catheters (PICCs) has increased rapidly for several reasons, including ease of insertion, variety of uses (e.g., drug administration and venous access), perceived safety, and cost-effectiveness compared with other CVCs (22, 23). This has made it easier to pull all CVCs in patients with candidemia without having to determine whether a catheter is colonized and taking the chance of leaving the possibly infected catheter in place. Despite these benefits, PICCs are associated with a higher risk of deep vein thrombosis than are CVCs, especially in patients who are critically ill or have a malignancy (24). These complications are important because they not only complicate and interrupt treatment but also increase costs, morbidity, and mortality (25). Because candidemia frequently develops in patients who are critically ill or have a malignancy, the decision to insert PICCs in such patients should be guided by weighing the risk of thrombosis against the benefit provided by these devices. In addition, our study included a limited number of patients with PICCs, so further studies are needed to clarify this issue.
Our study has a few limitations. First, it might be argued that some patients were classified as having CRC or not having CRC on the basis of clinical features, without microbiological confirmation. However, catheter tip culture alone is not sufficiently sensitive to rule out CRC, especially in patients who have long-term CVCs and have been exposed to antibiotics. For patients with long-term CVCs, intraluminal and extraluminal spread can infect the CVCs, resulting in more frequent negative results of catheter tip cultures. In addition, microbiological confirmation of the noncatheter site is not always possible in patients with candidemia. Therefore, most studies define catheter-related bloodstream infections based on clinical background and microbiological evidence (9, 11, 26). Second, despite the careful case definitions, the source of candidemia was not determined for 6% of our cohort. These patients had no clinical evidence of CRC or non-CRC because of a rapidly fatal course, as well as no microbiological evidence of CRC or non-CRC. To avoid misclassification bias, we excluded these patients. Although this exclusion might also lead to overestimating the diagnostic performance of the DTP, there are currently no accurate diagnostic tools for classifying these patients. To overcome this limitation, more accurate diagnostic tools for diagnosing CRC without catheter removal should be used as reference standards, such as simultaneous quantitative blood cultures for catheter-related bacteremia (8). However, this method is labor-intensive, expensive, and not widely used, and the optimal criterion for diagnosing CRC by means of simultaneous quantitative blood cultures is not yet known for patients with candidemia. Third, Bouza et al. (10) reported that among candidemic patients with a CVC, at least two positive blood cultures out of three blood samples were 100% sensitive for diagnosing CRC. They proposed that when only one blood culture is positive in a candidemic patient with a CVC in whom at least three blood cultures have been obtained, the probability that the catheter is the origin of the infection is extremely low, and other sources should be investigated (10). However, we included only patients with a CVC in whom blood cultures drawn simultaneously from the CVC and a peripheral vein were positive for the same Candida species. All our patients with candidemia had at least two positive blood cultures, and no patients had one positive blood culture; therefore, we were not able evaluate the diagnostic performance of at least two positive blood cultures out of three blood cultures for diagnosing CRC. Finally, only a small number of patients were included in the subgroup analysis; this hampered the statistical analysis, making the conclusions less certain in these subgroups.
In conclusion, our results suggest that a DTP of ≥120 min is a useful diagnostic tool for evaluating patients with candidemia who have an indwelling CVC, and it may help in deciding whether to remove or retain the catheter in these patients. When the cutoff of ≥150 min was selected among patients with non-glabrata Candida infections, DTP was a good rule-in test for diagnosing CRC. Further studies are required to assess the usefulness of DTP for determining catheter removal and the effect of this decision on the outcomes of patients with candidemia.
Supplementary Material
Footnotes
Published ahead of print 14 May 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.00605-14.
REFERENCES
- 1.Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20:133–163. 10.1128/CMR.00029-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blumberg HM, Jarvis WR, Soucie JM, Edwards JE, Patterson JE, Pfaller MA, Rangel-Frausto MS, Rinaldi MG, Saiman L, Wiblin RT, Wenzel RP, National Epidemiology of Mycoses Survey (NEMIS) Study Group 2001. Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. The National Epidemiology of Mycosis Survey. Clin. Infect. Dis. 33:177–186. 10.1086/321811 [DOI] [PubMed] [Google Scholar]
- 3.Nucci M, Colombo AL, Silveira F, Richtmann R, Salomão R, Branchini ML, Spector N. 1998. Risk factors for death in patients with candidemia. Infect. Control. Hosp. Epidemiol. 19:846–850. 10.1086/647743 [DOI] [PubMed] [Google Scholar]
- 4.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK. 2009. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 49:1–45. 10.1086/599376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maki DG, Weise CE, Sarafin HW. 1977. A semiquantitative culture method for identifying intravenous-catheter-related infection. N. Engl. J. Med. 296:1305–1309. 10.1056/NEJM197706092962301 [DOI] [PubMed] [Google Scholar]
- 6.Capdevila JA, Planes AM, Palomar M, Gasser I, Almirante B, Pahissa A, Crespo E, Martinez-Vázquez JM. 1992. Value of differential quantitative blood cultures in the diagnosis of catheter-related sepsis. Eur. J. Clin. Microbiol. Infect. Dis. 11:403–407. 10.1007/BF01961854 [DOI] [PubMed] [Google Scholar]
- 7.Fan ST, Teoh-Chan CH, Lau KF. 1989. Evaluation of central venous catheter sepsis by differential quantitative blood culture. Eur. J. Clin. Microbiol. Infect. Dis. 8:142–144. 10.1007/BF01963898 [DOI] [PubMed] [Google Scholar]
- 8.Raad I, Hanna HA, Alakech B, Chatzinikolaou I, Johnson MM, Tarrand J. 2004. Differential time to positivity: a useful method for diagnosing catheter-related bloodstream infections. Ann. Intern. Med. 140:18–25. 10.7326/0003-4819-140-1-200401060-00007 [DOI] [PubMed] [Google Scholar]
- 9.Blot F, Nitenberg G, Chachaty E, Raynard B, Germann N, Antoun S, Laplanche A, Brun-Buisson C, Tancrède C. 1999. Diagnosis of catheter-related bacteraemia: a prospective comparison of the time to positivity of hub-blood versus peripheral-blood cultures. Lancet 354:1071–1077. 10.1016/S0140-6736(98)11134-0 [DOI] [PubMed] [Google Scholar]
- 10.Bouza E, Alcalá L, Muñoz P, Martín-Rabadán P, Guembe M, Rodríguez-Créixems M, GEIDI and the COMIC Study Groups 2013. Can microbiologists help to assess catheter involvement in candidaemic patients before removal? Clin. Microbiol. Infect. 19:E129–E135. 10.1111/1469-0691.12096 [DOI] [PubMed] [Google Scholar]
- 11.Ben-Ami R, Weinberger M, Orni-Wasserlauff R, Schwartz D, Itzhaki A, Lazarovitch T, Bash E, Aharoni Y, Moroz I, Giladi M. 2008. Time to blood culture positivity as a marker for catheter-related candidemia. J. Clin. Microbiol. 46:2222–2226. 10.1128/JCM.00214-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horvath LL, Hospenthal DR, Murray CK, Dooley DP. 2003. Detection of simulated candidemia by the Bactec 9240 system with Plus Aerobic/F and Anaerobic/F blood culture bottles. J. Clin. Microbiol. 41:4714–4717. 10.1128/JCM.41.10.4714-4717.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodrigues CF, Silva S, Henriques M. 2014. Candida glabrata: a review of its features and resistance. Eur. J. Clin. Microbiol. Infect. Dis. 33:673–688. 10.1007/s10096-013-2009-3 [DOI] [PubMed] [Google Scholar]
- 14.Pappas PG, Kauffman CA, Andes D, Benjamin DK, Jr, Calandra TF, Edwards JE, Jr, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD, Infectious Disease Society of America 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 48:503–535. 10.1086/596757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luzzati R, Amalfitano G, Lazzarini L, Soldani F, Bellino S, Solbiati M, Danzi MC, Vento S, Todeschini G, Vivenza C, Concia E. 2000. Nosocomial candidemia in non-neutropenic patients at an Italian tertiary care hospital. Eur. J. Clin. Microbiol. Infect. Dis. 19:602–607. 10.1007/s100960000325 [DOI] [PubMed] [Google Scholar]
- 16.Rex JH, Bennett JE, Sugar AM, Pappas PG, Serody J, Edwards JE, Washburn RG. 1995. Intravascular catheter exchange and duration of candidemia. NIAID Mycoses Study Group and the Candidemia Study Group. Clin. Infect. Dis. 21:994–996 [DOI] [PubMed] [Google Scholar]
- 17.Rex JH, Pappas PG, Karchmer AW, Sobel J, Edwards JE, Hadley S, Brass C, Vazquez JA, Chapman SW, Horowitz HW, Zervos M, McKinsey D, Lee J, Babinchak T, Bradsher RW, Cleary JD, Cohen DM, Danziger L, Goldman M, Goodman J, Hilton E, Hyslop NE, Kett DH, Lutz J, Rubin RH, Scheld WM, Schuster M, Simmons B, Stein DK, Washburn RG, Mautner L, Chu TC, Panzer H, Rosenstein RB, Booth J, National Institute of Allergy and Infectious Diseases Mycoses Study Group 2003. A randomized and blinded multicenter trial of high-dose fluconazole plus placebo versus fluconazole plus amphotericin B as therapy for candidemia and its consequences in nonneutropenic subjects. Clin. Infect. Dis. 36:1221–1228. 10.1086/374850 [DOI] [PubMed] [Google Scholar]
- 18.Nucci M, Anaissie E. 2001. Revisiting the source of candidemia: skin or gut? Clin. Infect. Dis. 33:1959–1967. 10.1086/323759 [DOI] [PubMed] [Google Scholar]
- 19.Raad I, Hanna H, Boktour M, Girgawy E, Danawi H, Mardani M, Kontoyiannis D, Darouiche R, Hachem R, Bodey GP. 2004. Management of central venous catheters in patients with cancer and candidemia. Clin. Infect. Dis. 38:1119–1127. 10.1086/382874 [DOI] [PubMed] [Google Scholar]
- 20.Nucci M, Anaissie E, Betts RF, Dupont BF, Wu C, Buell DN, Kovanda L, Lortholary O. 2010. Early removal of central venous catheter in patients with candidemia does not improve outcome: analysis of 842 patients from 2 randomized clinical trials. Clin. Infect. Dis. 51:295–303. 10.1086/653935 [DOI] [PubMed] [Google Scholar]
- 21.Kontoyiannis DP, Vaziri I, Hanna HA, Boktour M, Thornby J, Hachem R, Bodey GP, Raad II. 2001. Risk factors for Candida tropicalis fungemia in patients with cancer. Clin. Infect. Dis. 33:1676–1681. 10.1086/323812 [DOI] [PubMed] [Google Scholar]
- 22.Tejedor SC, Tong D, Stein J, Payne C, Dressler D, Xue W, Steinberg JP. 2012. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter.” Infect. Control. Hosp. Epidemiol. 33:50–57. 10.1086/663645 [DOI] [PubMed] [Google Scholar]
- 23.Periard D, Monney P, Waeber G, Zurkinden C, Mazzolai L, Hayoz D, Doenz F, Zanetti G, Wasserfallen JB, Denys A. 2008. Randomized controlled trial of peripherally inserted central catheters vs. peripheral catheters for middle duration in-hospital intravenous therapy. J. Thromb. Haemost. 6:1281–1288. 10.1111/j.1538-7836.2008.03053.x [DOI] [PubMed] [Google Scholar]
- 24.Chopra V, Anand S, Hickner A, Buist M, Rogers MA, Saint S, Flanders SA. 2013. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet 382:311–325. 10.1016/S0140-6736(13)60592-9 [DOI] [PubMed] [Google Scholar]
- 25.Chopra V, Anand S, Krein SL, Chenoweth C, Saint S. 2012. Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am. J. Med. 125:733–741. 10.1016/j.amjmed.2012.04.010 [DOI] [PubMed] [Google Scholar]
- 26.Seifert H, Cornely O, Seggewiss K, Decker M, Stefanik D, Wisplinghoff H, Fätkenheuer G. 2003. Bloodstream infection in neutropenic cancer patients related to short-term nontunnelled catheters determined by quantitative blood cultures, differential time to positivity, and molecular epidemiological typing with pulsed-field gel electrophoresis. J. Clin. Microbiol. 41:118–123. 10.1128/JCM.41.1.118-123.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


